Genomic Insights into Pediococcus pentosaceus ENM104: A Probiotic with Potential Antimicrobial and Cholesterol-Reducing Properties
Abstract
:1. Introduction
2. Results and Discussion
2.1. Antibacterial Activity of Cell-Free Supernatants (CFSs) from Pediococcus pentosaceus ENM104
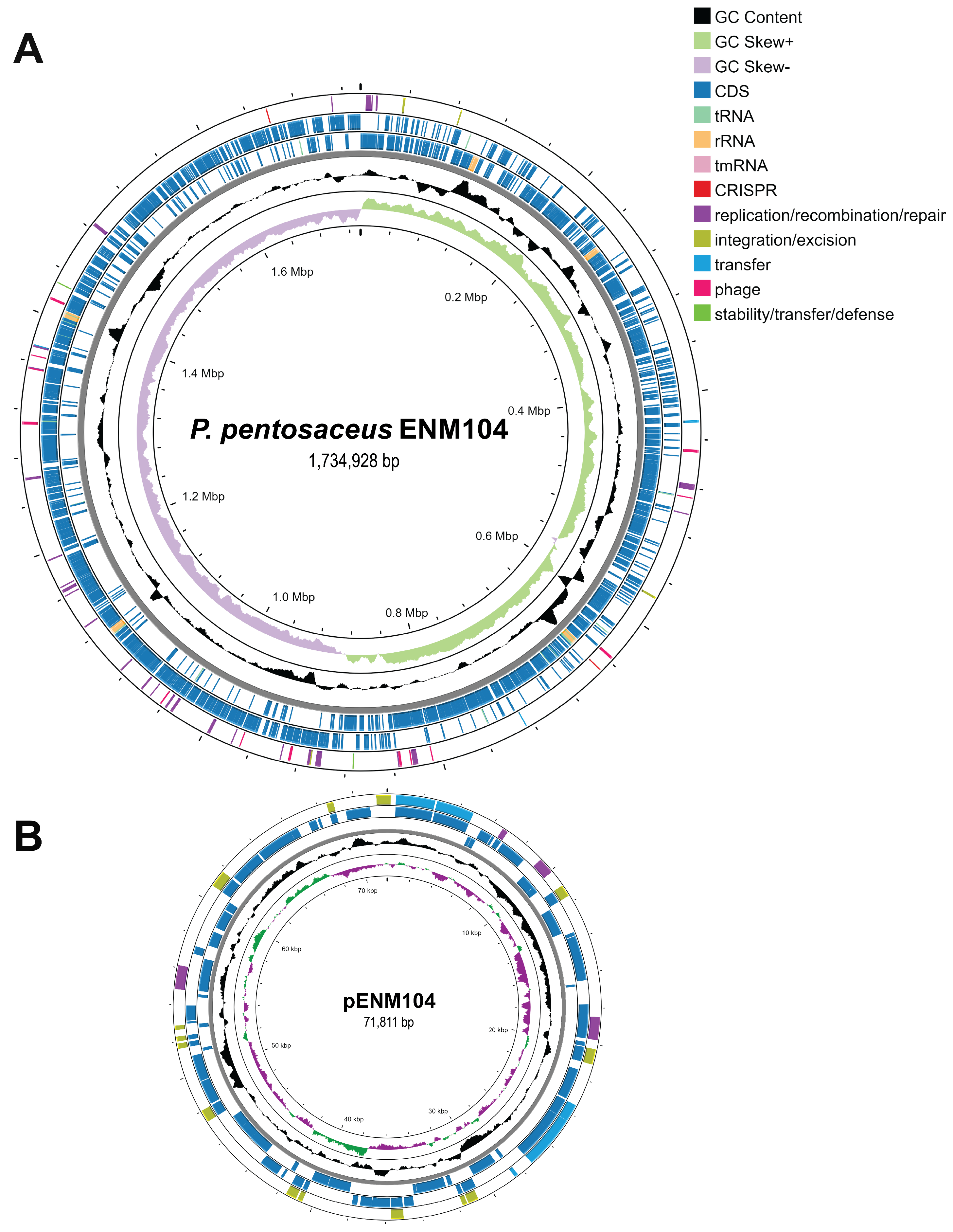
2.2. Genome and Plasmid Annotation of ENM104
2.3. Probiotic Properties of P. pentosaceus ENM104
2.4. Secondary Metabolite Identification
2.5. Cholesterol-Reducing Gene and GABA Synthesis Gene Identification
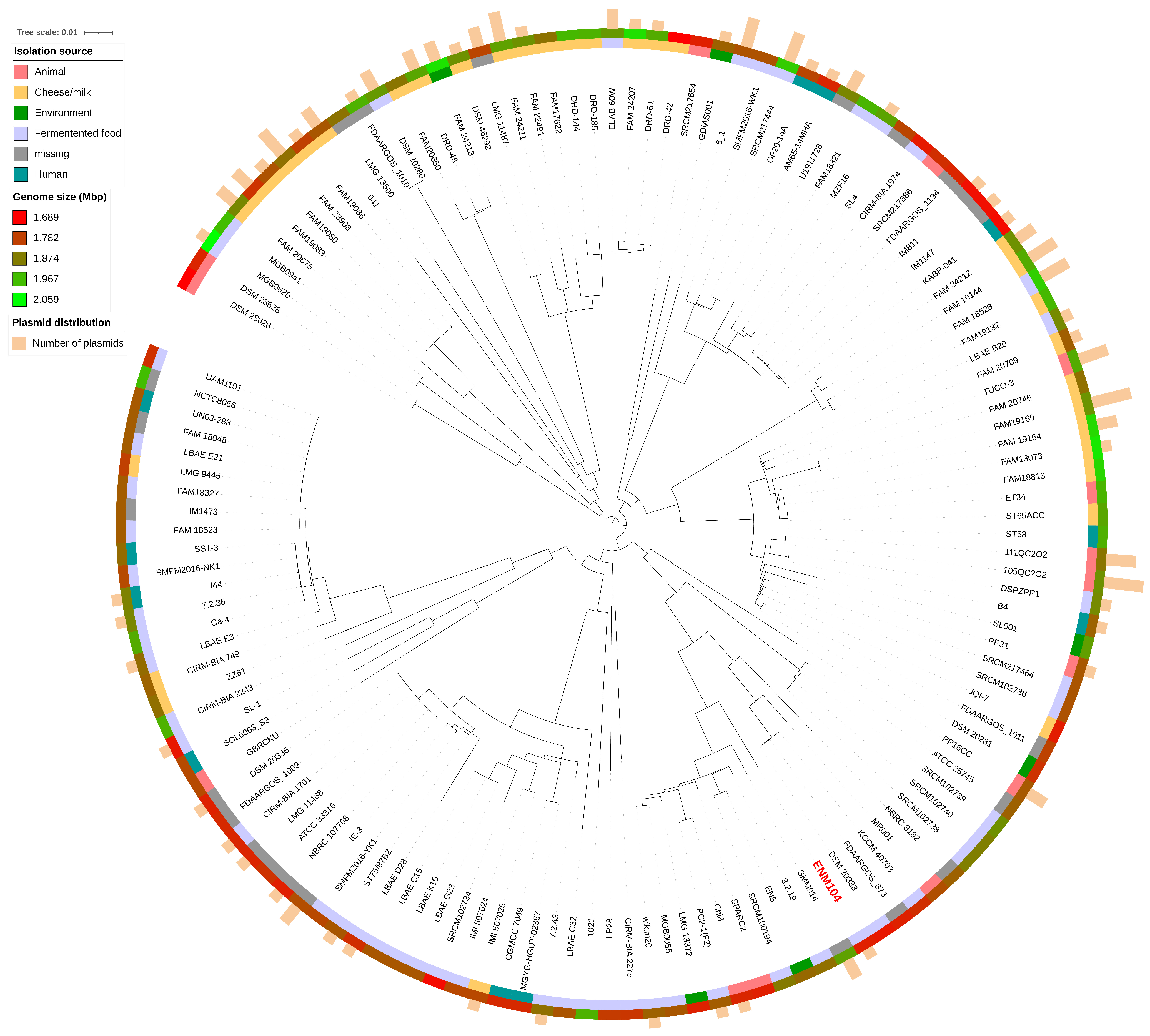
2.6. Pan-Genome Analysis
2.7. Comparative Genomic Analysis
3. Materials and Methods
3.1. Microbiological Characterization
3.1.1. Bacterial Strains
3.1.2. Preparation of P. pentosaceus Cell-Free Supernatant (CFS)
3.1.3. Antibacterial Activity Using the Agar Well Diffusion Assay
3.2. Genomic Characterization and Bioinformatics Analysis
3.2.1. Genomic DNA Extraction and Whole-Genome Sequencing
3.2.2. Genome Assembly, Annotation, and Visualization
3.2.3. In Silico Analysis of Probiotic Properties of P. pentosaceus ENM104
3.2.4. Bacteriocin Identification and Secondary Metabolite Identification
3.2.5. Cholesterol-Reducing Gene and γ-Aminobutyric Acid (GABA) Synthesis Gene Identification
3.2.6. Pan-Genome Analysis of Pediococcus Species
3.2.7. Comparative Genomic Analysis of ENM104
3.3. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cirat, R.; Capozzi, V.; Benmechernene, Z.; Spano, G.; Grieco, F.; Fragasso, M. LAB Antagonistic Activities and Their Significance in Food Biotechnology: Molecular Mechanisms, Food Targets, and Other Related Traits of Interest. Fermentation 2024, 10, 222. [Google Scholar] [CrossRef]
- Jiang, S.; Cai, L.; Lv, L.; Li, L. Pediococcus pentosaceus, a future additive or probiotic candidate. Microb. Cell Factories 2021, 20, 45. [Google Scholar] [CrossRef] [PubMed]
- Porto, M.C.W.; Kuniyoshi, T.M.; Azevedo, P.; Vitolo, M.; Oliveira, R.S. Pediococcus spp.: An important genus of lactic acid bacteria and pediocin producers. Biotechnol. Adv. 2017, 35, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-K.; Jin, H.; Song, N.-E.; Baik, S.-H. Probiotic Properties of Pediococcus pentosaceus JBCC 106 and Its Lactic Acid Fermentation on Broccoli Juice. Microorganisms 2023, 11, 1920. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, V.; Khan, M.S.; Jamal, Q.M.S.; Alzohairy, M.A.; Al Karaawi, M.A.; Siddiqui, M.U. Antimicrobial potential of bacteriocins: In therapy, agriculture and food preservation. Int. J. Antimicrob. Agents 2017, 49, 1–11. [Google Scholar] [CrossRef]
- Li, H.; Xie, X.; Li, Y.; Chen, M.; Xue, L.; Wang, J.; Zhang, J.; Wu, S.; Ye, Q.; Zhang, S. Pediococcus pentosaceus IM96 exerts protective effects against enterohemorrhagic Escherichia coli O157: H7 infection in vivo. Foods 2021, 10, 2945. [Google Scholar] [CrossRef]
- Thao, T.T.P.; Lan, T.T.P.; Phuong, T.V.; Truong, H.T.H.; Khoo, K.S.; Manickam, S.; Hoa, T.T.; Tram, N.D.Q.; Show, P.L.; Huy, N.D. Characterization halotolerant lactic acid bacteria Pediococcus pentosaceus HN10 and in vivo evaluation for bacterial pathogens inhibition. Chem. Eng. Process.-Process Intensif. 2021, 168, 108576. [Google Scholar] [CrossRef]
- Chiu, H.H.; Tsai, C.C.; Hsih, H.Y.; Tsen, H.Y. Screening from pickled vegetables the potential probiotic strains of lactic acid bacteria able to inhibit the Salmonella invasion in mice. J. Appl. Microbiol. 2008, 104, 605–612. [Google Scholar] [CrossRef]
- Kingcha, Y.; Tosukhowong, A.; Zendo, T.; Roytrakul, S.; Luxananil, P.; Chareonpornsook, K.; Valyasevi, R.; Sonomoto, K.; Visessanguan, W. Anti-listeria activity of Pediococcus pentosaceus BCC 3772 and application as starter culture for Nham, a traditional fermented pork sausage. Food Control 2012, 25, 190–196. [Google Scholar] [CrossRef]
- Uymaz, B.; Şimşek, Ö.; Akkoç, N.; Ataoğlu, H.; Akçelik, M. In vitro characterization of probiotic properties of Pediococcus pentosaceus BH105 isolated from human faeces. Ann. Microbiol. 2009, 59, 485–491. [Google Scholar] [CrossRef]
- Jitpakdee, J.; Kantachote, D.; Kanzaki, H.; Nitoda, T. Potential of lactic acid bacteria to produce functional fermented whey beverage with putative health promoting attributes. LWT 2022, 160, 113269. [Google Scholar] [CrossRef]
- Jitpakdee, J.; Kantachote, D.; Kanzaki, H.; Nitoda, T. Selected probiotic lactic acid bacteria isolated from fermented foods for functional milk production: Lower cholesterol with more beneficial compounds. LWT 2021, 135, 110061. [Google Scholar] [CrossRef]
- Atanasova, J.; Ivanova, I. Antibacterial peptides from goat and sheep milk proteins. Biotechnol. Biotechnol. Equip. 2010, 24, 1799–1803. [Google Scholar] [CrossRef]
- Reynolds, D.; Kollef, M. The epidemiology and pathogenesis and treatment of Pseudomonas aeruginosa infections: An update. Drugs 2021, 81, 2117–2131. [Google Scholar] [CrossRef]
- Alvarez-Sieiro, P.; Montalbán-López, M.; Mu, D.; Kuipers, O.P. Bacteriocins of lactic acid bacteria: Extending the family. Appl. Microbiol. Biotechnol. 2016, 100, 2939–2951. [Google Scholar] [CrossRef]
- Yi, E.-J.; Kim, A.-J. Antimicrobial and Antibiofilm Effect of Bacteriocin-Producing Pediococcus inopinatus K35 Isolated from Kimchi against Multidrug-Resistant Pseudomonas aeruginosa. Antibiotics 2023, 12, 676. [Google Scholar] [CrossRef] [PubMed]
- Yap, P.-C.; Ayuhan, N.; Woon, J.J.; Teh, C.S.J.; Lee, V.S.; Azman, A.S.; AbuBakar, S.; Lee, H.Y. Profiling of potential antibacterial compounds of lactic acid bacteria against extremely drug resistant (XDR) Acinetobacter baumannii. Molecules 2021, 26, 1727. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, F.S.; da Silva Rodrigues, R.; De Carvalho, A.F.; Nero, L.A. Genomic analyses of Pediococcus pentosaceus ST65ACC, a bacteriocinogenic strain isolated from artisanal raw-milk cheese. Probiotics Antimicrob. Proteins 2023, 15, 630–645. [Google Scholar] [CrossRef]
- Jiang, J.; Yang, B.; Ross, R.P.; Stanton, C.; Zhao, J.; Zhang, H.; Chen, W. Comparative genomics of Pediococcus pentosaceus isolated from different niches reveals genetic diversity in carbohydrate metabolism and immune system. Front. Microbiol. 2020, 11, 253. [Google Scholar] [CrossRef]
- Zaghloul, E.H.; Halfawy, N.M.E. Marine Pediococcus pentosaceus E3 Probiotic Properties, Whole-Genome Sequence Analysis, and Safety Assessment. In Probiotics and Antimicrobial Proteins; Springer: New York, NY, USA, 2024; pp. 1–12. [Google Scholar] [CrossRef]
- Zommiti, M.; Boukerb, A.M.; Feuilloley, M.G.; Ferchichi, M.; Connil, N. Draft genome sequence of Pediococcus pentosaceus MZF16, a bacteriocinogenic probiotic strain isolated from dried ossban in Tunisia. Microbiol. Resour. Announc. 2019, 8, 10–1128. [Google Scholar] [CrossRef]
- Blanco, I.R.; Pizauro, L.J.L.; dos Anjos Almeida, J.V.; Mendonça, C.M.N.; de Mello Varani, A.; de Souza Oliveira, R.P. Pan-genomic and comparative analysis of Pediococcus pentosaceus focused on the in silico assessment of pediocin-like bacteriocins. Comput. Struct. Biotechnol. J. 2022, 20, 5595–5606. [Google Scholar] [CrossRef] [PubMed]
- Finks, S.S.; Martiny, J.B. Plasmid-encoded traits vary across environments. MBio 2023, 14, e03191-22. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Huang, L.; Zeng, Y.; Li, W.; Zhou, D.; Xie, J.; Xie, J.; Tu, Q.; Deng, D.; Yin, J. Pediococcus pentosaceus: Screening and application as probiotics in food processing. Front. Microbiol. 2021, 12, 762467. [Google Scholar] [CrossRef] [PubMed]
- Anjana, A.; Tiwari, S.K. Bacteriocin-producing probiotic lactic acid bacteria in controlling dysbiosis of the gut microbiota. Front. Cell. Infect. Microbiol. 2022, 12, 851140. [Google Scholar] [CrossRef] [PubMed]
- Al Atya, A.K.; Belguesmia, Y.; Chataigne, G.; Ravallec, R.; Vachée, A.; Szunerits, S.; Boukherroub, R.; Drider, D. Anti-MRSA activities of enterocins DD28 and DD93 and evidences on their role in the inhibition of biofilm formation. Front. Microbiol. 2016, 7, 817. [Google Scholar] [CrossRef]
- Cavicchioli, V.Q.; Camargo, A.C.; Todorov, S.D.; Nero, L.A. Novel bacteriocinogenic Enterococcus hirae and Pediococcus pentosaceus strains with antilisterial activity isolated from Brazilian artisanal cheese. J. Dairy Sci. 2017, 100, 2526–2535. [Google Scholar] [CrossRef]
- Risdian, C.; Mozef, T.; Wink, J. Biosynthesis of polyketides in Streptomyces. Microorganisms 2019, 7, 124. [Google Scholar] [CrossRef]
- Tang, J.; Peng, X.; Liu, D.-M.; Xu, Y.-Q.; Xiong, J.; Wu, J.-J. Assessment of the safety and probiotic properties of Lactobacillus delbrueckii DMLD-H1 based on comprehensive genomic and phenotypic analysis. LWT 2023, 184, 115070. [Google Scholar] [CrossRef]
- De Smet, I.; Van Hoorde, L.; Vande Woestyne, M.; Christiaens, H.; Verstraete, W. Significance of bile salt hydrolytic activities of lactobacilli. J. Appl. Microbiol. 1995, 79, 292–301. [Google Scholar] [CrossRef]
- Kumar, M.; Nagpal, R.; Kumar, R.; Hemalatha, R.; Verma, V.; Kumar, A.; Chakraborty, C.; Singh, B.; Marotta, F.; Jain, S. Cholesterol-lowering probiotics as potential biotherapeutics for metabolic diseases. J. Diabetes Res. 2012, 2012, 902917. [Google Scholar] [CrossRef]
- Cui, Y.; Miao, K.; Niyaphorn, S.; Qu, X. Production of gamma-aminobutyric acid from lactic acid bacteria: A systematic review. Int. J. Mol. Sci. 2020, 21, 995. [Google Scholar] [CrossRef]
- Pennacchietti, E.; d’Alonzo, C.; Freddi, L.; Occhialini, A.; De Biase, D. The glutaminase-dependent acid resistance system: Qualitative and quantitative assays and analysis of its distribution in enteric bacteria. Front. Microbiol. 2018, 9, 2869. [Google Scholar] [CrossRef] [PubMed]
- Phuengjayaem, S.; Pakdeeto, A.; Kingkaew, E.; Tunvongvinis, T.; Somphong, A.; Tanasupawat, S. Genome sequences and functional analysis of Levilactobacillus brevis LSF9-1 and Pediococcus acidilactici LSF1-1 from fermented fish cake (Som-fak) with gamma-aminobutyric acid (GABA) production. Funct. Integr. Genom. 2023, 23, 158. [Google Scholar] [CrossRef]
- Xuan, J.; Han, X.; Che, J.; Zhuo, J.; Xu, J.; Lu, J.; Mu, H.; Wang, J.; Tu, J.; Liu, G. Production of γ—Aminobutyric acid—Enriched sourdough bread using an isolated Pediococcus pentosaceus strain JC30. Heliyon 2024, 10. [Google Scholar] [CrossRef]
- Carafa, I.; Nardin, T.; Larcher, R.; Viola, R.; Tuohy, K.; Franciosi, E. Identification and characterization of wild lactobacilli and pediococci from spontaneously fermented Mountain cheese. Food Microbiol. 2015, 48, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Thuy, D.T.B.; Nguyen, A.T.; Khoo, K.S.; Chew, K.W.; Cnockaert, M.; Vandamme, P.; Ho, Y.-C.; Huy, N.D.; Cocoletzi, H.H.; Show, P.L. Optimization of culture conditions for gamma-aminobutyric acid production by newly identified Pediococcus pentosaceus MN12 isolated from ‘mam nem’, a fermented fish sauce. Bioengineered 2021, 12, 54–62. [Google Scholar] [CrossRef]
- De Toro, M.; Pilar Garcillán-Barcia, M.; De La Cruz, F. Plasmid diversity and adaptation analyzed by massive sequencing of Escherichia coli plasmids. In Plasmids: Biology and Impact in Biotechnology and Discovery; ASM Press: Washington, DC, USA, 2015; pp. 219–235. [Google Scholar]
- Hayashi, T.; Tanaka, Y.; Sakai, N.; Okada, U.; Yao, M.; Watanabe, N.; Tamura, T.; Tanaka, I. SCO4008, a putative TetR transcriptional repressor from Streptomyces coelicolor A3 (2), regulates transcription of sco4007 by multidrug recognition. J. Mol. Biol. 2013, 425, 3289–3300. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Wang, Y.; Zhang, N.; Xiao, M.; Zhang, J.; Xing, X.; Zhang, Y.; Fan, Y.; Li, X.; Nan, B. Antioxidant mechanism of Lactiplantibacillus plantarum KM1 under H2O2 stress by proteomics analysis. Front. Microbiol. 2022, 13, 897387. [Google Scholar] [CrossRef]
- Declerck, N.; Vincent, F.; Hoh, F.; Aymerich, S.; Van Tilbeurgh, H. RNA recognition by transcriptional antiterminators of the BglG/SacY family: Functional and structural comparison of the CAT domain from SacY and LicT. J. Mol. Biol. 1999, 294, 389–402. [Google Scholar] [CrossRef]
- Chukamnerd, A.; Pomwised, R.; Chusri, S.; Singkhamanan, K.; Chumtong, S.; Jeenkeawpiam, K.; Sakunrang, C.; Saroeng, K.; Saengsuwan, P.; Wonglapsuwan, M. Antimicrobial Susceptibility and Molecular Features of Colonizing Isolates of Pseudomonas aeruginosa and the Report of a Novel Sequence Type (ST) 3910 from Thailand. Antibiotics 2023, 12, 165. [Google Scholar] [CrossRef]
- Kaewnirat, K.; Chuaychob, S.; Chukamnerd, A.; Pomwised, R.; Surachat, K.; Phoo, M.T.P.; Phaothong, C.; Sakunrang, C.; Jeenkeawpiam, K.; Hortiwakul, T. In vitro synergistic activities of fosfomycin in combination with other antimicrobial agents against carbapenem-resistant Escherichia coli harboring bla NDM-1 on the IncN2 plasmid and a study of the genomic characteristics of these pathogens. Infect. Drug Resist. 2022, 15, 1777–1791. [Google Scholar] [CrossRef] [PubMed]
- Chukamnerd, A.; Singkhamanan, K.; Chongsuvivatwong, V.; Palittapongarnpim, P.; Doi, Y.; Pomwised, R.; Sakunrang, C.; Jeenkeawpiam, K.; Yingkajorn, M.; Chusri, S. Whole-genome analysis of carbapenem-resistant Acinetobacter baumannii from clinical isolates in Southern Thailand. Comput. Struct. Biotechnol. J. 2022, 20, 545–558. [Google Scholar] [CrossRef] [PubMed]
- Chukamnerd, A.; Pomwised, R.; Jeenkeawpiam, K.; Sakunrang, C.; Chusri, S.; Surachat, K. Genomic insights into blaNDM-carrying carbapenem-resistant Klebsiella pneumoniae clinical isolates from a university hospital in Thailand. Microbiol. Res. 2022, 263, 127136. [Google Scholar] [CrossRef]
- Keeratikunakorn, K.; Kaewchomphunuch, T.; Kaeoket, K.; Ngamwongsatit, N. Antimicrobial activity of cell free supernatants from probiotics inhibits against pathogenic bacteria isolated from fresh boar semen. Sci. Rep. 2023, 13, 5995. [Google Scholar] [CrossRef]
- Wick, R.R.; Judd, L.M.; Gorrie, C.L.; Holt, K.E. Unicycler: Resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput. Biol. 2017, 13, e1005595. [Google Scholar] [CrossRef] [PubMed]
- Seemann, T. Prokka: Rapid prokaryotic genome annotation. Bioinformatics. 2014, 30, 2068–2069. [Google Scholar] [CrossRef]
- Aziz, R.K.; Bartels, D.; Best, A.A.; DeJongh, M.; Disz, T.; Edwards, R.A.; Formsma, K.; Gerdes, S.; Glass, E.M.; Kubal, M. The RAST Server: Rapid annotations using subsystems technology. BMC Genom. 2008, 9, 75. [Google Scholar] [CrossRef]
- Al-Emran, H.M.; Moon, J.F.; Miah, M.L.; Meghla, N.S.; Reuben, R.C.; Uddin, M.J.; Ibnat, H.; Sarkar, S.L.; Roy, P.C.; Rahman, M.S. Genomic analysis and in vivo efficacy of Pediococcus acidilactici as a potential probiotic to prevent hyperglycemia, hypercholesterolemia and gastrointestinal infections. Sci. Rep. 2022, 12, 20429. [Google Scholar] [CrossRef]
- Kim, J.-A.; Jang, H.-J.; Kim, D.-H.; Son, Y.K.; Kim, Y. Complete genome sequence of Pediococcus acidilactici CACC 537 isolated from canine. J. Anim. Sci. Technol. 2023, 65, 1105. [Google Scholar] [CrossRef]
- van Heel, A.J.; de Jong, A.; Song, C.; Viel, J.H.; Kok, J.; Kuipers, O.P. BAGEL4: A user-friendly web server to thoroughly mine RiPPs and bacteriocins. Nucleic Acids Res. 2018, 46, W278–W281. [Google Scholar] [CrossRef]
- Blin, K.; Shaw, S.; Augustijn, H.E.; Reitz, Z.L.; Biermann, F.; Alanjary, M.; Fetter, A.; Terlouw, B.R.; Metcalf, W.W.; Helfrich, E.J. antiSMASH 7.0: New and improved predictions for detection, regulation, chemical structures and visualisation. Nucleic Acids Res. 2023, 51, W46–W50. [Google Scholar] [CrossRef] [PubMed]
- Page, A.J.; Cummins, C.A.; Hunt, M.; Wong, V.K.; Reuter, S.; Holden, M.T.; Fookes, M.; Falush, D.; Keane, J.A.; Parkhill, J. Roary: Rapid large-scale prokaryote pan genome analysis. Bioinformatics 2015, 31, 3691–3693. [Google Scholar] [CrossRef] [PubMed]
- Wanna, W.; Surachat, K.; Kaitimonchai, P.; Phongdara, A. Evaluation of probiotic characteristics and whole genome analysis of Pediococcus pentosaceus MR001 for use as probiotic bacteria in shrimp aquaculture. Sci. Rep. 2021, 11, 18334. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.X.; Li, Y.D.; Hu, X.J.; Shi, H.Y.; Li, L.J. Whole-genome sequence assembly of Pediococcus pentosaceus LI05 (CGMCC 7049) from the human gastrointestinal tract and comparative analysis with representative sequences from three food-borne strains. Gut Pathog. 2014, 6, 36. [Google Scholar] [CrossRef]




| Clinical Strains | Inhibition Zones (mm) | |||||||
|---|---|---|---|---|---|---|---|---|
| Time (h) | Non-Adjusted | Adjusted | ||||||
| 12 | 24 | 36 | 48 | 12 | 24 | 36 | 48 | |
| Pseudomonas aeruginosa PA01 | 15.17 ± 0.34 * | 15.33 ± 1.25 * | 15.67 ± 0.47 * | 16.83 ± 0.29 * | N | N | N | N |
| Escherichia coli CREC003 | N | N | N | N | N | N | N | N |
| Acinetobacter baumannii CRAB-SK005 | 12.33 ± 0.47 * | 13.33 ± 0.47 * | 14.83 ± 0.62 * | 15.50 ± 0.50 * | N | N | N | N |
| Klebsiella pneumoniae CRKP10 | N | N | N | 12.17 ± 0.29 * | N | N | N | N |
| Attribute | Chromosome | Plasmid |
|---|---|---|
| Genome size (bp) | 1,734,928 | 71,811 |
| G + C content (%) | 37.2 | 38.1 |
| Number of CDS | 1689 | 85 |
| tRNA | 55 | 0 |
| rRNA | 15 | 0 |
| tmRNA | 1 | 0 |
| RAST subsystems | 194 | 5 |
| Gene | Function |
|---|---|
| Stress Resistance | |
| Heat stress | |
| htpX | Protease HtpX |
| hrcA | Heat-inducible transcription repressor HrcA |
| hslO | 33 kDa chaperonin |
| dnaK | Chaperone protein DnaK |
| dnaJ | Chaperone protein DnaJ |
| ctsR | Transcriptional regulator CtsR |
| grpE | Protein GrpE |
| clp operon | ATP-dependent Clp protease |
| hslV | ATP-dependent protease subunit HslV |
| Acid stress | |
| atp operon | ATP synthase subunit |
| nhaC | Na(+)/H(+) antiporter NhaC |
| Bile tolerance | |
| ppaC | Manganese-dependent inorganic pyrophosphatase |
| cfa | Cyclopropane-fatty-acyl-phospholipid synthase |
| Vitamin biosynthesis | |
| ribU | Riboflavin transporter FmnP |
| ribZ | Riboflavin transporter RibZ |
| ribF | Bifunctional riboflavin kinase/FMN adenylyltransferase |
| folT | Folate transporter FolT |
| btuD | Vitamin B12 import ATP-binding protein BtuD |
| ytrB | hypothetical protein |
| lnrL | Linearmycin resistance ATP-binding protein LnrL |
| Immunomodulation | |
| dltA | D-alanine-D-alanyl carrier protein ligase |
| dltC | D-alanyl carrier protein |
| dltD | Protein DltD |
| Bacteriocin | |
| pedA | Bacteriocin pediocin PA-1 |
| Secondary metabolite biosynthesis | |
| mtgA | Biosynthetic peptidoglycan transglycosylase |
| Pediococcus Strains | Isolation Sources | GABA Production | Reference |
|---|---|---|---|
| Pediococcus pentosaceus ENM104 | Fermented pork sausage | 4.49 ± 0.03 μg/mL | [12] |
| Pediococcus pentosaceus JC30 | Traditional kimchi | 3.32 ± 0.04 mg/g | [35] |
| Pediococcus acidilactici LSF1-1 | Fermented fish cake (Som fak) | 0.80 ± 0.00 g/L | [34] |
| Pediococcus pentosaceus 56 | Traditional Mountain Malga (TMM) cheese | 9.6 ± 0.9 mg/L | [36] |
| Pediococcus pentosaceus MN12 | Fermented fish sauce (mam nem) | 16.8 ± 0.00 mM | [37] |
| Molecular Function | |
|---|---|
| Gene | Function |
| sphR | Alkaline phosphatase synthesis transcriptional regulatory protein SphR |
| xylQ | lpha-xylosidase XylQ |
| bglC | Aryl-phospho-beta-D-glucosidase BglC |
| adhR | HTH-type transcriptional regulator AdhR |
| cmtB | Mannitol-specific cryptic phosphotransferase enzyme IIA component |
| lmrA | Multidrug resistance ABC transporter ATP-binding and permease protein |
| fryB | PTS system fructose-like EIIB component 1 |
| hprS | Sensor histidine kinase HprS |
| licT | Transcription antiterminator LicT |
| btuD | Vitamin B12 import ATP-binding protein BtuD |
| bglA | 6-phospho-beta-glucosidase BglA |
| recD2 | ATP-dependent RecD-like DNA helicase |
| lacM | Beta-galactosidase small subunit |
| lpdC | Gallate decarboxylase |
| mngB | Mannosylglycerate hydrolase |
| pox5 | Pyruvate oxidase |
| tkt | Transketolase |
| Biological process | |
| sphR | Alkaline phosphatase synthesis transcriptional regulatory protein SphR |
| xylQ | lpha-xylosidase XylQ |
| bglC | Aryl-phospho-beta-D-glucosidase BglC |
| adhR | HTH-type transcriptional regulator AdhR |
| cmtB | Mannitol-specific cryptic phosphotransferase enzyme IIA component |
| lmrA | Multidrug resistance ABC transporter ATP-binding and permease protein |
| fryB | PTS system fructose-like EIIB component 1 |
| hprS | Sensor histidine kinase HprS |
| licT | Transcription antiterminator LicT |
| btuD | Vitamin B12 import ATP-binding protein BtuD |
| csbX | Alpha-ketoglutarate permease |
| ulaA | Ascorbate-specific PTS system EIIC component |
| gsiB | Glucose starvation-inducible protein B |
| kup | Low affinity potassium transport system protein kup |
| agaC | N-acetylgalactosamine permease IIC component 1 |
| Strains | Specimen | Antibacterial Resistance Profiles | References | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β-Lactam Combination Agents | Carbapenems | Lipopeptide | Aminoglycosides | Fluoroquinolones | |||||||||
| TZP | C/T | IPM | MEM | DOR | CST | AMK | GEN | TOB | CIP | LVX | |||
| Pseudomonas aeruginosa PA01 | Rectum | S | S | R | R | R | R | S | S | S | S | S | [42] |
| Escherichia coli CREC003 | Rectum | - | - | R | R | R | - | S | S | S | R | R | [43] |
| Acinetobacter baumannii CRAB-SK005 | Rectum | - | - | R | R | R | - | - | - | - | - | - | [44] |
| Klebsiella pneumoniae CRKP10 | Sputum | R | - | R | R | R | I | R | R | R | R | R | [45] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kompramool, S.; Singkhamanan, K.; Pomwised, R.; Chaichana, N.; Suwannasin, S.; Wonglapsuwan, M.; Jitpakdee, J.; Kantachote, D.; Yaikhan, T.; Surachat, K. Genomic Insights into Pediococcus pentosaceus ENM104: A Probiotic with Potential Antimicrobial and Cholesterol-Reducing Properties. Antibiotics 2024, 13, 813. https://doi.org/10.3390/antibiotics13090813
Kompramool S, Singkhamanan K, Pomwised R, Chaichana N, Suwannasin S, Wonglapsuwan M, Jitpakdee J, Kantachote D, Yaikhan T, Surachat K. Genomic Insights into Pediococcus pentosaceus ENM104: A Probiotic with Potential Antimicrobial and Cholesterol-Reducing Properties. Antibiotics. 2024; 13(9):813. https://doi.org/10.3390/antibiotics13090813
Chicago/Turabian StyleKompramool, Siriwan, Kamonnut Singkhamanan, Rattanaruji Pomwised, Nattarika Chaichana, Sirikan Suwannasin, Monwadee Wonglapsuwan, Jirayu Jitpakdee, Duangporn Kantachote, Thunchanok Yaikhan, and Komwit Surachat. 2024. "Genomic Insights into Pediococcus pentosaceus ENM104: A Probiotic with Potential Antimicrobial and Cholesterol-Reducing Properties" Antibiotics 13, no. 9: 813. https://doi.org/10.3390/antibiotics13090813







