Measuring Temporal Trends and Patterns of Inpatient Antibiotic Use in Northwest China’s Hospitals: Data from the Center for Antibacterial Surveillance, 2012–2022
Abstract
1. Introduction
2. Results
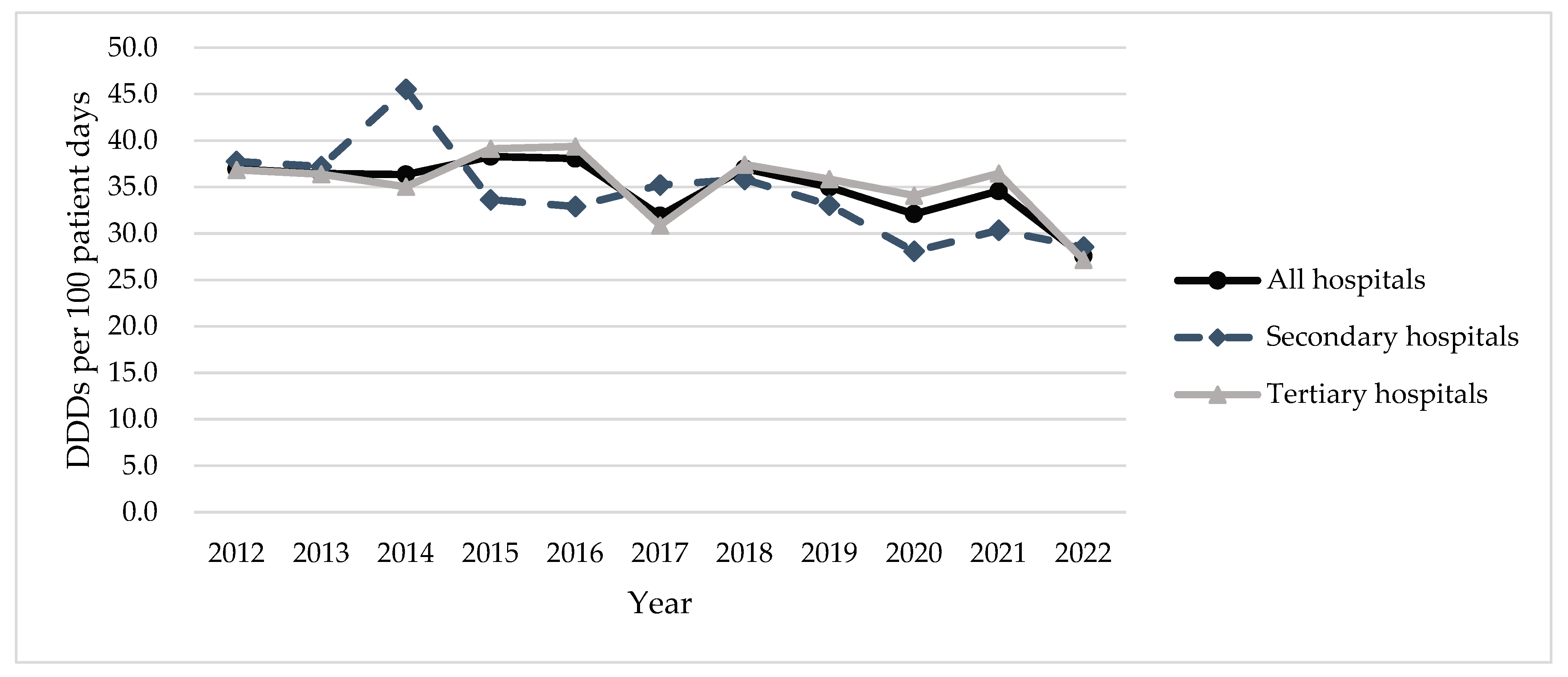
2.1. Total Antibiotic Usage
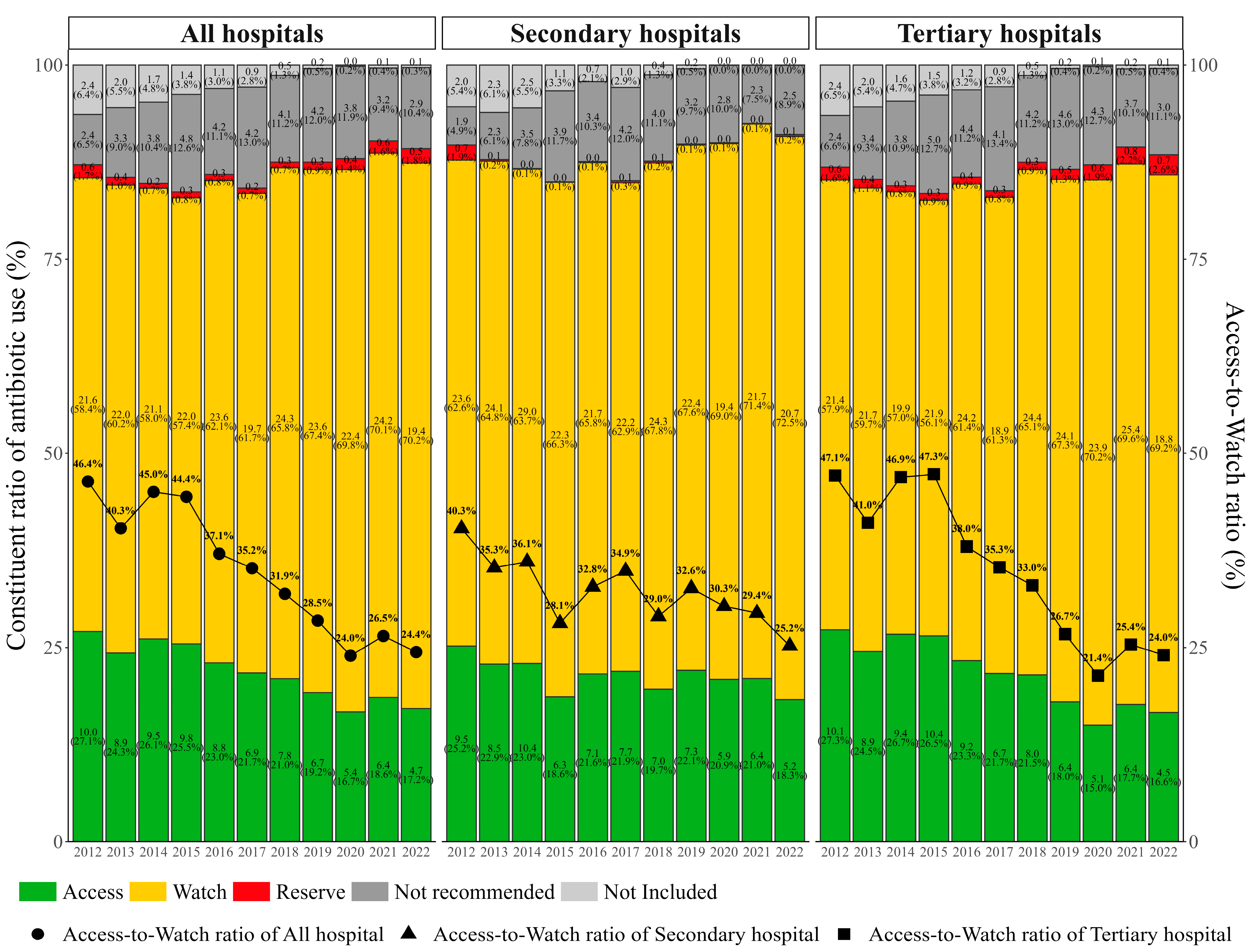
2.2. Antibiotic Usage by AWaRe Classification
2.3. DU90%
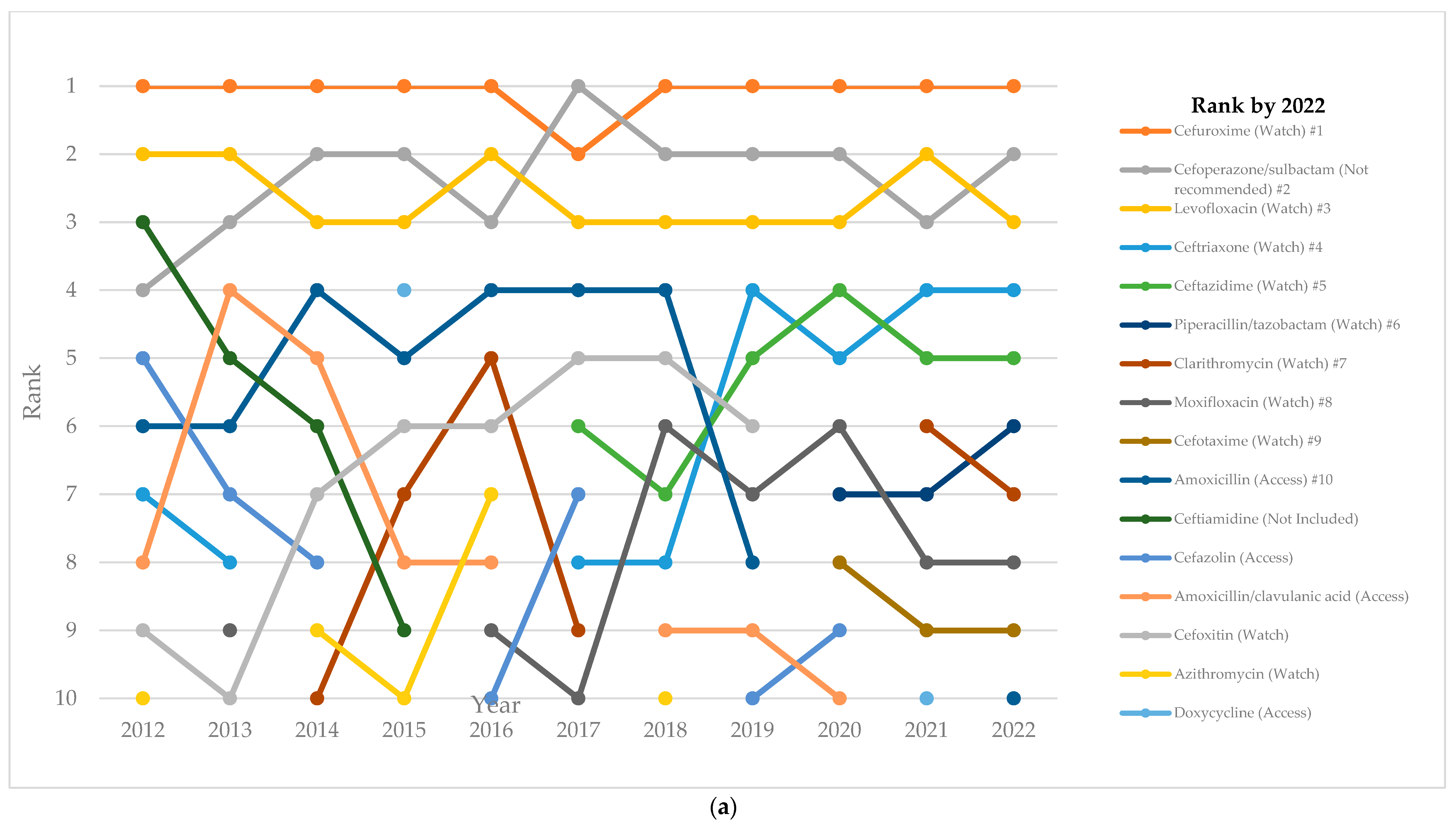
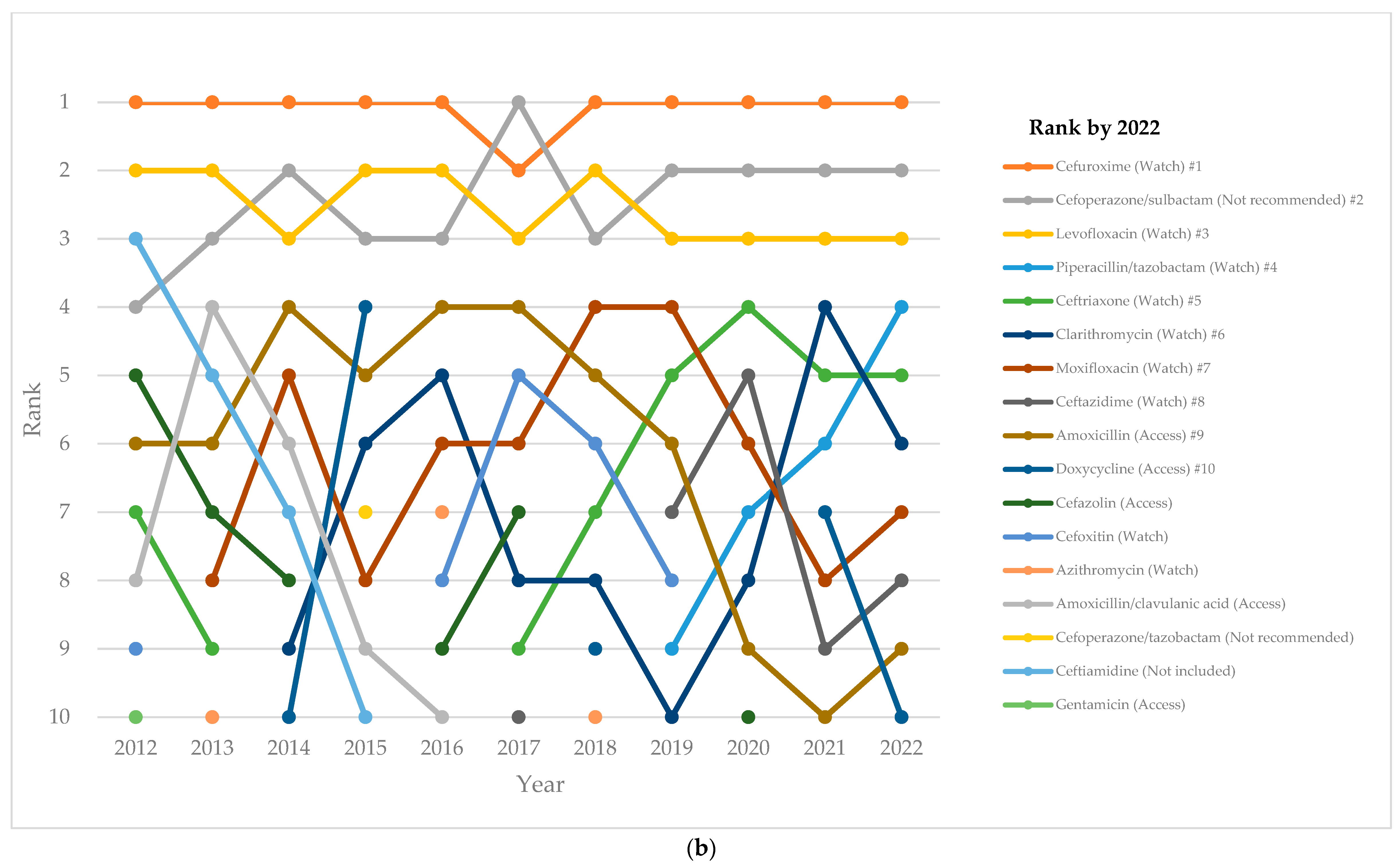
2.4. Top-10 Antibiotics
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Setting
4.3. Data Source
4.4. Data Collection
4.5. Data Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO Global Antimicrobial Resistance and Use Surveillance System (GLASS): Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report. 2022. Available online: https://www.who.int/publications/i/item/9789240062702 (accessed on 10 July 2024).
- Jonas, O.B.; Irwin, A.; Berthe, F.C.J.; Le Gall, F.G.; Marquez, P.V. Drug-Resistant Infections: A Threat to Our Economic Future (Vol. 2): Final Report (English). HNP/Agriculture Global Antimicrobial Resistance Initiative Washington, D.C.: World Bank Group. Available online: http://documents.worldbank.org/curated/en/323311493396993758/final-report (accessed on 11 July 2024).
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Optimizing Use of Antimicrobial Medicines. Available online: https://www.who.int/activities/optimizing-selection-and-use-of-antimicrobial-medicines (accessed on 26 March 2024).
- Laxminarayan, R.; Van Boeckel, T.; Frost, I.; Kariuki, S.; Khan, E.A.; Limmathurotsakul, D.; Larsson, D.G.J.; Levy-Hara, G.; Mendelson, M.; Outterson, K.; et al. The Lancet Infectious Diseases Commission on antimicrobial resistance: 6 years later. Lancet Infect. Dis. 2020, 20, e51–e60. [Google Scholar] [CrossRef] [PubMed]
- Holmes, A.H.; Moore, L.S.P.; Sundsfjord, A.; Steinbakk, M.; Regmi, S.; Karkey, A.; Guerin, P.J.; Piddock, L.J.V. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet 2016, 387, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wei, L.; Li, H.; Zhang, M.; Cao, B.; Bian, J.; Zhan, S. Appropriateness of antibiotic prescriptions in ambulatory care in China: A nationwide descriptive database study. Lancet Infect. Dis. 2021, 21, 847–857. [Google Scholar] [CrossRef] [PubMed]
- Tackling Drug-Resistant Infections Globally: Final Report and Recommendations; Wellcome Trust: London, UK, 2016; Available online: https://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf (accessed on 10 March 2022).
- Global Antimicrobial Resistance and Use Surveillance System (GLASS); World Health Organization: Geneva, Switzerland, 2015; Available online: https://www.who.int/initiatives/glass (accessed on 6 October 2022).
- GLASS Methodology for Surveillance of National Antimicrobial Consumption; World Health Organization: Geneva, Switzerland, 2020; Available online: https://iris.who.int/bitstream/handle/10665/336215/9789240012639-eng.pdf (accessed on 6 October 2022).
- GLASS Manual on the Management of Antimicrobial Consumption Data; World Health Organization: Geneva, Switzerland, 2020; Available online: https://www.who.int/publications/i/item/9789240010192 (accessed on 6 October 2022).
- GLASS Guide for National Surveillance Systems for Monitoring Antimicrobial Consumption in Hospitals; World Health Organization: Geneva, Switzerland, 2020; Available online: https://www.who.int/publications/i/item/9789240000421 (accessed on 6 October 2022).
- Xiao, Y.; Li, L. China’s national plan to combat antimicrobial resistance. Lancet Infect. Dis. 2016, 16, 1216–1218. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Hu, F. China’s new national action plan to combat antimicrobial resistance (2022-25). J. Antimicrob. Chemother. 2023, 78, 558–560. [Google Scholar] [CrossRef] [PubMed]
- Wushouer, H.; Zhou, Y.; Zhang, W.; Hu, L.; Du, K.; Yang, Y.; Yao, G.; Little, P.; Zheng, B.; Guan, X.; et al. Inpatient antibacterial use trends and patterns, China, 2013–2021. Bull. World Health Organ. 2023, 101, 248–261B. [Google Scholar] [CrossRef] [PubMed]
- Notice of the Ministry of Health on Strengthening the Clinical Application of Antibacterials; Ministry of Health: Beijing, China, 2012. Available online: http://www.gov.cn/gzdt/2012-06/11/content_2158064.htm (accessed on 8 August 2021). (In Chinese)
- Regulations for Clinical Use of Antimicrobials; Ministry of Health: Beijing, China, 2012. Available online: http://www.gov.cn/flfg/2012-05/08/content_2132174.htm (accessed on 8 March 2018). (In Chinese)
- National Action Plan for Containing Antimicrobial Resistance (2016–2020); National Health and Family Planning Commission: Beijing, China, 2016. Available online: http://www.gov.cn/xinwen/2016-08/25/content_5102348.htm (accessed on 2 March 2018). (In Chinese)
- Yan, K.; Xue, M.; Ye, D.; Yang, C.; Chang, J.; Jiang, M.; Zhao, M.; Zhang, H.; Fang, Y. Antibiotic prescribing practices in secondary and tertiary hospitals in Shaanxi province, western China, 2013–2015. PLoS ONE 2018, 13, e0207229. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Feng, H.; Ma, Y.; Zhao, D.; Hu, X. Long-term trend of antibiotic use at public health care institutions in northwest China, 2012–2020—A case study of Gansu Province. BMC Public Health 2023, 23, 27. [Google Scholar] [CrossRef] [PubMed]
- National Health and Family Planning Commission of the People’s Republic of China. Notice on Further Strengthening the Management of Clinical Application of Antimicrobial Drugs (27 August 2015). Available online: https://www.gov.cn/xinwen/2015-08/27/content_2920789.htm (accessed on 1 May 2024).
- Acharya, U.; Mahat, R.; Acharya, S.P.; Pahari, B.; Tripathi, P. Prescribing pattern and utilization of selected antimicrobials in a tertiary hospital of Nepal. Grande Med. J. 2019, 1, 96–102. [Google Scholar] [CrossRef]
- Khan, S.; Bond, S.E.; Lee-Milner, J.; Conway, B.R.; Lattyak, W.J.; Aldeyab, M.A. Antimicrobial consumption in an acute NHS Trust during the COVID-19 pandemic: Intervention time series analysis. JAC Antimicrob. Resist. 2024, 6, dlae013. [Google Scholar] [CrossRef] [PubMed]
- De Greeff, S.C.; Mouton, J.W.; Schoffelen, A.F.; Verduin, C.M. NethMap 2019: Consumption of Antimicrobial Agents and Antimicrobial Resistance among Medically Important Bacteria in the Netherlands/MARAN 2019: Monitoring of Antimicrobial Resistance and Antibiotic Usage in Animals in the Netherlands in 2018; Stichting Werkgroep Antibioticabeleid: Leiden, The Netherland, 2019; Available online: https://rivm.openrepository.com/handle/10029/623134 (accessed on 25 March 2024).
- Swedres Svarm. Sales of Antibiotics and Occurrence of Resistance in Sweden. Solna: Public Health Agency of Sweden and National Veterinary Institute. 2019. Available online: https://www.sva.se/media/0hihej1c/swedres-svarm-2019.pdf (accessed on 25 March 2024).
- Ministry of Health of the People’s Republic of China. Notification on Launching National Regulatory Campaign of Clinical Application of Antibacterial Drugs (19 April 2011). Available online: http://www.moh.gov.cn/mohyzs/s3586/201104/51376.shtml (accessed on 28 April 2017).
- World Health Organization. In the Face of Slow Progress, WHO Offers a New Tool and Sets a Target to Accelerate Action against Antimicrobial Resistance. 2019. Available online: https://www.who.int/news/item/18-06-2019-in-the-face-of-slow-progress-who-offers-a-new-tool-and-sets-a-target-to-accelerate-action-against-antimicrobial-resistance (accessed on 26 March 2024).
- Klein, E.Y.; Milkowska-Shibata, M.; Tseng, K.K.; Sharland, M.; Gandra, S.; Pulcini, C.; Laxminarayan, R. Assessment of WHO antibiotic consumption and access targets in 76 countries, 2000–2015: An analysis of pharmaceutical sales data. Lancet Infect. Dis. 2021, 21, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Wushouer, H.; Yang, Y.; Deng, Y.; Zheng, B.; Shi, L.; Guan, X. A comparative study of antibiotic use in China under Chinese restriction list and world health organization AWaRe classification. Clin. Microbiol. Infect. 2023, 29, 545–547. [Google Scholar] [CrossRef] [PubMed]
- Department of Medical Administration and Committee of Experts on Rational Drug Use; National Health and Family Planning Commission of the People’s Republic of China. National Antimicrobial Therapy Guidance, 1st ed.; People’s Medical Publishing House Co., Ltd.: Beijing, China, 2012. [Google Scholar]
- Kim, J.; Kim, H.J. Consistent Model Selection in Segmented Line Regression. J. Stat. Plan. Inference 2016, 170, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Gillis, D.; Edwards, B.P.M. The utility of joinpoint regression for estimating population parameters given changes in population structure. Heliyon 2019, 5, e02515. [Google Scholar] [CrossRef] [PubMed]
- National Health Commission. 2022 China Health Statistics Yearbook; Peking Union Medical College Press: Beijing, China, 2022; p. 14. [Google Scholar]
- Bureau of Statistics of Xinjiang Uygur Autonomous Region. Statistical Communiqué of Xinjiang Uygur Autonomous Region on the 2022 Economic and Social Development (24 March 2023). Available online: https://tjj.xinjiang.gov.cn/tjj/tjgn/202303/6fc01f2b37a84efaa38fd34363c0a84e.shtml?eqid=db33eaac0000009d00000003647e7bfb (accessed on 23 March 2024).
- World Health Organization. The 2023 WHO Aware Classification of Antibiotics for Evaluation and Monitoring of Use. Available online: https://qualityhealthservices.who.int/quality-toolkit/qt-catalog-item/the-2023-who-aware-classification-of-antibiotics-for-evaluation-and-monitoring-of-use (accessed on 17 June 2024).
- World Health Organization Collaborating Centre for Drug Statistics Methodology; Norwegian Institute of Public Health. ATC/DDD Index. Available online: https://www.whocc.no/atc_ddd_index/ (accessed on 1 May 2024).
- Clegg, L.X.; Hankey, B.F.; Tiwari, R.; Feuer, E.J.; Edwards, B.K. Estimating average annual per cent change in trend analysis. Stat. Med. 2009, 28, 3670–3682. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Chen, H.S.; Midthune, D.; Wheeler, B.; Buckman, D.W.; Green, D.; Byrne, J.; Luo, J.; Feuer, E.J. Data-Driven Choice of a Model Selection Method in Joinpoint Regression. J. Appl. Stat. 2023, 50, 1992–2013. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Chen, H.S.; Byrne, J.; Wheeler, B.; Feuer, E.J. Twenty Years Since Joinpoint 1.0: Two Major Enhancements, their Justification, and Impact. Stat. Med. 2022, 41, 3102–3130. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Fay, M.P.; Yu, B.; Barrett, M.J.; Feuer, E.J. Comparability of segmented line regression models. Biometrics 2004, 60, 1005–1014. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute Surveillance Research Projects. Joinpoint Help System, Pairwise Comparison. Available online: https://surveillance.cancer.gov/help/joinpoint/setting-parameters/advanced-analysis-tools-tab/pairwise-comparison (accessed on 17 July 2024).
- National Cancer Institute. Joinpoint Trend Analysis Software. Available online: https://surveillance.cancer.gov/joinpoint/ (accessed on 14 June 2024).

| ATC Codes (Level 3) | Rate of Antibiotic Use Defined as Daily Doses Per 100 Patient Days (% of Total Antibiotic Use) | AAPC, % | Lower 95% CI | Upper 95% CI | p-Value | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Year | |||||||||||||||
| 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | |||||
| All Hospitals | 36.9 (100.0) | 36.5 (100.0) | 36.3 (100.0) | 38.3 (100.0) | 38.1 (100.0) | 31.9 (100.0) | 37 (100.0) | 35 (100.0) | 32.1 (100.0) | 34.6 (100.0) | 27.6 (100.0) | −2.0 | −3.6 | −0.4 | 0.018 * |
| J01A | 0.1 (0.3) | 0.1 (0.4) | 1.1 (3.1) | 2.3 (6.1) | 0.8 (2.2) | 0.6 (2.0) | 1.0 (2.6) | 0.7 (1.9) | 0.6 (1.7) | 1.6 (4.6) | 0.9 (3.4) | 26.3 | −7.2 | 71.8 | 0.137 |
| J01B | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | - | - | - | - | ||||||
| J01C | 5.0 (13.5) | 5.3 (14.6) | 5.3 (14.6) | 5.2 (13.5) | 5.7 (15.1) | 4.3 (13.6) | 4.8 (13.0) | 4.5 (12.7) | 3.6 (11.1) | 4.1 (12.0) | 3.8 (13.7) | −3.6 | −5.5 | −1.6 | 0.003 ** |
| J01D | 20.2 (54.7) | 19.9 (54.6) | 19.1 (52.5) | 20.2 (52.7) | 20.0 (52.4) | 18.7 (58.7) | 20.6 (55.7) | 20.4 (58.2) | 19.7 (61.4) | 19.4 (56.0) | 15.3 (55.3) | −2.1 | −4.2 | 0.0 | 0.046 * |
| J01E | 0.1 (0.3) | 0.1 (0.2) | 0.3 (0.9) | 0.2 (0.5) | 0.2 (0.4) | 0.1 (0.4) | 0.3 (0.7) | 0.2 (0.5) | 0.1 (0.3) | 0.1 (0.4) | 0.1 (0.4) | −1.4 | −10.2 | 8.2 | 0.735 |
| J01F | 3.6 (9.7) | 3.3 (9.0) | 3.5 (9.6) | 3.4 (9.0) | 4.1 (10.9) | 2.9 (9.2) | 3.0 (8.1) | 2.6 (7.5) | 2.0 (6.3) | 3.2 (9.3) | 2.2 (8.1) | −4.3 | −7.5 | −0.9 | 0.018 * |
| J01G | 1.8 (5.0) | 1.2 (3.2) | 0.8 (2.2) | 0.6 (1.6) | 0.6 (1.5) | 0.6 (1.8) | 0.6 (1.5) | 0.4 (1.0) | 0.3 (1.0) | 0.2 (0.7) | 0.2 (0.6) | −18.3 | −21.3 | −15.1 | <0.001 *** |
| J01M | 4.2 (11.3) | 4.7 (12.9) | 4.4 (12.2) | 4.8 (12.4) | 5.1 (13.4) | 3.2 (9.9) | 5.4 (14.6) | 5.1 (14.7) | 4.6 (14.2) | 4.6 (13.3) | 4 (14.3) | 0.0 | −3.3 | 3.4 | 0.979 |
| J01X | 1.9 (5.2) | 1.9 (5.2) | 1.8 (4.9) | 1.6 (4.2) | 1.6 (4.1) | 1.4 (4.3) | 1.4 (3.7) | 1.2 (3.4) | 1.3 (4.0) | 1.3 (3.7) | 1.1 (4.0) | −5.4 | −6.4 | −4.3 | <0.001 *** |
| Tertiary Hospitals | 36.8 (100.0) | 36.4 (100.0) | 35 (100.0) | 39.1 (100.0) | 39.4 (100.0) | 30.9 (100.0) | 37.4 (100.0) | 35.9 (100.0) | 34.1 (100.0) | 36.5 (100.0) | 27.2 (100.0) | −1.6 | −3.7 | 0.5 | 0.111 |
| J01A | 0.1 (0.4) | 0.1 (0.4) | 1.3 (3.7) | 2.8 (7.0) | 1.1 (2.7) | 0.8 (2.7) | 1.3 (3.5) | 0.9 (2.6) | 0.8 (2.4) | 2.3 (6.2) | 1.2 (4.3) | 30.1 | −3.0 | 74.4 | 0.079 |
| J01B | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | - | - | - | - | ||||||
| J01C | 5.0 (13.6) | 5.3 (14.6) | 5.1 (14.6) | 5.2 (13.3) | 5.9 (15.0) | 4.0 (12.9) | 4.7 (12.5) | 4.2 (11.7) | 3.5 (10.4) | 4.1 (11.3) | 3.7 (13.6) | −3.9 | −6.1 | −1.6 | 0.004 ** |
| J01D | 19.9 (54.0) | 19.5 (53.5) | 18.0 (51.4) | 20.0 (51.2) | 19.9 (50.4) | 17.3 (56.0) | 19.5 (52.0) | 19.7 (55.1) | 20.0 (58.7) | 19.0 (52.1) | 14.2 (52.3) | −2.4 | −5.8 | 1.1 | 0.180 |
| J01E | 0.1 (0.2) | 0.1 (0.3) | 0.4 (1.0) | 0.2 (0.6) | 0.2 (0.5) | 0.2 (0.5) | 0.4 (1.0) | 0.2 (0.6) | 0.1 (0.4) | 0.2 (0.6) | 0.2 (0.6) | 10.1 | −8.9 | 33.1 | 0.321 |
| J01F | 3.6 (9.7) | 3.2 (8.9) | 3.2 (9.2) | 3.5 (8.9) | 4.4 (11.2) | 3.2 (10.3) | 3.3 (8.8) | 2.9 (8.2) | 2.3 (6.8) | 3.9 (10.6) | 2.4 (8.9) | −2.6 | −6.2 | 1.1 | 0.145 |
| J01G | 1.9 (5.3) | 1.3 (3.5) | 0.8 (2.3) | 0.6 (1.6) | 0.7 (1.7) | 0.6 (1.9) | 0.6 (1.6) | 0.4 (1.2) | 0.4 (1.2) | 0.3 (0.8) | 0.2 (0.7) | −17.0 | −20.5 | −13.5 | <0.001 *** |
| J01M | 4.3 (11.6) | 4.9 (13.5) | 4.5 (12.8) | 5.0 (12.8) | 5.6 (14.3) | 3.5 (11.2) | 6.2 (16.6) | 6.0 (16.8) | 5.3 (15.6) | 5.2 (14.2) | 4.1 (15) | 0.8 | −3.1 | 4.9 | 0.650 |
| J01X | 1.9 (5.2) | 1.9 (5.4) | 1.7 (5.0) | 1.7 (4.4) | 1.7 (4.2) | 1.4 (4.4) | 1.5 (4.0) | 1.3 (3.7) | 1.5 (4.5) | 1.5 (4.2) | 1.3 (4.7) | −3.6 | −5.2 | −1.9 | 0.001 ** |
| Secondary Hospitals | 37.8 (100.0) | 37.2 (100.0) | 45.5 (100.0) | 33.6 (100.0) | 32.9 (100.0) | 35.2 (100.0) | 35.8 (100.0) | 33.1 (100.0) | 28.1 (100.0) | 30.4 (100.0) | 28.5 (100.0) | −3.2 | −5.1 | −1.4 | 0.004 ** |
| J01A | 0.0 (0.1) | 0.0 (0.1) | 0.0 (0.1) | 0.0 (0.0) | 0.0 (0.0) | 0.1 (0.2) | 0.0 (0.1) | 0.1 (0.2) | 0.0 (0.1) | 0.0 (0.1) | 0.4 (1.4) | 17.6 | −6.2 | 47.6 | 0.140 |
| J01C | 4.6 (12.3) | 5.5 (14.9) | 6.7 (14.7) | 4.9 (14.7) | 5.0 (15.2) | 5.5 (15.6) | 5.2 (14.4) | 5.0 (15.2) | 3.6 (13.0) | 4.2 (13.8) | 4.0 (14.1) | −1.9 | −8.1 | 4.6 | 0.553 |
| J01D | 23.2 (61.6) | 23.5 (63.1) | 26.7 (58.6) | 21.0 (62.5) | 20.4 (61.9) | 23.4 (66.5) | 23.6 (65.9) | 21.8 (65.8) | 19.0 (67.8) | 20.1 (66.3) | 17.7 (62.0) | −2.5 | −4.3 | −0.7 | 0.013 * |
| J01E | 0.5 (1.4) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | −22.6 | −33.4 | −10.0 | 0.001 ** |
| J01F | 3.4 (9.0) | 3.4 (9.3) | 5.2 (11.3) | 3.1 (9.2) | 3.1 (9.4) | 2.1 (6.1) | 2.3 (6.3) | 2.0 (6.0) | 1.5 (5.2) | 1.8 (5.9) | 1.8 (6.5) | −9.2 | −13.1 | −5.2 | 0.001 ** |
| J01G | 0.9 (2.5) | 0.6 (1.5) | 0.8 (1.7) | 0.3 (1.0) | 0.2 (0.7) | 0.5 (1.3) | 0.4 (1.2) | 0.2 (0.6) | 0.1 (0.4) | 0.2 (0.5) | 0.1 (0.4) | −17.9 | −24.2 | −11.2 | <0.001 *** |
| J01M | 3.1 (8.2) | 2.7 (7.4) | 4.1 (9.0) | 3.3 (9.8) | 3.0 (9.2) | 2.2 (6.3) | 3.2 (8.9) | 3.2 (9.7) | 3.1 (10.9) | 3.3 (11.0) | 3.7 (13.0) | 0.7 | −2.8 | 4.3 | 0.670 |
| J01X | 1.9 (5.0) | 1.4 (3.7) | 2.1 (4.6) | 0.9 (2.8) | 1.2 (3.6) | 1.4 (4.0) | 1.1 (3.1) | 0.8 (2.6) | 0.8 (2.7) | 0.7 (2.3) | 0.7 (2.5) | −9.3 | −13.2 | −5.3 | 0.001 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aierken, A.; Zhu, X.; Wang, N.; Zhang, J.; Li, W.; Wushouer, H.; Abudukeremu, K. Measuring Temporal Trends and Patterns of Inpatient Antibiotic Use in Northwest China’s Hospitals: Data from the Center for Antibacterial Surveillance, 2012–2022. Antibiotics 2024, 13, 732. https://doi.org/10.3390/antibiotics13080732
Aierken A, Zhu X, Wang N, Zhang J, Li W, Wushouer H, Abudukeremu K. Measuring Temporal Trends and Patterns of Inpatient Antibiotic Use in Northwest China’s Hospitals: Data from the Center for Antibacterial Surveillance, 2012–2022. Antibiotics. 2024; 13(8):732. https://doi.org/10.3390/antibiotics13080732
Chicago/Turabian StyleAierken, Aizezijiang, Xiaochen Zhu, Ningning Wang, Jiangtao Zhang, Weibin Li, Haishaerjiang Wushouer, and Kaisaier Abudukeremu. 2024. "Measuring Temporal Trends and Patterns of Inpatient Antibiotic Use in Northwest China’s Hospitals: Data from the Center for Antibacterial Surveillance, 2012–2022" Antibiotics 13, no. 8: 732. https://doi.org/10.3390/antibiotics13080732
APA StyleAierken, A., Zhu, X., Wang, N., Zhang, J., Li, W., Wushouer, H., & Abudukeremu, K. (2024). Measuring Temporal Trends and Patterns of Inpatient Antibiotic Use in Northwest China’s Hospitals: Data from the Center for Antibacterial Surveillance, 2012–2022. Antibiotics, 13(8), 732. https://doi.org/10.3390/antibiotics13080732





