Complex Infection-Control Measures with Disinfectant Switch Help the Successful Early Control of Carbapenem-Resistant Acinetobacter baumannii Outbreak in Intensive Care Unit
Abstract
:1. Introduction
2. Results
2.1. Description of the Outbreak
2.2. Environmental Investigations
2.3. Infection-Control Measures during the Outbreak
2.4. Characteristics of CRAB Strains of This Outbreak
2.4.1. Biofilm-Forming Capacity
2.4.2. Antibiotic Susceptibility
2.4.3. Disinfectant Susceptibility of the Planktonic and Biofilm-Forming Phase of CRAB
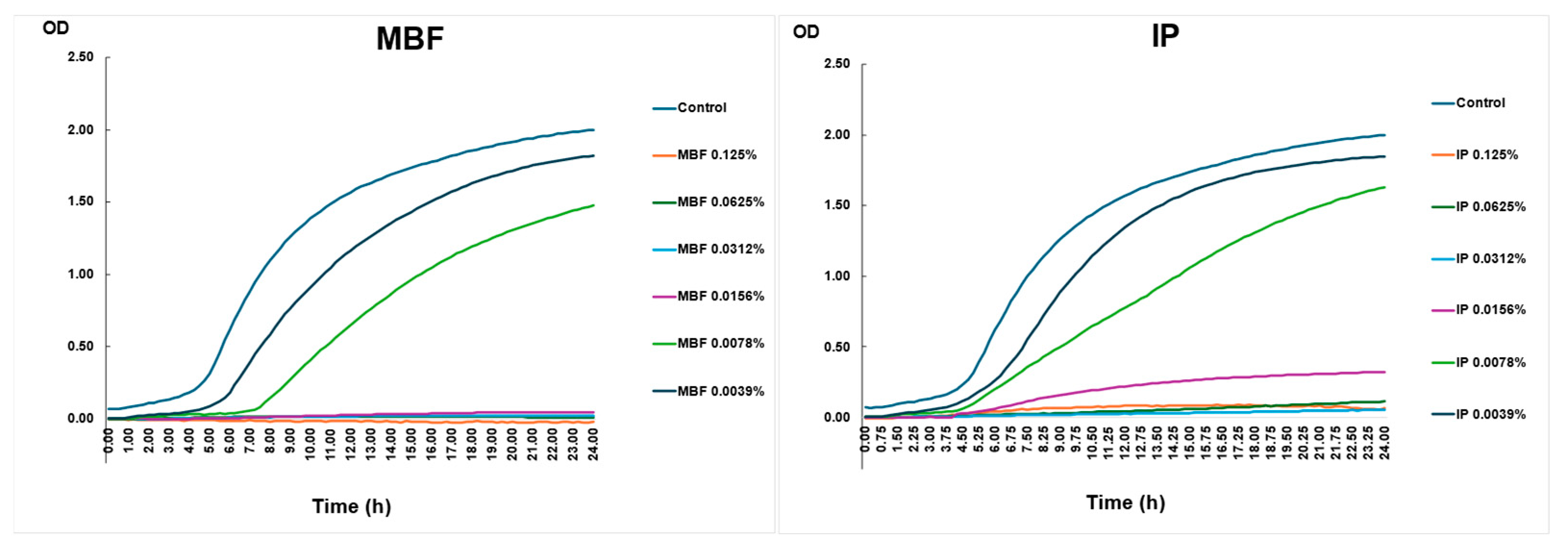
2.4.4. Killing Curve Assay with the Two Different Surface Disinfectants—MBF and IP—Used during the Outbreak
2.4.5. Genomic Analysis
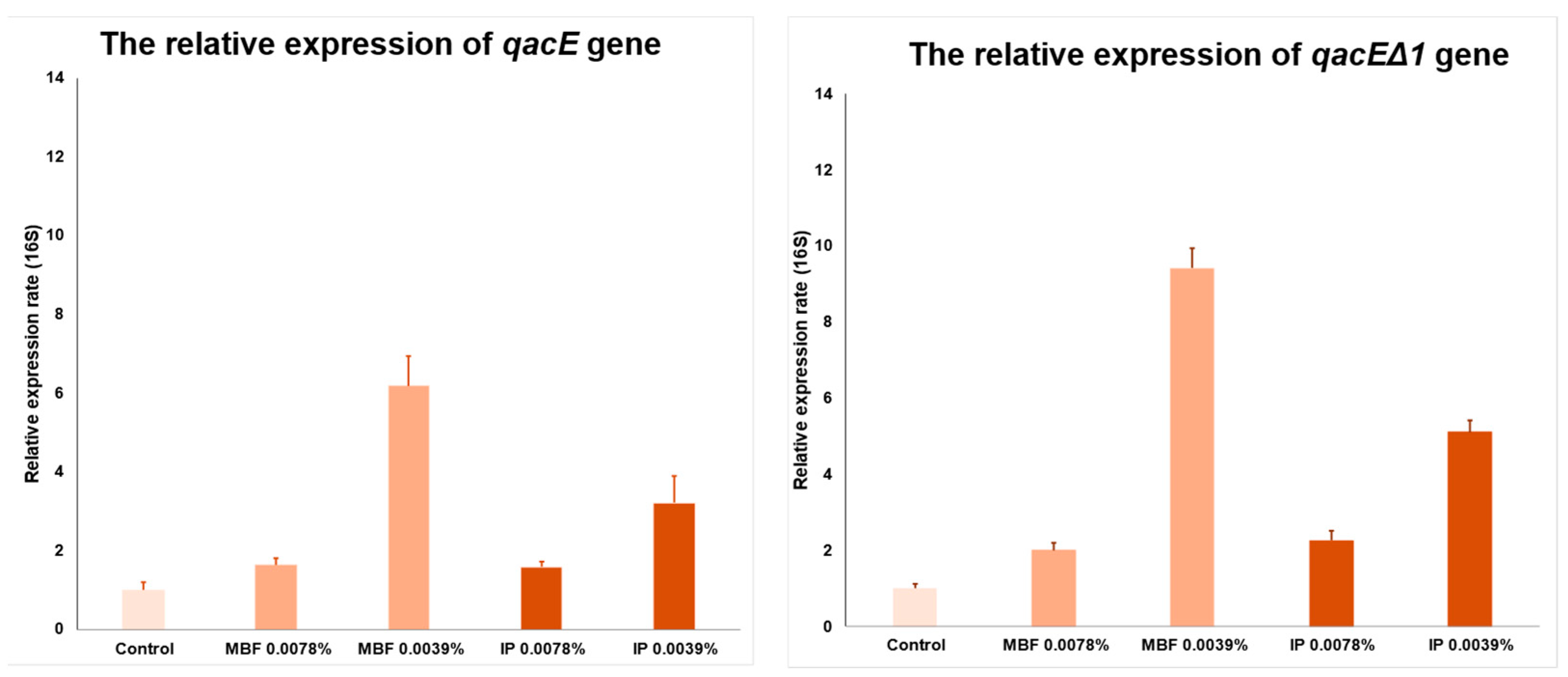
2.4.6. Real-Time PCR Assay to Determine the Relative Expression Rate of qacE and qacEΔ1
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Environmental Sampling
4.3. Bacterial Strain
4.4. Minimal Inhibitory Concentration and Minimal Bactericidal Concentration Determination
4.5. Killing Curve Assay
4.6. Investigation of Biofilm Production
4.7. Minimum Biofilm Inhibitory Concentration (MBIC) Determination
4.8. Whole-Genome Sequencing (WGS)
4.9. Analysis of Disinfectant Resistance Genes by Quantitative PCR
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Obenhuber, T.; Scheier, T.C.; Stutz, T.; Hug, M.; Fontein, D.; Kaiser, A.; Schoene, S.; Steiger, P.; Brugger, S.D.; Zingg, W.; et al. An outbreak of multi-drug-resistant Acinetobacter baumannii on a burns ICU and its control with multi-faceted containment measures. J. Hosp. Infect. 2024, 146, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Baleivanualala, S.C.; Matanitobua, S.; Soqo, V.; Smita, S.; Limaono, J.; Sharma, S.C.; Devi, S.V.; Boseiwaqa, L.V.; Vera, N.; Kumar, S.; et al. Molecular and clinical epidemiology of carbapenem resistant Acinetobacter baumannii, Pseudomonas aeruginosa and Enterobacterales in Fiji: A multicentre prospective observational study. Lancet Reg. Health West Pac. 2024, 47, 101095. [Google Scholar] [CrossRef] [PubMed]
- Su, P.W.; Yang, E.C.; Moi, S.H.; Yang, C.H.; Chuang, L.Y. Prevalence of Carbapenem Resistance Genes among Acinetobacter baumannii Isolated from a Teaching Hospital in Taiwan. Antibiotics 2023, 12, 1357. [Google Scholar] [CrossRef]
- Tickler, I.A.; Kawa, D.; Obradovich, A.E.; Fang, F.C.; Tenover, F.C.; The Healthcare Associated Infections Consortium. Characterization of Carbapenemase- and ESBL-Producing Gram-Negative Bacilli Isolated from Patients with Urinary Tract and Bloodstream Infections. Antibiotics 2023, 12, 1386. [Google Scholar] [CrossRef]
- Seifert, H.; Muller, C.; Stefanik, D.; Higgins, P.G.; Wohlfarth, E.; Kresken, M. In Vitro Activity of Cefiderocol against a Global Collection of Carbapenem-Resistant Acinetobacter baumannii Isolates. Antibiotics 2023, 12, 1172. [Google Scholar] [CrossRef]
- Ruekit, S.; Srijan, A.; Serichantalergs, O.; Margulieux, K.R.; Mc Gann, P.; Mills, E.G.; Stribling, W.C.; Pimsawat, T.; Kormanee, R.; Nakornchai, S.; et al. Molecular characterization of multidrug-resistant ESKAPEE pathogens from clinical samples in Chonburi, Thailand (2017–2018). BMC Infect. Dis. 2022, 22, 695. [Google Scholar] [CrossRef]
- Piperaki, E.T.; Tzouvelekis, L.S.; Miriagou, V.; Daikos, G.L. Carbapenem-resistant Acinetobacter baumannii: In pursuit of an effective treatment. Clin. Microbiol. Infect. 2019, 25, 951–957. [Google Scholar] [CrossRef]
- Bonomo, R.A.; Szabo, D. Mechanisms of multidrug resistance in Acinetobacter species and Pseudomonas aeruginosa. Clin. Infect. Dis. 2006, 43 (Suppl. S2), S49–S56. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, B.; Liu, C.; Sun, X.; Chu, Y. Acinetobacter baumannii infection in intensive care unit: Analysis of distribution and drug resistance. Mol. Biol. Rep. 2024, 51, 120. [Google Scholar] [CrossRef]
- Paul, M.; Carrara, E.; Retamar, P.; Tangden, T.; Bitterman, R.; Bonomo, R.A.; de Waele, J.; Daikos, G.L.; Akova, M.; Harbarth, S.; et al. European Society of Clinical Microbiology and Infectious Diseases (ESCMID) guidelines for the treatment of infections caused by multidrug-resistant Gram-negative bacilli (endorsed by European society of intensive care medicine). Clin. Microbiol. Infect. 2022, 28, 521–547. [Google Scholar] [CrossRef]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.R.; Arias, C.A. ESKAPE pathogens: Antimicrobial resistance, epidemiology, clinical impact and therapeutics. Nat. Rev. Microbiol. 2024, 22, 598–616. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Di, L.; Dong, S.; Tian, X.; Huang, D.; Zhao, Y.; Chen, J.; Xia, D.; Wang, S. Whole genome sequencing and genomic characteristics analysis of carbapenem-resistant Acinetobacter baumannii clinical isolates in two hospitals in China. Infect. Genet. Evol. 2024, 123, 105642. [Google Scholar] [CrossRef] [PubMed]
- Jauneikaite, E.; Baker, K.S.; Nunn, J.G.; Midega, J.T.; Hsu, L.Y.; Singh, S.R.; Halpin, A.L.; Hopkins, K.L.; Price, J.R.; Srikantiah, P.; et al. Genomics for antimicrobial resistance surveillance to support infection prevention and control in health-care facilities. Lancet Microbe 2023, 4, e1040–e1046. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.; Nielsen, T.B.; Bonomo, R.A.; Pantapalangkoor, P.; Luna, B.; Spellberg, B. Clinical and Pathophysiological Overview of Acinetobacter Infections: A Century of Challenges. Clin. Microbiol. Rev. 2017, 30, 409–447. [Google Scholar] [CrossRef] [PubMed]
- Hajihashemi, B.; Abbasi, A.; Shokri, D. Emergence of colistin resistant Acinetobacter baumannii clonal complex 2 (CC2) among hospitalized patients in Iran. Acta Microbiol. Immunol. Hung. 2023, 70, 213–219. [Google Scholar] [CrossRef]
- Seifert, H.; Blondeau, J.; Lucassen, K.; Utt, E.A. Global update on the in vitro activity of tigecycline and comparators against isolates of Acinetobacter baumannii and rates of resistant phenotypes (2016–2018). J. Glob. Antimicrob. Resist. 2022, 31, 82–89. [Google Scholar] [CrossRef]
- Attia, N.M.; Elbaradei, A. Fluoroquinolone resistance conferred by gyrA, parC mutations, and AbaQ efflux pump among Acinetobacter baumannii clinical isolates causing ventilator-associated pneumonia. Acta Microbiol. Immunol. Hung. 2019, 67, 234–238. [Google Scholar] [CrossRef]
- Namaki Kheljan, M.; Hassanzadeh, M.; Srdari Jabedar, M.; Mohammadi Gollou, A.; Ashouri, P.; Teimourpour, R.; Arzanlou, M. Characterization of disinfectant susceptibility profiles among clinical isolates of Acinetobacter baumannii in Ardabil, Iran. Acta Microbiol. Immunol. Hung. 2023, 70, 311–317. [Google Scholar] [CrossRef]
- McDonnell, G.; Russell, A.D. Antiseptics and disinfectants: Activity, action, and resistance. Clin. Microbiol. Rev. 1999, 12, 147–179. [Google Scholar] [CrossRef]
- Boyce, J.M. Quaternary ammonium disinfectants and antiseptics: Tolerance, resistance and potential impact on antibiotic resistance. Antimicrob. Resist. Infect. Control 2023, 12, 32. [Google Scholar] [CrossRef]
- Maillard, J.Y.; Centeleghe, I. How biofilm changes our understanding of cleaning and disinfection. Antimicrob. Resist. Infect. Control 2023, 12, 95. [Google Scholar] [CrossRef]
- Amodeo, D.; Manzi, P.; De Palma, I.; Puccio, A.; Nante, N.; Barcaccia, M.; Marini, D.; Pietrella, D. Efficacy of Violet-Blue (405 nm) LED Lamps for Disinfection of High-Environmental-Contact Surfaces in Healthcare Facilities: Leading to the Inactvation of Microorganisms and Reduction of MRSA Contamination. Pathogens 2023, 12, 1338. [Google Scholar] [CrossRef]
- Messina, G.; Rosadini, D.; Burgassi, S.; Messina, D.; Nante, N.; Tani, M.; Cevenini, G. Tanning the bugs—A pilot study of an innovative approach to stethoscope disinfection. J. Hosp. Infect. 2017, 95, 228–230. [Google Scholar] [CrossRef]
- Tanguy, M.; Kouatchet, A.; Tanguy, B.; Pichard, É.; Fanello, S.; Joly-Guillou, M.L. Management of an Acinetobacter baumannii outbreak in an intensive care unit. Med. Mal. Infect. 2017, 47, 409–414. [Google Scholar] [CrossRef]
- Gramatniece, A.; Silamikelis, I.; Zahare, I.; Urtans, V.; Zahare, I.; Dimina, E.; Saule, M.; Balode, A.; Radovica-Spalvina, I.; Klovins, J.; et al. Control of Acinetobacter baumannii outbreak in the neonatal intensive care unit in Latvia: Whole-genome sequencing powered investigation and closure of the ward. Antimicrob. Resist. Infect. Control 2019, 8, 84. [Google Scholar] [CrossRef]
- Ostorhazi, E.; Rozgonyi, F.; Sztodola, A.; Harmos, F.; Kovalszky, I.; Szabo, D.; Knappe, D.; Hoffmann, R.; Cassone, M.; Wade, J.D.; et al. Preclinical advantages of intramuscularly administered peptide A3-APO over existing therapies in Acinetobacter baumannii wound infections. J. Antimicrob. Chemother. 2010, 65, 2416–2422. [Google Scholar] [CrossRef]
- Dancer, S.J. Controlling hospital-acquired infection: Focus on the role of the environment and new technologies for decontamination. Clin. Microbiol. Rev. 2014, 27, 665–690. [Google Scholar] [CrossRef]
- Cassini, A.; Hogberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Cecchini, M.; et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: A population-level modelling analysis. Lancet Infect. Dis. 2019, 19, 56–66. [Google Scholar] [CrossRef]
- Landman, D.; Quale, J.M.; Mayorga, D.; Adedeji, A.; Vangala, K.; Ravishankar, J.; Flores, C.; Brooks, S. Citywide clonal outbreak of multiresistant Acinetobacter baumannii and Pseudomonas aeruginosa in Brooklyn, NY: The preantibiotic era has returned. Arch. Intern. Med. 2002, 162, 1515–1520. [Google Scholar] [CrossRef]
- Firoozeh, F.; Bakhshi, F.; Dadashi, M.; Badmasti, F.; Zibaei, M.; Omidinia, N. Detection of multidrug-resistant Acinetobacter baumannii from burn patients and healthcare workers in Iran. Acta Microbiol. Immunol. Hung. 2023, 70, 22–28. [Google Scholar] [CrossRef]
- Weinberg, S.E.; Villedieu, A.; Bagdasarian, N.; Karah, N.; Teare, L.; Elamin, W.F. Control and management of multidrug resistant Acinetobacter baumannii: A review of the evidence and proposal of novel approaches. Infect. Prev. Pract. 2020, 2, 100077. [Google Scholar] [CrossRef]
- Dijkshoorn, L.; Nemec, A.; Seifert, H. An increasing threat in hospitals: Multidrug-resistant Acinetobacter baumannii. Nat. Rev. Microbiol. 2007, 5, 939–951. [Google Scholar] [CrossRef]
- Tacconelli, E.; Cataldo, M.A.; Dancer, S.J.; De Angelis, G.; Falcone, M.; Frank, U.; Kahlmeter, G.; Pan, A.; Petrosillo, N.; Rodriguez-Bano, J.; et al. ESCMID guidelines for the management of the infection control measures to reduce transmission of multidrug-resistant Gram-negative bacteria in hospitalized patients. Clin. Microbiol. Infect. 2014, 20 (Suppl. S1), 1–55. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for the Prevention and Control of Carbapenem-Resistant Enterobacteriaceae, Acinetobacter baumannii and Pseudomonas aeruginosa in Health Care Facilities; World Health Organization: Geneva, Switzerland, 2017; Available online: https://apps.who.int/iris/handle/10665/259462 (accessed on 27 May 2022).
- Tacconelli, E.; Buhl, M.; Humphreys, H.; Malek, V.; Presterl, E.; Rodriguez-Bano, J.; Vos, M.C.; Zingg, W.; Mutters, N.T. Analysis of the challenges in implementing guidelines to prevent the spread of multidrug-resistant gram-negatives in Europe. BMJ Open 2019, 9, e027683. [Google Scholar] [CrossRef]
- Ben-Chetrit, E.; Wiener-Well, Y.; Lesho, E.; Kopuit, P.; Broyer, C.; Bier, L.; Assous, M.V.; Benenson, S.; Cohen, M.J.; McGann, P.T.; et al. An intervention to control an ICU outbreak of carbapenem-resistant Acinetobacter baumannii: Long-term impact for the ICU and hospital. Crit. Care 2018, 22, 319. [Google Scholar] [CrossRef]
- Gray, A.P.; Allard, R.; Pare, R.; Tannenbaum, T.; Lefebvre, B.; Levesque, S.; Mulvey, M.; Maalouf, L.; Perna, S.; Longtin, Y. Management of a hospital outbreak of extensively drug-resistant Acinetobacter baumannii using a multimodal intervention including daily chlorhexidine baths. J. Hosp. Infect. 2016, 93, 29–34. [Google Scholar] [CrossRef]
- Meschiari, M.; Lopez-Lozano, J.M.; Di Pilato, V.; Gimenez-Esparza, C.; Vecchi, E.; Bacca, E.; Orlando, G.; Franceschini, E.; Sarti, M.; Pecorari, M.; et al. A five-component infection control bundle to permanently eliminate a carbapenem-resistant Acinetobacter baumannii spreading in an intensive care unit. Antimicrob. Resist. Infect. Control 2021, 10, 123. [Google Scholar] [CrossRef]
- Metan, G.; Zarakolu, P.; Otlu, B.; Tekin, I.; Aytac, H.; Bolek, E.C.; Metin, B.C.; Arsava, E.M.; Unal, S. Emergence of colistin and carbapenem-resistant Acinetobacter calcoaceticus-Acinetobacter baumannii (CCR-Acb) complex in a neurological intensive care unit followed by successful control of the outbreak. J. Infect. Public Health 2020, 13, 564–570. [Google Scholar] [CrossRef]
- Valencia-Martin, R.; Gonzalez-Galan, V.; Alvarez-Marin, R.; Cazalla-Foncueva, A.M.; Aldabo, T.; Gil-Navarro, M.V.; Alonso-Araujo, I.; Martin, C.; Gordon, R.; Garcia-Nunez, E.J.; et al. A multimodal intervention program to control a long-term Acinetobacter baumannii endemic in a tertiary care hospital. Antimicrob. Resist. Infect. Control 2019, 8, 199. [Google Scholar] [CrossRef]
- Chung, Y.K.; Kim, J.S.; Lee, S.S.; Lee, J.A.; Kim, H.S.; Shin, K.S.; Park, E.Y.; Kang, B.S.; Lee, H.J.; Kang, H.J. Effect of daily chlorhexidine bathing on acquisition of carbapenem-resistant Acinetobacter baumannii (CRAB) in the medical intensive care unit with CRAB endemicity. Am. J. Infect. Control 2015, 43, 1171–1177. [Google Scholar] [CrossRef]
- Karampatakis, T.; Tsergouli, K.; Iosifidis, E.; Antachopoulos, C.; Karapanagiotou, A.; Karyoti, A.; Gritsi-Gerogianni, N.; Tsakris, A.; Roilides, E. Impact of active surveillance and infection control measures on carbapenem-resistant Gram-negative bacterial colonization and infections in intensive care. J. Hosp. Infect. 2018, 99, 396–404. [Google Scholar] [CrossRef]
- Fan, C.Y.; Lee, W.T.; Hsu, T.C.; Lee, C.H.; Wang, S.P.; Chen, W.S.; Huang, C.H.; Lee, C.C. Effect of chlorhexidine bathing on colonization or infection with Acinetobacter baumannii: A systematic review and meta-analysis. J. Hosp. Infect. 2019, 103, 284–292. [Google Scholar] [CrossRef]
- Enfield, K.B.; Huq, N.N.; Gosseling, M.F.; Low, D.J.; Hazen, K.C.; Toney, D.M.; Slitt, G.; Zapata, H.J.; Cox, H.L.; Lewis, J.D.; et al. Control of Simultaneous Outbreaks of Carbapenemase-Producing Enterobacteriaceae and Extensively Drug-ResistantAcinetobacter baumannii Infection in an Intensive Care Unit Using Interventions Promoted in the Centers for Disease Control and Prevention 2012 Carbapenemase-Resistant Enterobacteriaceae Toolkit. Infect. Control Hosp. Epidemiol. 2014, 35, 810–817. [Google Scholar]
- Eckardt, P.; Canavan, K.; Guran, R.; George, E.; Miller, N.; Avendano, D.H.; Kim, M.; Himed, K.; Ramirez, K.H.G. Containment of a carbapenem-resistant Acinetobacter baumannii complex outbreak in a COVID-19 intensive care unit. Am. J. Infect. Control 2022, 50, 477–481. [Google Scholar] [CrossRef]
- Lerner, A.O.; Abu-Hanna, J.; Carmeli, Y.; Schechner, V. Environmental contamination by carbapenem-resistant Acinetobacter baumannii: The effects of room type and cleaning methods. Infect. Control Hosp. Epidemiol. 2020, 41, 166–171. [Google Scholar] [CrossRef]
- Harris, A.D.; Johnson, J.K.; Pineles, L.; O’Hara, L.M.; Bonomo, R.A.; Thom, K.A. Patient-to-Patient Transmission of Acinetobacter baumannii Gastrointestinal Colonization in the Intensive Care Unit. Antimicrob. Agents Chemother. 2019, 63, e00392-19. [Google Scholar] [CrossRef]
- Cho, O.H.; Bak, M.H.; Baek, E.H.; Park, K.-H.; Kim, S.; Bae, I.G. Successful control of carbapenem-resistant Acinetobacter baumannii in a Korean university hospital: A 6-year perspective. Am. J. Infect. Control 2014, 42, 976–979. [Google Scholar] [CrossRef]
- Perez, S.; Innes, G.K.; Walters, M.; Mehr, J.; Arias, J.; Greeley, R.; Chew, D. Increase in Hospital-Acquired Carbapenem-Resistant Acinetobacter baumannii Infection and Colonization in an Acute Care Hospital during a Surge in COVID-19 Admissions—New Jersey, February–July 2020. MMWR. Morb. Mortal. Wkly. Rep. 2020, 69, 1827–1831. [Google Scholar] [CrossRef]
- Munoz-Price, L.S.; Carling, P.; Cleary, T.; Fajardo-Aquino, Y.; DePascale, D.; Jimenez, A.; Hughes, M.; Namias, N.; Pizano, L.; Kett, D.H.; et al. Control of a two-decade endemic situation with carbapenem-resistant Acinetobacter baumannii: Electronic dissemination of a bundle of interventions. Am. J. Infect. Control 2014, 42, 466–471. [Google Scholar] [CrossRef]
- Medioli, F.; Bacca, E.; Faltoni, M.; Burastero, G.J.; Volpi, S.; Menozzi, M.; Orlando, G.; Bedini, A.; Franceschini, E.; Mussini, C.; et al. Is It Possible to Eradicate Carbapenem-Resistant Acinetobacter baumannii (CRAB) from Endemic Hospitals? Antibiotics 2022, 11, 1015. [Google Scholar] [CrossRef]
- Gomaa, F.A.M.; Helal, Z.H.; Khan, M.I. High Prevalence of blaNDM-1, blaVIM, qacE, and qacEΔ1 genes and their association with decreased susceptibility to antibiotics and common hospital biocides in clinical isolates of Acinetobacter baumannii. Microorganisms 2017, 5, 18. [Google Scholar] [CrossRef]
- Elkhatib, W.F.; Khalil, M.A.F.; Ashour, H.M. Integrons and Antiseptic Resistance Genes Mediate Resistance of Acinetobacter baumannii and Pseudomonas aeruginosa Isolates from Intensive Care Unit Patients with Wound Infections. Curr. Mol. Med. 2019, 19, 286–293. [Google Scholar] [CrossRef]
- Slipski, C.J.; Jamieson-Datzkiw, T.R.; Zhanel, G.G.; Bay, D.C. Characterization of Proteobacterial Plasmid Integron-Encoded qac Efflux Pump Sequence Diversity and Quaternary Ammonium Compound Antiseptic Selection in Escherichia coli Grown Planktonically and as Biofilms. Antimicrob. Agents Chemother. 2021, 65, e0106921. [Google Scholar] [CrossRef]
- Stepanovic, S.; Vukovic, D.; Hola, V.; Di Bonaventura, G.; Djukic, S.; Cirkovic, I.; Ruzicka, F. Quantification of biofilm in microtiter plates: Overview of testing conditions and practical recommendations for assessment of biofilm production by staphylococci. APMIS 2007, 115, 891–899. [Google Scholar] [CrossRef]
- Overbeek, R.; Olson, R.; Pusch, G.D.; Olsen, G.J.; Davis, J.J.; Disz, T.; Edwards, R.A.; Gerdes, S.; Parrello, B.; Shukla, M.; et al. The SEED and the Rapid Annotation of microbial genomes using Subsystems Technology (RAST). Nucleic Acids Res. 2014, 42, D206–D214. [Google Scholar] [CrossRef]
- Grant, J.R.; Enns, E.; Marinier, E.; Mandal, A.; Herman, E.K.; Chen, C.Y.; Graham, M.; Van Domselaar, G.; Stothard, P. Proksee: In-depth characterization and visualization of bacterial genomes. Nucleic Acids Res. 2023, 51, W484–W492. [Google Scholar] [CrossRef]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef]
- Aziz, R.K.; Bartels, D.; Best, A.A.; DeJongh, M.; Disz, T.; Edwards, R.A.; Formsma, K.; Gerdes, S.; Glass, E.M.; Kubal, M.; et al. The RAST Server: Rapid annotations using subsystems technology. BMC Genom. 2008, 9, 75. [Google Scholar] [CrossRef]


| Variables | All Patients (n = 291) |
|---|---|
| Age (years) | 55.4 (38–85) |
| Gender, male | 135 (46%) |
| Neurosurgical disease | |
| CNS tumor | 175 (60%) |
| Subarachnoid hemorrhage | 62 (21%) |
| Other hemorrhages | 22 (46%) |
| Stroke | 32 (7%) |
| Surgical treatment | |
| Neurosurgical operation | 207 (71%) |
| Endovascular treatment | 84 (29%) |
| Underlying diseases | |
| Hypertension | 42 (14%) |
| Diabetes mellitus | 49 (16%) |
| Others | 64 (22%) |
| Infection | |
| Urinary tract infection | 34 (11.6%) |
| Pneumonia | 19 (7%) |
| Wound infection | 10 (3.4%) |
| Bloodstream infection | 7 (2%) |
| Meningitis | 3 (1%) |
| Death | 12 (4%) |
| Patient | Age (Years) | Gender | Underlying Diseases | Surgical Procedure | Multiresistant Bacteria | CRAB-Positive Specimens | Infection/Colonization | Antibiotic Treatment | Duration of Hospital Stay (Days) | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| P1 | 37 | male | intracranial hemorrhage, hypertension | craniotomy | CRAB, MRSA | trachea | colonization | imipenem/cilastatin | 29 | discharged |
| P2 | 63 | male | subarachnoid hemorrhage, hypertension | craniotomy | CRAB, VRE | urine, liquor, anorectal | infection: UTI, meningitis | colomycin, imipenem/cilastatin | 58 | discharged |
| P3 | 48 | female | stroke, hypertension | intervention | CRAB | bloodculture | infection: BSI | colomycin | 12 | discharged |
| P4 | 67 | female | AVM angiomatis, hypertension, diabetes mellitus, COPD | craniotomy | CRAB | trachea | colonization | imipenem/cilastatin, vancomycin, meropenem | 6 | discharged |
| P5 | 76 | female | cerebral metastasis, hypertension, hypothyreosis, breast cancer | craniotomy | CRAB, ESBL-KP, VRE | wound, nasal, bloodculture | infection: SSI, BSI | colomycin, meropenem, vancomycin, linezolid, gentamycin | 36 | exit |
| P6 | 53 | male | subarachnoid hemorrhage, hypertension, diabetes mellitus | craniotomy | CRAB, ESBL-KP, VRE | trachea, bloodculture, anorectal | infection: VAP, BSI | colomycin, ceftriaxone, cefatizidime, imipenem/cilastatin, meropenem, levofloxacin | 38 | discharged |
| Before CRAB Outbreak | During CRAB Outbreak | After CRAB Outbreak | |
|---|---|---|---|
| Enforcement of hand hygiene | |||
| Regular education programs on hand hygiene | yearly | weekly, including circulating staff | every six months |
| Audit of hand hygiene compliance | every six month | daily | weekly |
| Universal contact precautions | |||
| earing gloves, coat, apron, and shoe protector in the patient zone | in case of MDR-positive patients | in case of MDR-positive patients and their contacts | in case of MDR-positive patients and their contacts |
| Wearing of personal protective equipment by HCWs | yes | yes | yes |
| Access restrictions | yes | yes | yes |
| Review of invasive procedures | yes | yes | yes |
| Cohort isolation | |||
| cohort isolation of colonized patient | no | yes | yes |
| Healthcare worker cohorting (HCW), patient:HCW ratio | 3:1 | 2:1 | 3:1 |
| Surveillance cultures | |||
| Passive surveillance: antibiogram monitoring | yes | yes | yes |
| Active surveillance: screening cultures | no | initial (in 24 h) and in every 5 days | initial (in 24 h) and weekly |
| Environmental surveillance cultures | occasionally | once a week | every three months |
| Patient decolonization | |||
| Throat decolonizitation with chlorhexidine digluconate | no | yes | yes |
| Environmental cleaning and disinfection | |||
| Extensive management using checklists under the supervision of the unit manager | no | yes | yes |
| Patient zone | twice a day | three times a day, and necessery | three times a day, and necessery |
| Environment except the patient zone | twice a day | three times a day | three times a day |
| Cleaning personnel | two persons for the unit | three cohorting cleaning persons for the unit | three cohorting cleaning persons for the unit |
| Improved waste management | yes | yes | yes |
| Touchless environmental disinfection systems (e.g., vaporized hydrogen peroxide) | yes | yes | yes |
| Developing a purposeful disinfectant policy | |||
| Disinfection stewardship | no | Disinfection switch | yes |
| Antibiotic stewardship | yes | yes | yes |
| Genomic surveillance | |||
| Whole-genome sequencing | no | yes | no |
| Monitoring and feedback | |||
| Discussions on the performance of the infection-control strategy | monthly | daily | weekly |
| Disinfectants | Acinetobacter baumannii | Acinetobacter baumannii | ||||||
|---|---|---|---|---|---|---|---|---|
| Outbreak CRAB | BAA-1805 | |||||||
| MIC | MBC | MBC/MIC | MBIC | MIC | MBC | MBC/MIC | MBIC | |
| Benzalkonium chloride | 32 | 32 | 1 | 0.5 | 16 | 32 | 2 | <0.06 |
| 2-phenoxyethanol | 0.5 | 0.25 | 2 | 0.0078 | 0.25 | 0.25 | 1 | 0.0078 |
| Chlorhexidine digluconate | 16 | 32 | 2 | 0.25 | 32 | 64 | 2 | 8 |
| Multilocus sequence typing (Pasteur schema) | ST2 |
| Beta-lactamases | blaOXA-83 (OXA-51-like) |
| blaADC-30 | |
| Fluoroquinolone resistance determinants | gyrA: Ser81Leu parC: Ser84Leu |
| Aminoglycoside resistance genes | ant(3″)-II (aminoglycoside nucleotidyltransferase) aph(6)-Id (aminoglycoside O-phosphotransferase) aph(3″)-Ib (aminoglycoside O-phosphotransferase) aac(6’)-Ia (aminoglycoside 6′-N-acetyltransferase) |
| Efflux pumps | adeABC; adeFGH; adeIJK; adeRS; adeL; adeN |
| Disinfectant, antiseptic resistance determinants | qacE; qacEΔ1 |
| Biofilm formation | pgaABCD; csuA/B, csuABCDE; bfmR; bfmS; ompA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kelemen, J.; Sztermen, M.; Dakos, E.K.; Budai, J.; Katona, J.; Szekeressy, Z.; Sipos, L.; Papp, Z.; Stercz, B.; Dunai, Z.A.; et al. Complex Infection-Control Measures with Disinfectant Switch Help the Successful Early Control of Carbapenem-Resistant Acinetobacter baumannii Outbreak in Intensive Care Unit. Antibiotics 2024, 13, 869. https://doi.org/10.3390/antibiotics13090869
Kelemen J, Sztermen M, Dakos EK, Budai J, Katona J, Szekeressy Z, Sipos L, Papp Z, Stercz B, Dunai ZA, et al. Complex Infection-Control Measures with Disinfectant Switch Help the Successful Early Control of Carbapenem-Resistant Acinetobacter baumannii Outbreak in Intensive Care Unit. Antibiotics. 2024; 13(9):869. https://doi.org/10.3390/antibiotics13090869
Chicago/Turabian StyleKelemen, Jozsef, Marton Sztermen, Eva Krisztina Dakos, Jozsef Budai, Jozsef Katona, Zsuzsanna Szekeressy, Laszlo Sipos, Zoltan Papp, Balazs Stercz, Zsuzsanna A. Dunai, and et al. 2024. "Complex Infection-Control Measures with Disinfectant Switch Help the Successful Early Control of Carbapenem-Resistant Acinetobacter baumannii Outbreak in Intensive Care Unit" Antibiotics 13, no. 9: 869. https://doi.org/10.3390/antibiotics13090869







