Green Synthesis of Silver Nanoparticle Using Black Mulberry and Characterization, Phytochemical, and Bioactivity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of BM-AgNPs
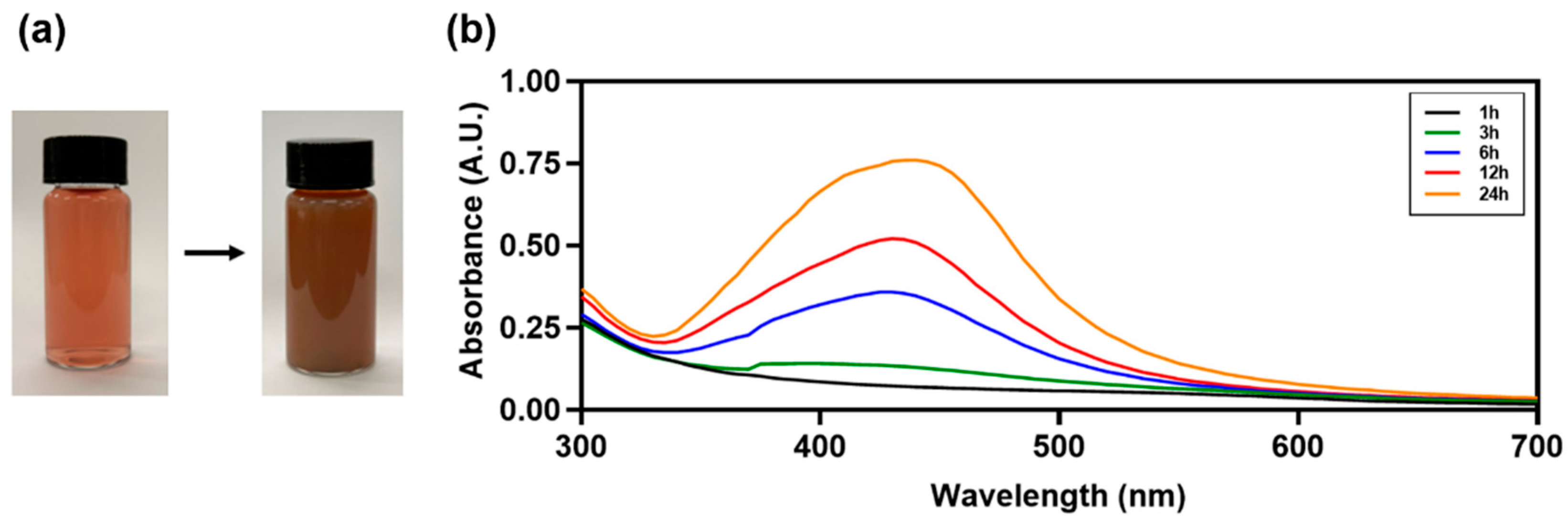
2.1.1. UV-Vis Absorbance Spectra and Color Change
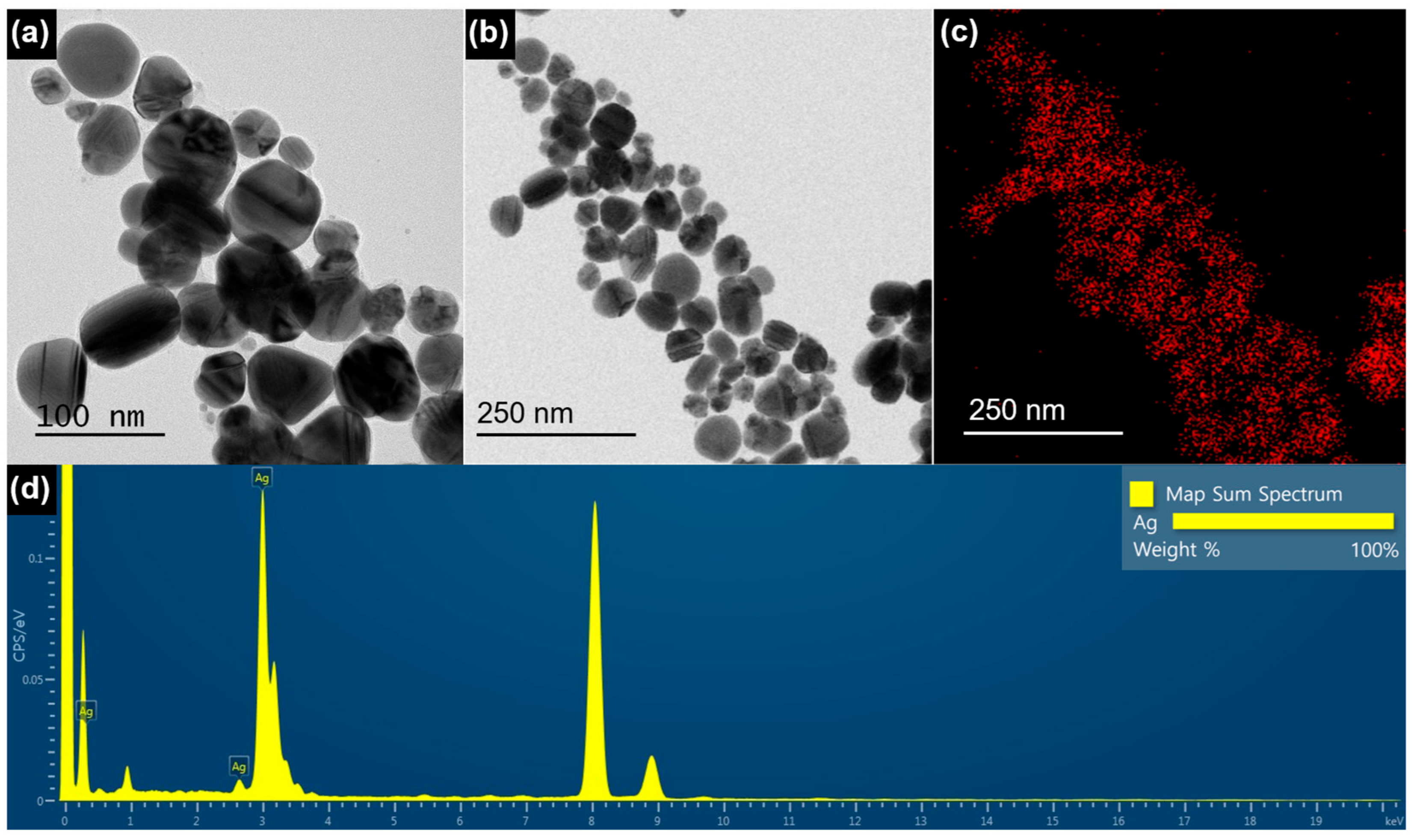
2.1.2. TEM
2.1.3. Particle Size, PDI and Zeta Potential
2.1.4. XRD
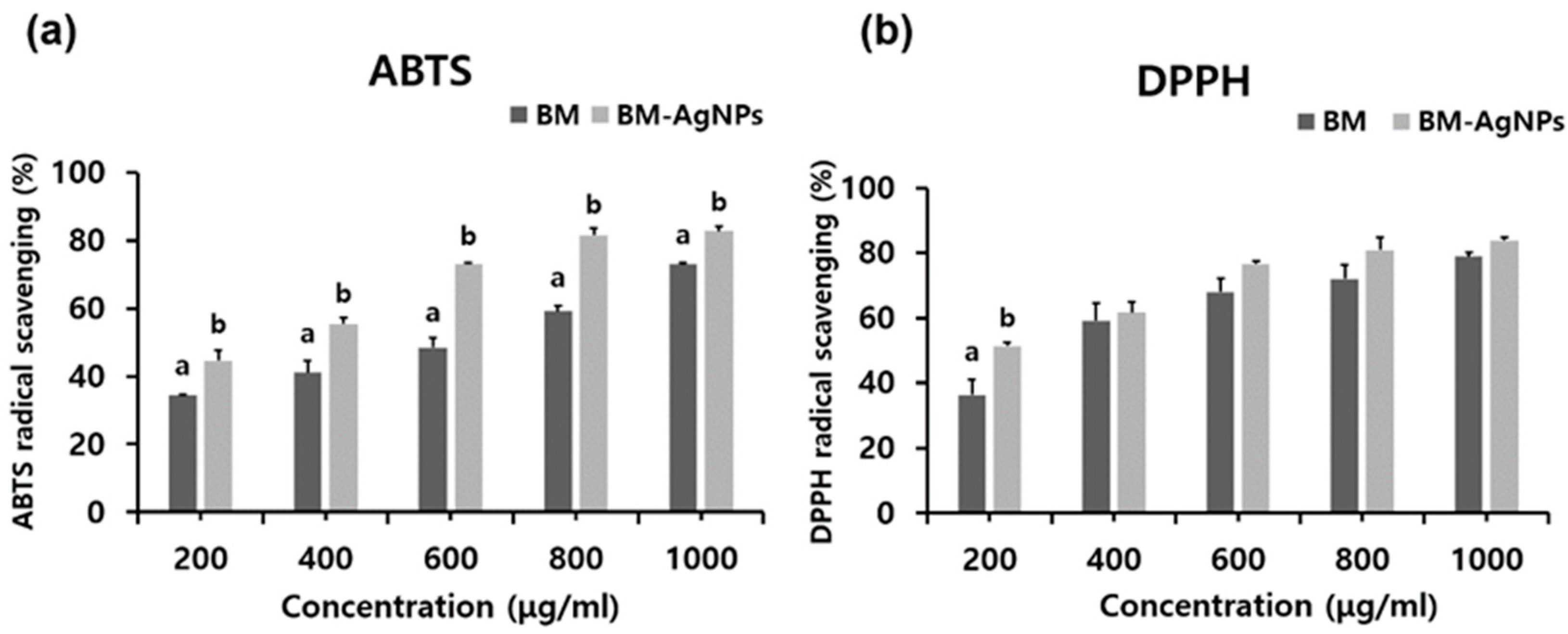
2.2. Phytochemical of BM-AgNPs
2.3. Antibacterial Activities
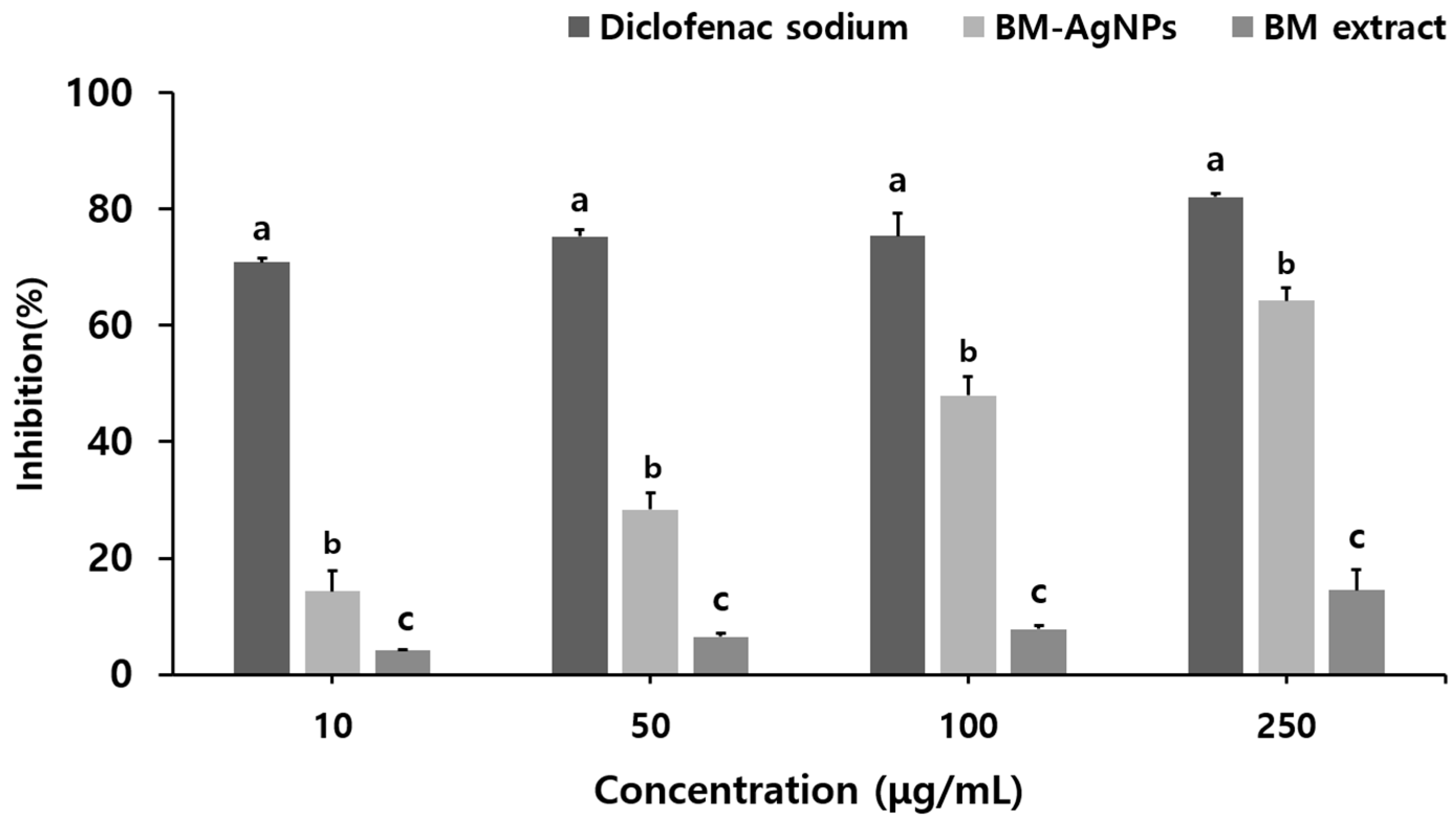
2.4. Anti-Inflammatory
2.5. Anti-Cancer
3. Materials and Methods
3.1. Materials
3.2. Preparation of BM Extract
3.3. Synthesis of BM-AgNPs
3.4. Characterization of BM-AgNPs
3.5. Phytochemical of BM-AgNPs
3.5.1. TFC
3.5.2. TPC
3.5.3. TAC
- A (absorbance) = (A 510nm − A 700nm) pH = 1.0 − (A 500nm − A 700 nm) pH = 4.5
- M (molecular weight) = 449.2 g/mol for C3G
- DF (dilution factor)
- 1000 = conversion from g to mg
- ε (molar absorptivity coefficient in L/mol/cm for C3G) = 26,900
- d (path length) = 1 cm
3.6. Antioxidant Activity
3.6.1. ABTS
3.6.2. DPPH
3.7. Antibacterial Activity
3.8. Anti-Inflammatory
3.9. Anti-Cancer
3.9.1. Cell Culture
3.9.2. Cell Viability (%)
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saxena, S.K.; Nyodu, R.; Kumar, S.; Maurya, V.K. Current advances in nanotechnology and medicine. In NanoBioMedicine; Springer: Singapore, 2020; pp. 3–16. [Google Scholar]
- Mani, M.; Okla, M.K.; Selvaraj, S.; Ram Kumar, A.; Kumaresan, S.; Muthukumaran, A.; Kaviyarasu, K.; El-Tayeb, M.A.; Elbadawi, Y.B.; Almaary, K.S.; et al. A novel biogenic Allium cepa leaf mediated silver nanoparticles for antimicrobial, antioxidant, and anticancer effects on MCF-7 cell line. Environ. Res. 2021, 198, 111199. [Google Scholar] [CrossRef] [PubMed]
- Marinescu, L.; Ficai, D.; Oprea, O.; Marin, A.; Ficai, A.; Andronescu, E.; Holban, A.-M. Optimized synthesis approaches of metal nanoparticles with antimicrobial applications. J. Nanomater. 2020, 2020, 6651207. [Google Scholar] [CrossRef]
- Alahmad, A.; Feldhoff, A.; Bigall, N.C.; Rusch, P.; Scheper, T.; Walter, J.-G. Hypericum perforatum L.-mediated green synthesis of silver nanoparticles exhibiting antioxidant and anticancer activities. Nanomaterials 2021, 11, 487. [Google Scholar] [CrossRef] [PubMed]
- Kumkoon, T.; Srisaisap, M.; Boonserm, P. Biosynthesized Silver Nanoparticles Using Morus alba (White Mulberry) Leaf Extract as Potential Antibacterial and Anticancer Agents. Molecules 2023, 28, 1213. [Google Scholar] [CrossRef] [PubMed]
- Wongpreecha, J.; Polpanich, D.; Suteewong, T.; Kaewsaneha, C.; Tangboriboonrat, P. One-pot, large-scale green synthesis of silver nanoparticles-chitosan with enhanced antibacterial activity and low cytotoxicity. Carbohydr. Polym. 2018, 199, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Haque, S.; Norbert, C.C.; Acharyya, R.; Mukherjee, S.; Kathirvel, M.; Patra, C.R. Biosynthesized silver nanoparticles for cancer therapy and in vivo bioimaging. Cancers 2021, 13, 6114. [Google Scholar] [CrossRef] [PubMed]
- Chugh, D.; Viswamalya, V.S.; Das, B. Green synthesis of silver nanoparticles with algae and the importance of capping agents in the process. J. Genet. Eng. Biotechnol. 2021, 19, 126. [Google Scholar] [CrossRef]
- Kumar, S.; Basumatary, I.B.; Sudhani, H.P.K.; Bajpai, V.K.; Chen, L.; Shukla, S.; Mukherjee, A. Plant extract mediated silver nanoparticles and their applications as antimicrobials and in sustainable food packaging: A state-of-the-art review. Trends Food Sci. Technol. 2021, 112, 651–666. [Google Scholar] [CrossRef]
- Liu, Y.; Hussain, M.; Memon, H.; Yasin, S. Solar irradiation and Nageia nagi extract assisted rapid synthesis of silver nanoparticles and their antibacterial activity. Dig. J. Nanomater. Biostruct. 2015, 10, 1019–1024. [Google Scholar]
- Ghatage, M.M.; Mane, P.A.; Gambhir, R.P.; Parkhe, V.S.; Kamble, P.A.; Lokhande, C.D.; Tiwari, A.P. Green synthesis of silver nanoparticles via Aloe barbadensis miller leaves: Anticancer, antioxidative, antimicrobial and photocatalytic properties. Appl. Surf. Sci. Adv. 2023, 16, 100426. [Google Scholar] [CrossRef]
- Jain, S.; Mehata, M.S. Medicinal plant leaf extract and pure flavonoid mediated green synthesis of silver nanoparticles and their enhanced antibacterial property. Sci. Rep. 2017, 7, 15867. [Google Scholar] [CrossRef] [PubMed]
- Raji, P.; Samrot, A.V.; Keerthana, D.; Karishma, S. Antibacterial activity of alkaloids, flavonoids, saponins and tannins mediated green synthesised silver nanoparticles against Pseudomonas aeruginosa and Bacillus subtilis. J. Clust. Sci. 2019, 30, 881–895. [Google Scholar] [CrossRef]
- Farshori, N.N.; Al-Oqail, M.M.; Al-Sheddi, E.S.; Al-Massarani, S.M.; Saquib, Q.; Siddiqui, M.A.; Wahab, R.; Al-Khedhairy, A.A. Green synthesis of silver nanoparticles using Phoenix dactylifera seed extract and its anticancer effect against human lung adenocarcinoma cells. J. Drug Deliv. Sci. Technol. 2022, 70, 103260. [Google Scholar] [CrossRef]
- Pirtarighat, S.; Ghannadnia, M.; Baghshahi, S. Green synthesis of silver nanoparticles using the plant extract of Salvia spinosa grown in vitro and their antibacterial activity assessment. J. Nanostruct. Chem. 2018, 9, 1–9. [Google Scholar] [CrossRef]
- Ahmed, S.; Ahmad, M.; Swami, B.L.; Ikram, S. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: A green expertise. J. Adv. Res. 2016, 7, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, S.; Siddique, F.; Ameer, K.; Ahmad, R.S.; Hameed, A.; Ebad, A.; Mohamed Ahmed, I.A.; Shibli, S. Effects of white mulberry powder fortification on antioxidant activity, physicochemical, microbial and sensorial properties of yogurt produced from buffalo milk. Food Sci. Nutr. 2023, 11, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Suriyaprom, S.; Kaewkod, T.; Promputtha, I.; Desvaux, M.; Tragoolpua, Y. Evaluation of Antioxidant and Antibacterial Activities of White Mulberry (Morus alba L.) Fruit Extracts. Plants 2021, 10, 2736. [Google Scholar] [CrossRef] [PubMed]
- Turan, E.; Simsek, A. Effects of lyophilized black mulberry water extract on lipid oxidation, metmyoglobin formation, color stability, microbial quality and sensory properties of beef patties stored under aerobic and vacuum packaging conditions. Meat Sci. 2021, 178, 108522. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Salcedo, E.M.; Mena, P.; García-Viguera, C.; Martínez, J.J.; Hernández, F. Phytochemical evaluation of white (Morus alba L.) and black (Morus nigra L.) mulberry fruits, a starting point for the assessment of their beneficial properties. J. Funct. Foods 2015, 12, 399–408. [Google Scholar] [CrossRef]
- Zhou, M.; Chen, Q.; Bi, J.; Wang, Y.; Wu, X. Degradation kinetics of cyanidin 3-O-glucoside and cyanidin 3-O-rutinoside during hot air and vacuum drying in mulberry (Morus alba L.) fruit: A comparative study based on solid food system. Food Chem. 2017, 229, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, X.; Ai, J.; Yue, Z.; Wang, Y.; Bao, B.; Tian, L.; Bai, W. Interaction between black mulberry pectin-rich fractions and cyanidin-3-O-glucoside under in vitro digestion. Food Hydrocoll. 2023, 134, 108110. [Google Scholar] [CrossRef]
- Deniz, G.; Laloglu, E.; Koc, K.; Nadaroglu, H.; Geyikoglu, F. The effect of black mulberry (Morus nigra) extract on carbon tetrachloride-induced liver damage. Arch. Biol. Sci. 2018, 70, 371–378. [Google Scholar] [CrossRef]
- Chen, Q.; Li, Z.; Bi, J.; Zhou, L.; Yi, J.; Wu, X. Effect of hybrid drying methods on physicochemical, nutritional and antioxidant properties of dried black mulberry. LWT 2017, 80, 178–184. [Google Scholar] [CrossRef]
- Manzoor, S.; Qayoom, K. Morus nigra L. (Black mulberry): A plant with potent medicinal value. South Asian J. Agric. Sci. 2023, 3, 60–63. [Google Scholar] [CrossRef]
- Kanimozhi, S.; Durga, R.; Sabithasree, M.; Kumar, A.V.; Sofiavizhimalar, A.; Kadam, A.A.; Rajagopal, R.; Sathya, R.; Azelee, N.I.W. Biogenic synthesis of silver nanoparticle using Cissus quadrangularis extract and its invitro study. J. King Saud Univ. Sci. 2022, 34, 101930. [Google Scholar] [CrossRef]
- Baghizadeh, A.; Ranjbar, S.; Gupta, V.K.; Asif, M.; Pourseyedi, S.; Karimi, M.J.; Mohammadinejad, R. Green synthesis of silver nanoparticles using seed extract of Calendula officinalis in liquid phase. J. Mol. Liq. 2015, 207, 159–163. [Google Scholar] [CrossRef]
- Alsammarraie, F.K.; Wang, W.; Zhou, P.; Mustapha, A.; Lin, M. Green synthesis of silver nanoparticles using turmeric extracts and investigation of their antibacterial activities. Colloids Surf. B Biointerfaces 2018, 171, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Mittal, J.; Jain, R.; Sharma, M.M. Phytofabrication of silver nanoparticles using aqueous leaf extract of Xanthium strumerium L. and their bactericidal efficacy. Adv. Nat. Sci. Nanosci. Nanotechnol. 2017, 8, 025011. [Google Scholar] [CrossRef]
- Mittal, J.; Singh, A.; Batra, A.; Sharma, M.M. Synthesis and characterization of silver nanoparticles and their antimicrobial efficacy. Part. Sci. Technol. 2017, 35, 338–345. [Google Scholar] [CrossRef]
- Azmi, S.N.H.; Al-Jassasi, B.M.H.; Al-Sawafi, H.M.S.; Al-Shukaili, S.H.G.; Rahman, N.; Nasir, M. Optimization for synthesis of silver nanoparticles through response surface methodology using leaf extract of Boswellia sacra and its application in antimicrobial activity. Environ. Monit. Assess. 2021, 193, 497. [Google Scholar] [CrossRef]
- Chand, K.; Jiao, C.; Lakhan, M.N.; Shah, A.H.; Kumar, V.; Fouad, D.E.; Chandio, M.B.; Maitlo, A.A.; Ahmed, M.; Cao, D. Green synthesis, characterization and photocatalytic activity of silver nanoparticles synthesized with Nigella Sativa seed extract. Chem. Phys. Lett. 2021, 763, 138218. [Google Scholar] [CrossRef]
- Wilson, B.K.; Prud’homme, R.K. Nanoparticle size distribution quantification from transmission electron microscopy (TEM) of ruthenium tetroxide stained polymeric nanoparticles. J. Colloid. Interface Sci. 2021, 604, 208–220. [Google Scholar] [CrossRef] [PubMed]
- Al-Khafaji, M.A.; Gaal, A.; Wacha, A.; Bota, A.; Varga, Z. Particle Size Distribution of Bimodal Silica Nanoparticles: A Comparison of Different Measurement Techniques. Materials 2020, 13, 3101. [Google Scholar] [CrossRef] [PubMed]
- Scherer, M.D.; Sposito, J.C.; Falco, W.F.; Grisolia, A.B.; Andrade, L.H.; Lima, S.M.; Machado, G.; Nascimento, V.A.; Gonçalves, D.A.; Wender, H. Cytotoxic and genotoxic effects of silver nanoparticles on meristematic cells of Allium cepa roots: A close analysis of particle size dependence. Sci. Total Environ. 2019, 660, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Tantra, R.; Schulze, P.; Quincey, P. Effect of nanoparticle concentration on zeta-potential measurement results and reproducibility. Particuology 2010, 8, 279–285. [Google Scholar] [CrossRef]
- Pochapski, D.J.; Carvalho dos Santos, C.; Leite, G.W.; Pulcinelli, S.H.; Santilli, C.V. Zeta potential and colloidal stability predictions for inorganic nanoparticle dispersions: Effects of experimental conditions and electrokinetic models on the interpretation of results. Langmuir 2021, 37, 13379–13389. [Google Scholar] [CrossRef] [PubMed]
- Riaz, M.; Mutreja, V.; Sareen, S.; Ahmad, B.; Faheem, M.; Zahid, N.; Jabbour, G.; Park, J. Exceptional antibacterial and cytotoxic potency of monodisperse greener AgNPs prepared under optimized pH and temperature. Sci. Rep. 2021, 11, 2866. [Google Scholar] [CrossRef] [PubMed]
- Chinni, S.V.; Gopinath, S.C.B.; Anbu, P.; Fuloria, N.K.; Fuloria, S.; Mariappan, P.; Krusnamurthy, K.; Veeranjaneya Reddy, L.; Ramachawolran, G.; Sreeramanan, S.; et al. Characterization and Antibacterial Response of Silver Nanoparticles Biosynthesized Using an Ethanolic Extract of Coccinia indica Leaves. Crystals 2021, 11, 97. [Google Scholar] [CrossRef]
- Yi, Z.; Xu, X.; Fang, Q.; Wang, Y.; Li, X.; Tan, X.; Luo, J.; Jiang, X.; Wu, W.; Yi, Y. Fabrication of silver nanosheets on quartz glass substrates through electroless plating approach. Appl. Phys. A 2014, 114, 485–493. [Google Scholar] [CrossRef]
- Ryu, S.; Nam, S.H.; Baek, J.S. Green Synthesis of Silver Nanoparticles (AgNPs) of Angelica Gigas Fabricated by Hot-Melt Extrusion Technology for Enhanced Antifungal Effects. Materials 2022, 15, 7231. [Google Scholar] [CrossRef] [PubMed]
- Noori, A.; Donnelly, T.; Colbert, J.; Cai, W.; Newman, L.A.; White, J.C. Exposure of tomato (Lycopersicon esculentum) to silver nanoparticles and silver nitrate: Physiological and molecular response. Int. J. Phytoremediation 2020, 22, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Shavalibor, A.; Esmaeilzadeh Bahabadi, S. Effect of biologically synthesized silver nanoparticles on Melissa officinalis L. Evaluation of growth parameters, secondary metabolites and antioxidant enzymes. Iran. J. Plant Physiol. 2021, 11, 3799–3809. [Google Scholar]
- Krajewska, J.B.; Dlugosz, O.; Salaga, M.; Banach, M.; Fichna, J. Silver nanoparticles based on blackcurrant extract show potent anti-inflammatory effect in vitro and in DSS-induced colitis in mice. Int. J. Pharm. 2020, 585, 119549. [Google Scholar] [CrossRef] [PubMed]
- Campos, A.M.; Lissi, E.A. Kinetics of the reaction between 2, 2′-azinobis (3-ethylbenzothiazoline-6-sulfonic acid (ABTS) derived radical cations and phenols. Int. J. Chem. Kinet. 1997, 29, 219–224. [Google Scholar] [CrossRef]
- Siakavella, I.K.; Lamari, F.; Papoulis, D.; Orkoula, M.; Gkolfi, P.; Lykouras, M.; Avgoustakis, K.; Hatziantoniou, S. Effect of Plant Extracts on the Characteristics of Silver Nanoparticles for Topical Application. Pharmaceutics 2020, 12, 1244. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Blas, M.; Maldonado-Luna, N.M.; Rivera-Quiñones, C.M.; Vega-Avila, A.L.; Roman-Velázquez, F.R.; Perales-Perez, O.J. Single Step Microwave Assisted Synthesis and Antimicrobial Activity of Silver, Copper and Silver-Copper Nanoparticles. J. Mater. Sci. Chem. Eng. 2020, 8, 13–29. [Google Scholar] [CrossRef]
- Sukeri, S.; Karem, A.A.; Kamarudin, E.; Bahari, M. Antimicrobial Activity of Methanolic and Aqueous Extract of Rhodomyrtus tomentosa Leaves against Staphylococcus aureus and Escherichia coli. J. Pure Appl. Microbiol. 2021, 15, 186–193. [Google Scholar] [CrossRef]
- Naghmouchi, S.; Al-Zaban, M.I.; Al-Zaben, M.; Alharbi, N.; Bahatheq, A.; Gnilitskyi, I. Generation and Characterization of Silver Nanoparticles in Mentha pulegium Extract and Evaluation of Biological Activities of the Prepared Extract. J. Nanomater. 2022, 2022, 5410274. [Google Scholar] [CrossRef]
- Nogueira, S.S.; de Araujo-Nobre, A.R.; Mafud, A.C.; Guimaraes, M.A.; Alves, M.M.M.; Placido, A.; Carvalho, F.A.A.; Arcanjo, D.D.R.; Mascarenhas, Y.; Costa, F.G.; et al. Silver nanoparticle stabilized by hydrolyzed collagen and natural polymers: Synthesis, characterization and antibacterial-antifungal evaluation. Int. J. Biol. Macromol. 2019, 135, 808–814. [Google Scholar] [CrossRef] [PubMed]
- Ontong, J.C.; Singh, S.; Nwabor, O.F.; Chusri, S.; Voravuthikunchai, S.P. Potential of antimicrobial topical gel with synthesized biogenic silver nanoparticle using Rhodomyrtus tomentosa leaf extract and silk sericin. Biotechnol. Lett. 2020, 42, 2653–2664. [Google Scholar] [CrossRef] [PubMed]
- Mahadevan, S.; Vijayakumar, S.; Arulmozhi, P. Green synthesis of silver nano particles from Atalantia monophylla (L) Correa leaf extract, their antimicrobial activity and sensing capability of H(2)O(2). Microb. Pathog. 2017, 113, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi-Derazkola, S.; Hosseinzadeh, M.; Yousefinia, A.; Naghizadeh, A. Green Synthesis and Investigation of Antibacterial Activity of Silver Nanoparticles Using Eryngium bungei Boiss Plant Extract. J. Polym. Environ. 2021, 29, 2978–2985. [Google Scholar] [CrossRef]
- Dong, C.; Cheng, F.; Zhang, X.; Wang, X.; Yang, X.; Bin, Y. Rapid and green synthesis of monodisperse silver nanoparticles using mulberry leaf extract. Rare Met. Mater. Eng. 2018, 47, 1089–1095. [Google Scholar]
- Khan, M.A.; Moghul, N.B.; Butt, M.A.; Kiyani, M.M.; Zafar, I.; Bukhari, A.I. Assessment of antibacterial and antifungal potential of Curcuma longa and synthesized nanoparticles: A comparative study. J. Basic Microbiol. 2021, 61, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Urnukhsaikhan, E.; Bold, B.-E.; Gunbileg, A.; Sukhbaatar, N.; Mishig-Ochir, T. Antibacterial activity and characteristics of silver nanoparticles biosynthesized from Carduus crispus. Sci. Rep. 2021, 11, 21047. [Google Scholar] [CrossRef] [PubMed]
- Salve, P.; Vinchurkar, A.; Raut, R.; Chondekar, R.; Lakkakula, J.; Roy, A.; Hossain, M.J.; Alghamdi, S.; Almehmadi, M.; Abdulaziz, O.; et al. An Evaluation of Antimicrobial, Anticancer, Anti-Inflammatory and Antioxidant Activities of Silver Nanoparticles Synthesized from Leaf Extract of Madhuca longifolia Utilizing Quantitative and Qualitative Methods. Molecules 2022, 27, 6404. [Google Scholar] [CrossRef] [PubMed]
- Ahiwale, A.V.; Anandh, S.; Gosavi, P. Effect of Therapeutic Exercises on Pre-Menopausal Symptoms in Sedentary Life Style Women. Indian J. Public Health Res. Dev. 2019, 10, 1. [Google Scholar] [CrossRef]
- Sathishkumar, G.; Gobinath, C.; Wilson, A.; Sivaramakrishnan, S. Dendrophthoe falcata (L.f) Ettingsh (Neem mistletoe): A potent bioresource to fabricate silver nanoparticles for anticancer effect against human breast cancer cells (MCF-7). Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 128, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Karuppaiah, A.; Siram, K.; Selvaraj, D.; Ramasamy, M.; Babu, D.; Sankar, V. Synergistic and enhanced anticancer effect of a facile surface modified non-cytotoxic silver nanoparticle conjugated with gemcitabine in metastatic breast cancer cells. Mater. Today Commun. 2020, 23, 100884. [Google Scholar] [CrossRef]
- Chang, C.-C.; Yang, M.-H.; Wen, H.-M.; Chern, J.-C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 3. [Google Scholar]
- Zhang, Q.; Zhang, J.; Shen, J.; Silva, A.; Dennis, D.A.; Barrow, C.J. A simple 96-well microplate method for estimation of total polyphenol content in seaweeds. J. Appl. Phycol. 2006, 18, 445–450. [Google Scholar] [CrossRef]
- Nguyen, N.-T.; Vo, T.-L.-H. Fabrication of Silver Nanoparticles Using Cordyline fruticosa L. Leave Extract Endowing Silk Fibroin Modified Viscose Fabric with Durable Antibacterial Property. Polymers 2022, 14, 2409. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.-E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Govindappa, M.; Farheen, H.; Chandrappa, C.; Rai, R.V.; Raghavendra, V.B. Mycosynthesis of silver nanoparticles using extract of endophytic fungi, Penicillium species of Glycosmis mauritiana, and its antioxidant, antimicrobial, anti-inflammatory and tyrokinase inhibitory activity. Adv. Nat. Sci. Nanosci. Nanotechnol. 2016, 7, 035014. [Google Scholar] [CrossRef]


| Particle Size (nm) | PDI (Index) | Zeta Potential (mV) | |
|---|---|---|---|
| BM-AgNPs | 170.17 ± 12.65 | 0.281 ± 0.07 | −56.6 ± 0.56 |
| Sample | TPC | TFC | TAC | ABTS | DPPH |
|---|---|---|---|---|---|
| GAE mg/g | QE mg/g | C3G mg/L | IC50 μg/mL | IC50 μg/mL | |
| BM extract | 24.57 ± 0.17 a | 8.07 ± 0.04 a | 1.04 ± 0.24 a | 312.18 ± 15.34 a | 335.35 ± 39.33 a |
| BM-AgNPs | 148.42 ± 2.33 b | 12.80 ± 0.65 b | 1.63 ± 0.60 a | 250.53 ± 24.28 a | 134.90 ± 26.29 b |
| Ascorbic acid | - | - | - | 12.76 ± 0.54 c | 11.36 ± 0.27 c |
| BM Extract | BM-AgNPs | |||
|---|---|---|---|---|
| MIC (μg/mL) | MBC (μg/mL) | MIC (μg/mL) | MBC (μg/mL) | |
| S. aureus | - | - | 600 | 1000 |
| E. coli | - | - | 600 | 1200 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeon, Y.-N.; Ryu, S.-J.; Lee, H.-Y.; Kim, J.-O.; Baek, J.-S. Green Synthesis of Silver Nanoparticle Using Black Mulberry and Characterization, Phytochemical, and Bioactivity. Antibiotics 2024, 13, 686. https://doi.org/10.3390/antibiotics13080686
Jeon Y-N, Ryu S-J, Lee H-Y, Kim J-O, Baek J-S. Green Synthesis of Silver Nanoparticle Using Black Mulberry and Characterization, Phytochemical, and Bioactivity. Antibiotics. 2024; 13(8):686. https://doi.org/10.3390/antibiotics13080686
Chicago/Turabian StyleJeon, Yoo-Na, Su-Ji Ryu, Ha-Yeon Lee, Jang-Oh Kim, and Jong-Suep Baek. 2024. "Green Synthesis of Silver Nanoparticle Using Black Mulberry and Characterization, Phytochemical, and Bioactivity" Antibiotics 13, no. 8: 686. https://doi.org/10.3390/antibiotics13080686







