Phylogenetic Diversity, Antibiotic Resistance, and Virulence of Escherichia coli Strains from Urinary Tract Infections in Algeria
Abstract
:1. Introduction
2. Results
2.1. Demographic Characteristics
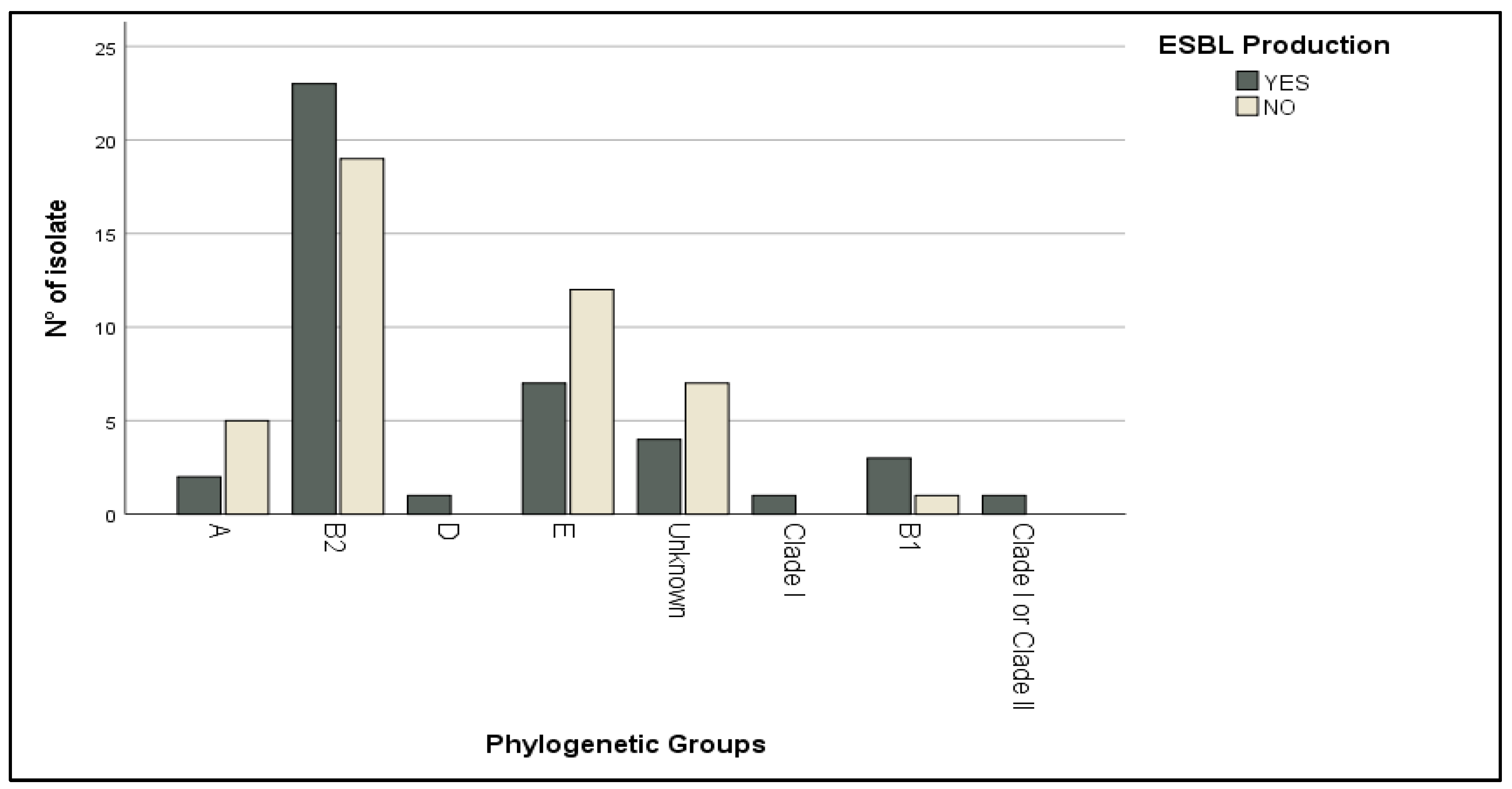
2.2. Phylogenetic Grouping
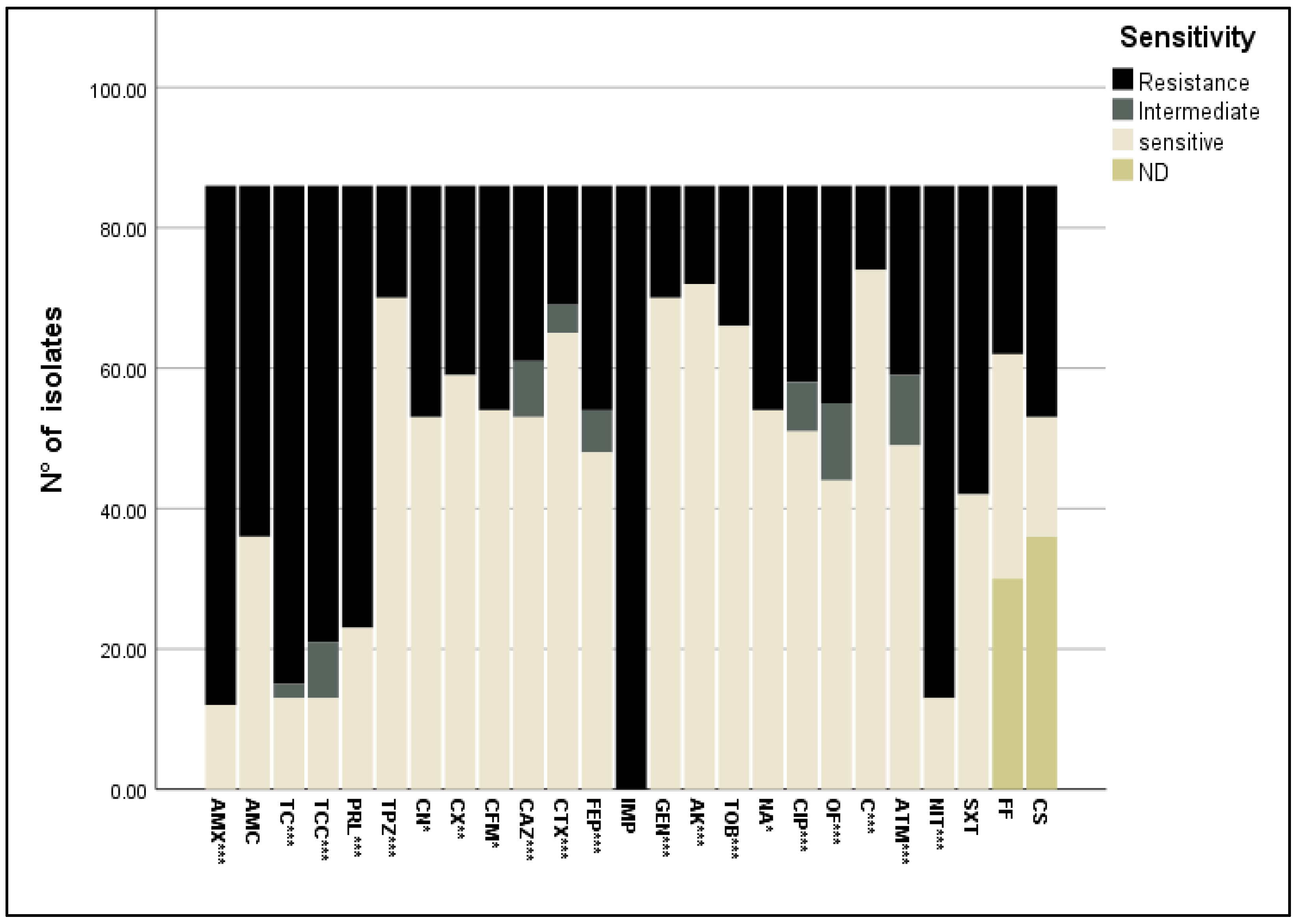
2.3. Antimicrobial Susceptibility
2.4. Extended β-Lactamase (ESBL) Production and Haemolysin Activity
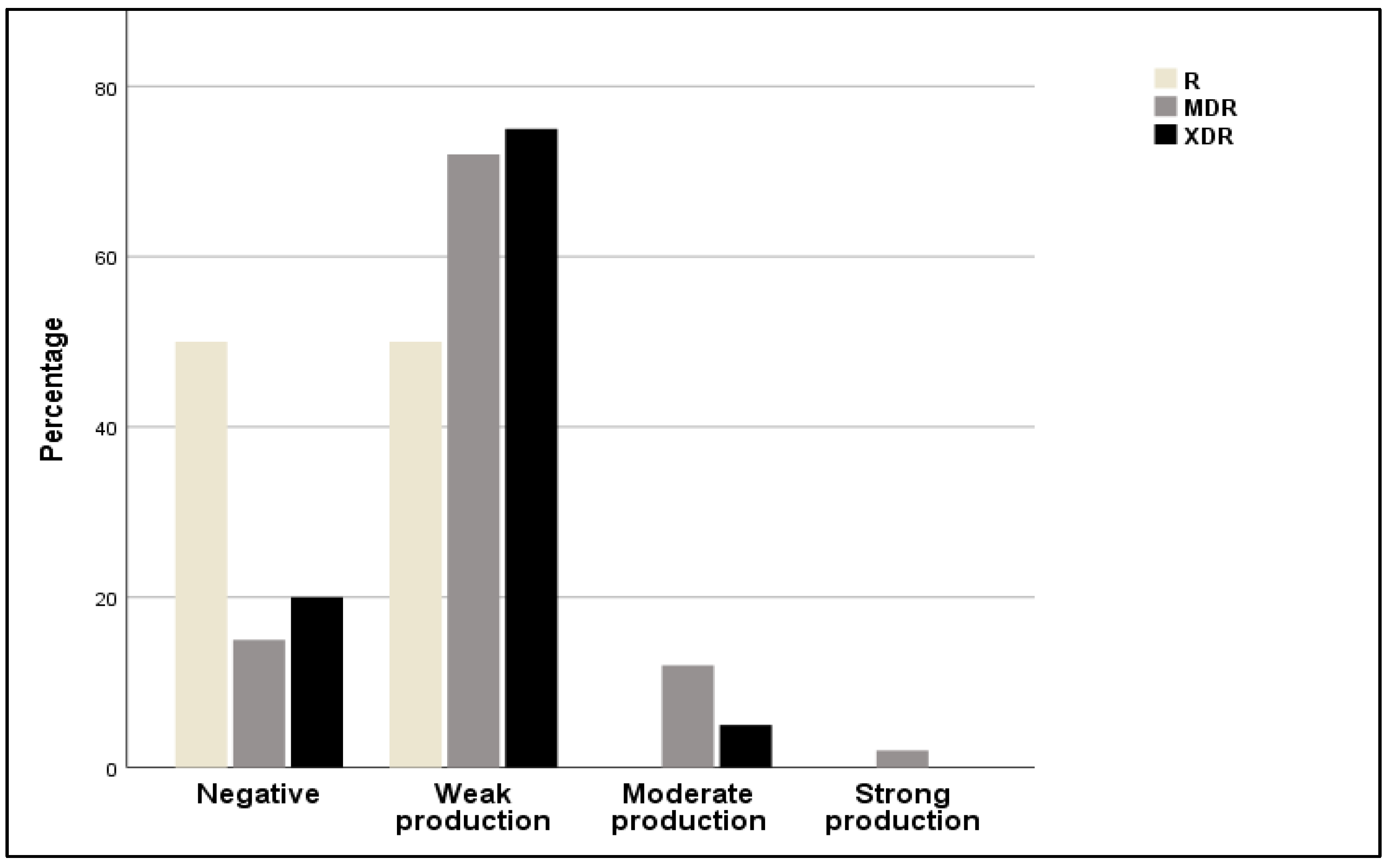
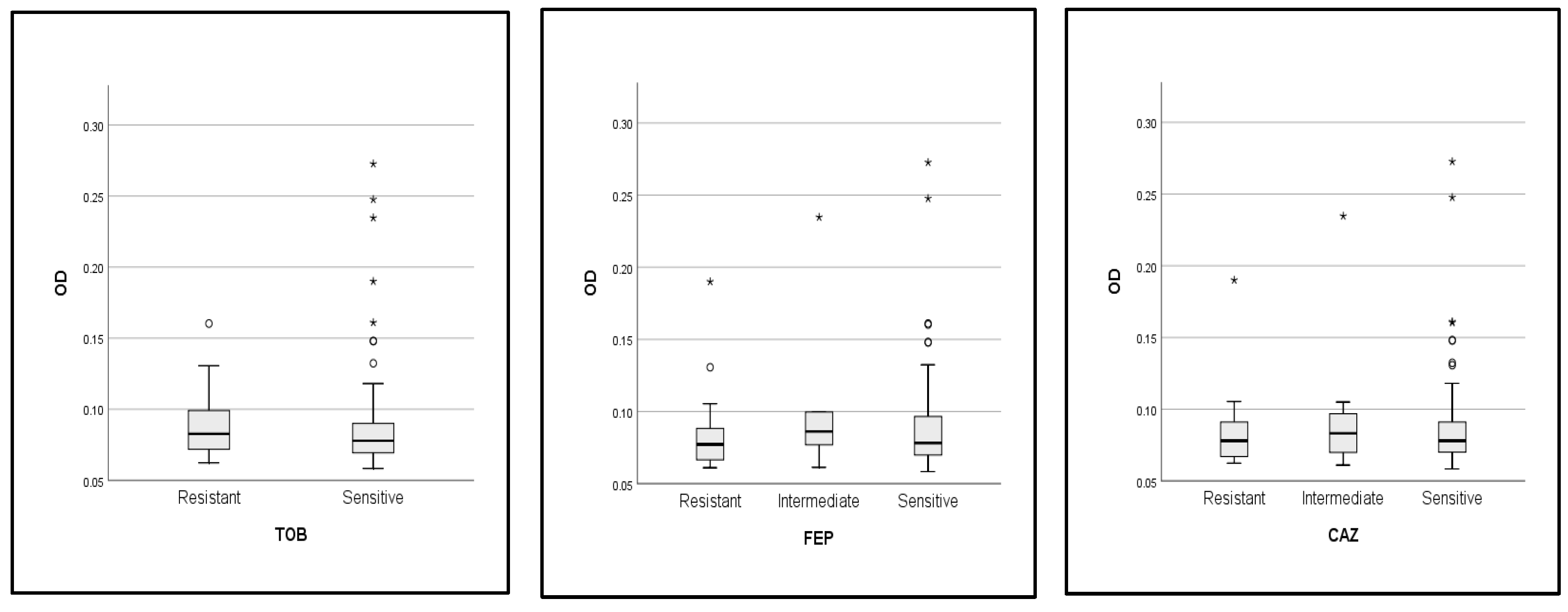
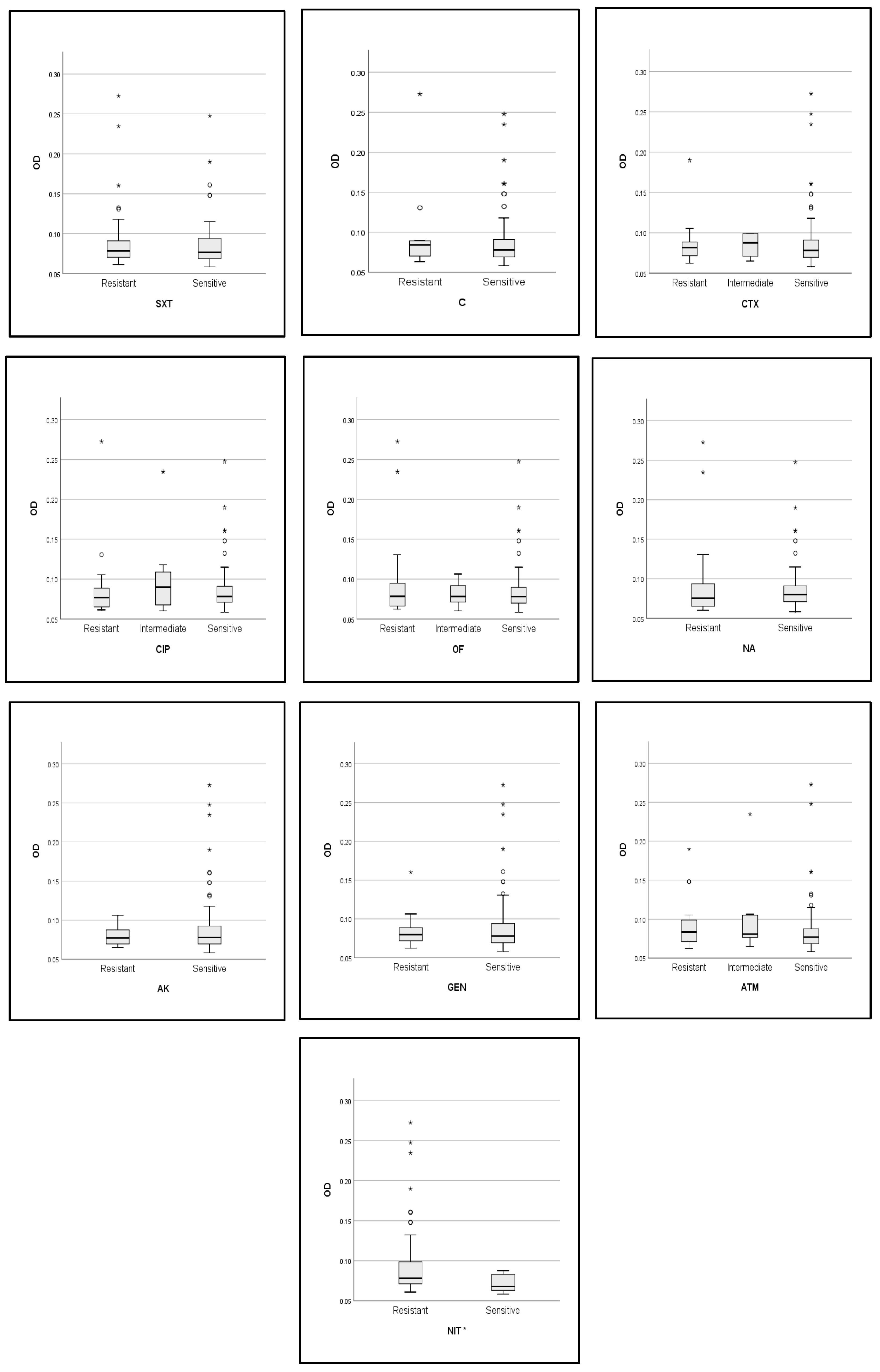
2.5. Correlations between Biofilm Activity, Phylogroup, and Antibiotic Resistance
3. Discussion
4. Materials and Methods
4.1. Origin of Isolates and Bacterial Strains
4.2. Antimicrobial Susceptibility Testing
4.3. Detection of Extended Spectrum β-Lactamases Production
4.4. DNA Extraction and Phylogenetic Grouping by Quadruplex PCR
4.5. Biofilm Production
4.6. Hemolysin Production
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Coura, F.M.; Diniz, A.N.; Oliveira Junior, C.A.; Lage, A.P.; Lobato, F.C.F.; Heinemann, M.B.; Silva, R.O.S. Detection of Virulence Genes and the Phylogenetic Groups of Escherichia coli Isolated from Dogs in Brazil. Ciênc. Rural 2018, 48, e20170478. [Google Scholar] [CrossRef]
- Denamur, E.; Clermont, O.; Bonacorsi, S.; Gordon, D. The Population Genetics of Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2021, 19, 37–54. [Google Scholar] [CrossRef]
- Bozorgomid, A.; Chegene Lorestani, R.; Rostamian, M.; Nemati Zargaran, F.; Shahvaisi-Zadeh, Z.; Akya, A. Antibiotic Resistance, Virulence Factors, and Phylogenetic Groups of Escherichia coli Isolated from Hospital Wastewater: A Case Study in the West of Iran. Environ. Health Eng. Manag. 2023, 10, 131–139. [Google Scholar] [CrossRef]
- Saralaya, V.; Shenoy, S.; Baliga, S.; Hegde, A.; Adhikari, P.; Chakraborty, A. Characterization of Escherichia coli Phylogenetic Groups Associated with Extraintestinal Infections in South Indian Population. Ann. Med. Health Sci. Res. 2015, 5, 241. [Google Scholar] [CrossRef] [PubMed]
- Boroumand, M.; Naghmachi, M.; Ghatee, M.A. Detection of Phylogenetic Groups and Drug Resistance Genes of Escherichia coli Causing Urinary Tract Infection in Southwest Iran. Jundishapur J. Microbiol. 2021, 14, e112547. [Google Scholar] [CrossRef]
- Rijavec, M.; Müller-Premru, M.; Zakotnik, B.; Žgur-Bertok, D. Virulence Factors and Biofilm Production among Escherichia coli Strains Causing Bacteraemia of Urinary Tract Origin. J. Med. Microbiol. 2008, 57, 1329–1334. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar, R.; Nazari, S.; Farahani, O. Phylogenetic Analysis and Antimicrobial Resistance Profiles of Escherichia coli Strains Isolated from UTI-Suspected Patients. Iran. J. Public Health 2020, 49, 1743. [Google Scholar] [CrossRef] [PubMed]
- Nemattalab, M.; Rohani, M.; Evazalipour, M.; Hesari, Z. Formulation of Cinnamon (Cinnamomum Verum) Oil Loaded Solid Lipid Nanoparticles and Evaluation of Its Antibacterial Activity against Multi-Drug Resistant Escherichia coli. BMC Complement. Med. Ther. 2022, 22, 289. [Google Scholar] [CrossRef]
- Nowrouzian, F.L.; Clermont, O.; Edin, M.; Östblom, A.; Denamur, E.; Wold, A.E.; Adlerberth, I. Escherichia coli B2 Phylogenetic Subgroups in the Infant Gut Microbiota: Predominance of Uropathogenic Lineages in Swedish Infants and Enteropathogenic Lineages in Pakistani Infants. Appl. Environ. Microbiol. 2019, 85, e01681-19. [Google Scholar] [CrossRef]
- Bakhtiari, S.; Mahmoudi, H.; Khosravi Seftjani, S.; Amirzargar, M.A.; Ghiasvand, S.; Ghaffari, M.E.; Adabi, M. Antibiotic Resistance Pattern and Phylogenetic Groups of the Uropathogenic Escherichia coli Isolates from Urinary Tract Infections in Hamedan, West of Iran. Iran. J. Microbiol. 2020, 12, 388. [Google Scholar] [CrossRef]
- Baponi, S.; Taravati, A.; Dilmagani, M. Determinationof phylogenetic groups of Escherichia coli isolated fromhuman urine in Urmia city. Crescent J. Med. Biol. Sci. 2016, 3, 97–99. [Google Scholar]
- Halaji, M.; Fayyazi, A.; Rajabnia, M.; Zare, D.; Pournajaf, A.; Ranjbar, R. Phylogenetic Group Distribution of Uropathogenic Escherichia coli and Related Antimicrobial Resistance Pattern: A Meta-Analysis and Systematic Review. Front. Cell. Infect. Microbiol. 2022, 12, 790184. [Google Scholar] [CrossRef]
- Staji, H.; Khoshgoftar, J.; Javaheri Vayeghan, A.; Salimi Bejestani, M.R. Phylogenetic Grouping and Assessment of Virulence Genotypes, With Antibiotic Resistance Patterns, of Escherichia coli Strains Implicated in Female Urinary Tract Infections. Int. J. Enteric Pathog. 2016, 4, e31609. [Google Scholar] [CrossRef]
- Silva, N.; Igrejas, G.; Gonçalves, A.; Poeta, P. Commensal Gut Bacteria: Distribution of Enterococcus Species and Prevalence of Escherichia coli Phylogenetic Groups in Animals and Humans in Portugal. Ann. Microbiol. 2012, 62, 449–459. [Google Scholar] [CrossRef]
- Stoppe, N.D.C.; Silva, J.S.; Carlos, C.; Sato, M.I.Z.; Saraiva, A.M.; Ottoboni, L.M.M.; Torres, T.T. Worldwide Phylogenetic Group Patterns of Escherichia coli from Commensal Human and Wastewater Treatment Plant Isolates. Front. Microbiol. 2017, 8, 2512. [Google Scholar] [CrossRef]
- Montealegre, M.C.; Talavera Rodríguez, A.; Roy, S.; Hossain, M.I.; Islam, M.A.; Lanza, V.F.; Julian, T.R. High Genomic Diversity and Heterogenous Origins of Pathogenic and Antibiotic-Resistant Escherichia coli in Household Settings Represent a Challenge to Reducing Transmission in Low-Income Settings. mSphere 2020, 5, e00704-19. [Google Scholar] [CrossRef] [PubMed]
- Fasugba, O.; Das, A.; Mnatzaganian, G.; Mitchell, B.G.; Collignon, P.; Gardner, A. Incidence of Single-Drug Resistant, Multidrug-Resistant and Extensively Drug-Resistant Escherichia coli Urinary Tract Infections: An Australian Laboratory-Based Retrospective Study. J. Glob. Antimicrob. Resist. 2019, 16, 254–259. [Google Scholar] [CrossRef]
- Al Nafeesah, A.; Al Fakeeh, K.; Chishti, S.; Hameed, T. E. coli. versus Non-E. coli. Urinary Tract Infections in Children: A Study from a Large Tertiary Care Center in Saudi Arabia. Int. J. Pediatr. Adolesc. Med. 2022, 9, 46–48. [Google Scholar] [CrossRef] [PubMed]
- Jalil, M.B.; Al Atbee, M.Y.N. The Prevalence of Multiple Drug Resistance Escherichia coli and Klebsiella Pneumoniae Isolated from Patients with Urinary Tract Infections. J. Clin. Lab. Anal. 2022, 36, e24619. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, Z.; Zheng, L.; Gong, Z.; Li, Y.; Jin, Y.; Huang, Y.; Chi, M. Urinary Tract Infections Caused by Uropathogenic Escherichia Coli: Mechanisms of Infection and Treatment Options. Int. J. Mol. Sci. 2023, 24, 10537. [Google Scholar] [CrossRef]
- Mohsin, A.S.; Alsakini, A.H.; Ali, M.R. Outbreak of Drug Resistance Escherichia coli Phylogenetic F Group Associated Urinary Tract Infection. Iran. J. Microbiol. 2022, 14, 341. [Google Scholar] [CrossRef] [PubMed]
- Shea, A.E.; Stocki, J.A.; Himpsl, S.D.; Smith, S.N.; Mobley, H.L.T. Loss of an Intimin-Like Protein Encoded on a Uropathogenic E. coli. Pathogenicity Island Reduces Inflammation and Affects Interactions with the Urothelium. Infect. Immun. 2022, 90, e00275-21. [Google Scholar] [CrossRef] [PubMed]
- Kabugo, D.; Kizito, S.; Ashok, D.D.; Kiwanuka, A.G.; Nabimba, R.; Namunana, S.; Kabaka, R.M.; Achan, B.; Najjuka, F.C. Factors Associated with Community-Acquired Urinary Tract Infections among Adults Attending Assessment Centre, Mulago Hospital Uganda. Afr. Health Sci. 2017, 16, 1131. [Google Scholar] [CrossRef] [PubMed]
- Dadi, B.R.; Abebe, T.; Zhang, L.; Mihret, A.; Abebe, W.; Amogne, W. Distribution of Virulence Genes and Phylogenetics of Uropathogenic Escherichia coli among Urinary Tract Infection Patients in Addis Ababa, Ethiopia. BMC Infect. Dis. 2020, 20, 108. [Google Scholar] [CrossRef]
- Hyun, M.; Lee, J.Y.; Kim, H.A. Differences of Virulence Factors, and Antimicrobial Susceptibility According to Phylogenetic Group in Uropathogenic Escherichia coli Strains Isolated from Korean Patients. Ann. Clin. Microbiol. Antimicrob. 2021, 20, 77. [Google Scholar] [CrossRef] [PubMed]
- Millán, Y.; Araque, M.; Ramírez, A. Distribución de Grupos Filogenéticos, Factores de Virulencia y Susceptibilidad Antimicrobiana En Cepas de Escherichia coli Uropatógena. Rev. Chil. Infectol. 2020, 37, 117–123. [Google Scholar] [CrossRef]
- Ahmed, A.E.; Abdelkarim, S.; Zenida, M.; Baiti, M.A.H.; Alhazmi, A.A.Y.; Alfaifi, B.A.H.; Majrabi, R.Q.M.; Khormi, N.Q.M.; Hakami, A.A.A.; Alqaari, R.A.M.; et al. Prevalence and Associated Risk Factors of Urinary Tract Infection among Diabetic Patients: A Cross-Sectional Study. Healthcare 2023, 11, 861. [Google Scholar] [CrossRef] [PubMed]
- Baldiris-Avila, R.; Montes-Robledo, A.; Buelvas-Montes, Y. Phylogenetic Classification, Biofilm-Forming Capacity, Virulence Factors, and Antimicrobial Resistance in Uropathogenic Escherichia coli (UPEC). Curr. Microbiol. 2020, 77, 3361–3370. [Google Scholar] [CrossRef]
- Elsayed Gawad, W.; Mohamed Helmy, O.; Mostafa Tawakkol, W.; Mohamed Hashem, A. Antimicrobial Resistance, Biofilm Formation, and Phylogenetic Grouping of Uropathogenic Escherichia coli Isolates in Egypt: The Role of Efflux Pump-Mediated Resistance. Jundishapur J. Microbiol. 2018, 11, e14444. [Google Scholar] [CrossRef]
- Agarwal, J.; Mishra, B.; Srivastava, S.; Srivastava, R. Genotypic Characteristics and Biofilm Formation among Escherichia coli Isolates from Indian Women with Acute Cystitis. Trans. R. Soc. Trop. Med. Hyg. 2013, 107, 183–187. [Google Scholar] [CrossRef]
- Moez, N.M.; Mashouf, R.Y.; Sedighi, I.; Shokoohizadeh, L.; Taheri, M. Phylogroup Classification and Investigation the Relationships between Phylogroups and Antibiotic Resistance Patterns of Uropathogenic E. coli. Isolated from Pediatric Urinary Tract Infection. Gene Rep. 2020, 20, 100758. [Google Scholar] [CrossRef]
- Hassuna, N.A.; Khairalla, A.S.; Farahat, E.M.; Hammad, A.M.; Abdel-Fattah, M. Molecular Characterization of Extended-Spectrum β Lactamase- Producing E. coli. Recovered from Community-Acquired Urinary Tract Infections in Upper Egypt. Sci. Rep. 2020, 10, 2772. [Google Scholar] [CrossRef]
- Farahat, E.M.; Hassuna, N.A.; Hammad, A.M.; Fattah, M.A.; Khairalla, A.S. Distribution of Integrons and Phylogenetic Groups among Escherichia coli Causing Community-Acquired Urinary Tract Infection in Upper Egypt. Can. J. Microbiol. 2021, 67, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, M.; Najar Peerayeh, S.; Mohabati Mobarez, A.; Bakhshi, B.; Karbalaei, M.; Abdolvand, Z. Phylogenetic Groups/B2 Subgroup Distributions, Serogrouping and Identification of Virulence Factors in Extended-Spectrum Cephalosporin-Resistant Escherichia coli Strains Isolated from the Stool of Healthy Children Under 10 Years Old. Arch. Pediatr. Infect. Dis. 2022, 10, e118889. [Google Scholar] [CrossRef]
- Hogins, J.; Xuan, Z.; Zimmern, P.E.; Reitzer, L. The Distinct Transcriptome of Virulence-Associated Phylogenetic Group B2 Escherichia coli. Microbiol. Spectr. 2023, 11, e02085-23. [Google Scholar] [CrossRef]
- Gunathilaka, G.A.D.K.K.; Dewasmika, W.A.P.M.; Sandaruwan, U.M.; Neelawala, N.G.D.A.K.; Madhumali, G.E.D.; Dissanayake, B.N.; Priyantha, M.A.R.; Prasada, D.V.P.; Dissanayake, D.R.A. Biofilm-Forming Ability, Antibiotic Resistance and Phylogeny of Escherichia coli Isolated from Extra Intestinal Infections of Humans, Dogs, and Chickens. Comp. Immunol. Microbiol. Infect. Dis. 2024, 105, 102123. [Google Scholar] [CrossRef]
- Suay-García, B.; Pérez-Gracia, M.T. Present and Future of Carbapenem-Resistant Enterobacteriaceae (CRE) Infections. Antibiotics 2019, 8, 122. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Lv, C.; Li, M.; Rahman, T.; Chang, Y.-F.; Guo, X.; Song, Z.; Zhao, Y.; Li, Q.; Ni, P.; et al. Carbapenem-Resistant Escherichia coli Exhibit Diverse Spatiotemporal Epidemiological Characteristics across the Globe. Commun. Biol. 2024, 7, 51. [Google Scholar] [CrossRef] [PubMed]
- Munkhdelger, Y.; Gunregjav, N.; Dorjpurev, A.; Juniichiro, N.; Sarantuya, J. Detection of Virulence Genes, Phylogenetic Group and Antibiotic Resistance of Uropathogenic Escherichia coli in Mongolia. J. Infect. Dev. Ctries. 2017, 11, 51–57. [Google Scholar] [CrossRef]
- Tewawong, N.; Kowaboot, S.; Pimainog, Y.; Watanagul, N.; Thongmee, T.; Poovorawan, Y. Distribution of Phylogenetic Groups, Adhesin Genes, Biofilm Formation, and Antimicrobial Resistance of Uropathogenic Escherichia coli Isolated from Hospitalized Patients in Thailand. PeerJ 2020, 8, e10453. [Google Scholar] [CrossRef]
- Mouanga Ndzime, Y.; Onanga, R.; Kassa Kassa, R.F.; Bignoumba, M.; Mbehang Nguema, P.P.; Gafou, A.; Lendamba, R.W.; Mbombe Moghoa, K.; Bisseye, C. Epidemiology of Community Origin Escherichia coli and Klebsiella Pneumoniae Uropathogenic Strains Resistant to Antibiotics in Franceville, Gabon. Infect. Drug Resist. 2021, 14, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Muvunyi, C.M.; Masaisa, F.; Musemakweri, A.; Muhirwa, G.; Claeys, G.W.; Bayingana, C.; Mutesa, L. Decreased Susceptibility to Commonly Used Antimicrobial Agents in Bacterial Pathogens Isolated from Urinary Tract Infections in Rwanda: Need for New Antimicrobial Guidelines. Am. J. Trop. Med. Hyg. 2011, 84, 923–928. [Google Scholar] [CrossRef] [PubMed]
- Jeanvoine, A.; Bouxom, H.; Leroy, J.; Gbaguidi-Haore, H.; Bertrand, X.; Slekovec, C. Resistance to Third-Generation Cephalosporins in Escherichia coli in the French Community: The Times They Are a-Changin’? Int. J. Antimicrob. Agents 2020, 55, 105909. [Google Scholar] [CrossRef] [PubMed]
- Naziri, Z.; Derakhshandeh, A.; Soltani Borchaloee, A.; Poormaleknia, M.; Azimzadeh, N. Treatment Failure in Urinary Tract Infections: A Warning Witness for Virulent Multi-Drug Resistant ESBL- Producing Escherichia Coli. Infect. Drug Resist. 2020, 13, 1839–1850. [Google Scholar] [CrossRef] [PubMed]
- Iranpour, D.; Hassanpour, M.; Ansari, H.; Tajbakhsh, S.; Khamisipour, G.; Najafi, A. Phylogenetic Groups of Escherichia coli Strains from Patients with Urinary Tract Infection in Iran Based on the New Clermont Phylotyping Method. BioMed Res. Int. 2015, 2015, 846219. [Google Scholar] [CrossRef] [PubMed]
- Calhau, V.; Domingues, S.; Ribeiro, G.; Mendonça, N.; Da Silva, G.J. Interplay between Pathogenicity Island Carriage, Resistance Profile and Plasmid Acquisition in Uropathogenic Escherichia Coli. J. Med. Microbiol. 2015, 64, 828–835. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Jia, S.; Cui, L.; Yang, H.; Yi, L. Phylogenetic Group, Biofilm Formation and Drug Resistance of Escherichia coli Isolated from Goose and Fish in Henan, China. Indian J. Anim. Res. 2023, 57, 1375–1379. [Google Scholar] [CrossRef]
- Norouzian, H.; Katouli, M.; Shahrokhi, N.; Sabeti, S.; Pooya, M.; Bouzari, S. The Relationship between Phylogenetic Groups and Antibiotic Susceptibility Patterns of Escherichia coli Strains Isolated from Feces and Urine of Patients with Acute or Recurrent Urinary Tract Infection. Iran. J. Microbiol. 2019, 11, 478–487. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.-C.; Fan, Y.-H.; Zhang, Y.-Z.; Bregente, C.J.B.; Lin, W.-H.; Chen, C.-A.; Lin, T.-P.; Kao, C.-Y. Characterization of Uropathogenic Escherichia coli Phylogroups Associated with Antimicrobial Resistance, Virulence Factor Distribution, and Virulence-Related Phenotypes. Infect. Genet. Evol. 2023, 114, 105493. [Google Scholar] [CrossRef]
- A Kadry, A.; M Al-Kashef, N.; M El-Ganiny, A. Distribution of Genes Encoding Adhesins and Biofilm Formation Capacity among Uropathogenic Escherichia coli Isolates in Relation to the Antimicrobial Resistance. Afr. Health Sci. 2020, 20, 238–247. [Google Scholar] [CrossRef]
- Poeta, P.; Radhouani, H.; Pinto, L.; Martinho, A.; Rego, V.; Rodrigues, R.; Gonçalves, A.; Rodrigues, J.; Estepa, V.; Torres, C.; et al. Wild Boars as Reservoirs of Extended-spectrum Beta-lactamase (ESBL) Producing Escherichia coli of Different Phylogenetic Groups. J. Basic Microbiol. 2009, 49, 584–588. [Google Scholar] [CrossRef] [PubMed]
- Mirkalantari, S.; Masjedian, F.; Irajian, G.; Siddig, E.E.; Fattahi, A. Determination of the Frequency of β-Lactamase Genes (Bla SHV, Bla TEM, Bla CTX-M) and Phylogenetic Groups among ESBL-Producing Uropathogenic Escherichia coli Isolated from Outpatients. J. Lab. Med. 2020, 44, 27–33. [Google Scholar] [CrossRef]
- Rahimifard, B.; Soheili, V.; Hashemitabar, G.; Askari Badouei, M. Possible Relationship of Novel Phylogenetic Structure With Antimicrobial Resistance, Biofilm Formation, and Hemolytic Activity in Uropathogenic Escherichia coli (UPEC). Int. J. Enteric Pathog. 2022, 10, 98–104. [Google Scholar] [CrossRef]
- Roy, R.; Tiwari, M.; Donelli, G.; Tiwari, V. Strategies for Combating Bacterial Biofilms: A Focus on Anti-Biofilm Agents and Their Mechanisms of Action. Virulence 2018, 9, 522–554. [Google Scholar] [CrossRef] [PubMed]
- Hashemizadeh, Z.; Kalantar-Neyestanaki, D.; Mansouri, S. Association between Virulence Profile, Biofilm Formation and Phylogenetic Groups of Escherichia coli Causing Urinary Tract Infection and the Commensal Gut Microbiota: A Comparative Analysis. Microb. Pathog. 2017, 110, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Maharjan, G.; Khadka, P.; Siddhi Shilpakar, G.; Chapagain, G.; Dhungana, G.R. Catheter-Associated Urinary Tract Infection and Obstinate Biofilm Producers. Can. J. Infect. Dis. Med. Microbiol. 2018, 2018, 7624857. [Google Scholar] [CrossRef]
- Soto, S.M.; Smithson, A.; Martinez, J.A.; Horcajada, J.P.; Mensa, J.; Vila, J. Biofilm Formation in Uropathogenic Escherichia coli Strains: Relationship With Prostatitis, Urovirulence Factors and Antimicrobial Resistance. J. Urol. 2007, 177, 365–368. [Google Scholar] [CrossRef] [PubMed]
- Javed, S.; Mirani, Z.A.; Pirzada, Z.A. Phylogenetic Group B2 Expressed Significant Biofilm Formation among Drug Resistant Uropathogenic Escherichia Coli. Libyan J. Med. 2021, 16, 1845444. [Google Scholar] [CrossRef]
- Matinfar, S.; Ahmadi, M.; Sisakht, A.M.; Sadeghi, J.; Javedansirat, S. Phylogenetic and Antibiotics Resistance in Extended-Spectrum B-Lactamase (ESBL) Uropathogenic Escherichia Coli: An Update Review. Gene Rep. 2021, 23, 101168. [Google Scholar] [CrossRef]
- Katongole, P.; Nalubega, F.; Florence, N.C.; Asiimwe, B.; Andia, I. Biofilm Formation, Antimicrobial Susceptibility and Virulence Genes of Uropathogenic Escherichia coli Isolated from Clinical Isolates in Uganda. BMC Infect. Dis. 2020, 20, 453. [Google Scholar] [CrossRef]
- Behzadi, P.; Urbán, E.; Gajdács, M. Association between Biofilm-Production and Antibiotic Resistance in Uropathogenic Escherichia coli (UPEC): An In Vitro Study. Diseases 2020, 8, 17. [Google Scholar] [CrossRef]
- Gajdács, M.; Kárpáti, K.; Nagy, Á.L.; Gugolya, M.; Stájer, A.; Burián, K. Association between Biofilm-Production and Antibiotic Resistance in Escherichia coli Isolates: A Laboratory-Based Case Study and a Literature Review. Acta Microbiol. Immunol. Hung. 2021, 68, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Cepas, V.; López, Y.; Muñoz, E.; Rolo, D.; Ardanuy, C.; Martí, S.; Xercavins, M.; Horcajada, J.P.; Bosch, J.; Soto, S.M. Relationship Between Biofilm Formation and Antimicrobial Resistance in Gram-Negative Bacteria. Microb. Drug Resist. 2019, 25, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Chlebicki, M. Urinary Tract Infections in Adults. Singapore Med. J. 2016, 57, 485–490. [Google Scholar] [CrossRef] [PubMed]
- European Committee on Antimicrobial Susceptibility Testing. 2022. Available online: https://www.sfm-microbiologie.org/wp-content/uploads/2022/05/CASFM2022_V1.0.pdf (accessed on 7 August 2024).
- Kaur, J. Modified Double Disc Synergy Test to Detect ESBL Production in Urinary Isolates of Escherichia coli and Klebsiella Pneumoniae. J. Clin. Diagn. Res. 2013, 7, 229. [Google Scholar] [CrossRef] [PubMed]
- Woodman, M.E.; Savage, C.R.; Arnold, W.K.; Stevenson, B. Direct PCR of Intact Bacteria (Colony PCR). Curr. Protoc. Microbiol. 2016, 42, A-3D. [Google Scholar] [CrossRef] [PubMed]
- Coy, M.R.; Hoffmann, M.; Kingdom Gibbard, H.N.; Kuhns, E.H.; Pelz-Stelinski, K.S.; Stelinski, L.L. Nested-Quantitative PCR Approach with Improved Sensitivity for the Detection of Low Titer Levels of Candidatus Liberibacter Asiaticus in the Asian Citrus Psyllid, Diaphorina Citri Kuwayama. J. Microbiol. Methods 2014, 102, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Clermont, O.; Bonacorsi, S.; Bingen, E. Rapid and Simple Determination of the Escherichia coli Phylogenetic Group. Appl. Environ. Microbiol. 2000, 66, 4555–4558. [Google Scholar] [CrossRef] [PubMed]
- Clermont, O.; Christenson, J.K.; Denamur, E.; Gordon, D.M. The Clermont Escherichia coli Phylo-typing Method Revisited: Improvement of Specificity and Detection of New Phylo-groups. Environ. Microbiol. Rep. 2013, 5, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Clermont, O.; Bonacorsi, S.; Bingen, E. Characterization of an Anonymous Molecular Marker Strongly Linked to Escherichia coli Strains Causing Neonatal Meningitis. J. Clin. Microbiol. 2004, 42, 1770–1772. [Google Scholar] [CrossRef]
- Lescat, M.; Clermont, O.; Woerther, P.L.; Glodt, J.; Dion, S.; Skurnik, D.; Djossou, F.; Dupont, C.; Perroz, G.; Picard, B.; et al. Commensal Escherichia coli Strains in Guiana Reveal a High Genetic Diversity with Host-dependant Population Structure. Environ. Microbiol. Rep. 2013, 5, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Clermont, O.; Lescat, M.; O’Brien, C.L.; Gordon, D.M.; Tenaillon, O.; Denamur, E. Evidence for a Human-specific Escherichia coli Clone. Environ. Microbiol. 2008, 10, 1000–1006. [Google Scholar] [CrossRef] [PubMed]
- Stepanović, S.; Vuković, D.; Hola, V.; Bonaventura, G.D.; Djukić, S.; Ćirković, I.; Ruzicka, F. Quantification of Biofilm in Microtiter Plates: Overview of Testing Conditions and Practical Recommendations for Assessment of Biofilm Production by Staphylococci. APMIS 2007, 115, 891–899. [Google Scholar] [CrossRef]
- Buxton, R. Blood Agar Plates and Hemolysis Protocols. 2005. Available online: https://asm.org/getattachment/7ec0de2b-bb16-4f6e-ba07-2aea25a43e76/protocol-28 (accessed on 7 August 2024).
- Mogrovejo-Arias, D.C.; Brill, F.H.H.; Wagner, D. Potentially Pathogenic Bacteria Isolated from Diverse Habitats in Spitsbergen, Svalbard. Environ. Earth Sci. 2020, 79, 109. [Google Scholar] [CrossRef]


| Gender | Inpatient | Outpatient | Total | |
|---|---|---|---|---|
| Adult | M | 3 (3.4%) | 11 (12.7%) | 14 (16.2%) |
| F | 3 (3.4%) | 38 (44.1%) | 41 (47.6%) | |
| Adult Total | 6 (6.9%) | 49 (57.9%) | 55 (63.9%) | |
| Children | M | 4 (4.6%) | 13 (15.1%) | 17 (19.7%) |
| F | 1 (1.1%) | 5 (5.8%) | 6 (6.9%) | |
| Children Total | 5 (6%) | 18 (20%) | 23 (26.7%) | |
| Elderly | M | 0 | 4 (4.6%) | 4 (4.6%) |
| F | 0 | 4 (4.6%) | 4 (4.6%) | |
| Elderly Total | 8 (9.3%) | 8 (9.3%) | ||
| Total | 11 (12.7%) | 75 (87.2%) | 86 (100%) |
| R (n = 6, 7%) | MDR (n = 60, 70%) | XDR (n = 20, 23%) | Total (n = 86, 100%) | |
|---|---|---|---|---|
| Gender | ||||
| M | 3 (3%) | 26 (30%) | 6 (7%) | 35 (41%) |
| F | 3 (3%) | 34 (40%) | 14 (16%) | 51 (59%) |
| Age | ||||
| Adult | 3 (3%) | 37 (43%) | 16 (19%) | 56 (65%) |
| Children | 2 (2%) | 18 (21%) | 2 (2%) | 22 (26%) |
| Elderly | 1 (1%) | 5 (6%) | 2 (2%) | 8 (9%) |
| Clinical status | ||||
| Inpatient | 0 | 10 (11%) | 1 (1%) | 11 (13%) |
| Outpatient | 6 (7%) | 50 (58%) | 19 (21%) | 75 (87%) |
| Phylogroups | ||||
| A | 1 (1%) | 4 (5%) | 2 (2%) | 7 (8%) |
| B1 | 0 | 4 (5%) | 0 | 4 (5%) |
| B2 | 4 (5%) | 27 (31%) | 11 (13%) | 42 (49%) |
| Clade I | 0 | 0 | 1 | 1 (1%) |
| Clade I or II | 0 | 1 (1%) | 0 | 1 (1%) |
| D | 0 | 1 (1%) | 0 | 1 (1%) |
| E | 0 | 14 (16%) | 5 (6%) | 19 (22%) |
| Unknown | 1 (1%) | 9 (10%) | 1 (1%) | 11 (13%) |
| ESBL production | ||||
| NO | 5 (6%) | 33 (38%) | 6 (7%) | 44 (51%) |
| YES | 1 (1%) | 27 (31%) | 14 (16%) | 42 (49%) |
| Hemolysin activity | ||||
| NO | 2 (2%) | 32 (37%) | 8 (9%) | 42 (49%) |
| α-hemolysin | 1 (1%) | 9 (10%) | 9 (10%) | 19 (22%) |
| β-hemolysin | 3 (3%) | 19 (22%) | 3 (3%) | 25 (29%) |
| Biofilm Production | ||||||
|---|---|---|---|---|---|---|
| No (n = 16, 19%) | Weak (n = 61, 71%) | Moderate (n = 8, 9%) | Strong (n = 1, 1%) | |||
| TOT | p Value | |||||
| Phylogroup | ||||||
| A | 2.3% | 4.6% | 1.1% | 0.0% | 8.1% | |
| B1 | 0.0% | 4.6% | 0.0% | 0.0% | 4.6% | |
| B2 | 11,6% | 31.3% | 5.8% | 0.0% | 48.8% | |
| Clade I | 0.0% | 1.1% | 0.0% | 0.0% | 1.1% | |
| Clade I or Clade II | 0.0% | 1.1% | 0.0% | 0.0% | 1.1% | |
| D | 0.0% | 1.1% | 0.0% | 0.0% | 1.1% | |
| E | 3.4% | 16.2% | 1.1% | 1.1% | 22.1% | |
| Unknown | 1.1% | 10.4% | 1.1% | 0.0% | 12.7% | |
| ESBL production | ||||||
| ESBL- negative | 13.9% | 29.1% | 6.9% | 1.1% | 51% | p = 0.02 |
| ESBL- producers | 4.7% | 41.9% | 2.4% | 0% | 49% | |
| Hemolytic activity | ||||||
| No hemolytic activity | 8.2% | 35% | 5.8% | 0% | 49% | |
| α-hemolysin | 3.5% | 17.5% | 1% | 0% | 22% | |
| β-hemolysin | 7% | 18.6% | 2.4% | 1% | 29% | |
| Phenotypic resistance | ||||||
| R | 3.5% | 3.5% | 0% | 0% | 7% | |
| MDR | 10.5% | 50% | 8.1% | 1.2% | 70% | |
| XDR | 4.7% | 17.4% | 1.2% | 0% | 23% | |
| Antibiotics | Biofilm Production | ||||
|---|---|---|---|---|---|
| Negative | Weak | Moderate | Strong | ||
| Penicillins | AMX | 69% | 92% | 75% | 100% |
| AMC | 50% | 61% | 50% | 100% | |
| TC | 69% | 87% | 75% | 100% | |
| TCC | 69% | 79% | 63% | 100% | |
| PRL | 50% | 80% | 63% | 100% | |
| TPZ | 25% | 16% | 25% | 0% | |
| Cephalosporins | CN | 44% | 41% | 0% | 100% |
| CX | 31% | 34% | 13% | 0% | |
| CFM | 50% | 39% | 0% | 0% | |
| CAZ | 31% | 33% | 0% | 0% | |
| CTX | 13% | 25% | 0% | 0% | |
| FEP | 44% | 39% | 13% | 0% | |
| Carbapenem | IMP | 100% | 100% | 100% | 100% |
| Aminoglycosides | GEN | 19% | 20% | 13% | 0% |
| AK | 13% | 20% | 0% | 0% | |
| TOB | 19% | 25% | 25% | 0% | |
| Quinolones | NA | 50% | 31% | 25% | 100% |
| CIP | 44% | 34% | 13% | 100% | |
| OF | 44% | 31% | 25% | 100% | |
| Phenicolate | C | 13% | 13% | 13% | 100% |
| Monobactams | ATM | 25% | 34% | 25% | 0% |
| Polymyxins | CS | 31% | 41% | 25% | 100% |
| Other | NIT | 63% | 89% | 100% | 100% |
| SXT | 38% | 54% | 50% | 100% | |
| FF | 25% | 31% | 13% | 0% | |
| Antibacterial Agents | MIC Dilution Range (mg/L) | Antibacterial Agents | MIC Dilution Range (mg/L) |
|---|---|---|---|
| Amoxicillin | 2–32 | Gentamycin | 1–16 |
| Amoxicillin + Clavulanic Acid | 16–256 | Tobramycin | 0.25–4 |
| Piperacillin | 4–64 | Nalidixic Acid | 8–128 |
| Piperacillin + Tazobactam | 4–64 | Ciprofloxacin | 0.125–2 |
| Cephalexin | 8–128 | Ofloxacin | 0.125–2 |
| Cefixime | 1–16 | Chloramphenicol | 2–32 |
| Ceftazidime | 0.25–4 | Aztreonam | 0.125–2 |
| Cefotaxime | 0.25–4 | Trimethoprim-Sulfamethoxazole | 2–32 |
| Target Gene | Primer ID | Amplicon Size (bp) | T °C | Reference |
|---|---|---|---|---|
| 16S rDNA | F1 R12 | 1500 | 56 | [68] |
| chuA | chuA.1b chuA.2 | 288 | 59 | [69] |
| yjaA | yjaA.1b yjaA.2b | 211 | 59 | [70] |
| tspE4.C2 | TspE4C2. 1b TspE4C2.2b | 152 | 59 | [70] |
| arpA | AceK.f ArpA1.r | 400 | 59 | [69,71] |
| arpA | ArpAgpE.f ArpAgpE.r | 301 | 57 | [72] |
| trpA | trpAgpC.1 trpAgpC.2 | 219 | 57 | [72] |
| trpA | trpBA.f trpBA.r | 489 | 57 | [73] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kara, A.; Massaro, C.; Giammanco, G.M.; Alduina, R.; Boussoualim, N. Phylogenetic Diversity, Antibiotic Resistance, and Virulence of Escherichia coli Strains from Urinary Tract Infections in Algeria. Antibiotics 2024, 13, 773. https://doi.org/10.3390/antibiotics13080773
Kara A, Massaro C, Giammanco GM, Alduina R, Boussoualim N. Phylogenetic Diversity, Antibiotic Resistance, and Virulence of Escherichia coli Strains from Urinary Tract Infections in Algeria. Antibiotics. 2024; 13(8):773. https://doi.org/10.3390/antibiotics13080773
Chicago/Turabian StyleKara, Anfal, Chiara Massaro, Giovanni M. Giammanco, Rosa Alduina, and Naouel Boussoualim. 2024. "Phylogenetic Diversity, Antibiotic Resistance, and Virulence of Escherichia coli Strains from Urinary Tract Infections in Algeria" Antibiotics 13, no. 8: 773. https://doi.org/10.3390/antibiotics13080773






