Impact of Empirical Antimicrobial Treatment on Patients with Ventilator-Associated Pneumonia Due to Stenotrophomonas maltophilia
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Setting
2.2. Bacterial Identification and Antimicrobial Susceptibility
2.3. Study Design and Data Collection
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brooke, J.S. Stenotrophomonas maltophilia: An emerging global opportunistic pathogen. Clin. Microbiol. Rev. 2012, 25, 2–41. [Google Scholar] [CrossRef] [PubMed]
- ibn Saied, W.; Merceron, S.; Schwebel, C.; Le Monnier, A.; Oziel, J.; Garrouste-Orgeas, M.; Marcotte, G.; Ruckly, S.; Souweine, B.; Darmon, M.; et al. Ventilator-associated pneumonia due to Stenotrophomonas maltophilia: Risk factors and outcome. J. Infect. 2020, 80, 279–285. [Google Scholar] [CrossRef]
- Denton, M.; Kerr, K.G. Microbiological and clinical aspects of infection associated with Stenotrophomonas maltophilia. Clin. Microbiol. Rev. 1998, 11, 57–80. [Google Scholar] [CrossRef]
- de Oliveira-Garcia, D.; Dall’Agnol, M.; Rosales, M.; Azzuz, A.C.G.S.; Martinez, M.B.; Girón, J.A. Characterization of flagella produced by clinical strains of Stenotrophomonas maltophilia. Emerg. Infect. Dis. 2002, 8, 918–923. [Google Scholar] [CrossRef]
- de Oliveira-Garcia, D.; Dall’Agnol, M.; Rosales, M.; Azzuz, A.C.G.S.; Alcántara, N.; Martinez, M.B.; Girón, J.A. Fimbriae and adherence of Stenotrophomonas maltophilia to epithelial cells and to abiotic surfaces. Cell Microbiol. 2003, 5, 625–636. [Google Scholar] [CrossRef] [PubMed]
- Waters, V.J.; Gómez, M.I.; Soong, G.; Amin, S.; Ernst, R.K.; Prince, A. Immunostimulatory properties of the emerging pathogen Stenotrophomonas maltophilia. Infect. Immun. 2007, 75, 1698–1703. [Google Scholar] [CrossRef] [PubMed]
- Ryan, R.P.; Fouhy, Y.; Garcia, B.F.; Watt, S.A.; Niehaus, K.; Yang, L.; Tolker-Nielsen, T.; Dow, J.M. Interspecies signaling via the Stenotrophomonas maltophilia diffusible signal factor influences biofilm formation and polymyxin tolerance in Pseudomonas aeruginosa. Mol. Microbiol. 2008, 68, 75–86. [Google Scholar] [CrossRef]
- Alonso, A.; Martínez, J.L. Multiple antibiotic resistance in Stenotrophomonas maltophilia. Antimicrob. Agents Chemother. 1997, 41, 1140–1142. [Google Scholar] [CrossRef] [PubMed]
- Nicodemo, A.C.; Paez, J.I.G. Antimicrobial therapy for Stenotrophomonas maltophilia infections. Eur. J. Clin. Microbiol. Infect. Dis. 2007, 26, 229–237. [Google Scholar] [CrossRef]
- Luna, C.M.; Vujacich, P.; Niederman, M.S.; Vay, C.; Gherardi, C.; Matera, J.; Jolly, E.C. Impact of BAL data on the therapy and outcome of ventilator-associated pneumonia. Chest 1997, 111, 676–685. [Google Scholar] [CrossRef]
- Gales, A.C.; Jones, R.N.; Sader, H.S. Global assessment of the antimicrobial activity of polymyxin B against 54 731 clinical isolates of Gram-negative bacilli: Report from the SENTRY antimicrobial surveillance programme (2001–2004). Clin. Microbiol. Infect. 2006, 12, 315–321. [Google Scholar] [CrossRef]
- Markou, N.; Fousteri, M.; Markantonis, S.L.; Boutzouka, E.; Tsigou, E.; Baltopoulo, G. Colistin penetration in the alveolar lining fluid of critically ill patients treated with IV colistimethate sodium. Chest 2011, 139, 232–233, author reply 233. [Google Scholar] [CrossRef] [PubMed]
- Raad, M.; Abou Haidar, M.; Ibrahim, R.; Rahal, R.; Abou Jaoude, J.; Harmouche, C.; Habr, B.; Ayoub, E.; Saliba, G.; Sleilaty, G.; et al. Stenotrophomonas maltophilia pneumonia in critical COVID-19 patients. Sci. Rep. 2023, 13, 3392. [Google Scholar] [CrossRef] [PubMed]
- Puech, B.; Canivet, C.; Teysseyre, L.; Miltgen, G.; Aujoulat, T.; Caron, M.; Combe, C.; Jabot, J.; Martinet, O.; Allyn, J.; et al. Effect of antibiotic therapy on the prognosis of ventilator-associated pneumonia caused by Stenotrophomonas maltophilia. Ann. Intensive Care 2021, 11, 160. [Google Scholar] [CrossRef] [PubMed]
- Kanchanasuwan, S.; Rongmuang, J.; Siripaitoon, P.; Kositpantawong, N.; Charoenmak, B.; Hortiwakul, T.; Nwabor, O.F.; Chusri, S. Clinical characteristics, outcomes, and risk factors for mortality in patients with Stenotrophomonas maltophilia bacteremia. J. Clin. Med. 2022, 11, 3085. [Google Scholar] [CrossRef] [PubMed]
- Kanchanasuwan, S.; Kositpantawong, N.; Singkhamanan, K.; Hortiwakul, T.; Charoenmak, B.; Ozioma, F.N.; Doi, Y.; Chusri, S. Outcomes of adjunctive therapy with intravenous cefoperazone-sulbactam for ventilator-associated Pneumonia due to carbapenem-resistant Acinetobacter baumannii. Infect. Drug Resist. 2021, 1255–1264. [Google Scholar] [CrossRef] [PubMed]
- Wayne, P. Performance Standards for Antimicrobial Susceptibility Testing, 20th Informational Supplement; CLSI Document m100; Clinical and Laboratory Standards Institute: Malvern, PA, USA, 2010; Volume S20. [Google Scholar]
- Knaus, W.A.; Draper, E.A.; Wagner, D.P.; Zimmerman, J.E. APACHE II: A severity of disease classification system. Crit. Care Med. 1985, 13, 818–829. [Google Scholar] [CrossRef] [PubMed]
- Insuwanno, W.; Kiratisin, P.; Jitmuang, A. Stenotrophomonas maltophilia infections: Clinical characteristics and factors associated with mortality of hospitalized patients. Infect. Drug Resist. 2020, 13, 1559–1566. [Google Scholar] [CrossRef] [PubMed]
- Xun, M.; Zhang, Y.; Li, B.L.; Wu, M.; Zong, Y.; Yin, Y.M. Clinical characteristics and risk factors of infections caused by Stenotrophomonas maltophilia in a hospital in northwest China. J. Infect. Dev. Ctries. 2014, 8, 1000–1005. [Google Scholar] [CrossRef]
- Hsu, J.F.; Chu, S.M.; Wang, H.C.; Liao, C.C.; Lai, M.Y.; Huang, H.R.; Chiang, M.C.; Fu, R.H.; Tsai, M.H. Multidrug-resistant healthcare-associated infections in neonates with severe respiratory failure and the impacts of inappropriate initial antibiotic therapy. Antibiotics 2021, 10, 459. [Google Scholar] [CrossRef]
- Koomanachai, P.; Srisompong, J.; Chayangsu, S.; Ruangkriengsin, D.; Thamlikitkul, V.; Wangchinda, W.; Sirijatuphat, R.; Rattanaumpawan, P. Implementation of clinical practice guidelines for empirical antibiotic therapy of bacteremia, urinary tract infection, and pneumonia: A multi-center quasi-experimental study. Antibiotics 2022, 11, 903. [Google Scholar] [CrossRef] [PubMed]
- Pajot, O.; Lakhal, K.; Lambert, J.; Gros, A.; Bruel, C.; Boulain, T.; Garot, D.; Das, V.; Timsit, J.F.; Cerf, C.; et al. Empirical antibiotic therapy for gram-negative bacilli ventilator-associated pneumonia: Observational study and pharmacodynamic assessment. Antibiotics 2022, 11, 1664. [Google Scholar] [CrossRef] [PubMed]
- Ghadimi, M.; Siemieniuk, R.A.C.; Guyatt, G.; Loeb, M.; Hazzan, A.A.; Aminaei, D.; Gomaa, H.; Wang, Y.; Yao, L.; Agarwal, A.; et al. Empiric antibiotic regimens in adults with non-ventilator-associated hospital-acquired pneumonia: A systematic review and network meta-analysis of randomized controlled trials. Clin. Microbiol. Infect. 2024; in press. [Google Scholar] [CrossRef] [PubMed]
- De Pascale, G.; Pintaudi, G.; Lisi, L.; De Maio, F.; Cutuli, S.L.; Tanzarella, E.S.; Carelli, S.; Lombardi, G.; Cesarano, M.; Gennenzi, V.; et al. Use of high-dose nebulized colistimethate in patients with colistin-only susceptible Acinetobacter baumannii VAP: Clinical, pharmacokinetic and microbiome features. Antibiotics 2023, 12, 125. [Google Scholar] [CrossRef]
- Tamma, P.D.; Aitken, S.L.; Bonomo, R.A.; Mathers, A.J.; van Duin, D.; Clancy, C.J. Infectious Diseases Society of America 2023 guidance on the treatment of antimicrobial resistant gram-negative infections. Clin. Infect. Dis. 2023, ciad428. [Google Scholar] [CrossRef]
| Antibiotics | No. of Resistant Isolates (%) |
|---|---|
| Chloramphenicol | 32 (13) |
| Colistin | 55 (23) |
| Ciprofloxacin | 11 (5) |
| Levofloxacin | 12 (5) |
| Trimethoprim-sulfamethoxazole | 7 (3) |
| Tigecycline | 9 (4) |
| Ceftazidime | 135 (56) |
| Parameter | Patients with VAP due to S. maltophilia (N = 240) |
|---|---|
| Demographics | |
| Age, median (IQR), [range] | 45 (41–74) [19–96] |
| Male sex, n (%) | 158 (66) |
| Comorbidities, n (%) | 217 (90) |
| Immunocompromised status, n (%) | 12 (5) |
| Obesity, n (%) | 176 (73) |
| Diabetes mellitus, n (%) | 82 (34) |
| Hypertension, n (%) | 124 (52) |
| Chronic kidney disease(s), n (%) | 30 (13) |
| Cardiovascular disease(s), n (%) | 48 (20) |
| Cerebrovascular disease(s), n (%) | 8 (3) |
| Chronic pulmonary disease(s), n (%) | 65 (27) |
| Solid organ malignancy, n (%) | 6 (3) |
| Hematologic malignancy, n (%) | 49 (2) |
| Charlson comorbidity index, median (IQR), [range] | 6 (5–8) [0–20] |
| Previous exposure to antibiotics | |
| Carbapenem, n (%) | 219 (91) |
| Cephalosporin, n (%) | 175 (73) |
| Fluoroquinolone, n (%) | 100 (42) |
| β-lactam/β-lactamase inhibitor, n (%) | 191 (80) |
| Aminoglycoside, n (%) | 49 (20) |
| Clinical characteristics | |
| Initial ICU admission, n (%) | 109 (45) |
| APACHE II score, median (IQR), [range] | 17 (13–21) [9–28] |
| Bloodstream infection due to S. maltophilia, n (%) | 121 (50) |
| Invasive medical devices, n (%) | 190 (79) |
| Intravascular device, n (%) | 123 (51) |
| Urinary catheterization, n (%) | 187 (78) |
| Treatment Empirical treatment including | |
| Carbapenem(s), n (%) | 234 (98) |
| Colistin, n (%) | 73 (30) |
| Trimethoprim-sulfamethoxazole, n (%) | 31 (13) |
| Fluoroquinolone(s), n (%) | 18 (8) |
| Ciprofloxacin, n (%) | 9 (4) |
| Levofloxacin, n (%) | 9 (4) |
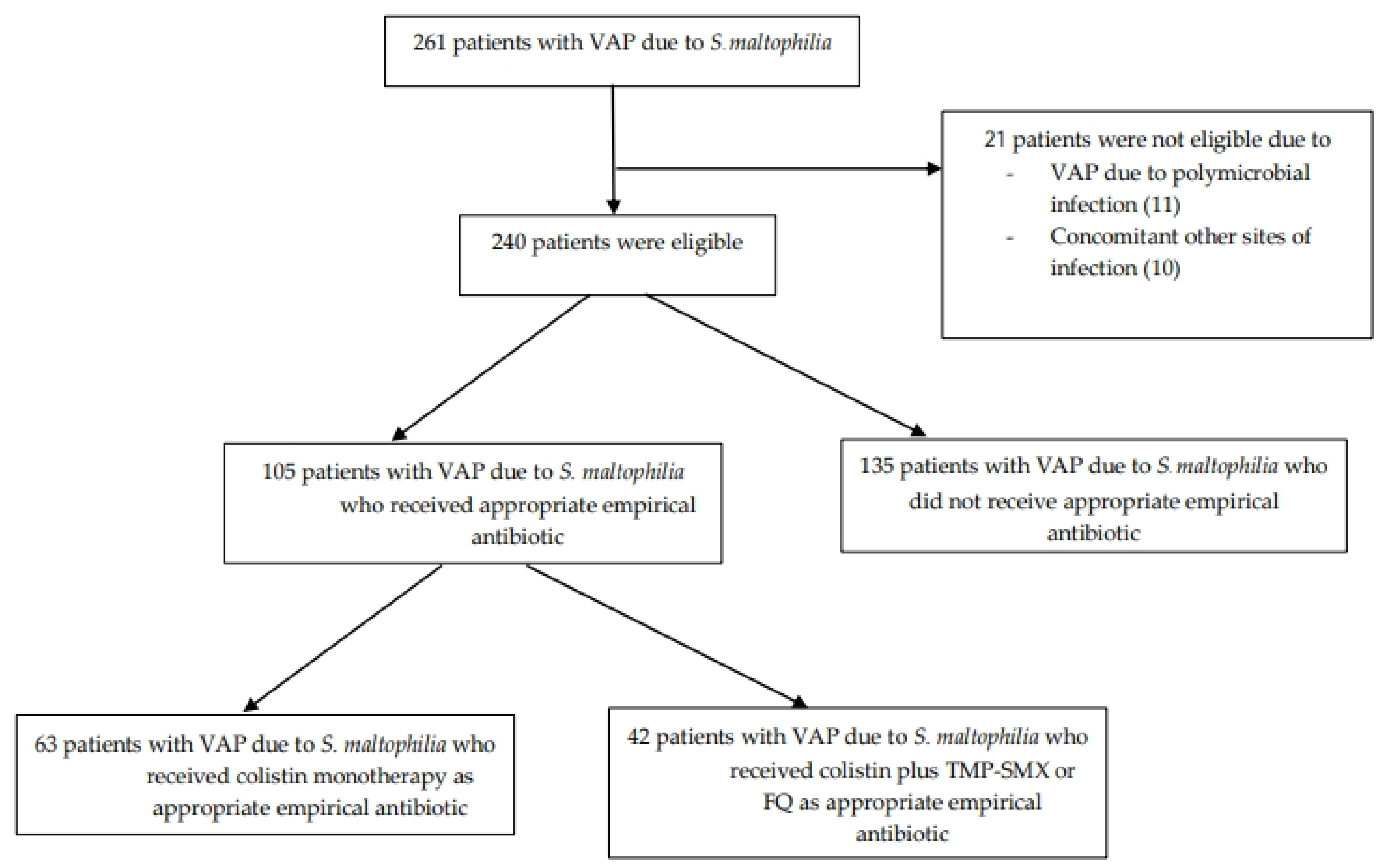
| Appropriate empirical antibiotics, n (%) | 105 (44) |
| Colistin monotherapy, n (%) | 63 (26) |
| Colistin plus trimethoprim-sulfamethoxazole or fluoroquinolone, n (%) | 42 (18) |
| Duration of empirical treatment (day), median (IQR), [range] | 3 (2–3) [2–4] |
| Definitive treatment regimen(s) | |
| Colistin plus trimethoprim-sulfamethoxazole, n (%) | 153 (64) |
| Colistin plus fluoroquinolone(s), n (%) | 19 (8) |
| Ciprofloxacin, n (%) | 10 (4) |
| Levofloxacin, n (%) | 9 (4) |
| Trimethoprim-sulfamethoxazole monotherapy, n (%) | 37 (15) |
| Fluoroquinolone monotherapy, n (%) | 10 (4) |
| Ciprofloxacin, n (%) | 7 (3) |
| Levofloxacin, n (%) | 3 (1) |
| Colistin monotherapy, n (%) | 21 (9) |
| Duration of definitive treatment (day), median (IQR), [range] | 14 (12–19) [7–21] |
| Parameter | S. maltophilia VAP Patients Who Did Not Receive Appropriate Empirical Antibiotics (N = 135) | S. maltophilia VAP Patients Who Received Appropriate Empirical Antibiotic with Colistin Monotherapy (N = 63) | S. maltophilia VAP Patients Who Received Appropriate Empirical Antibiotic with Colistin plus Trimethoprim-Sulfamethoxazole or Fluoroquinolone(s) (N = 42) | p-Value |
|---|---|---|---|---|
| Demographics | ||||
| Age, median (IQR) | 45 (41–68) | 45 (34–74) | 47 (42–74) | 0.639 |
| Male sex, n (%) | 82 (61) | 46 (73) | 30 (71) | 0.166 |
| Comorbidities, n (%) | 117 (87) | 58 (92) | 42 (100) | 0.033 |
| Immunocompromised status, n (%) | 6 (4) | 3 (5) | 3 (7) | 0.662 |
| Obesity, n (%) | 102 (76) | 41 (65) | 33 (79) | 0.210 |
| Diabetes mellitus, n (%) | 52 (39) | 17 (27) | 13 (31) | 0.250 |
| Hypertension, n (%) | 70 (52) | 32 (51) | 22 (52) | 0.899 |
| Chronic kidney disease, n (%) | 20 (15) | 7 (11) | 3 (7) | 0.392 |
| Cardiovascular disease, n (%) | 30 (22) | 9 (14) | 9 (21) | 0.072 |
| Cerebrovascular disease, n (%) | 4 (3) | 3 (5) | 1 (2) | 0.791 |
| Chronic pulmonary disease, n (%) | 38 (28) | 15 (24) | 12 (29) | 0.792 |
| Solid organ malignancy, n (%) | 4 (3) | 1 (2) | 1 (2) | 0.134 |
| Hematologic malignancy, n (%) | 22 (16) | 18 (29) | 9 (21) | 0.662 |
| Charlson comorbidity index, median (IQR) | 6 (4, 7) | 7 (5, 9) | 7 (6, 9) | 0.234 |
| Clinical characteristics | ||||
| Initial ICU admission, n (%) | 48 (36) | 37 (59) | 24 (57) | 0.002 |
| APACHE II score, median (IQR), [range] | 16 (14–18) [7−23] | 21 (14–23) [7−22] | 20 (13–22) [8−23] | <0.001 |
| Bloodstream infection due to S. maltophilia, n (%) | 67 (50) | 32 (51) | 22 (52) | 0.950 |
| Invasive medical devices, n (%) | 101 (75) | 53 (84) | 36 (86) | 0.167 |
| Previous exposure to carbapenems, n (%) | 124 (92) | 58 (92) | 37 (88) | 0.754 |
| Treatment | ||||
| Duration of empirical treatment (day), median (IQR) | 3 (2, 3) | 3 (2, 3) | 3 (2, 3) | 0.867 |
| Empirical treatment including carbapenems, n (%) | 132 (98) | 61 (98) | 41 (98) | 0.903 |
| Definitive treatment regimen(s) | ||||
| Colistin plus trimethoprim-sulfamethoxazole, n (%) | 86 (64) | 39 (62) | 28 (66) | 0.764 |
| Colistin plus fluoroquinolone(s), n (%) | 11 (8) | 4 (6) | 5 (12) | 0.064 |
| Trimethoprim-sulfamethoxazole monotherapy, n (%) | 22 (16) | 9 (14) | 6 (14) | 0.201 |
| Fluoroquinolone monotherapy, n (%) | 4 (3) | 3 (5) | 3 (7) | 0.074 |
| Colistin monotherapy, n (%) | 12 (9) | 5 (8) | 4 (10) | 0.815 |
| Duration of definitive treatment (day), median (IQR) | 15 (13–20) | 14 (11–18) | 14 (12–19) | 0.564 |
| Outcomes | ||||
| Mortality | ||||
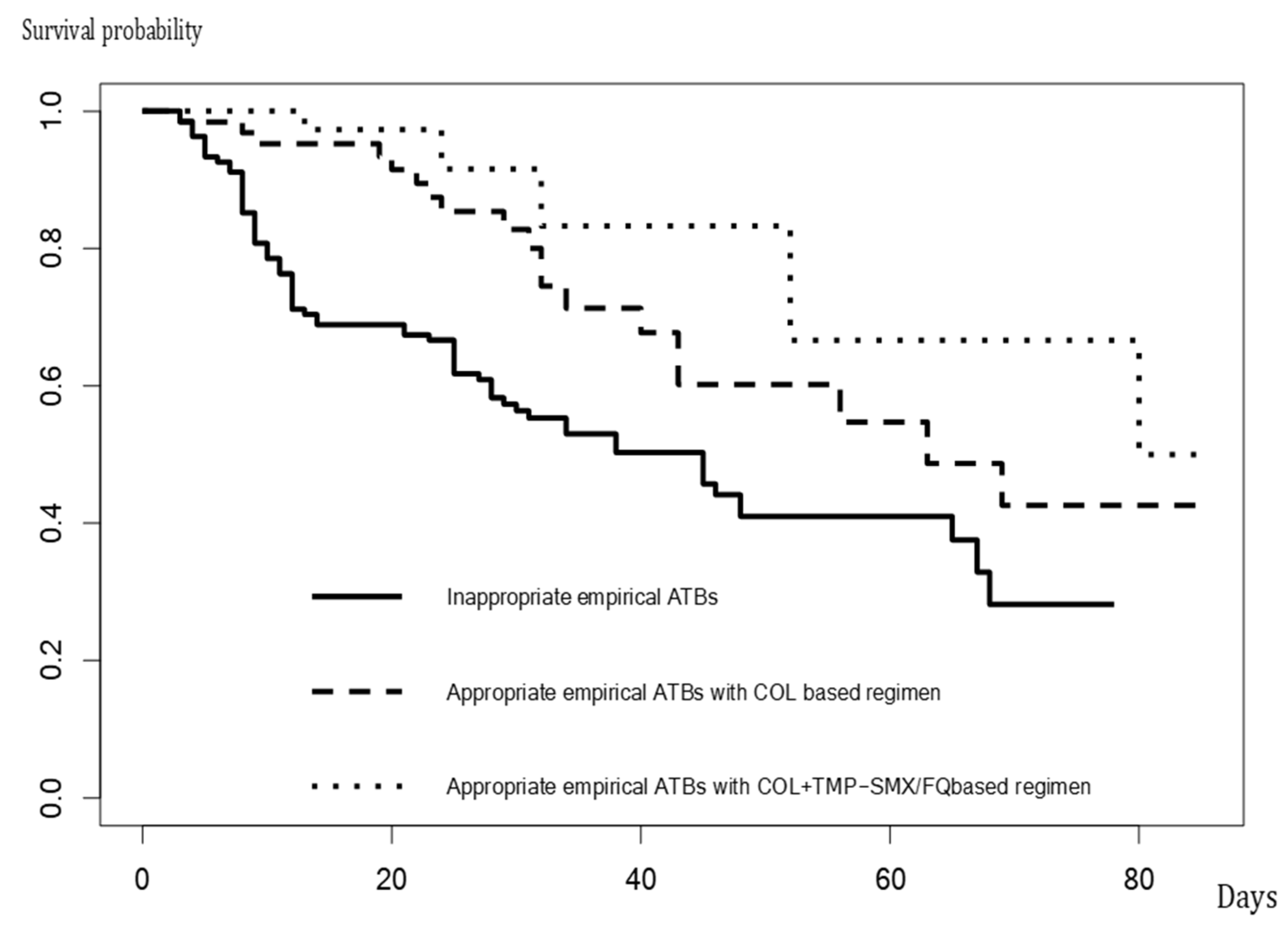
| 14-day, n (%) | 42 (31) | 13 (21) | 1 (2) | <0.001 |
| 30-day, n (%) | 60 (44) | 19 (30) | 2 (5) | <0.001 |
| In-hospital, n (%) | 71 (53) | 19 (30) | 5 (12) | <0.001 |
| Length of hospital stay after end of VAP treatment (days), median (IQR) | 28 (12–43) | 29 (21–43) | 21 (15–32) | 0.032 |
| No. of ventilator days since diagnosis of VAP (days), median (IQR) | 17 (14–21) | 15 (12–20) | 11 (9–17) | 0.041 |
| Hospital cost (Baht), median (IQR) | 200,716 (124,897–289,326) | 188,896 (115,345–232,886) | 156,564 (106,887–190,723) | 0.007 |
| Variables | Values | Crude ORs (95% CI) | Adjusted ORs (95% CI) | p-Values for Adjusted ORs | |
|---|---|---|---|---|---|
| Survivors (N = 145) | Non-Survivors (N = 95) | ||||
| Age (years), median (IQR) | 44 (41–67) | 48 (40–75) | 1.13 (0.98, 1.38) | 1.02 (0.97, 1.12) | 0.099 |
| Male sex, n (%) | 102 (70) | 56 (59) | 0.82 (0.44, 1.54) | 0.60 (0.35, 1.04) | 0.548 |
| Charlson comorbidity index, median (IQR) | 7 (5, 9) | 8 (6, 9) | 1.23 (0.88, 1.45) | 1.04 (0.81, 1.24) | 0.675 |
| Immunocompromised status, n (%) | 9 (6) | 3 (3) | 0.63 (0.15, 2.70) | 0.49 (0.12, 1.85) | 0.528 |
| Obesity, n (%) | 108 (75) | 68 (72) | 0.86 (0.48, 1.5) | 0.77 (0.38, 1.51) | 0.434 |
| APACHE II score [median (IQR)] | 14 (12–18) | 19 (15–22) | 1.88 (1.21, 3.02) | 1.32 (1.05, 2.43) | 0.041 |
| Bloodstream infection due to S. maltophilia, n (%) | 81 (56) | 40 (42) | 0.57 (0.34, 0.97) | 0.88 (0.56, 1.16) | 0.091 |
| Initial intensive care unit admission, n (%) | 69 (48) | 40 (42) | 0.80 (0.48, 1.35) | 1.41 (0.75, 2.63) | 0.286 |
| Appropriate empirical antibiotic(s) | |||||
| Colistin monotherapy, n (%) | 44 (30) | 19 (20) | 0.39 (0.21, 0.74) | 0.25 (0.12, 0.53) | <0.001 |
| Colistin plus trimethoprim-sulfamethoxazole or fluoroquinolone(s), n (%) | 37 (26) | 5 (5) | 0.12 (0.05, 0.33) | 0.08 (0.02, 0.19) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khunkit, P.; Siripaitoon, P.; Lertsrisatit, Y.; Watthanapaisal, D.; Kositpantawong, N.; Kanchanasuwan, S.; Cheh-oh, N.; Chittrakarn, S.; Jaroenmark, T.; Poonchuay, N.; et al. Impact of Empirical Antimicrobial Treatment on Patients with Ventilator-Associated Pneumonia Due to Stenotrophomonas maltophilia. Antibiotics 2024, 13, 729. https://doi.org/10.3390/antibiotics13080729
Khunkit P, Siripaitoon P, Lertsrisatit Y, Watthanapaisal D, Kositpantawong N, Kanchanasuwan S, Cheh-oh N, Chittrakarn S, Jaroenmark T, Poonchuay N, et al. Impact of Empirical Antimicrobial Treatment on Patients with Ventilator-Associated Pneumonia Due to Stenotrophomonas maltophilia. Antibiotics. 2024; 13(8):729. https://doi.org/10.3390/antibiotics13080729
Chicago/Turabian StyleKhunkit, Pirawan, Pisud Siripaitoon, Yongyut Lertsrisatit, Dissaya Watthanapaisal, Narongdet Kositpantawong, Siripen Kanchanasuwan, Nadia Cheh-oh, Sorawit Chittrakarn, Tanapat Jaroenmark, Natnicha Poonchuay, and et al. 2024. "Impact of Empirical Antimicrobial Treatment on Patients with Ventilator-Associated Pneumonia Due to Stenotrophomonas maltophilia" Antibiotics 13, no. 8: 729. https://doi.org/10.3390/antibiotics13080729
APA StyleKhunkit, P., Siripaitoon, P., Lertsrisatit, Y., Watthanapaisal, D., Kositpantawong, N., Kanchanasuwan, S., Cheh-oh, N., Chittrakarn, S., Jaroenmark, T., Poonchuay, N., & Chusri, S. (2024). Impact of Empirical Antimicrobial Treatment on Patients with Ventilator-Associated Pneumonia Due to Stenotrophomonas maltophilia. Antibiotics, 13(8), 729. https://doi.org/10.3390/antibiotics13080729





