Practical Application of Aztreonam-Avibactam as a Treatment Strategy for Ambler Class B Metallo-β-Lactamase Producing Enterobacteriaceae
Abstract
1. Introduction
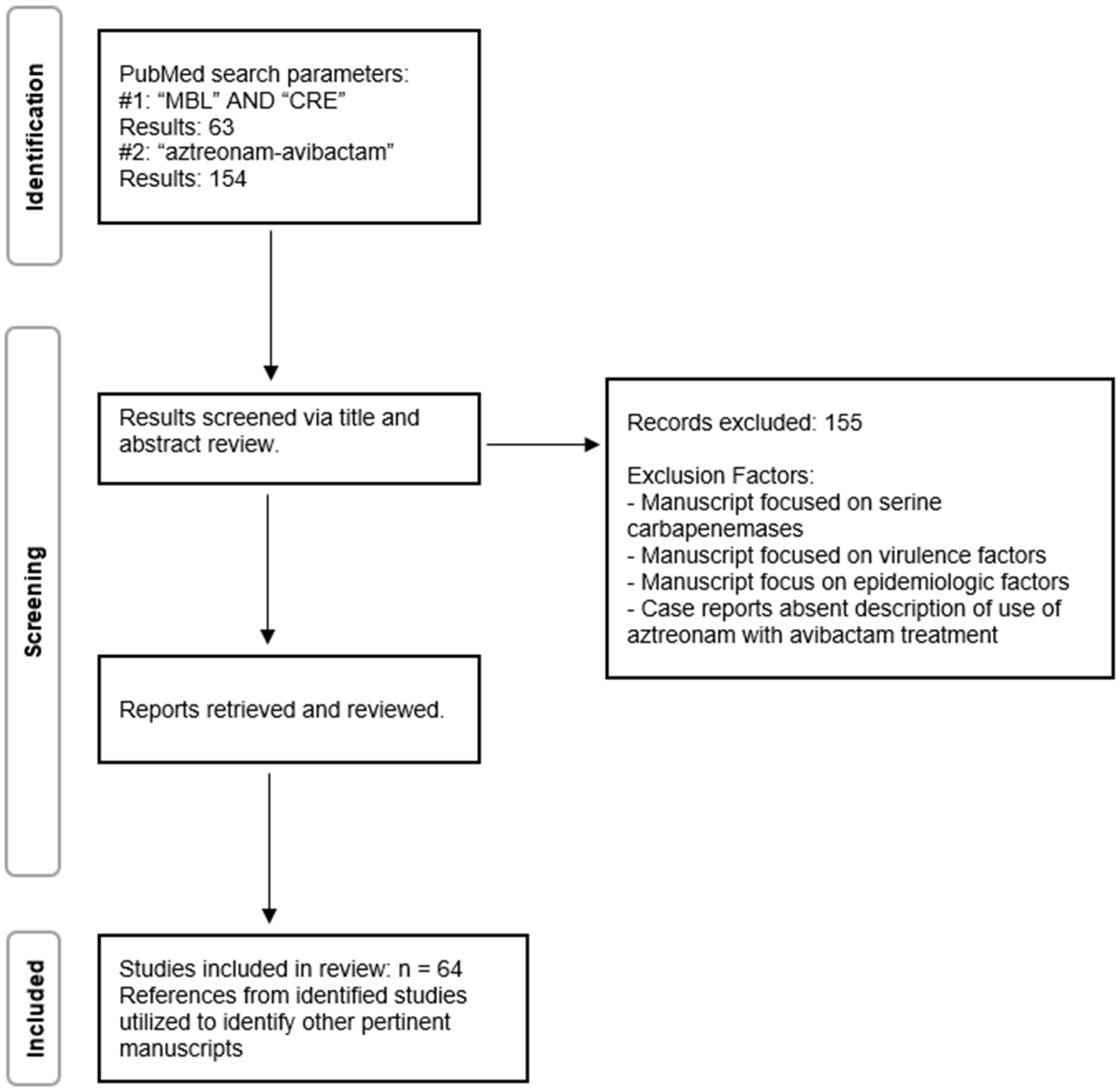
2. Methods
3. Systemic Review Findings
3.1. In Vitro Studies
3.2. In Vitro Susceptibility Testing
3.3. Limitations of Aztreonam-Avibactam Therapy
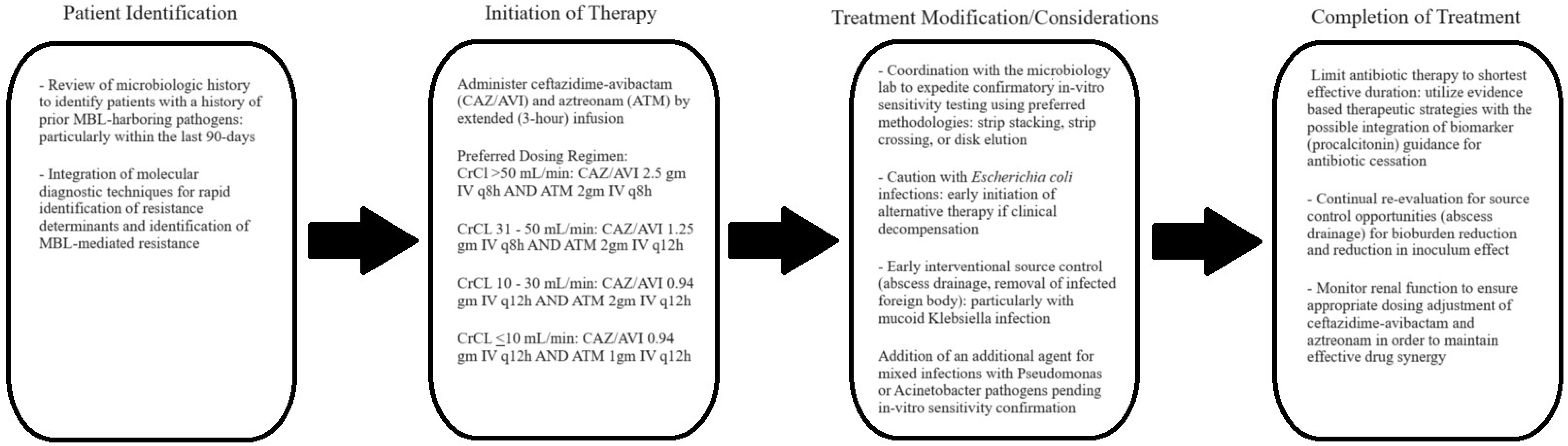
3.4. Clinical Experience with Aztreonam-Avibactam
4. Discussion
Funding
Data Availability Statement
Conflicts of Interest
References
- Hauck, C.; Cober, E.; Richter, S.S.; Perez, F.; Salata, R.A.; Kalayjian, R.C.; Watkins, R.R.; Scalera, N.M.; Doi, Y.; Kaye, K.S.; et al. Spectrum of excess mortality due to carbapenem-resistant Klebsiella pneumoniae infections. Clin. Microbiol. Infect. 2016, 22, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Gasink, L.B.; Edelstein, P.H.; Lautenbach, E.; Synnestvedt, M.; Fishman, N.O. Risk factors and clinical impact of Klebsiella pneumoniae carbapenemase-producing K. pneumoniae. Infect. Control. Hosp. Epidemiol. 2009, 30, 1180–1185. [Google Scholar] [CrossRef]
- Wong, D.; van Duin, D. Carbapenemase-producing organisms in solid organ transplantation. Curr. Opin. Organ Transplant. 2019, 24, 490–496. [Google Scholar] [CrossRef] [PubMed]
- van Duin, D.; Arias, C.A.; Komarow, L.; Chen, L.; Hanson, B.M.; Weston, G.; Cober, E.; Garner, O.B.; Jacob, J.T.; Satlin, M.J.; et al. Molecular and clinical epidemiology of carbapenem-resistant Enterobacterales in the USA (CRACKLE-2): A prospective cohort study. Lancet Infect. Dis. 2020, 20, 731–741. [Google Scholar] [CrossRef] [PubMed]
- Tamma, P.D.; Aitken, S.L.; Bonomo, R.A.; Mathers, A.J.; van Duin, D.; Clancy, C.J. Infectious Diseases Society of America 2022 Guidance on the Treatment of Extended-Spectrum β-lactamase Producing Enterobacterales (ESBL-E), Carbapenem-Resistant Enterobacterales (CRE), and Pseudomonas aeruginosa with Difficult-to-Treat Resistance (DTR-P. aeruginosa). Clin. Infect. Dis. 2022, 75, 187–212. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.; van Duin, D. Novel Beta-Lactamase Inhibitors: Unlocking Their Potential in Therapy. Drugs 2017, 77, 615–628. [Google Scholar] [CrossRef]
- Boyd, S.E.; Livermore, D.M.; Hooper, D.C.; Hope, W.W. Metallo-β-Lactamases: Structure, Function, Epidemiology, Treatment Options, and the Development Pipeline. Antimicrob. Agents Chemother. 2020, 64, e00397-20. [Google Scholar] [CrossRef]
- Johnson, A.P.; Woodford, N. Global spread of antibiotic resistance: The example of New Delhi metallo-β-lactamase (NDM)-mediated carbapenem resistance. J. Med. Microbiol. 2013, 62, 499–513. [Google Scholar] [CrossRef]
- Sader, S.H.; Castanheira, M.; Kimbrough, H.J.; Kantro, V.; Mendes, E.R. Aztreonam/avibactam activity against a large collection of carbapenem-resistant Enterobacterales (CRE) collected in hospitals from Europe, Asia and Latin America (2019–2021). JAC-Antimicrob. Resist. 2023, 5, dlad032. [Google Scholar] [CrossRef]
- Sonnevend, Á.; Ghazawi, A.; Darwish, D.; Barathan, G.; Hashmey, R.; Ashraf, T.; Rizvi, A.T.; Pál, T. In vitro efficacy of ceftazidime-avibactam, aztreonam-avibactam and other rescue antibiotics against carbapenem-resistant Enterobacterales from the Arabian Peninsula. Int. J. Infect. Dis. 2020, 99, 253–259. [Google Scholar] [CrossRef]
- Zhang, B.; Zhu, Z.; Jia, W.; Qu, F.; Huang, B.; Shan, B.; Yu, H.; Tang, Y.; Chen, L.; Du, H. In vitro activity of aztreonam-avibactam against metallo-β-lactamase-producing Enterobacteriaceae—A multicenter study in China. Int. J. Infect. Dis. 2020, 97, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Sader, S.H.; Mendes, E.R.; Carvalhaes, G.C.; Kimbrough, H.J.; Castanheira, M. Changing Epidemiology of Carbapenemases Among Carbapenem-Resistant Enterobacterales from United States Hospitals and the Activity of Aztreonam-Avibactam Against Contemporary Enterobacterales (2019–2021). Open Forum Infect. Dis. 2023, 10, ofad046. [Google Scholar] [CrossRef]
- Huang, Y.-S.; Chen, P.-Y.; Chou, P.-C.; Wang, J.-T. In Vitro Activities and Inoculum Effects of Cefiderocol and Aztreonam-Avibactam against Metallo-β-Lactamase-Producing Enterobacteriaceae. Microbiol. Spectr. 2023, 11, e0056923. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, A.; Boyd, S.; Sabour, S.; Bodnar, J.; Nazarian, E.; Peinovich, N.; Wagner, C.; Craft, B.; Vagnone, S.P.; Simpson, J.; et al. Aztreonam-Avibactam Susceptibility Testing Program for Metallo-Beta-Lactamase-Producing Enterobacterales in the Antibiotic Resistance Laboratory Network, March 2019 to December 2020. Antimicrob. Agents Chemother. 2021, 65, e0048621. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Ucha, C.J.; Alonso-Garcia, I.; Guijarro-Sánchez, P.; Lasarte-Monterrubio, C.; Álvarez-Fraga, L.; Cendón-Esteve, A.; Outeda, M.; Maceiras, R.; Peña-Escolano, A.; Martínez-Guitián, M.; et al. Activity of aztreonam in combination with novel β-lactamase inhibitors against metallo-β-lactamase-producing Enterobacterales from Spain. Int. J. Antimicrob. Agents 2023, 61, 106738. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, Y.; Jia, W.; Xu, X.; Sun, G.; Wang, T.; Li, J.; Zhang, G.; Jing, R.; Sun, H.; et al. In Vitro Activities of Aztreonam-Avibactam, Eravacycline, Cefoselis, and Other Comparators against Clinical Enterobacterales Isolates: A Multicenter Study in China, 2019. Microbiol. Spectr. 2023, 11, e0487322. [Google Scholar] [CrossRef] [PubMed]
- Dupont, H.; Gaillot, O.; Goetgheluck, A.-S.; Plassart, C.; Emond, J.-P.; Lecuru, M.; Gaillard, N.; Derdouri, S.; Lemaire, B.; Courtilles, D.G.M.; et al. Molecular Characterization of Carbapenem-Nonsusceptible Enterobacterial Isolates Collected during a Prospective Interregional Survey in France and Susceptibility to the Novel Ceftazidime-Avibactam and Aztreonam-Avibactam Combinations. Antimicrob. Agents Chemother. 2016, 60, 215–221. [Google Scholar] [CrossRef]
- Emeraud, C.; Escaut, L.; Boucly, A.; Fortineau, N.; Bonnin, A.R.; Naas, T.; Dortet, L. Aztreonam plus Clavulanate, Tazobactam, or Avibactam for Treatment of Infections Caused by Metallo-β-Lactamase-Producing Gram-Negative Bacteria. Antimicrob. Agents Chemother. 2019, 63, e00010-19. [Google Scholar] [CrossRef]
- Feng, K.; Jia, N.; Zhu, P.; Sy, S.; Liu, Y.; Dong, D.; Zhu, S.; Zhang, J.; Liu, Y.; Martins, S.F.; et al. Aztreonam/avibactam effect on pharmacodynamic indices for mutant selection of Escherichia coli and Klebsiella pneumoniae harbouring serine- and New Delhi metallo-β-lactamases. J. Antimicrob. Chemother. 2021, 76, 2875–2883. [Google Scholar] [CrossRef] [PubMed]
- Terrier, L.C.; Nordmann, P.; Poirel, L. In vitro activity of aztreonam in combination with newly developed β-lactamase inhibitors against MDR Enterobacterales and Pseudomonas aeruginosa producing metallo-β-lactamases. J. Antimicrob. Chemother. 2023, 78, 101–107. [Google Scholar] [CrossRef]
- Verschelden, G.; Noeparast, M.; Stoefs, A.; Honacker, V.E.; Vandoorslaer, K.; Vandervore, L.; Olbrecht, M.; Damme, V.K.; Demuyser, T.; Piérard, D.; et al. Aztreonam-avibactam synergy, a validation and comparison of diagnostic tools. Front. Microbiol. 2023, 14, 1322180. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Erickson, S.G.; Pettaway, C.; Arias, C.A.; Miller, W.R.; Bhatti, M.M. Evaluation of Susceptibility Testing Methods for Aztreonam and Ceftazidime-Avibactam Combination Therapy on Extensively Drug-Resistant Gram-Negative Organisms. Antimicrob. Agents Chemother. 2021, 65, e0084621. [Google Scholar] [CrossRef] [PubMed]
- Lima, O.D.K.; Lima, D.V.A.; Rocha, C.D.A.D.; Sampaio, F.C.S.; Cappellano, P.; Sampaio, M.L.J. A simple disk pre-diffusion test to predict in vitro aztreonam/avibactam activity against NDM-producing Klebsiella pneumoniae complex. J. Glob. Antimicrob. Resist. 2022, 28, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Kelley, J.C.; Kennedy-Mendez, A.; Walser, N.O.; Thwaites, T.M.; Arhin, F.F.; Pillar, M.C.; Hufnagel, A.D. Evaluation of Dilution Susceptibility Testing Methods for Aztreonam in Combination with Avibactam against Enterobacterales. Microbiol. Spectr. 2022, 10, e0360122. [Google Scholar] [CrossRef] [PubMed]
- Deschamps, M.; Dauwalder, O.; Dortet, L. Comparison of ETEST® superposition method and the MTS™ Aztreonam-avibactam strip with the reference method for aztreonam/avibactam susceptibility testing. J. Antimicrob. Chemother. 2023, 79, 685–687. [Google Scholar] [CrossRef] [PubMed]
- Emilie, M.C.; Alice, M.C.; Marine, G.; Farfour, E.; Pourbaix, A.; Dortet, L.; Lucie, L.; Marc, V. Evaluation of the MTS™ aztreonam-avibactam strip (Liofilchem) on New Delhi metallo-β-lactamase-producing Enterobacterales. Eur. J. Clin. Microbiol. Infect. Dis. 2024, 43, 777–784. [Google Scholar] [CrossRef]
- Ma, K.; Feng, Y.; Zong, Z. Aztreonam-avibactam may not replace ceftazidime/avibactam: The case of KPC-21 carbapenemase and penicillin-binding protein 3 with four extra amino acids. Int. J. Antimicrob. Agents 2022, 60, 106642. [Google Scholar] [CrossRef]
- Mushtaq, S.; Vickers, A.; Woodford, N.; Livermore, M.D. Activity of aztreonam/avibactam and ceftazidime/avibactam against Enterobacterales with carbapenemase-independent carbapenem resistance. Int. J. Antimicrob. Agents 2024, 63, 107081. [Google Scholar] [CrossRef]
- Livermore, M.D.; Mushtaq, S.; Vickers, A.; Woodford, N. Activity of aztreonam/avibactam against metallo-β-lactamase-producing Enterobacterales from the UK: Impact of penicillin-binding protein-3 inserts and CMY-42 β-lactamase in Escherichia coli. Int. J. Antimicrob. Agents 2023, 61, 106776. [Google Scholar] [CrossRef]
- Ma, K.; Zong, Z. Resistance to aztreonam-avibactam due to CTX-M-15 in the presence of penicillin-binding protein 3 with extra amino acids in Escherichia coli. Front. Microbiol. 2022, 13, 1047109. [Google Scholar] [CrossRef]
- Alm, A.R.; Johnstone, R.M.; Lahiri, D.S. Characterization of Escherichia coli NDM isolates with decreased susceptibility to aztreonam/avibactam: Role of a novel insertion in PBP3. J. Antimicrob. Chemother. 2015, 70, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Sadek, M.; Juhas, M.; Poirel, L.; Nordmann, P. Genetic Features Leading to Reduced Susceptibility to Aztreonam-Avibactam among Metallo-β-Lactamase-Producing Escherichia coli Isolates. Antimicrob. Agents Chemother. 2020, 64, e01659-20. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Lee, C.S.; Bae, M.; Sung, H.; Kim, M.-N.; Jung, J.; Kim, J.M.; Kim, S.-H.; Lee, S.-O.; Choi, S.-H.; et al. In Vitro Activities and Inoculum Effects of Ceftazidime-Avibactam and Aztreonam-Avibactam against Carbapenem-Resistant Enterobacterales Isolates from South Korea. Antibiotics 2020, 9, 912. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Yu, H.; Huang, X.; Long, S.; Zhang, J. In vitro activity of ceftazidime-avibactam, imipenem-relebactam, aztreonam-avibactam, and comparators toward carbapenem-resistant and hypervirulent Klebsiella pneumoniae isolates. Microbiol. Spectr. 2023, 11, e0280623. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Shen, P.; Chen, Y.; Zhou, K.; Chi, X.; Xiao, Y. Epidemiology and Genomic Characteristics of Bloodstream Infection Caused by Carbapenem-Resistant Klebsiella pneumoniae with Decreased Susceptibility to Aztreonam/Avibactam in China. Front. Cell. Infect. Microbiol. 2022, 12, 926209. [Google Scholar] [CrossRef] [PubMed]
- Niu, S.; Wei, J.; Zou, C.; Chavda, D.K.; Lv, J.; Zhang, H.; Du, H.; Tang, Y.-W.; Pitout, D.D.J.; Bonomo, A.R.; et al. In vitro selection of aztreonam/avibactam resistance in dual-carbapenemase-producing Klebsiella pneumoniae. J. Antimicrob. Chemother. 2020, 75, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Nordmann, P.; Yao, Y.; Falgenhauer, L.; Sadek, M.; Imirzalioglu, C.; Chakraborty, T. Recent Emergence of Aztreonam-Avibactam Resistance in NDM and OXA-48 Carbapenemase-Producing Escherichia coli in Germany. Antimicrob. Agents Chemother. 2021, 65, e0109021. [Google Scholar] [CrossRef]
- Wu, S.; Ma, K.; Feng, Y.; Zong, Z. Resistance to aztreonam-avibactam due to a mutation of SHV-12 in Enterobacter. Ann. Clin. Microbiol. Antimicrob. 2023, 22, 49. [Google Scholar] [CrossRef]
- Yasmin, M.; Fouts, E.D.; Jacobs, R.M.; Haydar, H.; Marshall, H.S.; White, R.; D’Souza, R.; Lodise, P.T.; Rhoads, D.D.; Hujer, M.A.; et al. Monitoring Ceftazidime-Avibactam and Aztreonam Concentrations in the Treatment of a Bloodstream Infection Caused by a Multidrug-Resistant Enterobacter sp. Carrying Both Klebsiella pneumoniae Carbapenemase–4 and New Delhi Metallo-β-Lactamase–1. Clin. Infect. Dis. 2020, 71, 1095–1098. [Google Scholar] [CrossRef]
- Shaw, E.; Rombauts, A.; Tubau, F.; Padullés, A.; Càmara, J.; Lozano, T.; Cobo-Sacristán, S.; Sabe, N.; Grau, I.; Rigo-Bonnin, R.; et al. Clinical outcomes after combination treatment with ceftazidime/avibactam and aztreonam for NDM-1/OXA-48/CTX-M-15-producing Klebsiella pneumoniae infection. J. Antimicrob. Chemother. 2018, 73, 1104–1106. [Google Scholar] [CrossRef]
- Davido, B.; Fellous, L.; Lawrence, C.; Maxime, V.; Rottman, M.; Dinh, A. Ceftazidime-Avibactam and Aztreonam, an Interesting Strategy to Overcome β-Lactam Resistance Conferred by Metallo-β-Lactamases in Enterobacteriaceae and Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2017, 61, e01008-17. [Google Scholar] [CrossRef] [PubMed]
- Rubnitz, Z.A.; Kunkel, V.N.; Baselski, V.S.; Summers, N.A. A Case of New Delhi Metallo-β-Lactamases (NDM) Citrobacter sedlakii Osteomyelitis Successfully Treated with Ceftazidime-Avibactam and Aztreonam. Cureus 2022, 14, e28855. [Google Scholar] [CrossRef]
- Marshall, S.; Hujer, M.A.; Rojas, J.L.; Papp-Wallace, M.K.; Humphries, M.R.; Spellberg, B.; Hujer, M.K.; Marshall, K.E.; Rudin, D.S.; Perez, F.; et al. Can Ceftazidime-Avibactam and Aztreonam Overcome β-Lactam Resistance Conferred by Metallo-β-Lactamases in Enterobacteriaceae? Antimicrob. Agents Chemother. 2017, 61, e02243-16. [Google Scholar] [CrossRef] [PubMed]
- Benchetrit, L.; Mathy, V.; Armand-Lefevre, L.; Bouadma, L.; Timsit, J.-F. Successful treatment of septic shock due to NDM-1-producing Klebsiella pneumoniae using ceftazidime/avibactam combined with aztreonam in solid organ transplant recipients: Report of two cases. Int. J. Antimicrob. Agents 2020, 55, 105842. [Google Scholar] [CrossRef]
- Timsit, J.F.; Wicky, P.H.; de Montmollin, E. Treatment of Severe Infections Due to Metallo-Betalactamases Enterobacterales in Critically Ill Patients. Antibiotics 2022, 11, 144. [Google Scholar] [CrossRef] [PubMed]
- Falcone, M.; Daikos, L.G.; Tiseo, G.; Bassoulis, D.; Giordano, C.; Galfo, V.; Leonildi, A.; Tagliaferri, E.; Barnini, S.; Sani, S.; et al. Efficacy of Ceftazidime-avibactam Plus Aztreonam in Patients with Bloodstream Infections Caused by Metallo-β-lactamase–Producing Enterobacterales. Clin. Infect. Dis. 2021, 72, 1871–1878. [Google Scholar] [CrossRef] [PubMed]
- Mauri, C.; Maraolo, E.A.; Bella, D.S.; Luzzaro, F.; Principe, L. The Revival of Aztreonam in Combination with Avibactam against Metallo-β-Lactamase-Producing Gram-Negatives: A Systematic Review of In Vitro Studies and Clinical Cases. Antibiotics 2021, 10, 1012. [Google Scholar] [CrossRef]
- Cornely, A.O.; Cisneros, M.J.; Torre-Cisneros, J.; Rodríguez-Hernández, J.M.; Tallón-Aguilar, L.; Calbo, E.; Horcajada, P.J.; Queckenberg, C.; Zettelmeyer, U.; Arenz, D.; et al. Pharmacokinetics and safety of aztreonam/avibactam for the treatment of complicated intra-abdominal infections in hospitalized adults: Results from the REJUVENATE study. J. Antimicrob. Chemother. 2020, 75, 618–627. [Google Scholar] [CrossRef]
- Lodise, T.P.; Smith, N.M.; O’Donnell, N.; Eakin, A.E.; Holden, P.N.; Boissonneault, K.R.; Zhou, J.; Tao, X.; Bulitta, J.B.; Fowler, V.G.; et al. Determining the optimal dosing of a novel combination regimen of ceftazidime/avibactam with aztreonam against NDM-1-producing Enterobacteriaceae using a hollow-fibre infection model. J. Antimicrob. Chemother. 2020, 75, 2622–2632. [Google Scholar] [CrossRef]
- Falcone, M.; Menichetti, F.; Cattaneo, D.; Tiseo, G.; Baldelli, S.; Galfo, V.; Leonildi, A.; Tagliaferri, E.; Paolo, D.A.; Pai, P.M. Pragmatic options for dose optimization of ceftazidime/avibactam with aztreonam in complex patients. J. Antimicrob. Chemother. 2021, 76, 1025–1031. [Google Scholar] [CrossRef]
- Lodise, P.T.; O’Donnell, N.J.; Raja, S.; Guptill, T.J.; Zaharoff, S.; Schwager, N.; Fowler, G.V.; Beresnev, T.; Wall, A.; Wiegand, K.; et al. Safety of Ceftazidime-Avibactam in Combination with Aztreonam (COMBINE) in a Phase I, Open-Label Study in Healthy Adult Volunteers. Antimicrob. Agents Chemother. 2022, 66, e0093522. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Riccobene, T.; Carrothers, J.T.; Wright, G.J.; Macpherson, M.; Cristinacce, A.; Mcfadyen, L.; Xie, R.; Luckey, A.; Raber, S. Dose selection for aztreonam-avibactam, including adjustments for renal impairment, for phase IIa and phase III evaluation. Eur. J. Clin. Pharmacol. 2024, 80, 529–543. [Google Scholar] [CrossRef] [PubMed]
- Pfizer. Phase 3 Studies of Pfizer’s Novel Antibiotic Combination Offer New Treatment Hope for Patients with Multidrug-Resistant Infections and Limited Treatment Options. Available online: https://www.pfizer.com/news/press-release/press-release-detail/phase-3-studies-pfizers-novel-antibiotic-combination-offer (accessed on 2 January 2024).
- Pfizer. Available online: https://clinicaltrials.gov/study/NCT03580044?tab=results (accessed on 2 January 2024).
- Timsit, J.F.; Paul, M.; Shields, R.K.; Echols, R.; Baba, T.; Yamano, Y.; Portsmouth, S. Cefiderocol for the Treatment of Infections Due to Metallo-B-lactamase-Producing Pathogens in the CREDIBLE-CR and APEKS-NP Phase 3 Randomized Studies. Clin. Infect. Dis. 2022, 75, 1081–1084. [Google Scholar] [CrossRef] [PubMed]
- Kazmierczak, M.K.; Tsuji, M.; Wise, G.M.; Hackel, M.; Yamano, Y.; Echols, R.; Sahm, F.D. In vitro activity of cefiderocol, a siderophore cephalosporin, against a recent collection of clinically relevant carbapenem-non-susceptible Gram-negative bacilli, including serine carbapenemase- and metallo-β-lactamase-producing isolates (SIDERO-WT-2014. Int. J. Antimicrob. Agents 2019, 53, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.W. Carriage of antibiotic resistant bacteria flora and its role in the guidance of clinical decision making. Pathog. Dis. 2020, 78, ftaa030. [Google Scholar] [CrossRef]
- Terrier, L.C.; Nordmann, P.; Sadek, M.; Poirel, L. In vitro activity of cefepime/zidebactam and cefepime/taniborbactam against aztreonam/avibactam-resistant NDM-like-producing Escherichia coli clinical isolates. J. Antimicrob. Chemother. 2023, 78, 1191–1194. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, D.W. Practical Application of Aztreonam-Avibactam as a Treatment Strategy for Ambler Class B Metallo-β-Lactamase Producing Enterobacteriaceae. Antibiotics 2024, 13, 766. https://doi.org/10.3390/antibiotics13080766
Wong DW. Practical Application of Aztreonam-Avibactam as a Treatment Strategy for Ambler Class B Metallo-β-Lactamase Producing Enterobacteriaceae. Antibiotics. 2024; 13(8):766. https://doi.org/10.3390/antibiotics13080766
Chicago/Turabian StyleWong, Darren W. 2024. "Practical Application of Aztreonam-Avibactam as a Treatment Strategy for Ambler Class B Metallo-β-Lactamase Producing Enterobacteriaceae" Antibiotics 13, no. 8: 766. https://doi.org/10.3390/antibiotics13080766
APA StyleWong, D. W. (2024). Practical Application of Aztreonam-Avibactam as a Treatment Strategy for Ambler Class B Metallo-β-Lactamase Producing Enterobacteriaceae. Antibiotics, 13(8), 766. https://doi.org/10.3390/antibiotics13080766





