Antimicrobial Activity of Green Synthesized Silver and Copper Oxide Nanoparticles against the Foodborne Pathogen Campylobacter jejuni
Abstract
:1. Introduction
2. Results
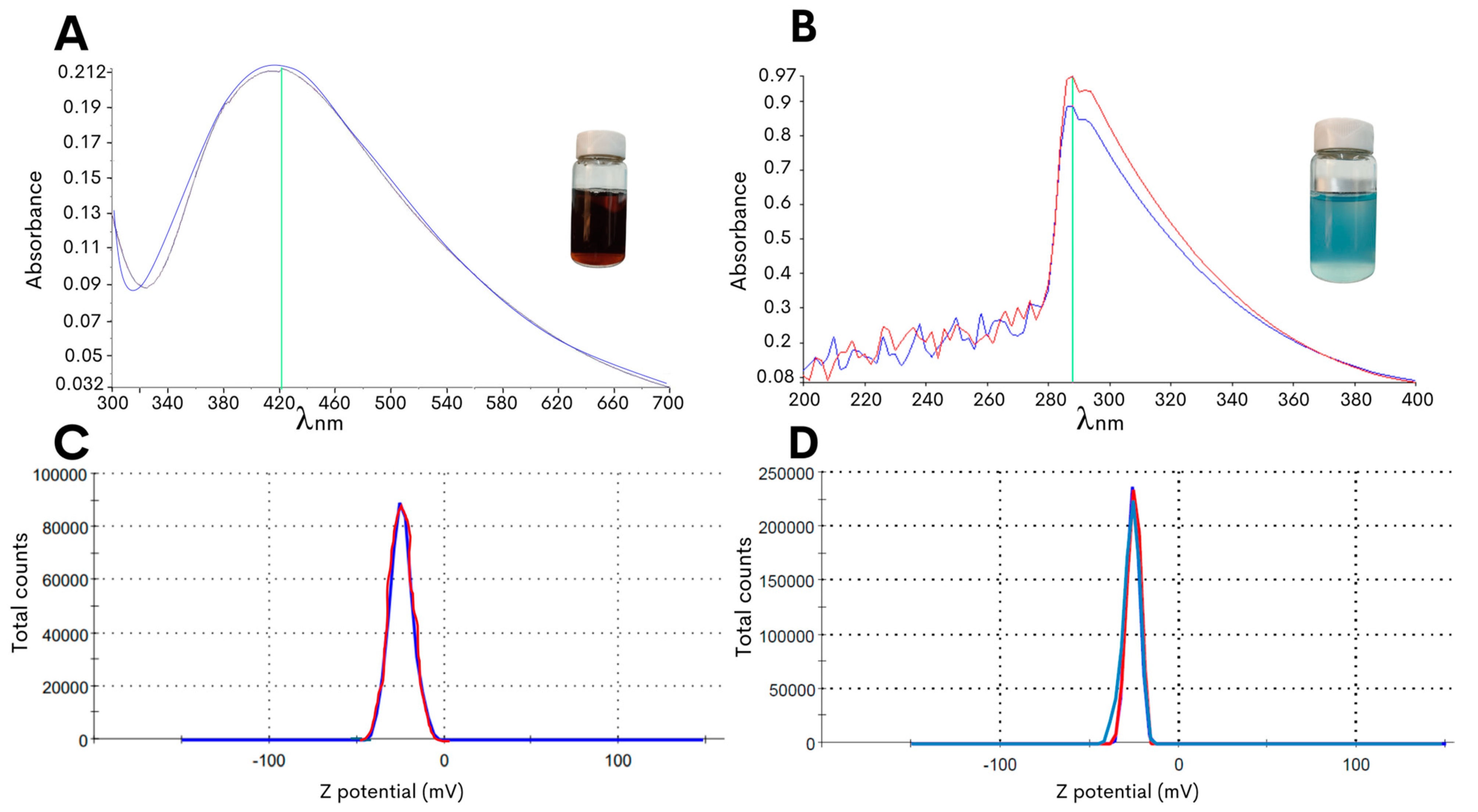
2.1. Optimized Biosynthesis of Silver and Copper Oxide NPs Derived from G. sessile
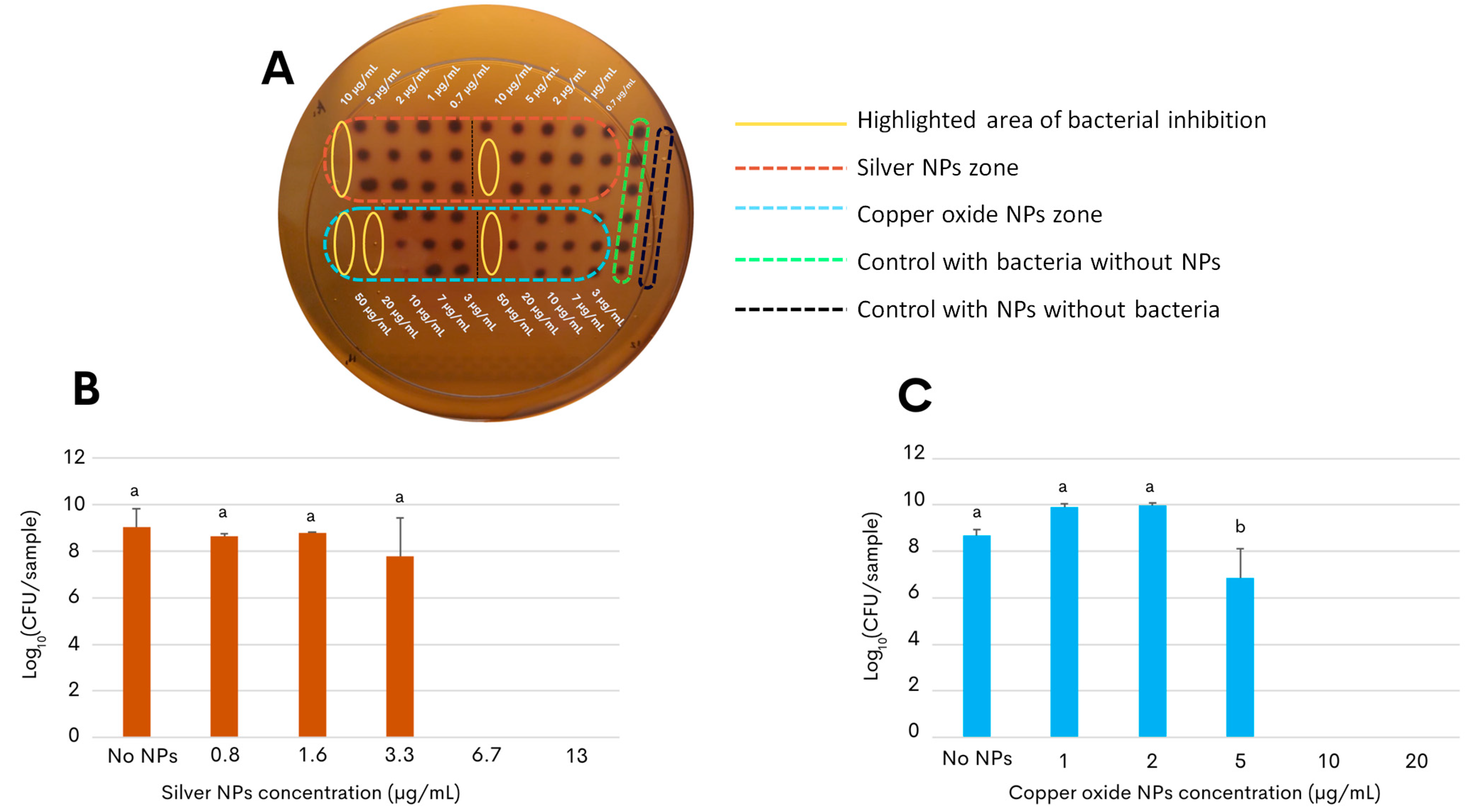
2.2. Antimicrobial Effects of the Silver and Copper Oxide NPs against the Foodborne Pathogen C. jejuni
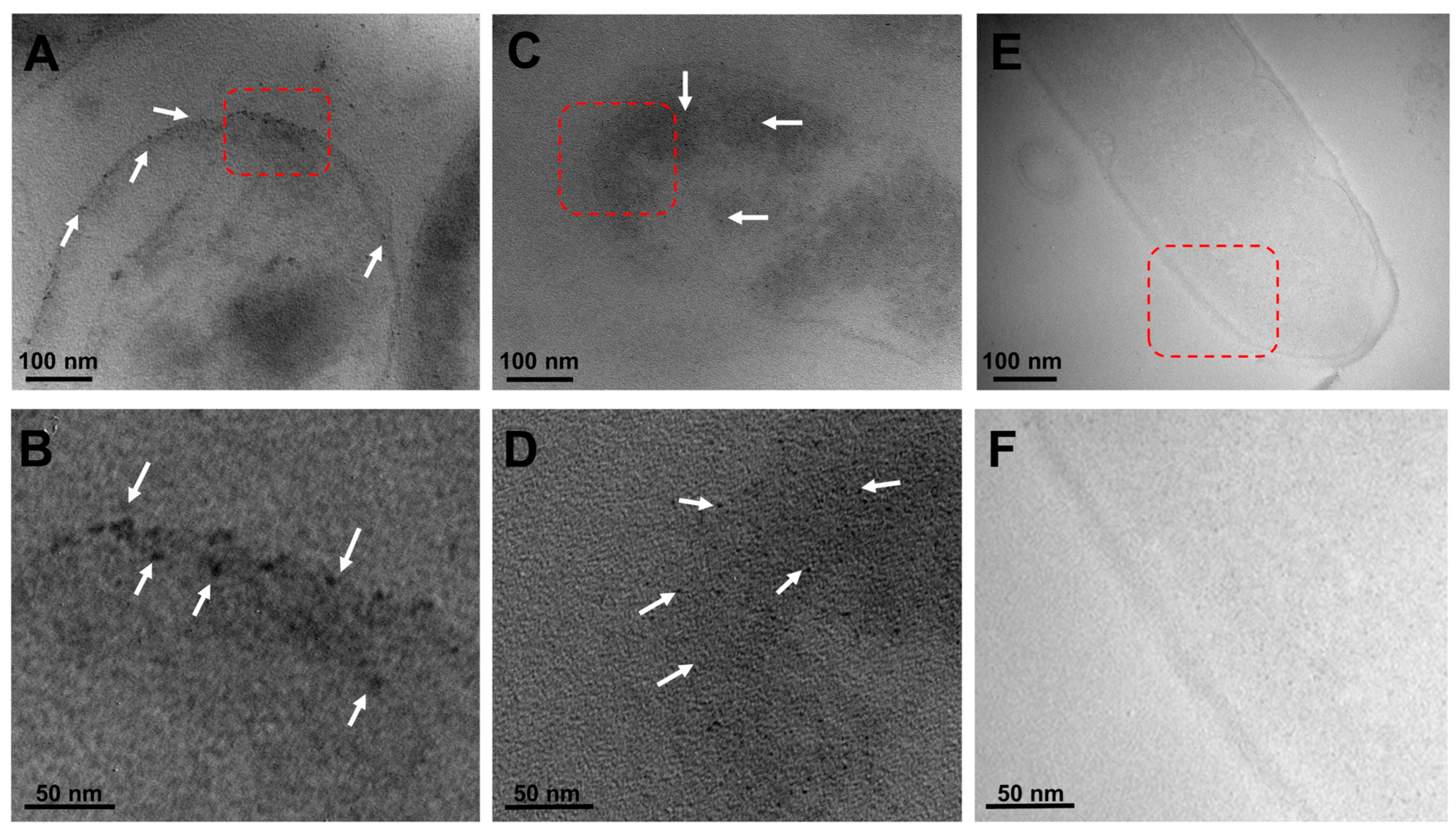
2.3. Visualization of the Localized Interactions between the G. sessile-Derived Metallic NPs and C. jejuni
3. Discussion
4. Materials and Methods
4.1. Growth Conditions and Extract Preparation of the Fungus G. sessile
4.2. Biosynthesis and Characterization of the Metallic NPs
4.3. Growth and Propagation of the Bacterial Foodborne Pathogen C. jejuni
4.4. In Vitro Assay to Assess Growth Inhibition of C. jejuni
4.5. In Vitro Test to Determine ROS Generation by C. jejuni
4.6. Transmission Electron Microscopy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alharbi, N.S.; Alsubhi, N.S.; Felimban, A.I. Green synthesis of silver nanoparticles using medicinal plants: Characterization and application. J. Radiat. Res. Appl. Sci. 2022, 15, 109–124. [Google Scholar] [CrossRef]
- Kandru, A. Nanotechnology: Application in Biology and Medicine. In Model Organisms to Study Biological Activities and Toxicity of Nanoparticles; Siddhardha, B., Dyavaiah, M., Kasinathan, K., Eds.; Springer: Singapore, 2020; pp. 1–18. [Google Scholar]
- Seil, J.T.; Webster, T.J. Antimicrobial applications of nanotechnology: Methods and literature. Int. J. Nanomedicine 2012, 7, 2767–2781. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.A. Introduction to nanotechnology and its applications to medicine. Surg. Neurol. 2004, 61, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Khorsandi, K.; Hosseinzadeh, R.; Sadat Esfahani, H.; Keyvani-Ghamsari, S.; Ur Rahman, S. Nanomaterials as drug delivery systems with antibacterial properties: Current trends and future priorities. Expert Rev. Anti Infect. Ther. 2021, 19, 1299–1323. [Google Scholar] [CrossRef] [PubMed]
- Yayehrad, A.T.; Wondie, G.B.; Marew, T. Different nanotechnology approaches for ciprofloxacin delivery against multidrug-resistant microbes. Infect. Drug Resist. 2022, 15, 413–426. [Google Scholar] [CrossRef] [PubMed]
- Vijayaram, S.; Razafindralambo, H.; Sun, Y.Z.; Vasantharaj, S.; Ghafarifarsani, H.; Hoseinifar, S.H.; Raeeszadeh, M. Applications of green synthesized metal nanoparticles - a review. Biol. Trace Elem. Res. 2024, 202, 360–386. [Google Scholar] [CrossRef] [PubMed]
- Bhavyasree, P.G.; Xavier, T.S. Green synthesised copper and copper oxide based nanomaterials using plant extracts and their application in antimicrobial activity: Review. Curr. Res. Green Sustain. Chem. 2022, 5, 100249. [Google Scholar] [CrossRef]
- Nam, N.H.; Luong, N.H. Nanoparticles: Synthesis and applications. In Materials for Biomedical Engineering: Inorganic Micro and Nanostructures; Elsevier: Amsterdam, The Netherlands, 2019; pp. 211–240. [Google Scholar] [CrossRef]
- Wu, G.-S.; Lu, J.-J.; Guo, J.-J.; Li, Y.-B.; Tan, W.; Dang, Y.-Y.; Zhong, Z.-F.; Xu, Z.-T.; Chen, X.-P.; Wang, Y.-T. Ganoderic acid DM, a natural triterpenoid, induces DNA damage, G1 cell cycle arrest and apoptosis in human breast cancer cells. Fitoterapia 2012, 83, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Loyd, A.L.; Richter, B.S.; Jusino, M.A.; Truong, C.; Smith, M.E.; Blanchette, R.A.; Smith, J.A. Identifying the “mushroom of immortality”: Assessing the Ganoderma species composition in commercial reishi products. Front. Microbiol. 2018, 9, 1557. [Google Scholar] [CrossRef]
- Sanodiya, S.B.; Thakur, S.G.; Baghel, K.R.; Prasad, B.K.S.G.; Bisen, S.P. Ganoderma lucidum: A potent pharmacological macrofungus. Curr. Pharm. Biotechnol. 2009, 10, 717–742. [Google Scholar] [CrossRef]
- Constantin, M.; Răut, I.; Suica-Bunghez, R.; Firinca, C.; Radu, N.; Gurban, A.-M.; Preda, S.; Alexandrescu, E.; Doni, M.; Jecu, L. Ganoderma lucidum-mediated green synthesis of silver nanoparticles with antimicrobial activity. Materials 2023, 16, 4261. [Google Scholar] [CrossRef] [PubMed]
- Cör Andrejč, D.; Knez, Ž.; Knez Marevci, M. Antioxidant, antibacterial, antitumor, antifungal, antiviral, anti-inflammatory, and nevro-protective activity of Ganoderma lucidum: An overview. Front. Pharmacol. 2022, 13, 934982. [Google Scholar] [CrossRef]
- Jan, R.H.; Lin, T.Y.; Hsu, Y.C.; Lee, S.S.; Lo, S.Y.; Chang, M.; Chen, L.K.; Lin, Y.L. Immuno-modulatory activity of Ganoderma lucidum-derived polysacharide on human monocytoid dendritic cells pulsed with Der p 1 allergen. BMC Immunol. 2011, 12, 31. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Li, Y.; Shang, H.; Luo, Y.; Tian, Y. Ganoderic acid A and its amide derivatives as potential anti-cancer agents by regulating the p53-MDM2 pathway: Synthesis and biological evaluation. Molecules 2023, 28, 2374. [Google Scholar] [CrossRef] [PubMed]
- Zhen, C.; Wu, X.; Zhang, J.; Liu, D.; Li, G.; Yan, Y.; He, X.; Miao, J.; Song, H.; Yan, Y.; et al. Ganoderma lucidum polysaccharides attenuates pressure-overload-induced pathological cardiac hypertrophy. Front. Pharmacol. 2023, 14, 1127123. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.L.; Chen, A.F.; Lin, Z.B. Ganoderma lucidum polysaccharides enhance the function of immunological effector cells in immunosuppressed mice. J. Ethnopharmacol. 2007, 111, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Torres-Torres, M.G.; Ryvarden, L.; Guzmán-Dávalos, L. Ganoderma subgenus Ganoderma in Mexico. Rev. Mex. Mic 2015, 41, 27–45. [Google Scholar]
- Viceconte, F.R.; Diaz, M.L.; Soresi, D.S.; Lencinas, I.B.; Carrera, A.; Prat, M.I.; Gurovic, M.S.V. Ganoderma sessile is a fast polysaccharide producer among Ganoderma species. Mycologia 2021, 113, 513–524. [Google Scholar] [CrossRef] [PubMed]
- Horn, D.; Rieger, J. Organic nanoparticles in the aqueous phase—Theory, experiment, and use. Angew. Chem. Int. Ed. 2001, 40, 4330–4361. [Google Scholar] [CrossRef]
- Flores-Rábago, K.M.; Rivera-Mendoza, D.; Vilchis-Nestor, A.R.; Juarez-Moreno, K.; Castro-Longoria, E. Antibacterial activity of biosynthesized copper oxide nanoparticles (CuONPs) using Ganoderma sessile. Antibiotics 2023, 12, 1251. [Google Scholar] [CrossRef]
- Murillo-Rábago, E.I.; Vilchis-Nestor, A.R.; Juarez-Moreno, K.; Garcia-Marin, L.E.; Quester, K.; Castro-Longoria, E. Optimized synthesis of small and stable silver nanoparticles using intracellular and extracellular components of fungi: An alternative for bacterial inhibition. Antibiotics 2022, 11, 800. [Google Scholar] [CrossRef] [PubMed]
- Rashidi, L.; Khosravi-Darani, K. The applications of nanotechnology in food industry. Crit. Rev. Food Sci. Nutr. 2011, 51, 723–730. [Google Scholar] [CrossRef]
- Rajeshkumar, S.; Malarkodi, C. In vitro antibacterial activity and mechanism of silver nanoparticles against foodborne pathogens. Bioinorg. Chem. Appl. 2014, 2014, 581890. [Google Scholar] [CrossRef] [PubMed]
- Zorraquín-Peña, I.; Cueva, C.; Bartolomé, B.; Moreno-Arribas, M.V. Silver nanoparticles against foodborne bacteria. Effects at intestinal level and health limitations. Microorganisms 2020, 8, 132. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority; European Centre for Disease Prevention Control. The European Union One Health 2019 Zoonoses Report. EFSA J. 2021, 19, e06406. [Google Scholar] [CrossRef]
- Kaakoush, N.O.; Castaño-Rodríguez, N.; Mitchell, H.M.; Man, S.M. Global epidemiology of Campylobacter infection. Clin. Microbiol. Rev. 2015, 28, 687–720. [Google Scholar] [CrossRef]
- Soto-Beltran, M.; Lee, B.G.; Amézquita-López, B.A.; Quiñones, B. Overview of methodologies for the culturing, recovery and detection of Campylobacter. Int. J. Environ. Health Res. 2023, 33, 307–323. [Google Scholar] [CrossRef] [PubMed]
- Vucic, S.; Kiernan, M.C.; Cornblath, D.R. Guillain-Barré syndrome: An update. J. Clin. Neurosci. 2009, 16, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, S. DLS and zeta potential - What they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef]
- Clogston, J.D.; Patri, A.K. Zeta potential measurement. In Characterization of Nanoparticles Intended for Drug Delivery; McNeil, S.E., Ed.; Humana Press: Totowa, NJ, USA, 2011; pp. 63–70. [Google Scholar]
- Abdal Dayem, A.; Hossain, M.K.; Lee, S.B.; Kim, K.; Saha, S.K.; Yang, G.M.; Choi, H.Y.; Cho, S.G. The role of reactive oxygen species (ROS) in the biological sctivities of metallic nanoparticles. Int. J. Mol. Sci. 2017, 18, 120. [Google Scholar] [CrossRef]
- Horst, A.M.; Vukanti, R.; Priester, J.H.; Holden, P.A. An assessment of fluorescence- and absorbance-based assays to study metal-oxide nanoparticle ROS production and effects on bacterial membranes. Small 2013, 9, 1753–1764. [Google Scholar] [CrossRef]
- Reiniers, M.J.; van Golen, R.F.; Bonnet, S.; Broekgaarden, M.; van Gulik, T.M.; Egmond, M.R.; Heger, M. Preparation and practical applications of 2′,7′-dichlorodihydrofluorescein in redox assays. Anal. Chem. 2017, 89, 3853–3857. [Google Scholar] [CrossRef] [PubMed]
- Allan-Wojtas, P.; Truelstrup Hansen, L.; Paulson, A.T. Microstructural studies of probiotic bacteria-loaded alginate microcapsules using standard electron microscopy techniques and anhydrous fixation. LWT-Food Sci. Technol. 2008, 41, 101–108. [Google Scholar] [CrossRef]
- Vázquez-Muñoz, R.; Avalos-Borja, M.; Castro-Longoria, E. Ultrastructural analysis of Candida albicans when exposed to silver nanoparticles. PLOS ONE 2014, 9, e108876. [Google Scholar] [CrossRef] [PubMed]
- Kreling, V.; Falcone, F.H.; Kehrenberg, C.; Hensel, A. Campylobacter sp.: Pathogenicity factors and prevention methods-new molecular targets for innovative antivirulence drugs? Appl. Microbiol. Biotechnol. 2020, 104, 10409–10436. [Google Scholar] [CrossRef]
- Neustaedter, C.M.; Robertson, K.; Tschritter, D.; Reid-Smith, R.J.; MacKinnon, M.C.; Murphy, C.P.; Chapman, B.; Neumann, N.F.; Otto, S.J.G. A scoping review of factors associated with antimicrobial-resistant Campylobacter species infections in humans. Epidemiol. Infect. 2023, 151, e100. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Mendoza, D.; Martínez-Flores, I.; Santamaría, R.I.; Lozano, L.; Bustamante, V.H.; Pérez-Morales, D. Genomic analysis reveals the genetic determinants associated with antibiotic resistance in the zoonotic pathogen Campylobacter spp. distributed globally. Front. Microbiol. 2020, 11, 513070. [Google Scholar] [CrossRef] [PubMed]
- Sproston, E.L.; Wimalarathna, H.M.L.; Sheppard, S.K. Trends in fluoroquinolone resistance in Campylobacter. Microb. Genom. 2018, 4, 198. [Google Scholar] [CrossRef]
- Wieczorek, K.; Osek, J. Antimicrobial resistance mechanisms among Campylobacter. BioMed Res. Int. 2013, 2013, 340605. [Google Scholar] [CrossRef]
- Klemm, E.J.; Wong, V.K.; Dougan, G. Emergence of dominant multidrug-resistant bacterial clades: Lessons from history and whole-genome sequencing. Proc. Natl. Acad. Sci. USA 2018, 115, 12872–12877. [Google Scholar] [CrossRef]
- Luangtongkum, T.; Jeon, B.; Han, J.; Plummer, P.; Logue, C.M.; Zhang, Q. Antibiotic resistance in Campylobacter: Emergence, transmission and persistence. Future Microbiol. 2009, 4, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Sahin, O.; Pavlovic, N.; LeJeune, J.; Carlson, J.; Wu, Z.; Dai, L.; Zhang, Q. Rising fluoroquinolone resistance in Campylobacter isolated from feedlot cattle in the United States. Sci. Rep. 2017, 7, 494. [Google Scholar] [CrossRef] [PubMed]
- Mba, I.E.; Nweze, E.I. Nanoparticles as therapeutic options for treating multidrug-resistant bacteria: Research progress, challenges, and prospects. World J. Microbiol. Biotechnol. 2021, 37, 108. [Google Scholar] [CrossRef] [PubMed]
- Gahlawat, G.; Choudhury, A.R. A review on the biosynthesis of metal and metal salt nanoparticles by microbes. RSC Adv. 2019, 9, 12944–12967. [Google Scholar] [CrossRef] [PubMed]
- Gericke, M.; Pinches, A. Biological synthesis of metal nanoparticles. Hydrometallurgy 2006, 83, 132–140. [Google Scholar] [CrossRef]
- Nasrullah, M.; Gul, F.Z.; Hanif, S.; Mannan, A.; Naz, S.; Ali, J.S.; Zia, M. Green and chemical syntheses of CdO NPs: A comparative study for yield attributes, biological characteristics, and toxicity concerns. ACS Omega 2020, 5, 5739–5747. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; He, Y.; Irwin Peter, L.; Jin, T.; Shi, X. Antibacterial activity and mechanism of action of zinc oxide nanoparticles against Campylobacter jejuni. Appl. Environ. Microbiol. 2011, 77, 2325–2331. [Google Scholar] [CrossRef] [PubMed]
- Duffy, L.L.; Osmond-McLeod, M.J.; Judy, J.; King, T. Investigation into the antibacterial activity of silver, zinc oxide and copper oxide nanoparticles against poultry-relevant isolates of Salmonella and Campylobacter. Food Control 2018, 92, 293–300. [Google Scholar] [CrossRef]
- Ali, M.; Hussain, R.; Tariq, F.; Noreen, Z.; Toufiq, A.M.; Bokhari, H.; Akhtar, N.; Rahman, S.u. Highly effective visible light-activated cobalt-doped TiO2 nanoparticles for antibacterial coatings against Campylobacter jejuni. Appl. Nanosci. 2020, 10, 1005–1012. [Google Scholar] [CrossRef]
- Silvan, J.M.; Zorraquin-Peña, I.; Gonzalez de Llano, D.; Moreno-Arribas, M.V.; Martinez-Rodriguez, A.J. Antibacterial activity of glutathione-stabilized silver nanoparticles against Campylobacter multidrug-resistant strains. Front. Microbiol. 2018, 9, 458. [Google Scholar] [CrossRef]
- Liu, F.; Xue, L.; Yuan, Y.; Pan, J.; Zhang, C.; Wang, H.; Brash, J.L.; Yuan, L.; Chen, H. Multifunctional nanoparticle–protein conjugates with controllable bioactivity and pH responsiveness. Nanoscale 2016, 8, 4387–4394. [Google Scholar] [CrossRef]
- Raza, M.A.; Kanwal, Z.; Rauf, A.; Sabri, A.N.; Riaz, S.; Naseem, S. Size- and shape-dependent antibacterial studies of silver nanoparticles synthesized by wet chemical routes. Nanomaterials 2016, 6, 74. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Kamle, M.; Shukla, S.; Mahato, D.K.; Chandra, P.; Hwang, S.K.; Kumar, P.; Huh, Y.S.; Han, Y.-K. Prospects of using nanotechnology for food preservation, safety, and security. J. Food Drug Anal. 2018, 26, 1201–1214. [Google Scholar] [CrossRef]
- Choi, O.; Hu, Z. Size dependent and reactive oxygen species related nanosilver toxicity to nitrifying bacteria. Environ. Sci. Technol. 2008, 42, 4583–4588. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, K.S.; Husen, A.; Rao, R.A.K. A review on biosynthesis of silver nanoparticles and their biocidal properties. J. Nanobiotechnol. 2018, 16, 14. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, B.; Parandhaman, T.; Das, S.K. Antibacterial effects of biosynthesized silver nanoparticles on surface ultrastructure and nanomechanical properties of gram-negative bacteria viz. Escherichia coli and Pseudomonas aeruginosa. ACS Appl. Mater. Interfaces 2016, 8, 4963–4976. [Google Scholar] [CrossRef]
- Sondi, I.; Salopek-Sondi, B. Silver nanoparticles as antimicrobial agent: A case study on E. coli as a model for Gram-negative bacteria. J. Colloid Interface Sci. 2004, 275, 177–182. [Google Scholar] [CrossRef]
- Chen, N.-F.; Liao, Y.-H.; Lin, P.-Y.; Chen, W.-F.; Wen, Z.-H.; Hsieh, S. Investigation of the characteristics and antibacterial activity of polymer-modified copper oxide nanoparticles. Int. J. Mol. Sci. 2021, 22, 12913. [Google Scholar] [CrossRef] [PubMed]
- Christena, L.R.; Mangalagowri, V.; Pradheeba, P.; Ahmed, K.B.A.; Shalini, B.I.S.; Vidyalakshmi, M.; Anbazhagan, V.; Sai subramanian, N. Copper nanoparticles as an efflux pump inhibitor to tackle drug resistant bacteria. RSC Adv. 2015, 5, 12899–12909. [Google Scholar] [CrossRef]
- Shehabeldine, A.M.; Amin, B.H.; Hagras, F.A.; Ramadan, A.A.; Kamel, M.R.; Ahmed, M.A.; Atia, K.H.; Salem, S.S. Potential antimicrobial and antibiofilm properties of copper oxide nanoparticles: Time-kill kinetic essay and ultrastructure of pathogenic bacterial cells. Appl. Biochem. Biotechnol. 2023, 195, 467–485. [Google Scholar] [CrossRef]
- Creighton, J.A.; Eadon, D.G. Ultraviolet–visible absorption spectra of the colloidal metallic elements. J. Chem. Soc. Faraday Trans. 1991, 87, 3881–3891. [Google Scholar] [CrossRef]
- McDermott, P.F.; Bodeis, S.M.; Aarestrup, F.M.; Brown, S.; Traczewski, M.; Fedorka-Cray, P.; Wallace, M.; Critchley, I.A.; Thornsberry, C.; Graff, S.; et al. Development of a standardized susceptibility test for campylobacter with quality-control ranges for ciprofloxacin, doxycycline, erythromycin, gentamicin, and meropenem. Microb. Drug Resist. 2004, 10, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. Available online: http://www.R-project.org/ (accessed on 1 May 2023).


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivera-Mendoza, D.; Quiñones, B.; Huerta-Saquero, A.; Castro-Longoria, E. Antimicrobial Activity of Green Synthesized Silver and Copper Oxide Nanoparticles against the Foodborne Pathogen Campylobacter jejuni. Antibiotics 2024, 13, 650. https://doi.org/10.3390/antibiotics13070650
Rivera-Mendoza D, Quiñones B, Huerta-Saquero A, Castro-Longoria E. Antimicrobial Activity of Green Synthesized Silver and Copper Oxide Nanoparticles against the Foodborne Pathogen Campylobacter jejuni. Antibiotics. 2024; 13(7):650. https://doi.org/10.3390/antibiotics13070650
Chicago/Turabian StyleRivera-Mendoza, Daniel, Beatriz Quiñones, Alejandro Huerta-Saquero, and Ernestina Castro-Longoria. 2024. "Antimicrobial Activity of Green Synthesized Silver and Copper Oxide Nanoparticles against the Foodborne Pathogen Campylobacter jejuni" Antibiotics 13, no. 7: 650. https://doi.org/10.3390/antibiotics13070650






