Pseudomonas aeruginosa Biofilm Lifecycle: Involvement of Mechanical Constraints and Timeline of Matrix Production
Abstract
:1. Introduction
- (i)
- Biofilms can develop on biotic surfaces, which are characterized by their biological origin such as living tissues, or onto abiotic nonorganic surfaces like metal, glass, plastic, or other synthetic materials. While abiotic-surface biofilms are often dominated by bacteria, biotic-surface biofilms seem to be more diverse in terms of microorganisms. Surface-associated biofilms are found in numerous environments, including water distribution systems, industrial cooling systems, and wastewater treatment equipment, where they are the leading causes of tube obstruction, material corrosion, and equipment deterioration. They are also associated with hospital-acquired infections, particularly those related to medical devices. These biofilms are described to be more resistant and more difficult to remove than other types of biofilms [2,4]. While numerous surface-associated biofilms have been described for their deleterious effects, they are also, and probably for most of them, beneficial, such as in symbiotic organisms like lichens and corals, which require biofilms for their survival and growth [5].
- (ii)
- The second type of biofilms are the so-called pellicles that develop at the air–liquid interface. They are commonly found in aquatic environments such as rivers and lakes forming flocs, but also in fountains and faucets, where they can cause obstructions and infections. They are generally thinner, less structured, and more mobile than surface-associated biofilms since bacteria within pellicles can easily detach and spread into the environment [6,7]. Such pellicles play major functions in contaminant degradation and biogeochemical cycle regulation [8]. They can cause infections when they are inhaled or through contaminated medical devices [9]. In the food industry, they are commonly used to produce beer, yogurt, or kombucha [10]. Lastly, bacteria can auto-aggregate or co-aggregate.
- (iii)
- Aggregates consist of a community in which bacteria bind to each other by physical interactions, such as cohesion, gravity, or turbulence, and chemical interactions with extracellular matrix exopolymers [11,12], without being attached to a surface or a pellicle. Aggregating biofilms are found in nutrient-rich environments, such as sediments and sludges. In hospitals, they can cause chronic infections, like in the lungs of cystic fibrosis (CF)-suffering patients, in the bladder leading to urinary tract chronic infections, or even on the skin, where they provoke chronic wound infections [13].
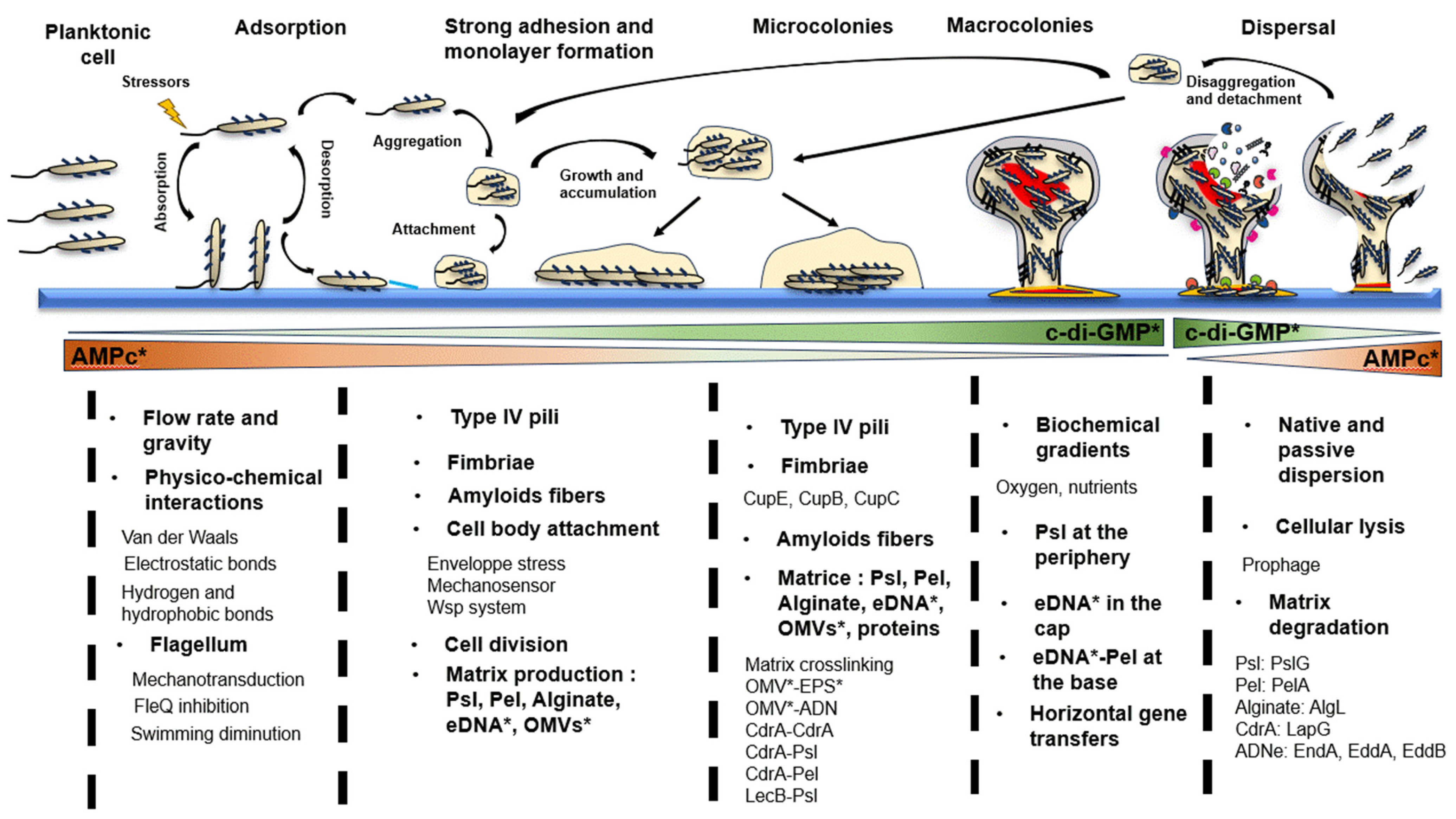
2. The Life Cycle of Pseudomonas aeruginosa Biofilm
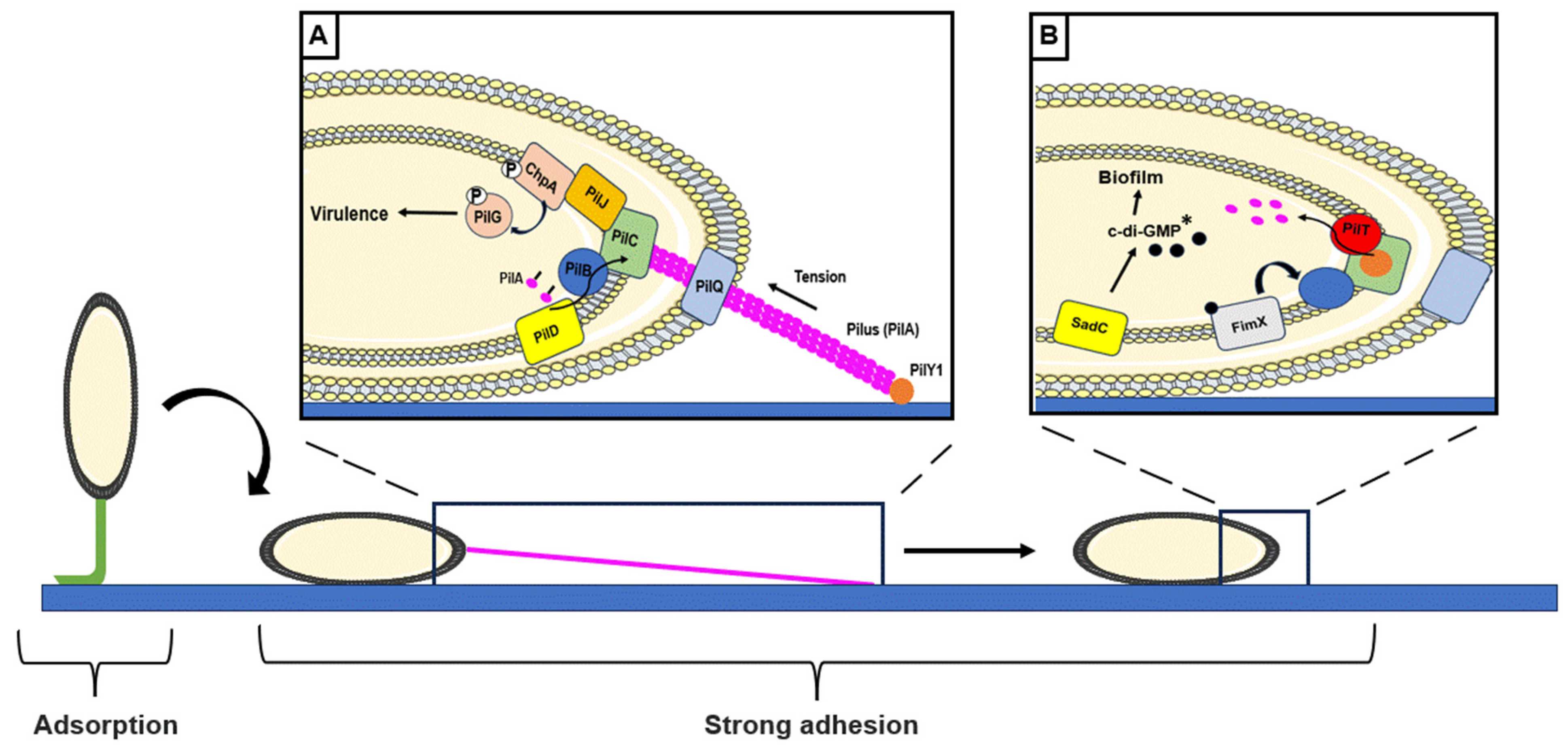
2.1. Step 1: Adsorption
2.2. Step 2: Strong Surface Adhesion and Monolayer Formation
2.2.1. Involvement of Appendages
2.2.2. Importance of Cell Attachment
2.2.3. Initiation of Extracellular Matrix Production
2.3. Step 3: Microcolonies Formation
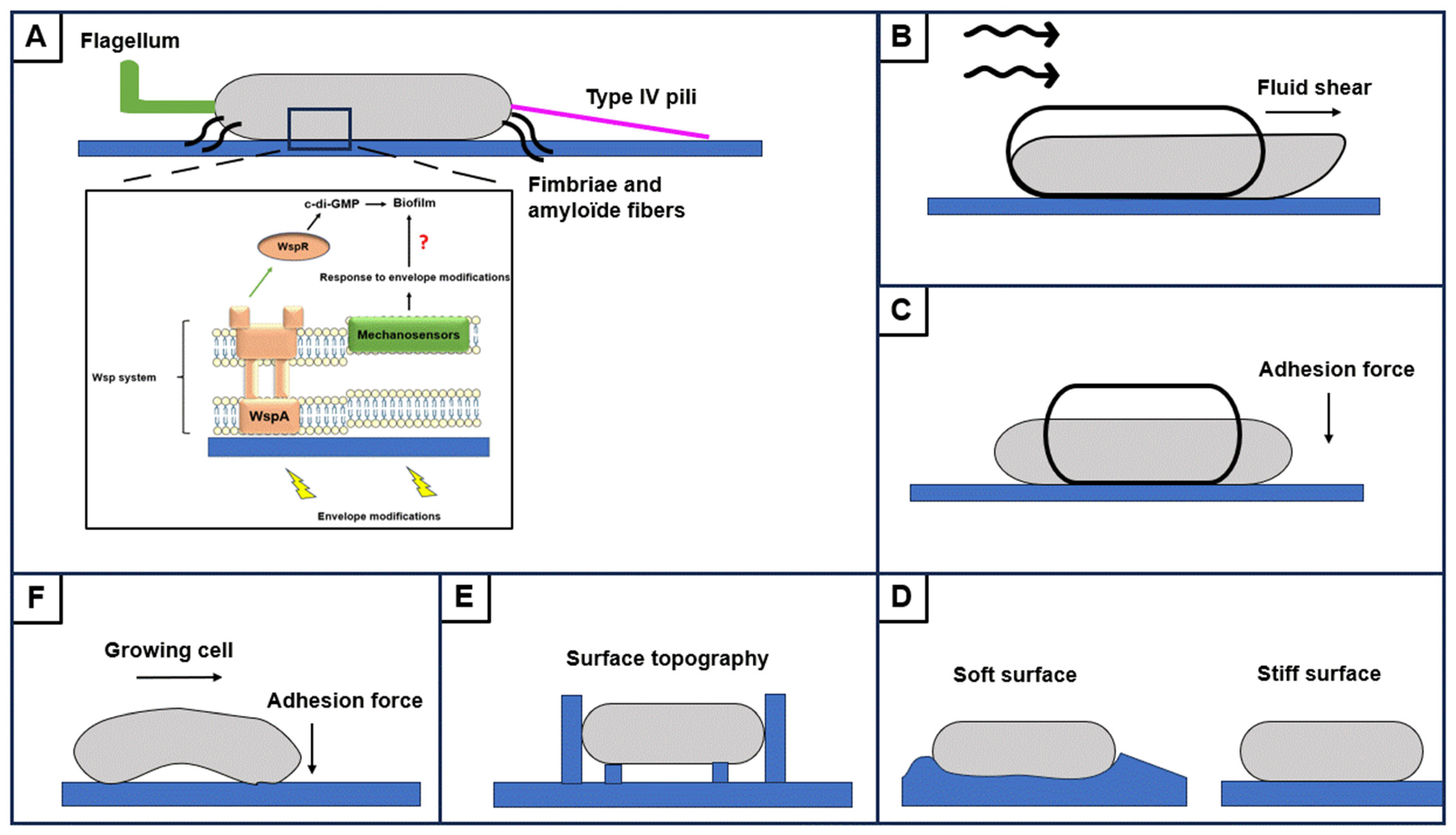
2.3.1. Physical and Mechanical Constraints
2.3.2. Appendages
2.3.3. Role of Matrix Compounds
2.4. Step 4: Macrocolonies Formation
2.4.1. Architecture and Biochemical Gradients
2.4.2. Influence of Mechanical Constraints on Biofilm Architecture
2.4.3. Matrix Production
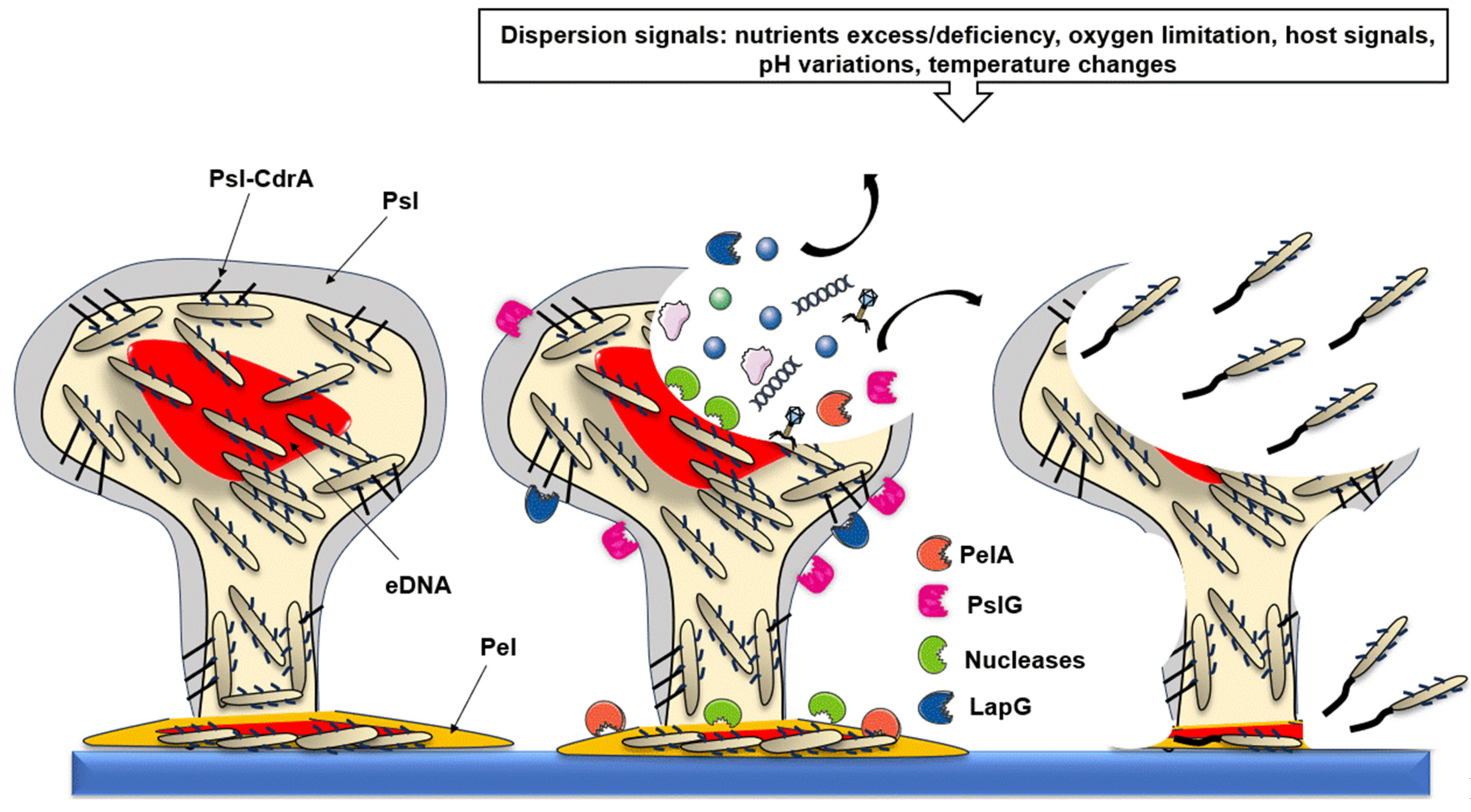
2.5. Step 5: Biofilm Dispersion
2.5.1. Native Dispersal
2.5.2. Passive Dispersal
2.5.3. Restructuring the Biofilm Architecture
3. Biofilm Treatment
3.1. Antibiotic Resistance and Tolerance
3.2. New Antibiotics
4. Concluding Remarks and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, Y.; Xiao, P.; Wang, Y.; Hao, Y. Mechanisms and control measures of mature biofilm resistance to antimicrobial Agents in the Clinical Context. ACS Omega 2020, 5, 22684–22690. [Google Scholar] [CrossRef]
- Flemming, H.-C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef]
- Cendra, M.D.M.; Torrents, E. Pseudomonas aeruginosa biofilms and their partners in crime. Biotechnol. Adv. 2021, 49, 107734. [Google Scholar] [CrossRef]
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef] [PubMed]
- Carr, E.C.; Harris, S.D.; Herr, J.R.; Riekhof, W.R. Lichens and biofilms: Common collective growth imparts similar developmental strategies. Algal Res. 2021, 54, 102217. [Google Scholar] [CrossRef]
- Armitano, J.; Méjean, V.; Jourlin-Castelli, C. Gram-negative bacteria can also form pellicles. Environ. Microbiol. Rep. 2014, 6, 534–544. [Google Scholar] [CrossRef] [PubMed]
- Krajnc, M.; Stefanic, P.; Kostanjšek, R.; Mandic-Mulec, I.; Dogsa, I.; Stopar, D. Systems view of Bacillus subtilis pellicle development. NPJ Biofilms Microbiomes 2022, 8, 25. [Google Scholar] [CrossRef]
- Maurya, A.; Kumar, R.; Raj, A. Biofilm-based technology for industrial wastewater treatment: Current technology, applications and future perspectives. World J. Microbiol. Biotechnol. 2023, 39, 112. [Google Scholar] [CrossRef]
- Golub, S.R.; Overton, T.W. Pellicle formation by Escherichia coli K-12: Role of adhesins and motility. J. Biosci. Bioeng. 2021, 131, 381–389. [Google Scholar] [CrossRef]
- Subbiahdoss, G.; Osmen, S.; Reimhult, E. Cellulosic biofilm formation of Komagataeibacter in kombucha at oil-water interfaces. Biofilm 2022, 4, 100071. [Google Scholar] [CrossRef]
- Burel, C.; Dreyfus, R.; Purevdorj-Gage, L. Physical mechanisms driving the reversible aggregation of Staphylococcus aureus and response to antimicrobials. Sci. Rep. 2021, 11, 15048. [Google Scholar] [CrossRef] [PubMed]
- Nwoko, E.-S.Q.A.; Okeke, I.N. Bacteria autoaggregation: How and why bacteria stick together. Biochem. Soc. Trans. 2021, 49, 1147–1157. [Google Scholar] [CrossRef] [PubMed]
- Sauer, K.; Stoodley, P.; Goeres, D.M.; Hall-Stoodley, L.; Burmølle, M.; Stewart, P.S.; Bjarnsholt, T. The biofilm life cycle: Expanding the conceptual model of biofilm formation. Nat. Rev. Microbiol. 2022, 20, 608–620. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Soto, I.; McTiernan, C.; Gonzalez-Gomez, M.; Ross, A.; Gupta, K.; Suuronen, E.J.; Mah, T.-F.; Griffith, M.; Alarcon, E.I. Mimicking biofilm formation and development: Recent progress in in vitro and in vivo biofilm models. Iscience 2021, 24, 102443. [Google Scholar] [CrossRef] [PubMed]
- Guilbaud, M.; Bruzaud, J.; Bouffartigues, E.; Orange, N.; Guillot, A.; Aubert-Frambourg, A.; Monnet, V.; Herry, J.-M.; Chevalier, S.; Bellon-Fontaone, M.-N. Proteomic response of Pseudomonas aeruginosa PAO1 adhering to solid surfaces. Front. Microbiol. 2017, 8, 1465. [Google Scholar] [CrossRef] [PubMed]
- Kimkes, T.E.P.; Heinemann, M. How bacteria recognise and respond to surface contact. FEMS Microbiol. Rev. 2020, 44, 106–122. [Google Scholar] [CrossRef] [PubMed]
- Mattei, M.R.; Frunzo, L.; D’Acunto, B.; Pechaud, Y.; Pirozzi, F.; Esposito, G. Continuum and discrete approach in modeling biofilm development and structure: A review. J. Math. Biol. 2018, 76, 945–1003. [Google Scholar] [CrossRef] [PubMed]
- Santore, M.M. Interplay of physico-chemical and mechanical bacteria-surface interactions with transport processes controls early biofilm growth: A review. Adv. Colloid. Interface Sci. 2022, 304, 102665. [Google Scholar] [CrossRef] [PubMed]
- Crabbé, A.; De Boever, P.; Van Houdt, R.; Moors, H.; Mergeay, M.; Cornelis, P. Use of the rotating wall vessel technology to study the effect of shear stress on growth behaviour of Pseudomonas aeruginosa PA01. Environ. Microbiol. 2008, 10, 2098–2110. [Google Scholar] [CrossRef]
- Lecuyer, S.; Rusconi, R.; Shen, Y.; Forsyth, A.; Vlamakis, H.; Kolter, R.; Stone, H.A. Shear stress increases the residence time of adhesion of Pseudomonas aeruginosa. Biophys. J. 2011, 100, 341–350. [Google Scholar] [CrossRef]
- McLean, R.J.; Cassanto, J.M.; Barnes, M.B.; Koo, J.H. Bacterial biofilm formation under microgravity conditions. FEMS Microbiol. Lett. 2001, 195, 115–119. [Google Scholar] [CrossRef]
- Bruzaud, J.; Tarrade, J.; Coudreuse, A.; Canette, A.; Herry, J.-M.; Taffin de Givenchy, E.; Darmanin, T.; Guittard, F.; Guilbaud, M.; Bellon-Fontaone, M.-N. Flagella but not type IV pili are involved in the initial adhesion of Pseudomonas aeruginosa PAO1 to hydrophobic or superhydrophobic surfaces. Colloids Surf. B Biointerfaces 2015, 131, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Berg, H.C. The rotary motor of bacterial flagella. Annu. Rev. Biochem. 2003, 72, 19–54. [Google Scholar] [CrossRef] [PubMed]
- Friedlander, R.S.; Vlamakis, H.; Kim, P.; Khan, M.; Kolter, R.; Aizenberg, J. Bacterial flagella explore microscale hummocks and hollows to increase adhesion. Proc. Natl. Acad. Sci. USA 2013, 110, 5624–5629. [Google Scholar] [CrossRef] [PubMed]
- Bucior, I.; Pielage, J.F.; Engel, J.N. Pseudomonas aeruginosa pili and flagella mediate distinct binding and signaling events at the apical and basolateral surface of airway epithelium. PLoS Pathog. 2012, 8, e1002616. [Google Scholar] [CrossRef]
- O’Toole, G.A.; Kolter, R. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol. Microbiol. 1998, 30, 295–304. [Google Scholar] [CrossRef]
- Gordon, V.D.; Wang, L. Bacterial mechanosensing: The force will be with you, always. J. Cell Sci. 2019, 132, jcs227694. [Google Scholar] [CrossRef]
- Chang, C.-Y. Surface sensing for biofilm formation in Pseudomonas aeruginosa. Front. Microbiol. 2018, 8, 2671. [Google Scholar] [CrossRef]
- Persat, A. Bacterial mechanotransduction. Curr. Opin. Microbiol. 2017, 36, 1–6. [Google Scholar] [CrossRef]
- Utada, A.S.; Bennett, R.R.; Fong, J.C.; Gibiansky, M.L.; Yildiz, F.H.; Golestanian, R.; Wong, G.C.L. Vibrio cholerae use pili and flagella synergistically to effect motility switching and conditional surface attachment. Nat. Commun. 2014, 5, 4913. [Google Scholar] [CrossRef]
- Dasgupta, N.; Arora, S.K.; Ramphal, R. fleN, a gene that regulates flagellar number in Pseudomonas aeruginosa. J. Bacteriol. 2000, 182, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Sultan, M.; Arya, R.; Kim, K.K. Roles of two-component systems in Pseudomonas aeruginosa virulence. Int. J. Mol. Sci. 2021, 22, 12152. [Google Scholar] [CrossRef] [PubMed]
- Hickman, J.W.; Harwood, C.S. Identification of FleQ from Pseudomonas aeruginosa as a c-di-GMP-responsive transcription factor. Mol. Microbiol. 2008, 69, 376–389. [Google Scholar] [CrossRef] [PubMed]
- Hengge, R. Principles of c-di-GMP signalling in bacteria. Nat. Rev. Microbiol. 2009, 7, 263–273. [Google Scholar] [CrossRef]
- Baker, A.E.; Webster, S.S.; Diepold, A.; Kuchma, S.L.; Bordeleau, E.; Armitage, J.P.; O’Toole, G.A.O. Flagellar stators stimulate c-di-GMP production by Pseudomonas aeruginosa. J. Bacteriol. 2019, 201, e00741-18. [Google Scholar] [CrossRef] [PubMed]
- Khong, N.Z.-J.; Zeng, Y.; Lai, S.-K.; Koh, C.-G.; Liang, Z.-X.; Chiam, K.-H.; Li, H.-Y. Dynamic swimming pattern of Pseudomonas aeruginosa near a vertical wall during initial attachment stages of biofilm formation. Sci. Rep. 2021, 11, 1952. [Google Scholar] [CrossRef]
- Kuchma, S.L.; Brothers, K.M.; Merritt, J.H.; Liberati, N.T.; Ausubel, F.M.; O’Toole, G.A. BifA, a cyclic-di-GMP phosphodiesterase, inversely regulates biofilm formation and swarming motility by Pseudomonas aeruginosa PA14. J. Bacteriol. 2007, 189, 8165–8178. [Google Scholar] [CrossRef] [PubMed]
- Petrova, O.E.; Cherny, K.E.; Sauer, K. The Pseudomonas aeruginosa diguanylate cyclase GcbA, a homolog of P. fluorescens GcbA, promotes initial attachment to surfaces, but not biofilm formation, via regulation of motility. J. Bacteriol. 2014, 196, 2827–2841. [Google Scholar] [CrossRef]
- Valentini, M.; Filloux, A. Biofilms and cyclic di-GMP (c-di-GMP) signaling: Lessons from Pseudomonas aeruginosa and other bacteria. J. Biol. Chem. 2016, 291, 12547–12555. [Google Scholar] [CrossRef]
- Wolska, K.I.; Grudniak, A.M.; Rudnicka, Z.; Markowska, K. Genetic control of bacterial biofilms. J. Appl. Genet. 2016, 57, 225–238. [Google Scholar] [CrossRef]
- Bouillet, S.; Ba, M.; Houot, L.; Iobbi-Nivol, C.; Bordi, C. Connected partner-switches control the life style of Pseudomonas aeruginosa through RpoS regulation. Sci. Rep. 2019, 9, 6496. [Google Scholar] [CrossRef]
- Köhler, T.; Curty, L.K.; Barja, F.; van Delden, C.; Pechère, J.C. Swarming of Pseudomonas aeruginosa is dependent on cell-to-cell signaling and requires flagella and pili. J. Bacteriol. 2000, 182, 5990–5996. [Google Scholar] [CrossRef] [PubMed]
- Koch, M.D.; Black, M.E.; Han, E.; Shaevitz, J.W.; Gitai, Z. Pseudomonas aeruginosa distinguishes surfaces by stiffness using retraction of type IV pili. Proc. Natl. Acad. Sci. USA 2022, 119, e2119434119. [Google Scholar] [CrossRef]
- Webster, S.S.; Mathelié-Guinlet, M.; Verissimo, A.F.; Schultz, D.; Viljoen, A.; Lee, C.K.; Schmidt, W.C.; Wong, G.C.L.; Dufrêne, V.F.; O’Toole, G.A. Force-induced changes of PilY1 drive surface sensing by Pseudomonas aeruginosa. mBio 2022, 13, e03754-21. [Google Scholar] [CrossRef]
- Rodesney, C.A.; Roman, B.; Dhamani, N.; Cooley, B.J.; Katira, P.; Touhami, A.; Gordon, V.D. Mechanosensing of shear by Pseudomonas aeruginosa leads to increased levels of the cyclic-di-GMP signal initiating biofilm development. Proc. Natl. Acad. Sci. USA 2017, 114, 5906–5911. [Google Scholar] [CrossRef]
- Bertrand, J.J.; West, J.T.; Engel, J.N. Genetic analysis of the regulation of type IV pilus function by the Chp Chemosensory system of Pseudomonas aeruginosa. J. Bacteriol. 2010, 192, 994–1010. [Google Scholar] [CrossRef] [PubMed]
- Geiger, C.J.; O’Toole, G.A. Evidence for the type IV pili retraction motor PilT as a component of the surface sensing system in Pseudomonas aeruginosa. J. Bacteriol. 2023, 205, e00179-23. [Google Scholar] [CrossRef] [PubMed]
- Talà, L.; Fineberg, A.; Kukura, P.; Persat, A. Pseudomonas aeruginosa orchestrates twitching motility by sequential control of type IV pili movements. Nat. Microbiol. 2019, 4, 774–780. [Google Scholar] [CrossRef]
- Luo, Y.; Zhao, K.; Baker, A.E.; Kuchma, S.L.; Coggan, K.A.; Wolfgang, M.C.; Wong, G.C.L.; O’Toole, G.A.O. A hierarchical cascade of second messengers regulates Pseudomonas aeruginosa surface behaviors. mBio 2015, 6, e02456-14. [Google Scholar] [CrossRef]
- Kasetty, S.; Katharios-Lanwermeyer, S.; O’Toole, G.A.; Nadell, C.D. Differential surface competition and biofilm invasion strategies of Pseudomonas aeruginosa PA14 and PAO1. J. Bacteriol. 2021, 203, e00265-21. [Google Scholar] [CrossRef]
- Jain, R.; Sliusarenko, O.; Kazmierczak, B.I. Interaction of the cyclic-di-GMP binding protein FimX and the Type 4 pilus assembly ATPase promotes pilus assembly. PLoS Pathog. 2017, 13, e1006594. [Google Scholar] [CrossRef] [PubMed]
- Fronzes, R.; Remaut, H.; Waksman, G. Architectures and biogenesis of non-flagellar protein appendages in Gram-negative bacteria. EMBO J. 2008, 27, 2271–2280. [Google Scholar] [CrossRef] [PubMed]
- Ruer, S.; Stender, S.; Filloux, A.; de Bentzmann, S. Assembly of fimbrial structures in Pseudomonas aeruginosa: Functionality and specificity of chaperone-usher machineries. J. Bacteriol. 2007, 189, 3547–3555. [Google Scholar] [CrossRef] [PubMed]
- Böhning, J.; Dobbelstein, A.W.; Sulkowski, N.; Eilers, K.; von Kügelgen, A.; Tarafder, A.K.; Peak-Chew, S.-Y.; Skehel, M.; Alva, V.; Filloux, A.; et al. Architecture of the biofilm-associated archaic Chaperone-Usher pilus CupE from Pseudomonas aeruginosa. PLoS Pathog. 2023, 19, e1011177. [Google Scholar] [CrossRef] [PubMed]
- Vallet, I.; Olson, J.W.; Lory, S.; Lazdunski, A.; Filloux, A. The chaperone/usher pathways of Pseudomonas aeruginosa: Identification of fimbrial gene clusters (cup) and their involvement in biofilm formation. Proc. Natl. Acad. Sci. USA 2001, 98, 6911–6916. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, H.; Ball, G.; Giraud, C.; Filloux, A. Expression of Pseudomonas aeruginosa CupD Fimbrial genes is antagonistically controlled by RcsB and the EAL-Containing PvrR response regulators. PLoS ONE 2009, 4, e6018. [Google Scholar] [CrossRef]
- Zeng, G.; Vad, B.S.; Dueholm, M.S.; Christiansen, G.; Nilsson, M.; Tolker-Nielsen, T.; Nielsen, P.H.; Meyer, R.I.; Otzen, D.E. Functional bacterial amyloid increases Pseudomonas biofilm hydrophobicity and stiffness. Front. Microbiol. 2015, 6, 1099. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Blanco, L.; Smith, D.R.; Chapman, M.R. Bacterial amyloids. Methods Mol. Biol. 2012, 849, 303–320. [Google Scholar] [PubMed]
- Dueholm, M.S.; Søndergaard, M.T.; Nilsson, M.; Christiansen, G.; Stensballe, A.; Overgaard, M.T.; Givsjov, M.; Tolker-Nielsen, T.; Otzen, D.E.; Nielsen, P.H. Expression of Fap amyloids in Pseudomonas aeruginosa, P. fluorescens, and P. putida results in aggregation and increased biofilm formation. Microbiolopen. 2013, 2, 365–382. [Google Scholar] [CrossRef]
- Beg, A.Z.; Rashid, F.; Talat, A.; Haseen, M.A.; Raza, N.; Akhtar, K.; Dueholm, M.K.D.; Khan, A.U. Functional amyloids in Pseudomonas aeruginosa are Essential for the proteome modulation that leads to pathoadaptation in pulmonary niches. Microbiol. Spectr. 2023, 11, e03071-22. [Google Scholar] [CrossRef]
- Yang, X.; Li, Z.; Zhao, L.; She, Z.; Gao, Z.; Sui, S.-F.; Dong, Y.; Li, Y. Structural insights into PA3488-mediated inactivation of Pseudomonas aeruginosa PldA. Nat. Commun. 2022, 13, 5979. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, M.M.; Whiteley, M. Pseudomonas aeruginosa attachment and biofilm development in dynamic environments. Mol. Microbiol. 2004, 53, 1075–1087. [Google Scholar] [CrossRef] [PubMed]
- O’Neal, L.; Baraquet, C.; Suo, Z.; Dreifus, J.E.; Peng, Y.; Raivio, T.L.; Wozniak, D.J.; Harwood, C.S.; Parsek, M.R. The Wsp system of Pseudomonas aeruginosa links surface sensing and cell envelope stress. Proc. Natl. Acad. Sci. USA 2022, 119, e2117633119. [Google Scholar] [CrossRef] [PubMed]
- Blanka, A.; Düvel, J.; Dötsch, A.; Klinkert, B.; Abraham, W.-R.; Kaever, V.; Ritter, C.; Narberhaus, F.; Häussler, S. Constitutive production of c-di-GMP is associated with mutations in a variant of Pseudomonas aeruginosa with altered membrane composition. Sci. Signal. 2015, 8, ra36. [Google Scholar] [CrossRef] [PubMed]
- Boechat, A.L.; Kaihami, G.H.; Politi, M.J.; Lépine, F.; Baldini, R.L. A novel role for an ECF sigma factor in fatty acid biosynthesis and membrane fluidity in Pseudomonas aeruginosa. PLoS ONE 2013, 8, e84775. [Google Scholar] [CrossRef]
- Fléchard, M.; Duchesne, R.; Tahrioui, A.; Bouffartigues, E.; Depayras, S.; Hardouin, J.; Lagy, C.; Maillot, O.; Tortuel, D.; Azuama, C.O.; et al. The absence of SigX results in impaired carbon metabolism and membrane fluidity in Pseudomonas aeruginosa. Sci. Rep. 2018, 8, 17212. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Wang, H.; Sauer, K.; Ren, D. Cyclic-di-GMP and oprF are involved in the response of Pseudomonas aeruginosa to substrate material stiffness during attachment on polydimethylsiloxane (PDMS). Front. Microbiol. 2018, 9, 110. [Google Scholar] [CrossRef] [PubMed]
- Bouffartigues, E.; Si Hadj Mohand, I.; Maillot, O.; Tortuel, D.; Omnes, J.; David, A.; Tahrioui, A.; Duchesne, R.; Azuama, C.O.; Nusser, M.; et al. The temperature-regulation of Pseudomonas aeruginosa cmaX-cfrX-cmpX operon reveals an intriguing molecular network involving the sigma factors AlgU and SigX. Front. Microbiol. 2020, 11, 579495. [Google Scholar] [CrossRef] [PubMed]
- Sankaran, J.; Tan, N.J.H.J.; But, K.P.; Cohen, Y.; Rice, S.A.; Wohland, T. Single microcolony diffusion analysis in Pseudomonas aeruginosa biofilms. npj Biofilms Microbiomes 2019, 5, 35. [Google Scholar] [CrossRef]
- Thi, M.T.T.; Wibowo, D.; Rehm, B.H.A. Pseudomonas aeruginosa biofilms. Int. J. Mol. Sci. 2020, 21, 8671. [Google Scholar] [CrossRef]
- Tuon, F.F.; Dantas, L.R.; Suss, P.H.; Tasca Ribeiro, V.S. Pathogenesis of the Pseudomonas aeruginosa biofilm: A Review. Pathogens 2022, 11, 300. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Ma, L.Z. Biofilm matrix and its regulation in Pseudomonas aeruginosa. Int. J. Mol. Sci. 2013, 14, 20983–21005. [Google Scholar] [CrossRef] [PubMed]
- Byrd, M.S.; Sadovskaya, I.; Vinogradov, E.; Lu, H.; Sprinkle, A.B.; Richardson, S.H.; Ma, L.; Ralston, B.; Parsek, M.R.; Anderson, E.M.; et al. Genetic and biochemical analyses of the Pseudomonas aeruginosa Psl exopolysaccharide reveal overlapping roles for polysaccharide synthesis enzymes in Psl and LPS production. Mol. Microbiol. 2009, 73, 622–638. [Google Scholar] [CrossRef]
- Ma, L.; Conover, M.; Lu, H.; Parsek, M.R.; Bayles, K.; Wozniak, D.J. Assembly and development of the Pseudomonas aeruginosa biofilm matrix. PLoS Pathog. 2009, 5, e1000354. [Google Scholar] [CrossRef] [PubMed]
- Colvin, K.M.; Irie, Y.; Tart, C.S.; Urbano, R.; Whitney, J.C.; Ryder, C.; Howell, P.L.; Wozniak, D.J.; Parsek, M.R. The Pel and Psl polysaccharides provide Pseudomonas aeruginosa structural redundancy within the biofilm matrix. Environ. Microbiol. 2012, 8, 1913–1928. [Google Scholar] [CrossRef]
- Zhao, K.; Tseng, B.S.; Beckerman, B.; Jin, F.; Gibiansky, M.L.; Harrison, J.J.; Luijten, E.; Parsek, M.R.; Wong, G.C.L. Psl trails guide exploration and microcolony formation in Pseudomonas aeruginosa biofilms. Nature 2013, 497, 388–391. [Google Scholar] [CrossRef]
- Zhang, J.; He, J.; Zhai, C.; Ma, L.Z.; Gu, L.; Zhao, K. Effects of PslG on the surface movement of Pseudomonas aeruginosa. Appl. Environ. Microbiol. 2018, 84, e00219-18. [Google Scholar] [CrossRef] [PubMed]
- Le Mauff, F.; Razvi, E.; Reichhardt, C.; Sivarajah, P.; Parsek, M.R.; Howell, P.L.; Sheppard, D.C. The Pel polysaccharide is predominantly composed of a dimeric repeat of α-1,4 linked galactosamine and N-acetylgalactosamine. Commun. Biol. 2022, 5, 502. [Google Scholar] [CrossRef]
- Ghafoor, A.; Hay, I.D.; Rehm, B.H.A. Role of exopolysaccharides in Pseudomonas aeruginosa biofilm formation and architecture. Appl. Environ. Microbiol. 2011, 77, 5238–5246. [Google Scholar] [CrossRef]
- Yang, L.; Hu, Y.; Liu, Y.; Zhang, J.; Ulstrup, J.; Molin, S. Distinct roles of extracellular polymeric substances in Pseudomonas aeruginosa biofilm development. Environ. Microbiol. 2011, 13, 1705–1717. [Google Scholar] [CrossRef]
- Vasseur, P.; Vallet-Gely, I.; Soscia, C.; Genin, S.; Filloux, A. The pel genes of the Pseudomonas aeruginosa PAK strain are involved at early and late stages of biofilm formation. Microbiology 2005, 151, 985–997. [Google Scholar] [CrossRef] [PubMed]
- Ryder, C.; Byrd, M.; Wozniak, D.J. Role of polysaccharides in Pseudomonas aeruginosa biofilm development. Curr. Opin. Microbiol. 2007, 10, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Wozniak, D.J.; Wyckoff, T.J.O.; Starkey, M.; Keyser, R.; Azadi, P.; O’Toole, G.A.; Parsek, M.R. Alginate is not a significant component of the extracellular polysaccharide matrix of PA14 and PAO1 Pseudomonas aeruginosa biofilms. Proc. Natl. Acad. Sci. USA 2003, 100, 7907–7912. [Google Scholar] [CrossRef] [PubMed]
- Allesen-Holm, M.; Barken, K.B.; Yang, L.; Klausen, M.; Webb, J.S.; Kjelleberg, S.; Molin, S.; Givskov, M.; Tolker-Nielsen, T. A characterization of DNA release in Pseudomonas aeruginosa cultures and biofilms. Mol. Microbiol. 2006, 59, 1114–1128. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S. Release mechanisms and molecular interactions of Pseudomonas aeruginosa extracellular DNA. Appl. Microbiol. Biotechnol. 2020, 104, 6549–6564. [Google Scholar] [CrossRef] [PubMed]
- Diggle, S.P.; Griffin, A.S.; Campbell, G.S.; West, S.A. Cooperation and conflict in quorum-sensing bacterial populations. Nature 2007, 450, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.; Cámara, M. Quorum sensing and environmental adaptation in Pseudomonas aeruginosa: A tale of regulatory networks and multifunctional signal molecules. Curr. Opin. Microbiol. 2009, 12, 182–191. [Google Scholar] [CrossRef]
- Mukherjee, S.; Bassler, B.L. Bacterial quorum sensing in complex and dynamically changing environments. Nat. Rev. Microbiol. 2019, 17, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Miranda, S.W.; Asfahl, K.L.; Dandekar, A.A.; Greenberg, E.P. Pseudomonas aeruginosa quorum sensing. Adv. Exp. Med. Biol. 2022, 1386, 95–115. [Google Scholar]
- Das, T.; Manefield, M. Pyocyanin promotes extracellular DNA release in Pseudomonas aeruginosa. PLoS ONE 2012, 7, e46718. [Google Scholar] [CrossRef]
- Zeng, B.; Wang, C.; Zhang, P.; Guo, Z.; Chen, L.; Duan, K. Heat shock protein DnaJ in Pseudomonas aeruginosa affects biofilm formation via pyocyanin production. Microorganisms 2020, 8, 395. [Google Scholar] [CrossRef] [PubMed]
- Okshevsky, M.; Meyer, R.L. The role of extracellular DNA in the establishment, maintenance and perpetuation of bacterial biofilms. Critical Rev. Microbiol. 2015, 41, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Gloag, E.S.; Turnbull, L.; Huang, A.; Vallotton, P.; Wang, H.; Nolan, L.M.; Mililli, L.; Hunt, C.; Lu, J.; Osvath, S.R.; et al. Self-organization of bacterial biofilms is facilitated by extracellular, D.N.A. Proc. Natl. Acad. Sci. USA 2013, 110, 11541–11546. [Google Scholar] [CrossRef]
- Eberlein, C.; Baumgarten, T.; Starke, S.; Heipieper, H.J. Immediate response mechanisms of Gram-negative solvent-tolerant bacteria to cope with environmental stress: Cis-trans isomerization of unsaturated fatty acids and outer membrane vesicle secretion. Appl. Microbiol. Biotechnol. 2018, 102, 2583–2593. [Google Scholar] [CrossRef]
- Manning, A.J.; Kuehn, M.J. Contribution of bacterial outer membrane vesicles to innate bacterial defense. BMC Microbiol. 2011, 11, 258. [Google Scholar] [CrossRef]
- Beveridge, T.J.; Makin, S.A.; Kadurugamuwa, J.L.; Li, Z. Interactions between biofilms and the environment. FEMS Microbiol. Rev. 1997, 20, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Duvernoy, M.-C.; Mora, T.; Ardré, M.; Croquette, V.; Bensimon, D.; Quilliet, C.; Ghigo, J.-M.; Balland, M.; Beloin, C.; Lecuyer, S.; et al. Asymmetric adhesion of rod-shaped bacteria controls microcolony morphogenesis. Nat. Commun. 2018, 9, 1120. [Google Scholar] [CrossRef]
- Beroz, F.; Yan, J.; Meir, Y.; Sabass, B.; Stone, H.A.; Bassler, B.L.; Wingreen, N.S.; Meir, Y. Verticalization of bacterial biofilms. Nat. Phys. 2018, 14, 954–960. [Google Scholar] [CrossRef]
- Hartmann, R.; Singh, P.K.; Pearce, P.; Mok, R.; Song, B.; Díaz-Pascual, F.; Dunkel, J.; Drescher, K. Emergence of three-dimensional order and structure in growing biofilms. Nat. Phys. 2019, 15, 251–256. [Google Scholar] [CrossRef]
- Ozer, E.; Yaniv, K.; Chetrit, E.; Boyarski, A.; Meijler, M.M.; Berkovich, R.; Kushmaro, A.; Alfonta, L. An inside look at a biofilm: Pseudomonas aeruginosa flagella biotracking. Sci. Adv. 2021, 7, eabg8581. [Google Scholar] [CrossRef]
- Barken, K.B.; Pamp, S.J.; Yang, L.; Gjermansen, M.; Bertrand, J.J.; Klausen, M.; Givskov, M.; Whitchurch, C.B.; Engel, N.; Tolker-Nielsen, T. Roles of type IV pili, flagellum-mediated motility and extracellular DNA in the formation of mature multicellular structures in Pseudomonas aeruginosa biofilms. Environ. Microbiol. 2008, 10, 2331–2343. [Google Scholar] [CrossRef] [PubMed]
- Giraud, C.; Bernard, C.S.; Calderon, V.; Yang, L.; Filloux, A.; Molin, S.; Fichant, G.; Bordi, C.; de Bentzmann, S. The PprA–PprB two-component system activates CupE, the first non-archetypal Pseudomonas aeruginosa chaperone–usher pathway system assembling fimbriae. Environ. Microbiol. 2011, 13, 666–683. [Google Scholar] [CrossRef]
- Herbst, F.A.; Sondergaard, M.T.; Kjeldal, H.; Stensballe, A.; Nielsen, P.H.; Duelholm, M.S. Major proteomic changes associated with amyloid-induced biofilm formation in Pseudomonas aeruginosa. J. Proteome Res. 2015, 14, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Chew, S.C.; Kundukad, B.; Seviour, T.; van der Maarel, J.R.C.; Yang, L.; Rice, S.A.; Doyle, P.; Kjelleberg, S. Dynamic remodeling of microbial biofilms by functionally distinct exopolysaccharides. mBio 2014, 5, e01536-14. [Google Scholar] [CrossRef] [PubMed]
- Cooley, B.J.; Thatcher, T.W.; Hashmi, S.M.; L’her, G.; Le, H.H.; Hurwitz, D.A.; Proventzano, D.; Touhami, A.; Gordon, V.D. The extracellular polysaccharide Pel makes the attachment of P. aeruginosa to surfaces symmetric and short-ranged. Soft Matter. 2013, 9, 3871–3876. [Google Scholar] [CrossRef]
- Morris, A.J.; Yau, Y.C.W.; Park, S.; Eisha, S.; McDonald, N.; Parsek, M.R.; Howell, P.L.; Hoffman, L.R.; Nguyen, D.; DiGiandomenica, A.; et al. Pseudomonas aeruginosa aggregation and Psl expression in sputum is associated with antibiotic eradication failure in children with cystic fibrosis. Sci. Rep. 2022, 12, 21444. [Google Scholar] [CrossRef]
- Mishra, M.; Byrd, M.S.; Sergeant, S.; Azad, A.K.; Parsek, M.R.; McPhail, L.; Schlesinger, L.S.; Woznaik, D.J. Pseudomonas aeruginosa Psl polysaccharide reduces neutrophil phagocytosis and the oxidative response by limiting complement-mediated opsonization. Cell Microbiol. 2012, 14, 95–106. [Google Scholar] [CrossRef]
- Turnbull, L.; Toyofuku, M.; Hynen, A.L.; Kurosawa, M.; Pessi, G.; Petty, N.K.; Osvath, S.R.; Carcamo-Oyarce, G.; Gloag, E.S.; Shimoni, R.; et al. Explosive cell lysis as a mechanism for the biogenesis of bacterial membrane vesicles and biofilms. Nat. Commun. 2016, 7, 11220. [Google Scholar] [CrossRef]
- Petrova, O.E.; Schurr, J.R.; Schurr, M.J.; Sauer, K. The novel Pseudomonas aeruginosa two-component regulator BfmR controls bacteriophage-mediated lysis and DNA release during biofilm development through PhdA. Mol. Microbiol. 2011, 81, 767–783. [Google Scholar] [CrossRef]
- Das, T.; Sehar, S.; Koop, L.; Wong, Y.K.; Ahmed, S.; Siddiqui, K.S.; Manefield, M. Influence of calcium in extracellular DNA mediated bacterial aggregation and biofilm Formation. PLoS ONE 2014, 9, e91935. [Google Scholar] [CrossRef]
- Cooke, A.C.; Nello, A.V.; Ernst, R.K.; Schertzer, J.W. Analysis of Pseudomonas aeruginosa biofilm membrane vesicles supports multiple mechanisms of biogenesis. PLoS ONE 2019, 14, e0212275. [Google Scholar] [CrossRef] [PubMed]
- Toyofuku, M.; Roschitzki, B.; Riedel, K.; Eberl, L. Identification of proteins associated with the Pseudomonas aeruginosa biofilm extracellular matrix. J. Proteome Res. 2012, 11, 4906–4915. [Google Scholar] [CrossRef] [PubMed]
- Johnston, E.L.; Zavan, L.; Bitto, N.J.; Petrovski, S.; Hill, A.F.; Kaparakis-Liaskos, M. Planktonic and biofilm-derived Pseudomonas aeruginosa outer membrane vesicles facilitate horizontal gene transfer of plasmid DNA. Microbiol. Spectr. 2023, 11, e05179-22. [Google Scholar] [CrossRef]
- Nolan, L.M.; Turnbull, L.; Katrib, M.; Osvath, S.R.; Losa, D.; Lazenby, J.J.; Whitchurch, C.B. Pseudomonas aeruginosa is capable of natural transformation in biofilms. Microbiology 2020, 166, 995–1003. [Google Scholar] [CrossRef]
- Michaelis, C.; Grohmann, E. Horizontal gene transfer of antibiotic resistance genes in biofilms. Antibiotics 2023, 12, 328. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Wang, L.; Miao, J.; Zhang, Z.; Ruan, J.; Xu, L.; Guo, H.; Zhang, M.; Qiao, W. Regulation of the formation and structure of biofilms by quorum sensing signal molecules packaged in outer membrane vesicles. Sci. Total Environ. 2022, 806, 151403. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, H.M.; Jagannadham, M.V. Biogenesis and multifaceted roles of outer membrane vesicles from Gram-negative bacteria. Microbiology 2014, 160, 2109–2121. [Google Scholar] [CrossRef] [PubMed]
- Reichhardt, C.; Wong, C.; Passos da Silva, D.; Wozniak, D.J.; Parsek, M.R. CdrA Interactions within the Pseudomonas aeruginosa biofilm matrix safeguard it from proteolysis and promote cellular packing. mBio 2018, 9, e01376-18. [Google Scholar] [CrossRef]
- Borlee, B.R.; Goldman, A.D.; Murakami, K.; Samudrala, R.; Wozniak, D.J.; Parsek, M.R. Pseudomonas aeruginosa uses a cyclic-di-GMP-regulated adhesin to reinforce the biofilm extracellular matrix. Mol. Microbiol. 2010, 75, 827–842. [Google Scholar] [CrossRef]
- Reichhardt, C.; Jacobs, H.M.; Matwichuk, M.; Wong, C.; Wozniak, D.J.; Parsek, M.R. The versatile Pseudomonas aeruginosa biofilm matrix protein CdrA promotes aggregation through different extracellular exopolysaccharide interactions. J. Bacteriol. 2020, 202, e00216-20. [Google Scholar] [CrossRef]
- Diggle, S.P.; Stacey, R.E.; Dodd, C.; Cámara, M.; Williams, P.; Winzer, K. The galactophilic lectin, LecA, contributes to biofilm development in Pseudomonas aeruginosa. Environ. Microbiol. 2006, 8, 1095–1104. [Google Scholar] [CrossRef] [PubMed]
- Passos da Silva, D.; Matwichuk, M.L.; Townsend, D.O.; Reichhardt, C.; Lamba, D.; Wozniak, D.J.; Parsek, M.R. The Pseudomonas aeruginosa lectin LecB binds to the exopolysaccharide Psl and stabilizes the biofilm matrix. Nat. Commun. 2019, 10, 2183. [Google Scholar] [CrossRef] [PubMed]
- Bedi, B.; Maurice, N.M.; Sadikot, R.T. Microarchitecture of Pseudomonas aeruginosa biofilms: A biological perspective. Biomed. Biotechnol. Res. J. 2018, 2, 227. [Google Scholar]
- Ghanbari, A.; Dehghany, J.; Schwebs, T.; Müsken, M.; Häussler, S.; Meyer-Hermann, M. Inoculation density and nutrient level determine the formation of mushroom-shaped structures in Pseudomonas aeruginosa biofilms. Sci. Rep. 2016, 6, 32097. [Google Scholar] [CrossRef] [PubMed]
- Klausen, M.; Aaes-Jørgensen, A.; Molin, S.; Tolker-Nielsen, T. Involvement of bacterial migration in the development of complex multicellular structures in Pseudomonas aeruginosa biofilms. Mol. Microbiol. 2003, 50, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Wood, S.R.; Kirkham, J.; Marsh, P.D.; Shore, R.C.; Nattress, B.; Robinson, C. Architecture of intact natural human plaque biofilms studied by confocal laser scanning microscopy. J. Dent. Res. 2000, 79, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Rabin, N.; Zheng, Y.; Opoku-Temeng, C.; Du, Y.; Bonsu, E.; Sintim, H.O. Biofilm formation mechanisms and targets for developing antibiofilm agents. Future MedChem. 2015, 7, 493–512. [Google Scholar] [CrossRef] [PubMed]
- Heacock-Kang, Y.; Sun, Z.; Zarzycki-Siek, J.; McMillan, I.A.; Norris, M.H.; Bluhm, A.P.; Cabanas, D.; Fogen, D.; Vo, H.; Donachie, S.P.; et al. Spatial transcriptomes within the Pseudomonas aeruginosa biofilm architecture. Mol. Microbiol. 2017, 106, 976–985. [Google Scholar] [CrossRef]
- Davey, M.E.; Caiazza, N.C.; O’Toole, G.A. Rhamnolipid surfactant production affects biofilm architecture in Pseudomonas aeruginosa PAO1. J Bacteriol. 2003, 185, 1027–1036. [Google Scholar] [CrossRef]
- Stewart, P.S. Diffusion in biofilms. J. Bacteriol. 2003, 185, 1485–1491. [Google Scholar] [CrossRef]
- Peterson, B.W.; He, Y.; Ren, Y.; Zerdoum, A.; Libera, M.R.; Sharma, P.K.; van Winkelhoff, A.-J.; Neut, D.; Stoodley, P.; van der Mei, H.; et al. Viscoelasticity of biofilms and their recalcitrance to mechanical and chemical challenges. FEMS Microbiol. Rev. 2015, 39, 234–245. [Google Scholar] [CrossRef] [PubMed]
- Recupido, F.; Toscano, G.; Tatè, R.; Petala, M.; Caserta, S.; Karapantsios, T.D.; Guido, S. The role of flow in bacterial biofilm morphology and wetting properties. Colloids Surf. B Biointerfaces 2020, 192, 111047. [Google Scholar] [CrossRef] [PubMed]
- Paul, E.; Ochoa, J.C.; Pechaud, Y.; Liu, Y.; Liné, A. Effect of shear stress and growth conditions on detachment and physical properties of biofilms. Water Res. 2012, 46, 5499–5508. [Google Scholar] [CrossRef] [PubMed]
- Fish, K.; Osborn, A.M.; Boxall, J.B. Biofilm structures (EPS and bacterial communities) in drinking water distribution systems are conditioned by hydraulics and influence discolouration. Sci. Total Environ. 2017, 593–594, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Miao, L.; Hou, J.; Wang, P.; Qian, J.; Dai, S. The effect of flow velocity on the distribution and composition of extracellular polymeric substances in biofilms and the detachment mechanism of biofilms. Water Sci. Technol. 2013, 69, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Sileika, T.S.; Chen, C.; Liu, Y.; Lee, J.; Packman, A.I. A novel planar flow cell for studies of biofilm heterogeneity and flow–biofilm interactions. Biotechnol. Bioeng. 2011, 108, 2571–2582. [Google Scholar] [CrossRef] [PubMed]
- David, A.; Louis, M.; Tahrioui, A.; Rodrigues, S.; Labbé, C.; Maillot, O.; Barreau, M.; Lesouhaitier, O.; Cornelis, P.; Chevalier, S.; et al. cmpX overexpression in Pseudomonas aeruginosa affects biofilm formation and cell morphology in response to shear stress. Biofilm 2024, 7, 100191. [Google Scholar] [CrossRef] [PubMed]
- Purevdorj, B.; Costerton, J.W.; Stoodley, P. Influence of hydrodynamics and cell signaling on the structure and behavior of Pseudomonas aeruginosa biofilms. Appl. Environ. Microbiol. 2002, 68, 4457–4464. [Google Scholar] [CrossRef] [PubMed]
- Dingemans, J.; Monsieurs, P.; Yu, S.-H.; Crabbé, A.; Förstner, K.U.; Malfroot, A.; Cornelis, P.; van houdt, R. Effect of shear stress on Pseudomonas aeruginosa isolated from the cystic fibrosis Lung. mBio 2016, 7, e00813-16. [Google Scholar] [CrossRef]
- Stoodley, P.; Lewandowski, Z.; Boyle, J.D.; Lappin-Scott, H.M. Structural deformation of bacterial biofilms caused by short-term fluctuations in fluid shear: An in situ investigation of biofilm rheology. Biotechnol. Bioeng. 1999, 65, 83–92. [Google Scholar] [CrossRef]
- Salek, M.M.; Jones, S.M.; Martinuzzi, R.J. The influence of flow cell geometry related shear stresses on the distribution, structure and susceptibility of Pseudomonas aeruginosa PAO1 biofilms. Biofouling 2009, 25, 711–725. [Google Scholar] [CrossRef]
- Zhang, Y.; Silva, D.M.; Young, P.; Traini, D.; Li, M.; Ong, H.X.; Cheng, S. Understanding the effects of aerodynamic and hydrodynamic shear forces on Pseudomonas aeruginosa biofilm growth. Biotechnol. Bioeng. 2022, 119, 1483–1497. [Google Scholar] [CrossRef]
- Sanfilippo, J.E.; Lorestani, A.; Koch, M.D.; Bratton, B.P.; Siryaporn, A.; Stone, H.A.; Gitai, Z. Microfluidic-based transcriptomics reveal force-independent bacterial rheosensing. Nat. Microbiol. 2019, 4, 1274–1281. [Google Scholar] [CrossRef]
- Ma, L.; Jackson, K.D.; Landry, R.M.; Parsek, M.R.; Wozniak, D.J. Analysis of Pseudomonas aeruginosa conditional Psl variants reveals roles for the Psl polysaccharide in adhesion and maintaining biofilm structure postattachment. J. Bacteriol. 2006, 188, 8213–8221. [Google Scholar] [CrossRef] [PubMed]
- Colvin, K.M.; Gordon, V.D.; Murakami, K.; Borlee, B.R.; Wozniak, D.J.; Wong, G.C.L.; Parsek, M.R. The Pel polysaccharide can serve a structural and protective role in the biofilm matrix of Pseudomonas aeruginosa. PLoS Pathog. 2011, 7, e1001264. [Google Scholar] [CrossRef] [PubMed]
- Tielen, P.; Strathmann, M.; Jaeger, K.-E.; Flemming, H.-C.; Wingender, J. Alginate acetylation influences initial surface colonization by mucoid Pseudomonas aeruginosa. Microbiol. Res. 2005, 160, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Mann, E.E.; Wozniak, D.J. Pseudomonas biofilm matrix composition and niche biology. FEMS Microbiol. Rev. 2012, 36, 893–916. [Google Scholar] [CrossRef]
- van Schaik, E.J.; Giltner, C.L.; Audette, G.F.; Keizer, D.W.; Bautista, D.L.; Slupsky, C.M.; Sykes, B.D.; Irvin, R.T. DNA binding: A novel function of Pseudomonas aeruginosa type IV pili. J. Bacteriol. 2005, 187, 1455–1464. [Google Scholar] [CrossRef]
- Panlilio, H.; Rice, C.V. The role of extracellular DNA in the formation, architecture, stability, and treatment of bacterial biofilms. Biotechnol. Bioeng. 2021, 118, 2129–2141. [Google Scholar] [CrossRef]
- Wang, S.; Liu, X.; Liu, H.; Zhang, L.; Guo, Y.; Yu, S.; Wozniak, D.J.; Ma, L.Z. The exopolysaccharide Psl–eDNA interaction enables the formation of a biofilm skeleton in Pseudomonas aeruginosa. Environ. Microbiol. Rep. 2015, 7, 330–340. [Google Scholar] [CrossRef]
- Jennings, L.K.; Storek, K.M.; Ledvina, H.E.; Coulon, C.; Marmont, L.S.; Sadovskaya, I.; Secor, P.R.; Tseng, B.S.; Scian, M.; Filloux, A.; et al. Pel is a cationic exopolysaccharide that cross-links extracellular DNA in the Pseudomonas aeruginosa biofilm matrix. Proc. Natl. Acad. Sci. USA 2015, 112, 11353–11358. [Google Scholar] [CrossRef] [PubMed]
- Finkel, S.E.; Kolter, R. DNA as a nutrient: Novel role for bacterial competence gene homologs. J. Bacteriol. 2001, 183, 6288–6293. [Google Scholar] [CrossRef]
- Guilhen, C.; Forestier, C.; Balestrino, D. Biofilm dispersal: Multiple elaborate strategies for dissemination of bacteria with unique properties. Mol. Microbiol. 2017, 105, 188–210. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, J.B. Biofilm dispersal: Mechanisms, clinical implications, and potential therapeutic uses. J. Dent. Res. 2010, 89, 205–218. [Google Scholar] [CrossRef]
- Jo, J.; Price-Whelan, A.; Dietrich, L.E.P. Gradients and consequences of heterogeneity in biofilms. Nat. Rev. Microbiol. 2022, 20, 593–607. [Google Scholar] [CrossRef] [PubMed]
- Rumbaugh, K.P.; Sauer, K. Biofilm dispersion. Nat. Rev. Microbiol. 2020, 18, 571–586. [Google Scholar] [CrossRef] [PubMed]
- Webb, J.S.; Thompson, L.S.; James, S.; Charlton, T.; Tolker-Nielsen, T.; Koch, B.; Givskov, M.; Kjelleberg, S. Cell death in Pseudomonas aeruginosa biofilm development. J. Bacteriol. 2003, 185, 4585–4592. [Google Scholar] [CrossRef]
- Huynh, T.T.; McDougald, D.; Klebensberger, J.; Qarni, B.A.; Barraud, N.; Rice, S.A.; Kjelleberg, S.; Schleheck, D. Glucose starvation-induced dispersal of Pseudomonas aeruginosa biofilms is cAMP and energy dependent. PLoS ONE 2012, 7, e42874. [Google Scholar] [CrossRef] [PubMed]
- Sauer, K.; Cullen, M.C.; Rickard, A.H.; Zeef, L.A.H.; Davies, D.G.; Gilbert, P. Characterization of nutrient-induced dispersion in Pseudomonas aeruginosa PAO1 biofilm. J. Bacteriol. 2004, 186, 7312–7326. [Google Scholar] [CrossRef]
- Barraud, N.; Hassett, D.J.; Hwang, S.-H.; Rice, S.A.; Kjelleberg, S.; Webb, J.S. Involvement of nitric oxide in biofilm dispersal of Pseudomonas aeruginosa. J. Bacteriol. 2006, 188, 7344–7353. [Google Scholar] [CrossRef]
- Sanchez, L.M.; Cheng, A.T.; Warner, C.J.A.; Townsley, L.; Peach, K.C.; Navarro, G.; Shikuma, N.J.; Bray, W.M.; Riener, R.M.; Yildiz, F.H.; et al. Biofilm formation and detachment in Gram-negative pathogens is modulated by select bile acids. PLoS ONE 2016, 11, e0149603. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.-K.; Duong, H.T.T.; Selvanayagam, R.; Boyer, C.; Barraud, N. Iron oxide nanoparticle-mediated hyperthermia stimulates dispersal in bacterial biofilms and enhances antibiotic efficacy. Sci. Rep. 2015, 5, 18385. [Google Scholar] [CrossRef] [PubMed]
- Wille, J.; Coenye, T. Biofilm dispersion: The key to biofilm eradication or opening Pandora’s box? Biofilm 2020, 2, 100027. [Google Scholar] [CrossRef]
- Choi, Y.C.; Morgenroth, E. Monitoring biofilm detachment under dynamic changes in shear stress using laser-based particle size analysis and mass fractionation. Water Sci. Technol. 2003, 47, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Su, T.; Wu, H.; Liu, S.; Wang, D.; Zhao, T.; Jin, Z.; Du, W.; Zhu, M.-J.; Chua, S.L.; et al. PslG, a self-produced glycosyl hydrolase, triggers biofilm disassembly by disrupting exopolysaccharide matrix. Cell Res. 2015, 25, 1352–1367. [Google Scholar] [CrossRef] [PubMed]
- Cherny, K.E.; Sauer, K. Untethering and degradation of the polysaccharide matrix are essential steps in the dispersion response of Pseudomonas aeruginosa biofilms. J. Bacteriol. 2020, 202, 3. [Google Scholar] [CrossRef] [PubMed]
- Nickel, J.C.; Ruseska, I.; Wright, J.B.; Costerton, J.W. Tobramycin resistance of Pseudomonas aeruginosa cells growing as a biofilm on urinary catheter material. Antimicrob. Agents Chemother. 1985, 4, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Liao, J.; Petrova, O.E.; Cherny, K.E.; Sauer, K. Elevated levels of the second messenger c-di-GMP contribute to antimicrobial resistance of Pseudomonas aeruginosa. Mol. Microbiol. 2014, 92, 488–506. [Google Scholar] [CrossRef] [PubMed]
- Maharjan, R.P.; Ferenci, T. A shifting mutational landscape in 6 nutritional states: Stress-induced mutagenesis as a series of distinct stress input–mutation output relationships. PLoS Biol. 2017, 15, e2001477. [Google Scholar] [CrossRef]
- Pallen, M.J.; Wren, B.W. Bacterial pathogenomics. Nature 2007, 449, 835–842. [Google Scholar] [CrossRef]
- Levin-Reisman, I.; Ronin, I.; Gefen, O.; Braniss, I.; Shoresh, N.; Balaban, N.Q. Antibiotic tolerance facilitates the evolution of resistance. Science 2017, 355, 826–830. [Google Scholar] [CrossRef] [PubMed]
- Goltermann, L.; Tolker-Nielsen, T. Importance of the exopolysaccharide matrix in antimicrobial tolerance of Pseudomonas aeruginosa aggregates. Antimicrob. Agents Chemother. 2017, 61, e02696-16. [Google Scholar] [CrossRef] [PubMed]
- Morris, A.J.; Jackson, L.; CWYau, Y.; Reichhardt, C.; Beaudoin, T.; Uwumarenogie, S.; Guttman, K.; Howell, P.L.; Parsek, M.R.; Hoffman, L.R.; et al. The role of Psl in the failure to eradicate Pseudomonas aeruginosa biofilms in children with cystic fibrosis. npj Biofilms Microbiomes 2021, 7, 63. [Google Scholar] [CrossRef] [PubMed]
- Mulcahy, H.; Charron-Mazenod, L.; Lewenza, S. Extracellular DNA chelates cations and induces antibiotic resistance in Pseudomonas aeruginosa biofilms. PLoS Pathog. 2008, 4, e1000213. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, M.K.; Jagannadham, M.V. Vesicles-mediated resistance to antibiotics in bacteria. Front. Microbiol. 2015, 6, 758. [Google Scholar] [CrossRef] [PubMed]
- Ciofu, O.; Moser, C.; Jensen, P.Ø.; Høiby, N. Tolerance and resistance of microbial biofilms. Nat. Rev. Microbiol. 2022, 20, 621–635. [Google Scholar] [CrossRef] [PubMed]
- Ciofu, O.; Rojo-Molinero, E.; Macià, M.D.; Oliver, A. Antibiotic treatment of biofilm infections. APMIS 2017, 125, 304–319. [Google Scholar] [CrossRef] [PubMed]
- Antonoplis, A.; Zang, X.; Huttner, M.A.; Chong, K.K.L.; Lee, Y.B.; Co, J.Y.; Amieva, M.R.; Kline, K.A.; Wender, P.A.; Cegelski, L. A dual-function antibiotic-transporter conjugate exhibits superior activity in sterilizing MRSA biofilms and killing persister cells. J. Am. Chem. Soc. 2018, 140, 16140–16151. [Google Scholar] [CrossRef] [PubMed]
- Verderosa, A.D.; Totsika, M.; Fairfull-Smith, K.E. Bacterial biofilm eradication agents: A current review. Front. Chem. 2019, 7, 495483. [Google Scholar] [CrossRef]
- Verderosa, A.D.; Dhouib, R.; Fairfull-Smith, K.E.; Totsika, M. Profluorescent fluoroquinolone-nitroxides for investigating antibiotic-bacterial interactions. Antibiotics 2019, 8, 19. [Google Scholar] [CrossRef]
- Sanz-García, F.; Hernando-Amado, S.; Martínez, J.L. Evolution under low antibiotic concentrations: A risk for the selection of Pseudomonas aeruginosa multidrug-resistant mutants in nature. Environ. Microbiol. 2022, 24, 1279–1293. [Google Scholar] [CrossRef]
- Rojo-Molinero, E.; Macia, M.D.; Rubio, R.; Moya, B.; Cabot, G.; Lopez-Causape, C.; Perez, J.L.; Canton, R.; Oliver, A. Sequential treatment of biofilms with aztreonam and tobramycin is a novel strategy for combating Pseudomonas aeruginosa chronic respiratory infections. Antimicrob. Agents Chemother. 2016, 60, 2912–2922. [Google Scholar] [CrossRef]
- Antoniu, S. Novel inhaled combined antibiotic formulations in the treatment of Pseudomonas aeruginosa airways infections in cystic fibrosis. Expert. Rev. Anti Infect. Ther. 2015, 13, 897–905. [Google Scholar] [CrossRef]
- Andersson, D.I.; Hughes, D. Microbiological effects of sublethal levels of antibiotics. Nat. Rev. Microbiol. 2014, 12, 465–478. [Google Scholar] [CrossRef]
- Kaplan, J.B. Antibiotic-induced biofilm formation. Int. J. Artif. Organs 2011, 34, 737–751. [Google Scholar] [CrossRef]
- Song, T.; Duperthuy, M.; Wai, S.N. Sub-optimal treatment of bacterial biofilms. Antibiotics 2016, 5, 23. [Google Scholar] [CrossRef]
- Tahrioui, A.; Duchesne, R.; Bouffartigues, E.; Rodrigues, S.; Maillot, O.; Tortuel, D.; Hardouin, J.; Taupin, L.; Groleau, M.-C.; Dufour, A.; et al. Extracellular DNA release, quorum sensing, and PrrF1/F2 small RNAs are key players in Pseudomonas aeruginosa tobramycin-enhanced biofilm formation. NPJ Biofilms Microbiomes 2019, 5, 15. [Google Scholar] [CrossRef]
- David, A.; Tahrioui, A.; Duchesne, R.; Tareau, A.-S.; Maillot, O.; Barreau, M.; Feuilloley, M.G.J.; Lesouhaitier, O.; Cornelis, P.; Bouffartigues, E.; et al. Membrane fluidity homeostasis is required for tobramycin-enhanced biofilm in Pseudomonas aeruginosa. Microbiol. Spectr. 2024, 12, 0230323. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
David, A.; Tahrioui, A.; Tareau, A.-S.; Forge, A.; Gonzalez, M.; Bouffartigues, E.; Lesouhaitier, O.; Chevalier, S. Pseudomonas aeruginosa Biofilm Lifecycle: Involvement of Mechanical Constraints and Timeline of Matrix Production. Antibiotics 2024, 13, 688. https://doi.org/10.3390/antibiotics13080688
David A, Tahrioui A, Tareau A-S, Forge A, Gonzalez M, Bouffartigues E, Lesouhaitier O, Chevalier S. Pseudomonas aeruginosa Biofilm Lifecycle: Involvement of Mechanical Constraints and Timeline of Matrix Production. Antibiotics. 2024; 13(8):688. https://doi.org/10.3390/antibiotics13080688
Chicago/Turabian StyleDavid, Audrey, Ali Tahrioui, Anne-Sophie Tareau, Adrien Forge, Mathieu Gonzalez, Emeline Bouffartigues, Olivier Lesouhaitier, and Sylvie Chevalier. 2024. "Pseudomonas aeruginosa Biofilm Lifecycle: Involvement of Mechanical Constraints and Timeline of Matrix Production" Antibiotics 13, no. 8: 688. https://doi.org/10.3390/antibiotics13080688




