Arcobacteraceae: An Exploration of Antibiotic Resistance Featuring the Latest Research Updates
Abstract
1. Introduction
2. Antibiotic Resistance of Arcobacter Spp. Isolated from Food and Related Land Animals
| Species | Antibiotic | Class | Sources | Refs. |
|---|---|---|---|---|
| A. butzleri and A. cryaerophilus | Nalidixic acid | Quinolone | Meat and related animals | [21,22,24] |
| A. butzleri, A. cryaerophilus, and A. skirrowii | Cefotaxime | Cephalosporin | Meat and related animals | [21,27] |
| A. butzleri, A. cryaerophilus, and A. skirrowii | Cefoperazone | Cephalosporin | Meat and related animals | [23] |
| A. butzleri, A. cryaerophilus, and A. skirrowii | Ampicillin | Penicillin | Meat and related animals | [24,25,27] |
| A. butzleri | Aztreonam | Monobactams | Meat and related animals | [24] |
| A. butzleri | Cephalothin | Cephalosporin | Meat and related animals | [24] |
| A. butzleri and A. cryaerophilus | Clindamycin | Lincosamide | Meat and related animals | [24] |
| A. butzleri and A. cryaerophilus | Oxacillin | Penicillin | Meat and related animals | [24] |
| A. butzleri and A. cryaerophilus | Penicillin G | Penicillin | Meat and related animals | [24] |
| A. butzleri | Erythromycin | Macrolide | Meat and related animals, food processing plant surfaces | [25,28,35,37] |
| A. butzleri and A. cryaerophilus | Tetracycline | Tetracycline | Meat and related animals | [21,25,28] |
| A. butzleri | Amoxicillin | Penicillin | Meat and related animals, food processing plant surfaces | [28,37] |
| A. butzleri and A. cryaerophilus | Cefoxitin | Cephamycin | Meat and related animals | [28] |
| A. butzleri, A. cryaerophilus, and A. skirrowii | Ampicillin–sulbactam | Penicillin and beta-lactamase inhibitors | Meat and related animals | [27] |
| A. butzleri | Amoxicillin–clavulanic acid | Penicillin and beta-lactamase inhibitors | Milk, dairy products, meat and related animals, food processing plant surfaces | [29,37] |
| A. butzleri | Tetracycline | Tetracycline | Milk and dairy products, meat and related animals, fresh vegetables | [29,30] |
| A. butzleri | Nalidixic acid | Quinolone | Food processing plant surfaces | [35,38] |
| A. butzleri | Ampicillin | Penicillin | Meat and related animals, food processing plant surfaces, pigs, ducks, quails, and sheep | [31,32,35,37] |
| A. butzleri | Azithromycin | Macrolide | Meat and related animals, food processing plant surfaces | [37] |
| A. butzleri | Clarithromycin | Macrolide | Meat and related animals, food processing plant surfaces | [37] |
| A. butzleri | Gentamicin | Aminoglycoside | Meat and related animals, food processing plant surfaces | [37] |
| A. butzleri | Cefotaxime | Cephalosporin | Meat and related animals, fresh vegetables | [30] |
| A. butzleri | Cefoperazone–sulbactam | Cephalosporin and beta-lactamase inhibitors | Pigs | [31] |
| A. butzleri | Chloramphenicol | Amphenicol | Pigs, ducks, quails, and sheep | [32] |
| A. butzleri | Penicillin | Penicillin | Pigs, ducks, quails, and sheep | [32] |
3. Antibiotic Resistance of Arcobacter Spp. Isolated from Water and Water Animals
| Antibiotic | Class | Sources | Refs. |
|---|---|---|---|
| Ampicillin | Penicillin | Surface water, aquatic environments, wastewater, mussels and clams | [15,24,40,42] |
| Azithromycin | Macrolide | Surface water | [15] |
| Ciprofloxacin | Fluoroquinolone | Surface water | [15] |
| Cephalothin | Cephalosporin | Aquatic environments, wastewater, seafood | [24,40,43] |
| Cefotaxime | Cephalosporin | Aquatic environments | [40] |
| Nalidixic acid | Quinolone | Aquatic environments, wastewater, Catla catla | [24,40,44] |
| Tetracycline | Tetracycline | Aquatic environments, sushi, mussels and clams | [30,40,42] |
| Aztreonam | Monobactam | Wastewater | [24] |
| Clindamycin | Lincomycin | Wastewater | [24] |
| Oxacillin | Penicillin | Wastewater | [24] |
| Penicillin G | Penicillin | Wastewater | [24] |
| Clindamycin | Lincosamide | Agricultural surface water | [41] |
| Chloramphenicol | Amphenicol | Agricultural surface water | [41] |
| Cefotaxime | Cephalosporin | Sushi, mussels and clams | [30,42] |
| Penicillin | Penicillin | Mussels and clams, Catla catla | [42,44] |
| Erythromycin | Macrolide | Mussels and clams, Catla catla | [42,44] |
| Cefoxitin | Cephalosporin | Seafood | [43] |
| Sulphamethizole | Sulfonamide | Seafood | [43] |
| Cefixime | Cephalosporin | Catla catla | [44] |
4. Antibiotic Resistance of Arcobacter Spp. Isolated from Humans
| Species | Antibiotic | Class | Refs. |
|---|---|---|---|
| A. butzleri and A. cryaerophilus | Ampicillin | Penicillin | [24,30,46,49,50] |
| A. butzleri | Amoxicillin–clavulanic acid | Penicillin and beta-lactamase inhibitors | [30,50] |
| A. butzleri | Aztreonam | Beta-lactam | [24] |
| A. butzleri, A. cryaerophilus, and A. skirrowii | Cefalotin | Cephalosporin | [30,51] |
| A. butzleri, A. cryaerophilus, and A. skirrowii | Cefazolin | Cephalosporin | [51] |
| A. butzleri | Cefotaxime | Cephalosporin | [30] |
| A. butzleri, A. cryaerophilus, and A. skirrowii | Ceftazidime | Cephalosporin | [51] |
| A. butzleri | Chloramphenicol | Amphenicol | [24,51] |
| A. butzleri | Ciprofloxacin | Fluoroquinolone | [30] |
| A. butzleri | Clindamycin | Lincomycin | [24] |
| A. butzleri | Erythromycin | Macrolide | [30] |
| A. butzleri | Gentamicin | Aminoglycoside | [30] |
| A. butzleri, A. cryaerophilus, and A. skirrowii | Nalidixic acid | Quinolone | [24,30,51] |
| A. butzleri | Oxacillin | Penicillin | [24] |
| A. butzleri | Penicillin G | Penicillin | [24] |
| A. butzleri | Streptomycin | Aminoglycoside | [30] |
| A. butzleri | Tetracycline | Tetracycline | [30,50] |
5. Genomic Traits Related to Antibiotic Resistance
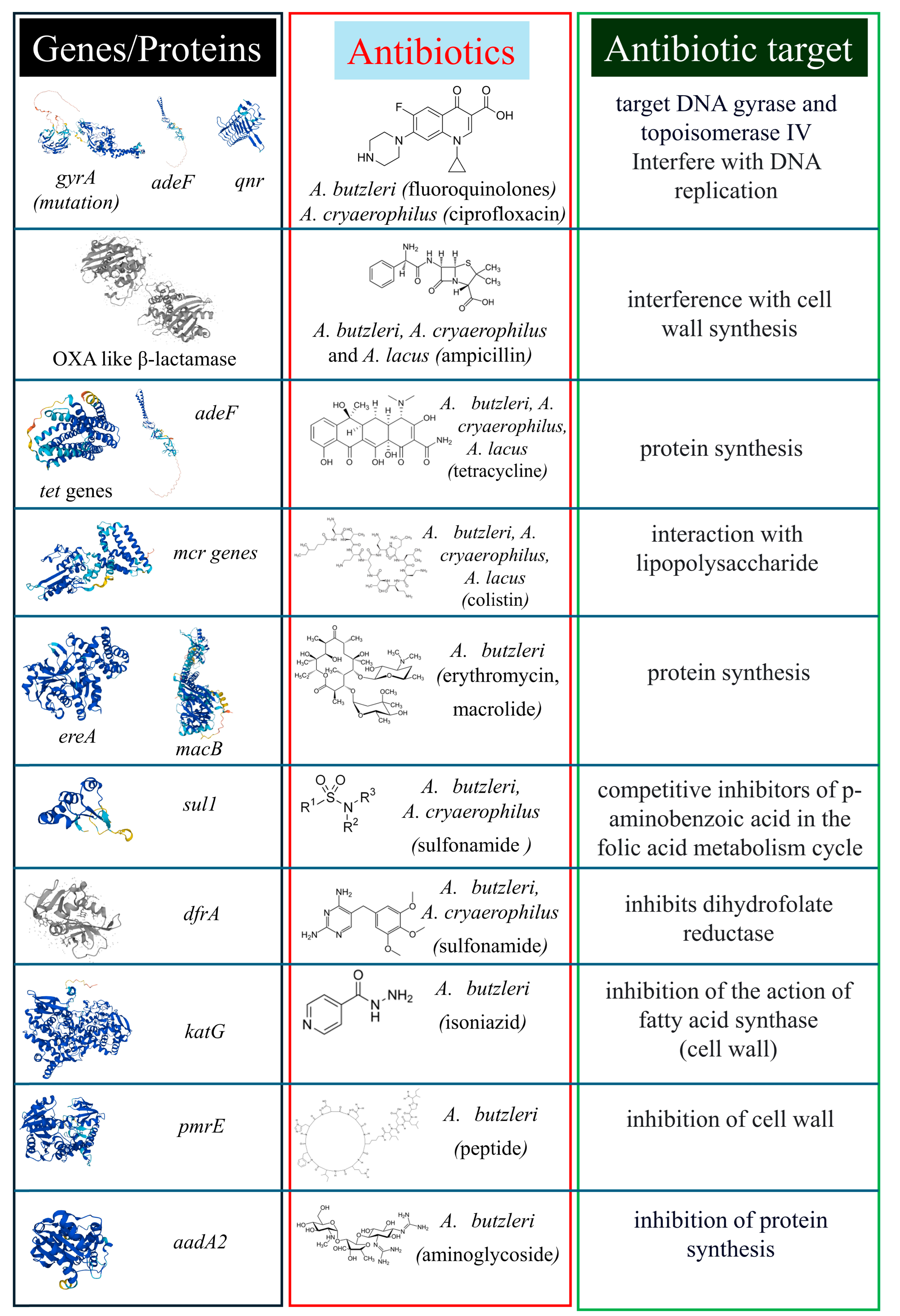
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Buzzanca, D.; Kerkhof, P.; Alessandria, V.; Rantsiou, K.; Houf, K. Arcobacteraceae comparative genome analysis demonstrates genome heterogeneity and reduction in species isolated from animals and associated with human illness. Heliyon 2023, 9, e17652. [Google Scholar] [CrossRef] [PubMed]
- Figueras, M.J.; Levican, A.; Pujol, I.; Ballester, F.; Quilez, M.J.R.; Gomez-Bertomeu, F. A severe case of persistent diarrhoea associated with Arcobacter cryaerophilus but attributed to Campylobacter sp. and a review of the clinical incidence of Arcobacter spp. New Microbes New Infect. 2014, 2, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Ramees, T.P.; Dhama, K.; Karthik, K.; Rathore, R.S.; Kumar, A.; Saminathan, M.; Tiwari, R.; Malik, Y.S.; Singh, R.K. Arcobacter: An emerging food-borne zoonotic pathogen, its public health concerns and advances in diagnosis and control—A comprehensive review. Vet. Q. 2017, 37, 136–161. [Google Scholar] [CrossRef]
- Vandamme, P.; Falsen, E.; Rossau, R.; Hoste, B.; Segers, P.; Tytgat, R.; De Ley, J. Revision of Campylobacter, Helicobacter, and Wolinella taxonomy: Emendation of generic descriptions and proposal of Arcobacter Gen. Nov. Int. J. Syst. Bacteriol. 1991, 41, 88–103. [Google Scholar] [CrossRef]
- Vandamme, P.; Vancanneyt, M.; Pot, B.; Mels, L.; Hoste, B.; Dewettinck, D.; Vlaes, L.; van den Borre, C.; Higgins, R.; Hommez, J. Polyphasic taxonomic study of the emended genus Arcobacter with Arcobacter butzleri Comb. Nov. and Arcobacter skirrowii Sp. Nov., an Aerotolerant bacterium isolated from veterinary specimens. Int. J. Syst. Bacteriol. 1992, 42, 344–356. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Atabay, H.I.; Corry, J.E.L. The prevalence of Campylobacters and Arcobacters in broiler chickens. J. Appl. Microbiol. 1997, 83, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Atabay, H.; Waino, M.; Madsen, M. Detection and diversity of various Arcobacter species in Danish poultry. Int. J. Food Microbiol. 2006, 109, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Çelik, E.; Otlu, S. Isolation of Arcobacter spp. and identification of isolates by multiplex PCR from various domestic poultry and wild avian species. Ann. Microbiol. 2020, 70, 60. [Google Scholar] [CrossRef]
- Houf, K.; On, S.L.W.; Coenye, T.; Debruyne, L.; De Smet, S.; Vandamme, P. Arcobacter thereius Sp. Nov., isolated from pigs and ducks. Int. J. Syst. Evol. Microbiol. 2009, 59, 2599–2604. [Google Scholar] [CrossRef]
- Ho, H.; Lipman, L.; Gaastra, W. Arcobacter, what is known and unknown about a potential foodborne zoonotic agent! Vet. Microbiol. 2006, 115, 1–13. [Google Scholar] [CrossRef]
- Pérez-Cataluña, A.; Salas-Massó, N.; Diéguez, A.L.; Balboa, S.; Lema, A.; Romalde, J.L.; Figueras, M.J. Revisiting the taxonomy of the genus Arcobacter: Getting order from the chaos. Front. Microbiol. 2018, 9, 2077. [Google Scholar] [CrossRef]
- González, A.; Ferrús, M.A. Study of Arcobacter Spp. Contamination in fresh lettuces detected by different cultural and molecular methods. Int. J. Food Microbiol. 2011, 145, 311–314. [Google Scholar] [CrossRef]
- Mottola, A.; Ciccarese, G.; Sinisi, C.; Savarino, A.E.; Marchetti, P.; Terio, V.; Tantillo, G.; Barrasso, R.; Di Pinto, A. Occurrence and characterization of Arcobacter spp. from ready-to-eat vegetables produced in Southern Italy. Ital. J. Food Saf. 2021, 10, 8585. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.H.; Park, S.M.; Kim, H.W.; Cho, T.J.; Kim, S.H.; Choi, C.; Rhee, M.S. Prevalence of pathogenic Arcobacter species in South Korea: Comparison of two protocols for isolating the bacteria from foods and examination of nine putative virulence genes. Food Microbiol. 2019, 78, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Uljanovas, D.; Gölz, G.; Brückner, V.; Grineviciene, A.; Tamuleviciene, E.; Alter, T.; Malakauskas, M. Prevalence, Antimicrobial susceptibility and virulence gene profiles of Arcobacter species isolated from human stool samples, foods of animal origin, ready-to-eat salad mixes and environmental water. Gut Pathog. 2021, 13, 76. [Google Scholar] [CrossRef]
- Chieffi, D.; Fanelli, F.; Fusco, V. Arcobacter butzleri: Up-to-date taxonomy, ecology, and pathogenicity of an emerging pathogen. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2071–2109. [Google Scholar] [CrossRef]
- WHO Antibiotic Resistance. World Health Organization Web Site 2020. pp. 1–9. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 2 April 2024).
- Jehanne, Q.; Bénéjat, L.; Ducournau, A.; Bessède, E.; Lehours, P. Molecular cut-off values for Aliarcobacter butzleri susceptibility testing. Microbiol. Spectr. 2022, 10, e01003-22. [Google Scholar] [CrossRef]
- Palladini, G.; Garbarino, C.; Luppi, A.; Russo, S.; Filippi, A.; Arrigoni, N.; Massella, E.; Ricchi, M. Comparison between broth microdilution and agar disk diffusion methods for antimicrobial susceptibility testing of bovine mastitis pathogens. J. Microbiol. Methods 2023, 212, 106796. [Google Scholar] [CrossRef]
- Lazou, T.P.; Chaintoutis, S.C. Comparison of disk diffusion and broth microdilution methods for antimicrobial susceptibility testing of Campylobacter isolates of meat origin. J. Microbiol. Methods 2023, 204, 106649. [Google Scholar] [CrossRef] [PubMed]
- Vicente-Martins, S.; Oleastro, M.; Domingues, F.C.; Ferreira, S. Arcobacter Spp. at retail food from Portugal: Prevalence, genotyping and antibiotics resistance. Food Control 2018, 85, 107–112. [Google Scholar] [CrossRef]
- Isidro, J.; Ferreira, S.; Pinto, M.; Domingues, F.; Oleastro, M.; Gomes, J.P.; Borges, V. Virulence and antibiotic resistance plasticity of Arcobacter butzleri: Insights on the genomic diversity of an emerging human pathogen. Infect. Genet. Evol. 2020, 80, 104213. [Google Scholar] [CrossRef] [PubMed]
- Yesilmen, S.; Vural, A.; Erkan, M.E.; Yildirim, I.H.; Guran, H.S. Prevalence and antibiotic resistance of Arcobacter spp. isolates from meats, meat products, and giblets. Acta Vet. Eurasia 2022, 48, 128–134. [Google Scholar] [CrossRef]
- Šilha, D.; Pejchalová, M.; Šilhová, L. Susceptibility to 18 drugs and multidrug resistance of Arcobacter isolates from different sources within the Czech Republic. J. Glob. Antimicrob. Resist. 2017, 9, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Kanaan, M.H.G. Prevalence, resistance to antimicrobials, and antibiotypes of Arcobacter species recovered from retail meat in wasit marketplaces in iraq. Int. J. One Health 2021, 7, 142–150. [Google Scholar] [CrossRef]
- Hänel, I.; Hotzel, H.; Tomaso, H.; Busch, A. Antimicrobial susceptibility and genomic structure of Arcobacter skirrowii isolates. Front. Microbiol. 2018, 9, 3067. [Google Scholar] [CrossRef] [PubMed]
- Ghaffar, L.A.; Eissa, A.E.; Elhariri, M.; Abdelsalam, M.; Al-Amry, K. Comparative analysis of 16S rRNA based phylogeny, antibiotic susceptibility, and virulence traits of Arcobacter species recovered from domestic fowl and the Nile Tilapia. Egypt. J. Aquat. Biol. Fish. 2021, 25, 263–279. [Google Scholar] [CrossRef]
- Jasim, S.A.; Al-abodi, H.R.; Ali, W.S. Resistance rate and novel virulence factor determinants of Arcobacter spp., from cattle fresh meat products from Iraq. Microb. Pathog. 2021, 152, 104649. [Google Scholar] [CrossRef]
- Lameei, A.; Rahimi, E.; Shakerian, A.; Momtaz, H. Genotyping, antibiotic resistance and prevalence of Arcobacter species in milk and dairy products. Vet. Med. Sci. 2022, 8, 1841–1849. [Google Scholar] [CrossRef]
- Gabucci, C.; Baldelli, G.; Amagliani, G.; Schiavano, G.F.; Savelli, D.; Russo, I.; Di Lullo, S.; Blasi, G.; Napoleoni, M.; Leoni, F.; et al. Widespread multidrug resistance of Arcobacter butzleri isolated from clinical and food sources in Central Italy. Antibiotics 2023, 12, 1292. [Google Scholar] [CrossRef]
- Chaiyasaen, N.; Direksin, K.; Thitima, N.; Nopwinyoowong, S. Prevalence, antibiograms, antibiotic resistance genes, and virulence genes of Arcobacter butzleri isolated from healthy pigs in Mid-Northeastern Thailand. Vet. Integr. Sci. 2023, 21, 309–331. [Google Scholar] [CrossRef]
- Paintsil, E.K.; Ofori, L.A.; Akenten, C.W.; Zautner, A.E.; Mbwana, J.; Khan, N.A.; Lusingu, J.P.A.; Kaseka, J.; Minja, D.T.R.; Gesase, S.; et al. Antibiotic-resistant Arcobacter spp. in commercial and smallholder farm animals in Asante Akim North Municipality, Ghana and Korogwe Town Council, Tanzania: A Cross-Sectional Study. Gut Pathog. 2023, 15, 63. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Malaxetxebarria, I.; Girbau, C.; Salazar-Sánchez, A.; Baztarrika, I.; Martínez-Ballesteros, I.; Laorden, L.; Alonso, R.; Fernández-Astorga, A. genetic characterization and biofilm formation of potentially pathogenic foodborne Arcobacter isolates. Int. J. Food Microbiol. 2022, 373, 109712. [Google Scholar] [CrossRef] [PubMed]
- Šilha, D.; Sirotková, S.; Švarcová, K.; Hofmeisterová, L.; Koryčanová, K.; Šilhová, L. Biofilm formation ability of Arcobacter-like and Campylobacter strains under different conditions and on food processing materials. Microorganisms 2021, 9, 2017. [Google Scholar] [CrossRef] [PubMed]
- Elmali, M.; Can, H.Y. Occurence and antimicrobial resistance of Arcobacter species in food and slaughterhouse samples. Food Sci. Technol. 2017, 37, 280–285. [Google Scholar] [CrossRef]
- Botta, C.; Buzzanca, D.; Chiarini, E.; Chiesa, F.; Rubiola, S.; Ferrocino, I.; Fontanella, E.; Rantsiou, K.; Houf, K.; Alessandria, V. Microbial contamination pathways in a poultry abattoir provided clues on the distribution and persistence of Arcobacter spp. Appl. Environ. Microbiol. 2024, 90, e00296-24. [Google Scholar] [CrossRef]
- Chiarini, E.; Buzzanca, D.; Chiesa, F.; Botta, C.; Rantsiou, K.; Houf, K.; Alessandria, V. Exploring multi-antibiotic resistance in Arcobacter butzleri isolates from a poultry processing plant in Northern Italy: An in-depth inquiry. Food Control 2024, 163, 110500. [Google Scholar] [CrossRef]
- Ferreira, S.; Oleastro, M.; Domingues, F.C. Occurrence, genetic diversity and antibiotic resistance of Arcobacter Sp. in a dairy plant. J. Appl. Microbiol. 2017, 123, 1019–1026. [Google Scholar] [CrossRef]
- Xiang, S.; Wang, X.; Ma, W.; Liu, X.; Zhang, B.; Huang, F.; Liu, F.; Guan, X. Response of microbial communities of karst river water to antibiotics and microbial source tracking for antibiotics. Sci. Total Environ. 2020, 706, 135730. [Google Scholar] [CrossRef]
- Sciortino, S.; Arculeo, P.; Alio, V.; Cardamone, C.; Nicastro, L.; Arculeo, M.; Alduina, R.; Costa, A. Occurrence and antimicrobial resistance of Arcobacter spp. recovered from aquatic environments. Antibiotics 2021, 10, 288. [Google Scholar] [CrossRef]
- Khan, I.U.H.; Chen, W.; Cloutier, M.; Lapen, D.R.; Craiovan, E.; Wilkes, G. Pathogenicity assessment of Arcobacter butzleri isolated from Canadian agricultural surface water. BMC Microbiol. 2024, 24, 17. [Google Scholar] [CrossRef]
- Fanelli, F.; Di Pinto, A.; Mottola, A.; Mule, G.; Chieffi, D.; Baruzzi, F.; Tantillo, G.; Fusco, V. Genomic characterization of Arcobacter butzleri isolated from shellfish: Novel insight into antibiotic resistance and virulence determinants. Front. Microbiol. 2019, 10, 670. [Google Scholar] [CrossRef]
- Rathlavath, S.; Kohli, V.; Singh, A.S.; Lekshmi, M.; Tripathi, G.; Kumar, S.; Nayak, B.B. Virulence genotypes and antimicrobial susceptibility patterns of Arcobacter butzleri isolated from seafood and its environment. Int. J. Food Microbiol. 2017, 263, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Nelapati, S.; Tumati, S.R.; Thirtham, M.R.; Ramani Pushpa, R.N.; Kamisetty, A.K.; Ch, B.K. Occurrence, virulence gene and antimicrobial susceptibility profiles of Arcobacter Sp. isolated from Catla (Catla Catla) in India. Lett. Appl. Microbiol. 2020, 70, 365–371. [Google Scholar] [CrossRef]
- Novak, A.; Vuko-Tokić, M.; Žitko, V.; Tonkić, M. Prolonged watery diarrhea and malnutrition caused by Aliarcobacter butzleri (formerly Arcobacter butzleri): The first pediatric case in Croatia and a literature review. Infez. Med. 2024, 32, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Brückner, V.; Fiebiger, U.; Ignatius, R.; Friesen, J.; Eisenblätter, M.; Höck, M.; Alter, T.; Bereswill, S.; Gölz, G.; Heimesaat, M.M. Prevalence and antimicrobial susceptibility of Arcobacter species in human stool samples derived from out- and inpatients: The prospective German Arcobacter prevalence study arcopath. Gut Pathog. 2020, 12, 21. [Google Scholar] [CrossRef] [PubMed]
- Rathore, A.; Patel, F.; Gupta, N.; Asiimwe, D.D.; Rollini, F.; Ravi, M. First case of Arcobacter species isolated in pericardial fluid in an HIV and COVID-19 patient with worsening cardiac tamponade. IDCases 2023, 32, e01771. [Google Scholar] [CrossRef]
- Kerkhof, P.-J.; Van den Abeele, A.-M.; Strubbe, B.; Vogelaers, D.; Vandamme, P.; Houf, K. Diagnostic approach for detection and identification of emerging enteric pathogens revisited: The (Ali)Arcobacter lanthieri case. New Microbes New Infect. 2021, 39, 100829. [Google Scholar] [CrossRef]
- Van den Abeele, A.-M.; Vogelaers, D.; Vanlaere, E.; Houf, K. Antimicrobial susceptibility testing of Arcobacter butzleri and Arcobacter cryaerophilus strains isolated from Belgian patients. J. Antimicrob. Chemother. 2016, 71, 1241–1244. [Google Scholar] [CrossRef]
- Baztarrika, I.; Salazar-Sánchez, A.; Hernaez Crespo, S.; López Mirones, J.I.; Canut, A.; Alonso, R.; Martínez-Ballesteros, I.; Martinez-Malaxetxebarria, I. Virulence genotype and phenotype of two clinical isolates of Arcobacter butzleri obtained from patients with different pathologies. Arch. Microbiol. 2023, 205, 369. [Google Scholar] [CrossRef]
- Khalili Dermani, S.; Akbari, M.; Arjomandzadegan, M.; Ahmadi, A. Prevalence, Comparison of diagnostic methods, antibiogram, and genotyping of Arcobacter spp. in diarrheal cases referring to clinical centers in Iran. Infect. Epidemiol. Microbiol. 2022, 8, 107–119. [Google Scholar] [CrossRef]
- Müller, E.; Hotzel, H.; Ahlers, C.; Hänel, I.; Tomaso, H.; Abdel-Glil, M.Y. Genomic analysis and antimicrobial resistance of Aliarcobacter cryaerophilus strains from german water poultry. Front. Microbiol. 2020, 11, 1549. [Google Scholar] [CrossRef] [PubMed]
- Barel, M.; Yildirim, Y. Arcobacter species isolated from various seafood and water sources; virulence genes, antibiotic resistance genes and molecular characterization. World J. Microbiol. Biotechnol. 2023, 39, 183. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Ju, C.; Zhou, G.; Yu, M.; Chen, H.; He, J.; Zhang, M.; Duan, Y. Genetic characteristics, antimicrobial resistance, and prevalence of Arcobacter Spp. isolated from various sources in Shenzhen, China. Front. Microbiol. 2022, 13, 1004224. [Google Scholar] [CrossRef] [PubMed]
- Parisi, A.; Capozzi, L.; Bianco, A.; Caruso, M.; Latorre, L.; Costa, A.; Giannico, A.; Ridolfi, D.; Bulzacchelli, C.; Santagada, G. Identification of virulence and antibiotic resistance factors in Arcobacter butzleri isolated from bovine milk by whole genome sequencing. Ital. J. Food Saf. 2019, 8, 7840. [Google Scholar] [CrossRef] [PubMed]
- Zautner, A.E.; Riedel, T.; Bunk, B.; Spröer, C.; Boahen, K.G.; Akenten, C.W.; Dreyer, A.; Färber, J.; Kaasch, A.J.; Overmann, J.; et al. Molecular characterization of Arcobacter butzleri isolates from poultry in rural Ghana. Front. Cell. Infect. Microbiol. 2023, 13, 1094067. [Google Scholar] [CrossRef] [PubMed]
- Bateman, A.; Martin, M.-J.; Orchard, S.; Magrane, M.; Ahmad, S.; Alpi, E.; Bowler-Barnett, E.H.; Britto, R.; Bye-A-Jee, H.; Cukura, A.; et al. UniProt: The universal protein knowledgebase in 2023. Nucleic Acids Res. 2023, 51, D523–D531. [Google Scholar] [CrossRef]
- Sultan, I.; Rahman, S.; Jan, A.T.; Siddiqui, M.T. Antibiotics, resistome and resistance mechanisms: A bacterial perspective. Front. Microbiol. 2018, 9, 2066. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buzzanca, D.; Chiarini, E.; Alessandria, V. Arcobacteraceae: An Exploration of Antibiotic Resistance Featuring the Latest Research Updates. Antibiotics 2024, 13, 669. https://doi.org/10.3390/antibiotics13070669
Buzzanca D, Chiarini E, Alessandria V. Arcobacteraceae: An Exploration of Antibiotic Resistance Featuring the Latest Research Updates. Antibiotics. 2024; 13(7):669. https://doi.org/10.3390/antibiotics13070669
Chicago/Turabian StyleBuzzanca, Davide, Elisabetta Chiarini, and Valentina Alessandria. 2024. "Arcobacteraceae: An Exploration of Antibiotic Resistance Featuring the Latest Research Updates" Antibiotics 13, no. 7: 669. https://doi.org/10.3390/antibiotics13070669
APA StyleBuzzanca, D., Chiarini, E., & Alessandria, V. (2024). Arcobacteraceae: An Exploration of Antibiotic Resistance Featuring the Latest Research Updates. Antibiotics, 13(7), 669. https://doi.org/10.3390/antibiotics13070669







