Medical Device-Associated Infections Caused by Biofilm-Forming Microbial Pathogens and Controlling Strategies
Abstract
:1. Introduction
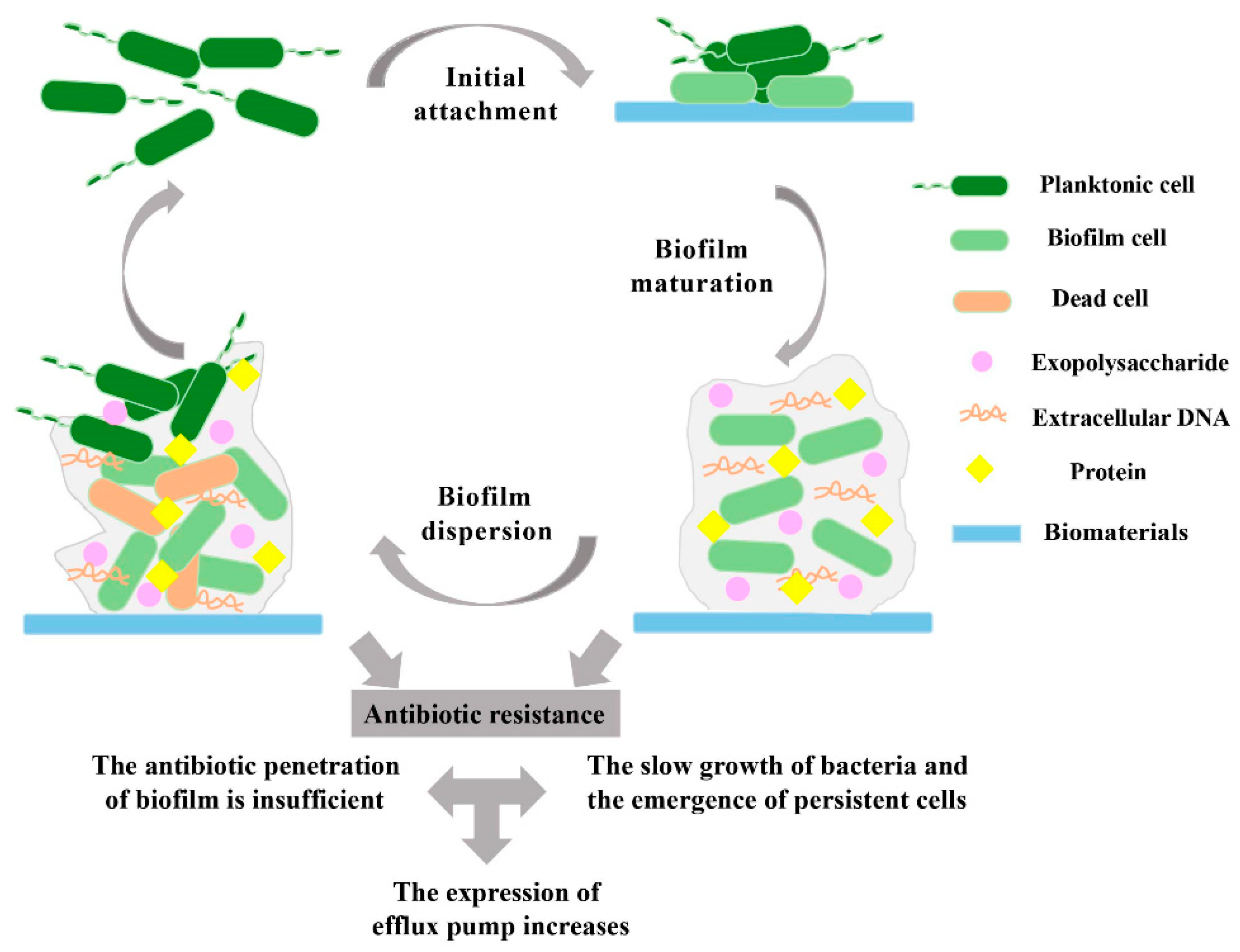
2. Role of Biofilms in HAIs
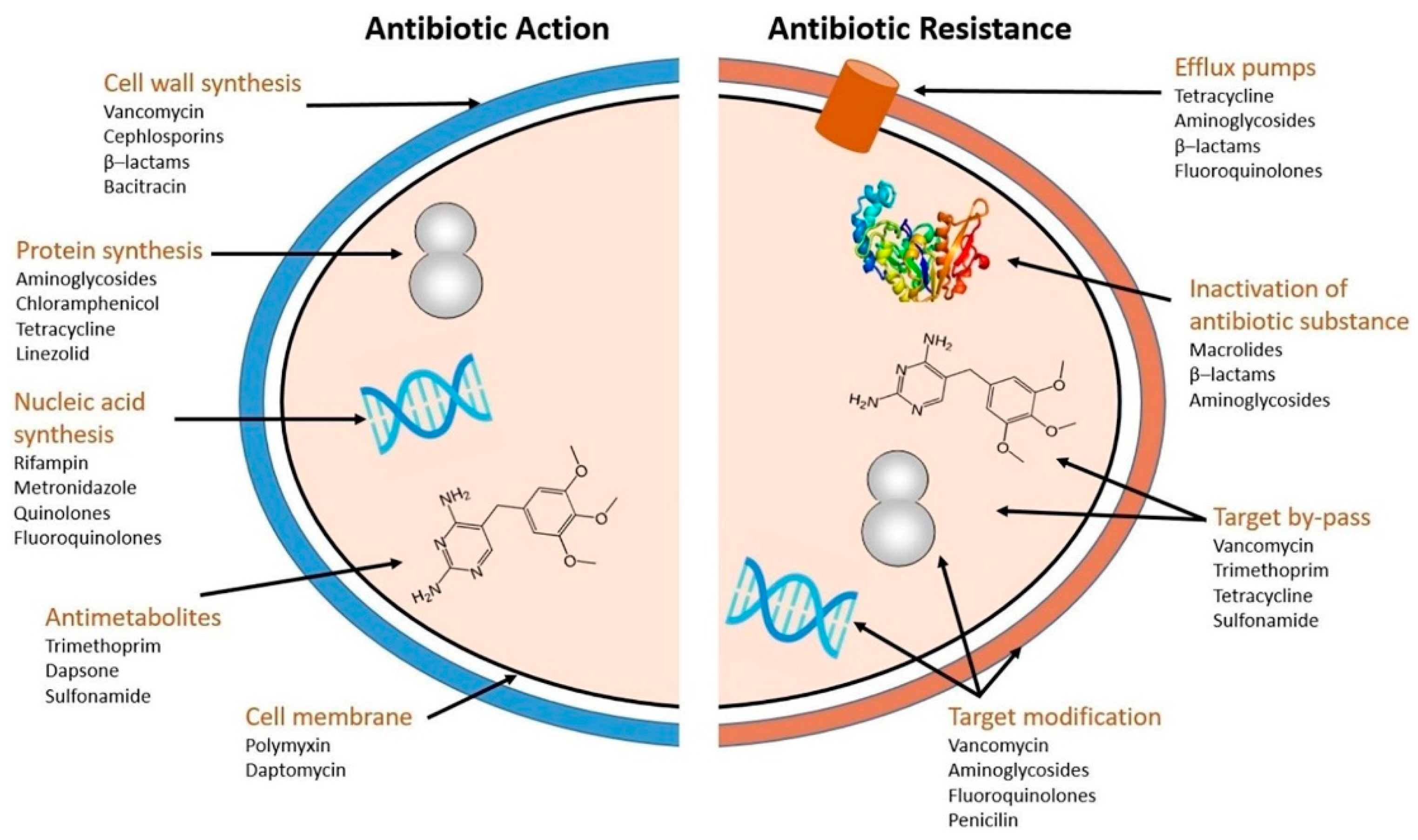
3. Pathogenesis of Biofilm-forming Microbes
| Medical Device | Common Pathogen | References |
|---|---|---|
| Orthopedic devices | Staphylococci, Gram-negative bacilli | [49] |
| Endotracheal tubes | Pseudomonas aeruginosa, Staphylococcus aureus | [50] |
| Contact lenses | Pseudomonas aeruginosa, Staphylococci | [51] |
| Intravascular catheters | Staphylococci, Enterococci, Gram-negative bacilli | [52] |
| Valves, pacemaker | Staphylococci, Streptococci | [53] |
| Respiratory equipment, Indwelling catheters | Acinetobacter baumanii | [54] |
| Bronchoscopy | Klebsiella pneumoniae | [55] |
| UTIs devices, Intravascular medical device | Enterobacter spp. | [56] |
| Respiratory device | Aspergillus fumigatus | [57] |
| Cardiac medical device | Cryptococcus neoformans | [58] |
| Urinary catheters | Enterococcus faecalis | [59] |
| Catheters, prosthetic devices | Aspergillus fumigatus | [60] |
| Central venous catheter | Staphylococcus epidermidis | [31] |
| Cerebrospinal shunts | Staphylococcus aureus, Propionibacterium | [61] |
| Dental implants | Prevotella intermedia, Actinobacillus | [62] |
4. Establishment of the Biofilm on Biomedical Device Surfaces
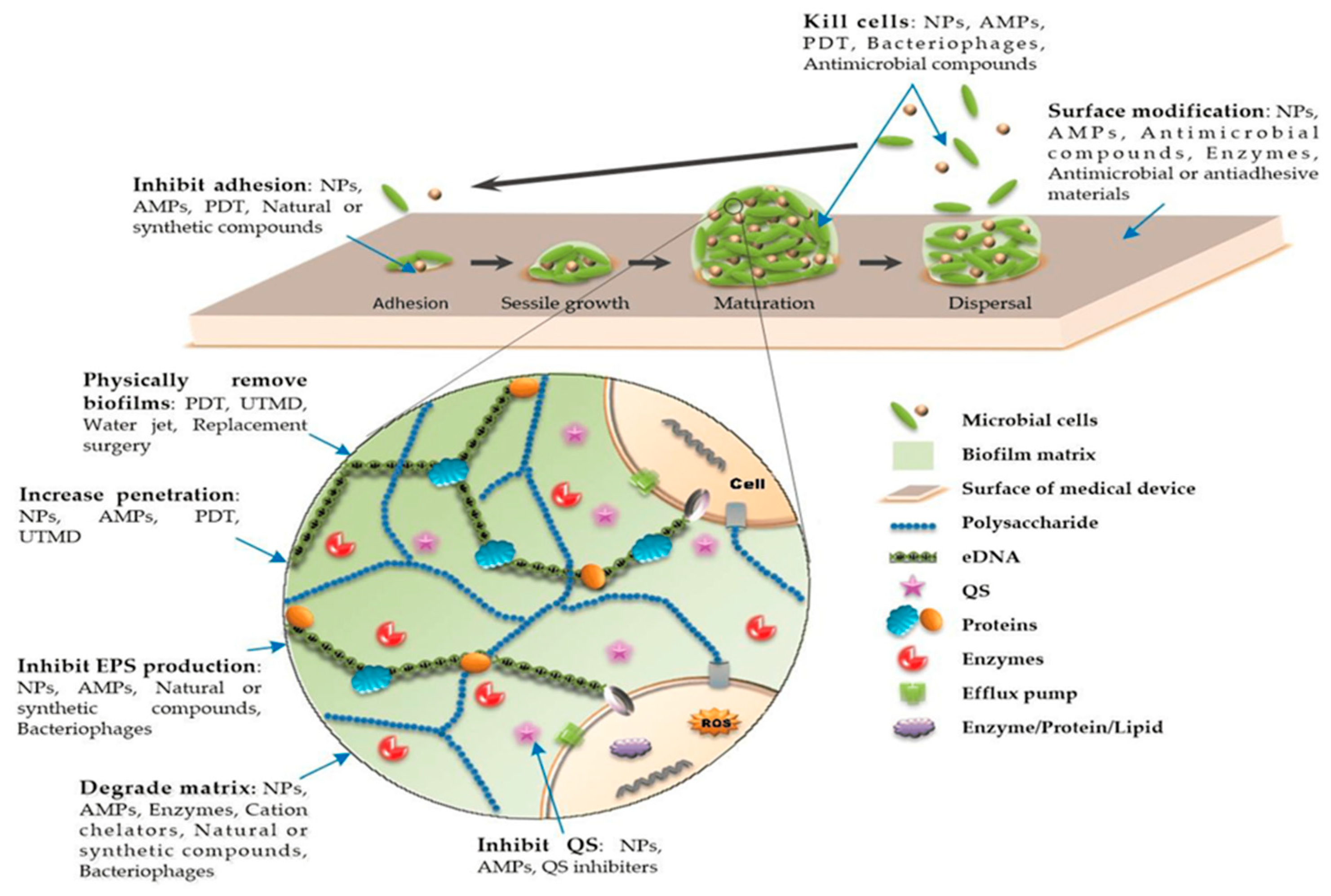
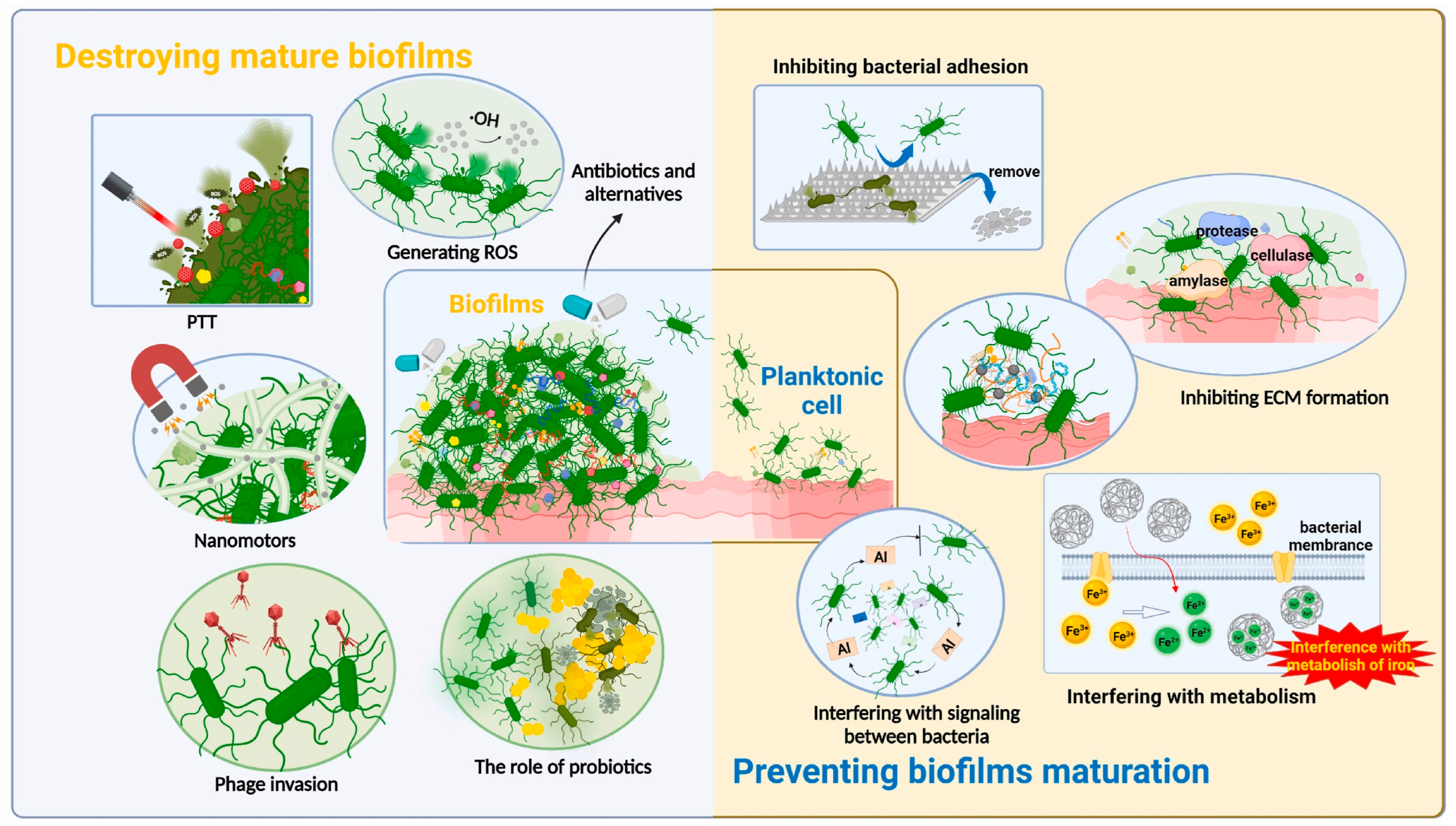
5. Recent Advancements in Antiadhesive or Antifouling Coating on the Surface of Biomedical Devices
| Name of Biomedical Devices | Antibacterial Materials | Antiadhesive or Antibiofilm Coating Agents | Types of Pathogens | Efficacy (Killing of Attached Cells in %) | References |
|---|---|---|---|---|---|
| Polydimethylsiloxane (PDMS), stainless steel (SS) surface | Antibacterial surfaces that are smart and photothermally activated | Tannic acid(TA) and Fe3+ ion | Escherichia coli | >99 | [96] |
| Blood-contacting medical devices | metal–phenolic and catecholamine | CuII–GA/CySA coatings copper ions (CuII)-gallic acid (GA)/ cystamine (CySA) | Escherichia coli, Staphylococcus aureus | ∼99 | [97] |
| Surfaces made of a variety of materials, including silicone, glass, poly(methyl methacrylate) (PMMA) plates, metal surfaces, polypropylene fiber, and filter paper | Electrostatic attraction, physically rupturing cell walls | Coating with positively-charged Zeolitic imidazolate frameworks(ZIF) nano-dagger arrays | Staphylococcus aureus | NA | [98] |
| Silicon (Si), PDMS, and SS | Gold nanoparticle layer(GNPL) | Regenerable smart antibacterial surfaces | Escherichia coli | >99 | [99] |
| Implantable device | Hydroxyethyl methacrylate(HEMA) and quercetin(Qe) | Dual-functional anti-biofilms surface | Pseudomonas aeruginosa, Staphylococcus aureus | NA | [100] |
| Metal materials surfaces | Poly (2-hydroxyethyl methacrylate) hydrophilic polymer with Quaternary ammonium salt(QAS) | Intelligent composite material surface with titanium content | Staphylococcus aureus, Escherichia coli | 99.86 and 97.08 | [101] |
| Biomedical catheters | Sulfamethoxazole (SMZ) and trimethoprim (TMP) | Polyethylene glycol(PEG) | Staphylococcus aureus, Escherichia coli | 80 (E. coli and S. aureus cells) | [102] |
| Urinary catheter | Poly(styrene sulfonate) (PSS), quaternary ammonium, H2O2 enzyme | New zwitterionic copolymers (PTMAEMA-co-PSPE) with different proportions of sulfobetaine | Staphylococcus aureus | 60 | [103] |
| Foley catheters | α-aminoisobutyric acid | Biocompatible amino acids | Escherichia coli, Bacillus subtilis | NA | [104] |
| Catheters, stents, and dialysis equipment | Silver nanoparticles | 2-Methacryloyloxyethyl phosphorylcholine | Escherichia coli, Escherichia coli K-12 | >99 | [105] |
| Dental implant | Quaternized polyethyleneimine | poly(glycidyl methacrylate) | Staphylococcus aureus | 95.6 | [106] |
| Implant medical devices | Gold nanoparticles | built a mixed-metal-organic network nanocluster | Escherichia coli | NA | [107] |
| Soft contact lens | Polyelectrolytes | Diclofenac sodium salt, doxifloxacin hydrochloride, and chlorhexidine diacetate monohydrate | Pseudomonas aeruginosa, Staphylococcus aureus | NA | [108] |
| Dental implants | Zinc (Zn) nanoparticles Electrohydrodynamic deposition | Nanoparticles of hydroxyapatite (nHA) and zinc oxide (nZnO) | Streptococcus spp. | NA | [109] |
| Sinus Stents | Ciprofloxacin nanoparticle | Poly-l-lactic acid (PLLA), ciprofloxacin | Pseudomonas aeruginosa | NA | [110] |
| Bone implant devices | Combined vancomycin and Melittin | Chitosan, bioactive glass, and melittin | Staphylococcus aureus | NA | [111] |
| Orthopedic implants | Liposome-encapsulated photosensitizers (PS), IR780, and perfluorohexane (PFH), | Lecithin, cholesterol and PEGylated DSPE | Escherichia coli, Staphylococcus aureus | 99.62 and 99.63 | [112] |
| Subcutaneous implants | Nitric oxide (NO) | NO-releasing xerogel coatings of silicone rubber | Staphylococcus aureus | 82 | [113] |
| Biomedical Implants | Black phosphorus | Black phosphorus nanosheets with N,N′- dimethyl propylene urea (DMPU) | Bacillus subtilis | 99.69 | [114] |
| Implantable medical devices | Titanium, titanium binding peptides (TiBP) | Chimeric peptide (TiBP(S)1–3 and E14LKK/H14LKK motifs.) | Streptococcus mutans, Staphylococcus epidermidis, Escherichia coli | NA | [115] |
| Biomedical implants | Nanoparticles of (silver and zinc pyrithione, ZnP) | Silver-based organomodified layered silicate additives nanoclays | Staphylococcus aureus | NA | [116] |
6. Conclusions and Future Perspectives
- Early biofilm detection will undoubtedly aid patient treatment and reduce costs. However, this is only possible if detection procedures and techniques are continuously improved, which might be accomplished with the use of artificial intelligence tools [117].
- Mixed-species biofilms exhibit notable differences in growth rate, gene expression, living habits, and structural appearance compared with those of single species. These differences are primarily manifested in enhanced biofilm metabolic capacity, resilience to environmental stress, and community-level signaling. Further studies on mixed-species biofilms are required [118].
- Mixed-species biofilms predominate in nature and are common in human hosts, such as the lungs and oral cavities of individuals with cystic fibrosis. Therefore, further studies are required to define the interactions within multispecies biofilms and the consequences of these interactions on biofilm community growth, makeup, and longevity [119].
- An antibiofilm or antiadhesive coating could be developed to prevent biofilm formation that works upon three lines of defense with antiadhesive, bactericidal, and anti-quorum sensing properties, adapting to the bacterial biofilm formation mechanism. Further research is required on such antibiofilm coatings [120].
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Solis-Velazquez, O.A.; Gutiérrez-Lomelí, M.; Guerreo-Medina, P.J.; Rosas-García, M.D.L.; Iñiguez-Moreno, M.; Avila-Novoa, M.G. Nosocomial pathogen biofilms on biomaterials: Different growth medium conditions and components of biofilms produced in vitro. J. Microbiol. Immunol. Infect. 2021, 54, 1038–1047. [Google Scholar] [CrossRef] [PubMed]
- Devanga Ragupathi, N.K.; Veeraraghavan, B.; Karunakaran, E.; Monk, P.N. Editorial: Biofilm-mediated nosocomial infections and its association with antimicrobial resistance: Detection, prevention, and management. Front. Med. 2022, 9, 987011. [Google Scholar] [CrossRef] [PubMed]
- Walter, J.; Haller, S.; Quinten, C.; Kärki, T.; Zacher, B.; Eckmanns, T.; Abu Sin, M.; Plachouras, D.; Kinross, P.; Suetens, C. Healthcare-associated pneumonia in acute care hospitals in European Union/European Economic Area countries: An analysis of data from a point prevalence survey, 2011 to 2012. Eurosurveillance 2018, 23, 1700843. [Google Scholar] [CrossRef] [PubMed]
- Dadi, N.C.T.; Radochová, B.; Vargová, J.; Bujdáková, H. Impact of Healthcare-Associated Infections Connected to Medical Devices-An Update. Microorganisms 2021, 9, 2332. [Google Scholar] [CrossRef] [PubMed]
- Boev, C.; Kiss, E. Hospital-Acquired Infections. Crit. Care Nurs. Clin. N. Am. 2017, 29, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.-C.; Chung, W.-S.; Lo, Y.-C.; Shih, W.-H.; Chou, C.-H.; Chen, C.-Y.; Tu, C.-Y.; Ho, M.-W. Changing epidemiology and prognosis of nosocomial bloodstream infection: A single-center retrospective study in Taiwan. J. Microbiol. Immunol. Infect. 2022, 55, 1293–1300. [Google Scholar] [CrossRef]
- Lemiech-Mirowska, E.; Kiersnowska, Z.; Michałkiewicz, M.; Depta, A.; Marczak, M. Nosocomial infections as one of the most important problems of healthcare system. Ann. Agric. Environ. Med. 2021, 28, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Di Franco, S.; Alfieri, A.; Pace, M.C.; Sansone, P.; Pota, V.; Fittipaldi, C.; Fiore, M.; Passavanti, M.B. Blood Stream Infections from MDR Bacteria. Life 2021, 11, 575. [Google Scholar] [CrossRef]
- Elliott, T.S.; Moss, H.A.; Tebbs, S.E.; Wilson, I.C.; Bonser, R.S.; Graham, T.R.; Burke, L.P.; Faroqui, M.H. Novel approach to investigate a source of microbial contamination of central venous catheters. Eur. J. Clin. Microbiol. Infect. Dis. 1997, 16, 210–213. [Google Scholar] [CrossRef]
- Pandey, V.K.; Srivastava, K.R.; Ajmal, G.; Thakur, V.K.; Gupta, V.K.; Upadhyay, S.N.; Mishra, P.K. Differential Susceptibility of Catheter Biomaterials to Biofilm-Associated Infections and Their Remedy by Drug-Encapsulated Eudragit RL100 Nanoparticles. Int. J. Mol. Sci. 2019, 20, 5110. [Google Scholar] [CrossRef]
- Sharma, S.; Mohler, J.; Mahajan, S.D.; Schwartz, S.A.; Bruggemann, L.; Aalinkeel, R. Microbial Biofilm: A Review on Formation, Infection, Antibiotic Resistance, Control Measures, and Innovative Treatment. Microorganisms 2023, 11, 1614. [Google Scholar] [CrossRef]
- Papenfort, K.; Bassler, B.L. Quorum sensing signal-response systems in Gram-negative bacteria. Nat. Rev. Microbiol. 2016, 14, 576–588. [Google Scholar] [CrossRef]
- Khan, F.; Javaid, A.; Kim, Y.M. Functional Diversity of Quorum Sensing Receptors in Pathogenic Bacteria: Interspecies, Intraspecies and Interkingdom Level. Curr. Drug Targets 2019, 20, 655–667. [Google Scholar] [CrossRef]
- Zaitseva, Y.V.; Koksharova, O.A.; Lipasova, V.A.; Plyuta, V.A.; Demidyuk, I.V.; Chernin, L.S.; Khmel, I.A. SprI/SprR Quorum Sensing System of Serratia proteamaculans 94. Biomed. Res. Int. 2019, 2019, 3865780. [Google Scholar] [CrossRef]
- Oppenheimer-Shaanan, Y.; Steinberg, N.; Kolodkin-Gal, I. Small molecules are natural triggers for the disassembly of biofilms. Trends Microbiol. 2013, 21, 594–601. [Google Scholar] [CrossRef]
- Gupta, P.; Sarkar, S.; Das, B.; Bhattacharjee, S.; Tribedi, P. Biofilm, pathogenesis and prevention—A journey to break the wall: A review. Arch. Microbiol. 2016, 198, 1–15. [Google Scholar] [CrossRef]
- Bjarnsholt, T. The role of bacterial biofilms in chronic infections. APMIS 2013, 121, 1–58. [Google Scholar] [CrossRef]
- Joo, H.-S.; Otto, M. Molecular Basis of In Vivo Biofilm Formation by Bacterial Pathogens. Chem. Biol. 2012, 19, 1503–1513. [Google Scholar] [CrossRef]
- Li, P.; Yin, R.; Cheng, J.; Lin, J. Bacterial Biofilm Formation on Biomaterials and Approaches to Its Treatment and Prevention. Int. J. Mol. Sci. 2023, 24, 11680. [Google Scholar] [CrossRef]
- Zheng, Y.; He, L.; Asiamah, T.K.; Otto, M. Colonization of medical devices by staphylococci. Environ. Microbiol. 2018, 20, 3141–3153. [Google Scholar] [CrossRef]
- Mack, D.; Rohde, H.; Harris, L.G.; Davies, A.P.; Horstkotte, M.A.; Knobloch, J.K. Biofilm formation in medical device-related infection. Int. J. Artif. Organs 2006, 29, 343–359. [Google Scholar] [CrossRef] [PubMed]
- Veerachamy, S.; Yarlagadda, T.; Manivasagam, G.; Yarlagadda, P.K. Bacterial adherence and biofilm formation on medical implants: A review. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2014, 228, 1083–1099. [Google Scholar] [CrossRef] [PubMed]
- Vertes, A.; Hitchins, V.; Phillips, K.S. Analytical Challenges of Microbial Biofilms on Medical Devices. Anal. Chem. 2012, 84, 3858–3866. [Google Scholar] [CrossRef]
- Wu, H.; Moser, C.; Wang, H.-Z.; Høiby, N.; Song, Z.-J. Strategies for combating bacterial biofilm infections. Int. J. Oral Sci. 2015, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lebeaux, D.; Ghigo, J.-M.; Beloin, C. Biofilm-Related Infections: Bridging the Gap between Clinical Management and Fundamental Aspects of Recalcitrance toward Antibiotics. Microbiol. Mol. Biol. Rev. 2014, 78, 510–543. [Google Scholar] [CrossRef] [PubMed]
- Khan, J.; Tarar, S.M.; Gul, I.; Nawaz, U.; Arshad, M. Challenges of antibiotic resistance biofilms and potential combating strategies: A review. 3 Biotech 2021, 11, 169. [Google Scholar] [CrossRef] [PubMed]
- Uluseker, C.; Kaster, K.M.; Thorsen, K.; Basiry, D.; Shobana, S.; Jain, M.; Kumar, G.; Kommedal, R.; Pala-Ozkok, I. A Review on Occurrence and Spread of Antibiotic Resistance in Wastewaters and in Wastewater Treatment Plants: Mechanisms and Perspectives. Front. Microbiol. 2021, 12, 717809. [Google Scholar] [CrossRef]
- Anderson, G.G.; O’Toole, G.A. Innate and induced resistance mechanisms of bacterial biofilms. Curr. Top. Microbiol. Immunol. 2008, 322, 85–105. [Google Scholar] [CrossRef] [PubMed]
- Mah, T.F. Biofilm-specific antibiotic resistance. Future Microbiol. 2012, 7, 1061–1072. [Google Scholar] [CrossRef]
- Coenye, T.; Bové, M.; Bjarnsholt, T. Biofilm antimicrobial susceptibility through an experimental evolutionary lens. Npj Biofilms Microbiomes 2022, 8, 82. [Google Scholar] [CrossRef]
- Khatoon, Z.; McTiernan, C.D.; Suuronen, E.J.; Mah, T.-F.; Alarcon, E.I. Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon 2018, 4, e01067. [Google Scholar] [CrossRef] [PubMed]
- Zhao, A.; Sun, J.; Liu, Y. Understanding bacterial biofilms: From definition to treatment strategies. Front. Cell. Infect. Microbiol. 2023, 13, 1137947. [Google Scholar] [CrossRef] [PubMed]
- Donlan, R. Biofilms and Device-Associated Infections. Emerg. Infect. Dis. 2001, 7, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Carniello, V.; Peterson, B.W.; van der Mei, H.C.; Busscher, H.J. Physico-chemistry from initial bacterial adhesion to surface-programmed biofilm growth. Adv. Colloid Interface Sci. 2018, 261, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Werneburg, G.T. Catheter-Associated Urinary Tract Infections: Current Challenges and Future Prospects. Res. Rep. Urol. 2022, 14, 109–133. [Google Scholar] [CrossRef]
- Roberts, C.G. The role of biofilms in reprocessing medical devices. Am. J. Infect. Control 2013, 41, S77–S80. [Google Scholar] [CrossRef]
- Di Domenico, E.G.; Oliva, A.; Guembe, M. The Current Knowledge on the Pathogenesis of Tissue and Medical Device-Related Biofilm Infections. Microorganisms 2022, 10, 1259. [Google Scholar] [CrossRef]
- Weinstein, R.A.; Darouiche, R.O. Device-Associated Infections: A Macroproblem That Starts with Microadherence. Clin. Infect. Dis. 2001, 33, 1567–1572. [Google Scholar] [CrossRef]
- Perry, E.K.; Tan, M.W. Bacterial biofilms in the human body: Prevalence and impacts on health and disease. Front. Cell Infect Microbiol. 2023, 13, 1237164. [Google Scholar] [CrossRef]
- Dewasthale, S.; Mani, I.; Vasdev, K. Microbial biofilm: Current challenges in health care industry. J. Appl. Biotechnol. Bioeng. 2018, 5, 160–164. [Google Scholar] [CrossRef]
- Mirzaei, R.; Mohammadzadeh, R.; Alikhani, M.Y.; Shokri Moghadam, M.; Karampoor, S.; Kazemi, S.; Barfipoursalar, A.; Yousefimashouf, R. The biofilm-associated bacterial infections unrelated to indwelling devices. IUBMB Life 2020, 72, 1271–1285. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Yuan, Z.; He, X.; Du, Z.; Wang, Y. The impact of negative pressure wound therapy on surgical wound infection, hospital stay and postoperative complications after spinal surgery: A meta-analysis. Int. Wound J. 2024, 21, e14378. [Google Scholar] [CrossRef]
- Zimmerli, W.; Moser, C. Pathogenesis and treatment concepts of orthopaedic biofilm infections. FEMS Immunol. Med. Microbiol. 2012, 65, 158–168. [Google Scholar] [CrossRef]
- Lerche, C.J.; Schwartz, F.; Theut, M.; Fosbøl, E.L.; Iversen, K.; Bundgaard, H.; Høiby, N.; Moser, C. Anti-biofilm Approach in Infective Endocarditis Exposes New Treatment Strategies for Improved Outcome. Front. Cell Dev. Biol. 2021, 9, 643335. [Google Scholar] [CrossRef] [PubMed]
- Kolpen, M.; Kragh, K.N.; Enciso, J.B.; Faurholt-Jepsen, D.; Lindegaard, B.; Egelund, G.B.; Jensen, A.V.; Ravn, P.; Mathiesen, I.H.M.; Gheorge, A.G.; et al. Bacterial biofilms predominate in both acute and chronic human lung infections. Thorax 2022, 77, 1015–1022. [Google Scholar] [CrossRef]
- Khan, F.; Tabassum, N.; Kim, Y.-M. A strategy to control colonization of pathogens: Embedding of lactic acid bacteria on the surface of urinary catheter. Appl. Microbiol. Biotechnol. 2020, 104, 9053–9066. [Google Scholar] [CrossRef] [PubMed]
- Staats, A.; Li, D.; Sullivan, A.C.; Stoodley, P. Biofilm formation in periprosthetic joint infections. Ann. Jt. 2021, 6, 43. [Google Scholar] [CrossRef]
- Zorgani, A.; Abofayed, A.; Glia, A.; Albarbar, A.; Hanish, S. Prevalence of Device-associated Nosocomial Infections Caused by Gram-negative Bacteria in a Trauma Intensive Care Unit in Libya. Oman Med. J. 2015, 30, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Beam, E.; Osmon, D. Prosthetic Joint Infection Update. Infect. Dis. Clin. N. Am. 2018, 32, 843–859. [Google Scholar] [CrossRef]
- Danin, P.-E.; Girou, E.; Legrand, P.; Louis, B.; Fodil, R.; Christov, C.; Devaquet, J.; Isabey, D.; Brochard, L. Description and Microbiology of Endotracheal Tube Biofilm in Mechanically Ventilated Subjects. Respir. Care 2015, 60, 21–29. [Google Scholar] [CrossRef]
- Urwin, L.; Okurowska, K.; Crowther, G.; Roy, S.; Garg, P.; Karunakaran, E.; MacNeil, S.; Partridge, L.J.; Green, L.R.; Monk, P.N. Corneal Infection Models: Tools to Investigate the Role of Biofilms in Bacterial Keratitis. Cells 2020, 9, 2450. [Google Scholar] [CrossRef] [PubMed]
- Selby, L.M.; Rupp, M.E.; Cawcutt, K.A. Prevention of Central-Line Associated Bloodstream Infections. Infect. Dis. Clin. N. Am. 2021, 35, 841–856. [Google Scholar] [CrossRef] [PubMed]
- Klug, D.; Balde, M.; Pavin, D.; Hidden-Lucet, F.o.; Clementy, J.; Sadoul, N.; Rey, J.L.; Lande, G.; Lazarus, A.; Victor, J.; et al. Risk Factors Related to Infections of Implanted Pacemakers and Cardioverter-Defibrillators. Circulation 2007, 116, 1349–1355. [Google Scholar] [CrossRef] [PubMed]
- Rebic, V.; Masic, N.; Teskeredzic, S.; Aljicevic, M.; Abduzaimovic, A.; Rebic, D. The Importance of Acinetobacter Species in the Hospital Environment. Med. Arch. 2018, 72, 330. [Google Scholar] [CrossRef] [PubMed]
- Galdys, A.L.; Marsh, J.W.; Delgado, E.; Pasculle, A.W.; Pacey, M.; Ayres, A.M.; Metzger, A.; Harrison, L.H.; Muto, C.A. Bronchoscope-associated clusters of multidrug-resistant Pseudomonas aeruginosa and carbapenem-resistant Klebsiella pneumoniae. Infect. Control. Hosp. Epidemiol. 2019, 40, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Peng, B.; Qu, J.; Zheng, J. Are Bacterial Persisters Dormant Cells Only? Front. Microbiol. 2022, 12, 708580. [Google Scholar] [CrossRef] [PubMed]
- McGuire, C.N.; Walter, D.J. Cryptococcus neoformans endocarditis in an immunocompetentpatient a case report. BMC Cardiovasc. Disord. 2022, 22, 565. [Google Scholar] [CrossRef]
- Garvey, M. Medical Device-Associated Healthcare Infections: Sterilization and the Potential of Novel Biological Approaches to Ensure Patient Safety. Int. J. Mol. Sci. 2023, 25, 201. [Google Scholar] [CrossRef] [PubMed]
- Josephs-Spaulding, J.; Singh, O.V. Medical Device Sterilization and Reprocessing in the Era of Multidrug-Resistant (MDR) Bacteria: Issues and Regulatory Concepts. Front. Med. Technol. 2021, 2, 587352. [Google Scholar] [CrossRef] [PubMed]
- Giles, C.; Lamont-Friedrich, S.J.; Michl, T.D.; Griesser, H.J.; Coad, B.R. The importance of fungal pathogens and antifungal coatings in medical device infections. Biotechnol. Adv. 2018, 36, 264–280. [Google Scholar] [CrossRef]
- Pietrocola, G.; Campoccia, D.; Motta, C.; Montanaro, L.; Arciola, C.R.; Speziale, P. Colonization and Infection of Indwelling Medical Devices by Staphylococcus aureus with an Emphasis on Orthopedic Implants. Int. J. Mol. Sci. 2022, 23, 5958. [Google Scholar] [CrossRef]
- Rismanchian, M.; Babashahi, A.; Goroohi, H.; Shahabouee, M.; Yaghini, J.; Badrian, H. Microflora around teeth and dental implants. Dent. Res. J. 2012, 9, 215. [Google Scholar] [CrossRef]
- Arciola, C.R.; Campoccia, D.; Montanaro, L. Implant infections: Adhesion, biofilm formation and immune evasion. Nat. Rev. Microbiol. 2018, 16, 397–409. [Google Scholar] [CrossRef]
- Abdallah, M.; Benoliel, C.; Drider, D.; Dhulster, P.; Chihib, N.-E. Biofilm formation and persistence on abiotic surfaces in the context of food and medical environments. Arch. Microbiol. 2014, 196, 453–472. [Google Scholar] [CrossRef]
- Rosenthal, V.D.; Bat-Erdene, I.; Gupta, D.; Belkebir, S.; Rajhans, P.; Zand, F.; Myatra, S.N.; Afeef, M.; Tanzi, V.L.; Muralidharan, S.; et al. International Nosocomial Infection Control Consortium (INICC) report, data summary of 45 countries for 2012-2017: Device-associated module. Am. J. Infect. Control 2020, 48, 423–432. [Google Scholar] [CrossRef]
- Al Bataineh, M.T.; Alazzam, A. Transforming medical device biofilm control with surface treatment using microfabrication techniques. PLoS ONE 2023, 18, e0292647. [Google Scholar] [CrossRef]
- Lourenço, B.N.; Marchioli, G.; Song, W.; Reis, R.L.; van Blitterswijk, C.A.; Karperien, M.; van Apeldoorn, A.; Mano, J.F. Wettability Influences Cell Behavior on Superhydrophobic Surfaces with Different Topographies. Biointerphases 2012, 7, 46. [Google Scholar] [CrossRef]
- Vetrivel, A.; Ramasamy, M.; Vetrivel, P.; Natchimuthu, S.; Arunachalam, S.; Kim, G.-S.; Murugesan, R. Pseudomonas aeruginosa Biofilm Formation and Its Control. Biologics 2021, 1, 312–336. [Google Scholar] [CrossRef]
- Thi, M.T.T.; Wibowo, D.; Rehm, B.H.A. Pseudomonas aeruginosa Biofilms. Int. J. Mol. Sci. 2020, 21, 8671. [Google Scholar] [CrossRef]
- Crivello, G.; Fracchia, L.; Ciardelli, G.; Boffito, M.; Mattu, C. In Vitro Models of Bacterial Biofilms: Innovative Tools to Improve Understanding and Treatment of Infections. Nanomaterials 2023, 13, 904. [Google Scholar] [CrossRef]
- Kurmoo, Y.; Hook, A.L.; Harvey, D.; Dubern, J.-F.; Williams, P.; Morgan, S.P.; Korposh, S.; Alexander, M.R. Real time monitoring of biofilm formation on coated medical devices for the reduction and interception of bacterial infections. Biomater. Sci. 2020, 8, 1464–1477. [Google Scholar] [CrossRef] [PubMed]
- Wi, Y.M.; Patel, R. Understanding Biofilms and Novel Approaches to the Diagnosis, Prevention, and Treatment of Medical Device-Associated Infections. Infect. Dis. Clin. N. Am. 2018, 32, 915–929. [Google Scholar] [CrossRef]
- Li, X.; Sun, L.; Zhang, P.; Wang, Y. Novel Approaches to Combat Medical Device-Associated BioFilms. Coatings 2021, 11, 294. [Google Scholar] [CrossRef]
- Mitra, D.; Kang, E.-T.; Neoh, K.G. Polymer-Based Coatings with Integrated Antifouling and Bactericidal Properties for Targeted Biomedical Applications. ACS Appl. Polym. Mater. 2021, 3, 2233–2263. [Google Scholar] [CrossRef]
- Chug, M.K.; Brisbois, E.J. Recent Developments in Multifunctional Antimicrobial Surfaces and Applications toward Advanced Nitric Oxide-Based Biomaterials. ACS Mater. Au 2022, 2, 525–551. [Google Scholar] [CrossRef]
- Khan, S.A.; Shakoor, A. Recent Strategies and Future Recommendations for the Fabrication of Antimicrobial, Antibiofilm, and Antibiofouling Biomaterials. Int. J. Nanomed. 2023, 18, 3377–3405. [Google Scholar] [CrossRef]
- Uneputty, A.; Dávila-Lezama, A.; Garibo, D.; Oknianska, A.; Bogdanchikova, N.; Hernández-Sánchez, J.F.; Susarrey-Arce, A. Strategies applied to modify structured and smooth surfaces: A step closer to reduce bacterial adhesion and biofilm formation. Colloid Interface Sci. Commun. 2022, 46, 100560. [Google Scholar] [CrossRef]
- Yang, W.J.; Cai, T.; Neoh, K.-G.; Kang, E.-T.; Teo, S.L.-M.; Rittschof, D. Barnacle Cement as Surface Anchor for “Clicking” of Antifouling and Antimicrobial Polymer Brushes on Stainless Steel. Biomacromolecules 2013, 14, 2041–2051. [Google Scholar] [CrossRef]
- Yang, W.J.; Cai, T.; Neoh, K.-G.; Kang, E.-T.; Dickinson, G.H.; Teo, S.L.-M.; Rittschof, D. Biomimetic Anchors for Antifouling and Antibacterial Polymer Brushes on Stainless Steel. Langmuir 2011, 27, 7065–7076. [Google Scholar] [CrossRef]
- Zhang, B.; Yan, Q.; Yuan, S.; Zhuang, X.; Zhang, F. Enhanced Antifouling and Anticorrosion Properties of Stainless Steel by Biomimetic Anchoring PEGDMA-Cross-Linking Polycationic Brushes. Ind. Eng. Chem. Res. 2019, 58, 7107–7119. [Google Scholar] [CrossRef]
- Xu, G.; Liu, P.; Pranantyo, D.; Xu, L.; Neoh, K.-G.; Kang, E.-T. Antifouling and Antimicrobial Coatings from Zwitterionic and Cationic Binary Polymer Brushes Assembled via “Click” Reactions. Ind. Eng. Chem. Res. 2017, 56, 14479–14488. [Google Scholar] [CrossRef]
- Wang, M.; Zheng, Y.; Yin, C.; Dai, S.; Fan, X.; Jiang, Y.; Liu, X.; Fang, J.; Yi, B.; Zhou, Q.; et al. Recent Progress in antibacterial hydrogel coatings for targeting biofilm to prevent orthopedic implant-associated infections. Front. Microbiol. 2023, 14, 1343202. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Ma, Y.; Shi, H.; Song, Y.; Guo, S.; Yang, S. Mg-, Zn-, and Fe-Based Alloys With Antibacterial Properties as Orthopedic Implant Materials. Front. Bioeng. Biotechnol. 2022, 10, 888084. [Google Scholar] [CrossRef]
- Egghe, T.; Morent, R.; Hoogenboom, R.; De Geyter, N. Substrate-independent and widely applicable deposition of antibacterial coatings. Trends Biotechnol. 2023, 41, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zheng, X.; Jian, R.; Bai, W.; Zheng, G.; Xie, Z.; Lin, Q.; Lin, F.; Xu, Y. In Situ Reduction of Silver Nanoparticles/Urushiol-Based Polybenzoxazine Composite Coatings with Enhanced Antimicrobial and Antifouling Performances. Polymers 2024, 16, 1167. [Google Scholar] [CrossRef]
- Simovic, S.; Barnes, T.J.; Tan, A.; Prestidge, C.A. Assembling nanoparticle coatings to improve the drug delivery performance of lipid based colloids. Nanoscale 2012, 4, 1220–1230. [Google Scholar] [CrossRef]
- Luchini, A.; Vitiello, G. Understanding the Nano-bio Interfaces: Lipid-Coatings for Inorganic Nanoparticles as Promising Strategy for Biomedical Applications. Front. Chem. 2019, 7, 343. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Jiménez, C.; Manzano, M.; Vallet-Regí, M. Nanoparticles Coated with Cell Membranes for Biomedical Applications. Biology 2020, 9, 406. [Google Scholar] [CrossRef]
- Mashaghi, S.; Jadidi, T.; Koenderink, G.; Mashaghi, A. Lipid Nanotechnology. Int. J. Mol. Sci. 2013, 14, 4242–4282. [Google Scholar] [CrossRef]
- Kang, M.G.; Khan, F.; Tabassum, N.; Cho, K.J.; Jo, D.M.; Kim, Y.M. Inhibition of Biofilm and Virulence Properties of Pathogenic Bacteria by Silver and Gold Nanoparticles Synthesized from Lactiplantibacillus sp. Strain C1. ACS Omega 2023, 8, 9873–9888. [Google Scholar] [CrossRef]
- Tabassum, N.; Khan, F.; Kang, M.G.; Jo, D.M.; Cho, K.J.; Kim, Y.M. Inhibition of Polymicrobial Biofilms of Candida albicans-Staphylococcus aureus/Streptococcus mutans by Fucoidan-Gold Nanoparticles. Mar. Drugs 2023, 21, 123. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.G.; Khan, F.; Jo, D.M.; Oh, D.; Tabassum, N.; Kim, Y.M. Antibiofilm and Antivirulence Activities of Gold and Zinc Oxide Nanoparticles Synthesized from Kimchi-Isolated Leuconostoc sp. Strain C2. Antibiotics 2022, 11, 1524. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Kang, M.G.; Jo, D.M.; Chandika, P.; Jung, W.K.; Kang, H.W.; Kim, Y.M. Phloroglucinol-Gold and -Zinc Oxide Nanoparticles: Antibiofilm and Antivirulence Activities towards Pseudomona saeruginosa PAO1. Mar. Drugs 2021, 19, 601. [Google Scholar] [CrossRef]
- Guerrero Correa, M.; Martínez, F.B.; Vidal, C.P.; Streitt, C.; Escrig, J.; de Dicastillo, C.L. Antimicrobial metal-based nanoparticles: A review on their synthesis, types and antimicrobial action. Beilstein J. Nanotechnol. 2020, 11, 1450–1469. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Yang, X.; He, Y.; Guo, C.; Li, Y.; Ji, H.; Qin, Y.; Wu, L. Strategies and materials for the prevention and treatment of biofilms. Mater. Today Bio 2023, 23, 100827. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, T.; Qu, Y.; Zhou, Y.; Zheng, Y.; Huang, C.; Zhang, Y.; Yu, Q.; Chen, H. Smart, Photothermally Activated, Antibacterial Surfaces with Thermally Triggered Bacteria-Releasing Properties. ACS Appl. Mater. Interfaces 2020, 12, 21283–21291. [Google Scholar] [CrossRef]
- Yang, Z.; Tu, Q.; Shen, X.; Liu, Y.; Zhang, Q.; Zhao, X.; Maitz, M.; Liu, T.; Hua, Q.; Wang, J.; et al. A facile metal-phenolic-amine strategy for dual-functionalization of blood-contacting devices with antibacterial and anticoagulant properties. Mater. Chem. Front. 2018, 3, 265–275. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, Y. Enhanced biomimic bactericidal surfaces by coating with positively-charged ZIF nano-dagger arrays. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 2199–2207. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Wei, T.; Zhao, J.; Jiang, S.; Yang, P.; Yu, Q.; Chen, H. Regenerable smart antibacterial surfaces: Full removal of killed bacteria via a sequential degradable layer. J. Mater. Chem. B 2018, 6, 3946–3955. [Google Scholar] [CrossRef]
- Zou, Y.; Lu, K.; Lin, Y.; Wu, Y.; Wang, Y.; Li, L.; Huang, C.; Zhang, Y.; Brash, J.L.; Chen, H.; et al. Dual-Functional Surfaces Based on an Antifouling Polymer and a Natural Antibiofilm Molecule: Prevention of Biofilm Formation without Using Biocides. ACS Appl. Mater. Interfaces 2021, 13, 45191–45200. [Google Scholar] [CrossRef]
- Lin, J.; Hu, J.; Wang, W.; Liu, K.; Zhou, C.; Liu, Z.; Kong, S.; Lin, S.; Deng, Y.; Guo, Z. Thermo and light-responsive strategies of smart titanium-containing composite material surface for enhancing bacterially anti-adhesive property. Chem. Eng. J. 2021, 407, 125783. [Google Scholar] [CrossRef]
- Yu, M.; Ding, X.; Zhu, Y.; Wu, S.; Ding, X.; Li, Y.; Yu, B.; Xu, F.-J. Facile Surface Multi-Functionalization of Biomedical Catheters with Dual-Microcrystalline Broad-Spectrum Antibacterial Drugs and Antifouling Poly(ethylene glycol) for Effective Inhibition of Bacterial Infections. ACS Appl. Bio Mater. 2019, 2, 1348–1356. [Google Scholar] [CrossRef]
- Vaterrodt, A.; Thallinger, B.; Daumann, K.; Koch, D.; Guebitz, G.M.; Ulbricht, M. Antifouling and Antibacterial Multifunctional Polyzwitterion/Enzyme Coating on Silicone Catheter Material Prepared by Electrostatic Layer-by-Layer Assembly. Langmuir 2016, 32, 1347–1359. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.; Kushwaha, P.; Kumar, S.; Kumar, R. Lysine and α-Aminoisobutyric Acid Conjugated Bioinspired Polydopamine Surfaces for the Enhanced Antibacterial Performance of the Foley Catheter. ACS Appl. Bio Mater. 2019, 2, 5799–5809. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, A.V.; Ritz, S.; Pütz, S.; Mailänder, V.; Landfester, K.; Ziener, U. Bioinspired phosphorylcholine containing polymer films with silver nanoparticles combining antifouling and antibacterial properties. Biomater. Sci. 2013, 1, 470. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhao, Y.-Q.; Zeng, Q.; Wu, Y.-W.; Hu, Y.; Duan, S.; Tang, Z.; Xu, F.-J. Dual-Functional Implants with Antibacterial and Osteointegration-Promoting Performances. ACS Appl. Mater. Interfaces 2019, 11, 36449–36457. [Google Scholar] [CrossRef] [PubMed]
- Chu, G.; Zhang, C.; Liu, Y.; Cao, Z.; Wang, L.; Chen, Y.; Zhou, W.; Gao, G.; Wang, K.; Cui, D. A Gold Nanocluster Constructed Mixed-Metal Metal–Organic Network Film for Combating Implant-Associated Infections. ACS Nano 2020, 14, 15633–15645. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.; Sousa, H.C.d.; Gil, M.H.; Santos, L.F.; Moutinho, G.M.; Serro, A.P.; Saramago, B. Antibacterial layer-by-layer coatings to control drug release from soft contact lenses material. Int. J. Pharm. 2018, 553, 186–200. [Google Scholar] [CrossRef] [PubMed]
- Abdulkareem, E.H.; Memarzadeh, K.; Allaker, R.P.; Huang, J.; Pratten, J.; Spratt, D. Anti-biofilm activity of zinc oxide and hydroxyapatite nanoparticles as dental implant coating materials. J. Dent. 2015, 43, 1462–1469. [Google Scholar] [CrossRef]
- Lim, D.J.; Skinner, D.; McLemore, J.; Rivers, N.; Elder, J.B.; Allen, M.; Koch, C.; West, J.; Zhang, S.; Thompson, H.M.; et al. In-vitro evaluation of a ciprofloxacin and azithromycin sinus stent for Pseudomonas aeruginosa biofilms. Int. Forum Allergy Rhinol. 2020, 10, 121–127. [Google Scholar] [CrossRef]
- Zarghami, V.; Ghorbani, M.; Bagheri, K.P.; Shokrgozar, M.A. Prevention the formation of biofilm on orthopedic implants by melittin thin layer on chitosan/bioactive glass/vancomycin coatings. J. Mater. Sci. Mater. Med. 2021, 32, 75. [Google Scholar] [CrossRef]
- Wang, X.; Tan, L.; Liu, X.; Cui, Z.; Yang, X.; Yeung, K.W.K.; Chu, P.K.; Wu, S. Construction of perfluorohexane/IR780@liposome coating on Ti for rapid bacteria killing under permeable near infrared light. Biomater. Sci. 2018, 6, 2460–2471. [Google Scholar] [CrossRef]
- Nablo, B.J.; Prichard, H.L.; Butler, R.D.; Klitzman, B.; Schoenfisch, M.H. Inhibition of implant-associated infections via nitric oxide release. Biomaterials 2005, 26, 6984–6990. [Google Scholar] [CrossRef]
- Xiong, Z.; Zhang, X.; Zhang, S.; Lei, L.; Ma, W.; Li, D.; Wang, W.; Zhao, Q.; Xing, B. Bacterial toxicity of exfoliated black phosphorus nanosheets. Ecotoxicol. Environ. Saf. 2018, 161, 507–514. [Google Scholar] [CrossRef]
- Yazici, H.; O’Neill, M.B.; Kacar, T.; Wilson, B.R.; Oren, E.E.; Sarikaya, M.; Tamerler, C. Engineered Chimeric Peptides as Antimicrobial Surface Coating Agents toward Infection-Free Implants. ACS Appl. Mater. Interfaces 2016, 8, 5070–5081. [Google Scholar] [CrossRef]
- Monte-Serrano, M.; Fernandez-Saiz, P.; Ortí-Lucas, R.M. Effective Antimicrobial Coatings Containing Silver-Based Nanoclays and Zinc Pyrithione. J. Microb. Biochem. Technol. 2015, 7, 398–403. [Google Scholar] [CrossRef]
- Cruz, A.; Condinho, M.; Carvalho, B.; Arraiano, C.M.; Pobre, V.; Pinto, S.N. The Two Weapons against Bacterial Biofilms: Detection and Treatment. Antibiotics 2021, 10, 1482. [Google Scholar] [CrossRef]
- Xu, T.; Xiao, Y.; Wang, H.; Zhu, J.; Lee, Y.; Zhao, J.; Lu, W.; Zhang, H. Characterization of Mixed-Species Biofilms Formed by Four Gut Microbiota. Microorganisms 2022, 10, 2332. [Google Scholar] [CrossRef]
- Elias, S.; Banin, E. Multi-species biofilms: Living with friendly neighbors. FEMS Microbiol. Rev. 2012, 36, 990–1004. [Google Scholar] [CrossRef]
- Zou, Y.; Liu, C.; Zhang, H.; Wu, Y.; Lin, Y.; Cheng, J.; Lu, K.; Li, L.; Zhang, Y.; Chen, H.; et al. Three lines of defense: A multifunctional coating with anti-adhesion, bacteria-killing and anti-quorum sensing properties for preventing biofilm formation of Pseudomonas aeruginosa. Acta Biomater. 2022, 151, 254–263. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mishra, A.; Aggarwal, A.; Khan, F. Medical Device-Associated Infections Caused by Biofilm-Forming Microbial Pathogens and Controlling Strategies. Antibiotics 2024, 13, 623. https://doi.org/10.3390/antibiotics13070623
Mishra A, Aggarwal A, Khan F. Medical Device-Associated Infections Caused by Biofilm-Forming Microbial Pathogens and Controlling Strategies. Antibiotics. 2024; 13(7):623. https://doi.org/10.3390/antibiotics13070623
Chicago/Turabian StyleMishra, Akanksha, Ashish Aggarwal, and Fazlurrahman Khan. 2024. "Medical Device-Associated Infections Caused by Biofilm-Forming Microbial Pathogens and Controlling Strategies" Antibiotics 13, no. 7: 623. https://doi.org/10.3390/antibiotics13070623
APA StyleMishra, A., Aggarwal, A., & Khan, F. (2024). Medical Device-Associated Infections Caused by Biofilm-Forming Microbial Pathogens and Controlling Strategies. Antibiotics, 13(7), 623. https://doi.org/10.3390/antibiotics13070623







