Silver Nanoparticles: A Versatile Tool Against Infectious and Non-Infectious Diseases
Abstract
1. Introduction
2. AgNP Synthesis
3. AgNPs in Infectious Pathologies
3.1. Antimicrobial Effects
3.1.1. Effects of AgNPs Against Bacteria
3.1.2. Activity of AgNPs Against Fungus
3.1.3. Activity of AgNPs Against Protozoa
3.1.4. Activity of AgNPs Against Viruses
- Temporary interactions between antiviral agents, like AgNPs, and viral surface ligands or host cell receptors, which sterically hinder virion–cell interactions
- Competitive binding of AgNPs to cellular receptor sites, effectively clocking these regions and preventing viral adhesion [41].
3.2. AgNPs to Combat Antibiotic Resistance
3.3. Anti-Biofilm Activity of AgNPs
4. AgNPs in Non-Infectious Pathologies
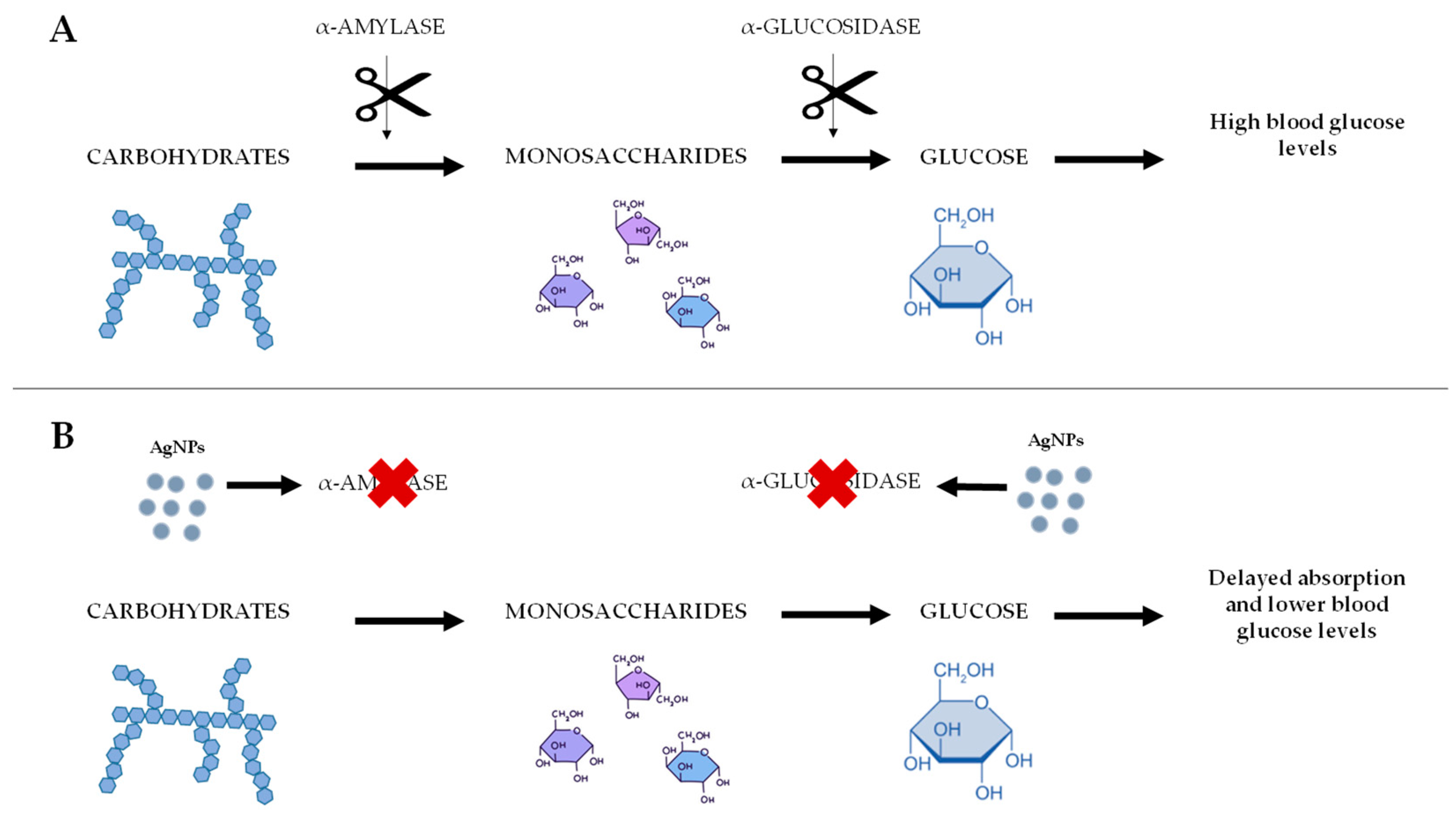
4.1. Diabetes
4.2. Wound Healing
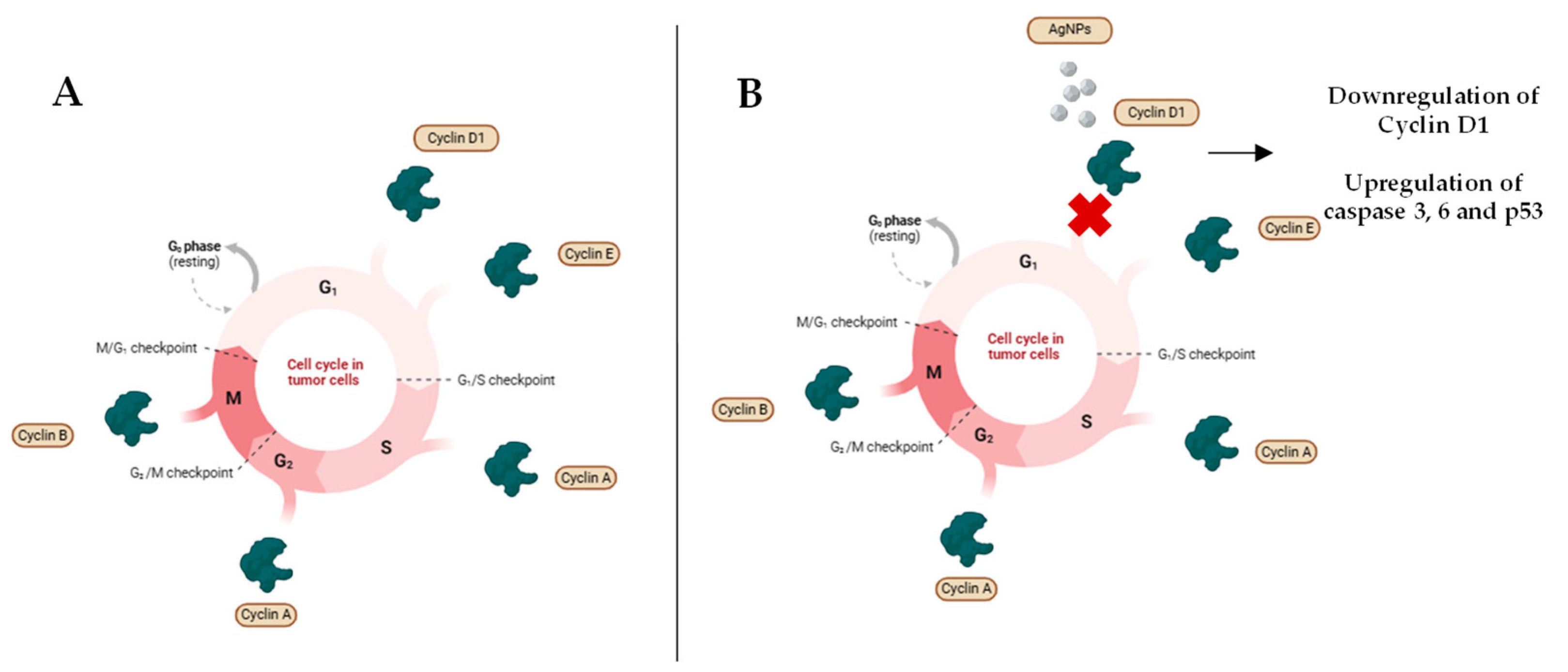
4.3. Cancer
4.3.1. Antitumor Properties of AgNPs
4.3.2. Combination of AgNPs with Chemotherapy and Radiotherapy
5. Biosensing
6. Toxicity of AgNPs for the Environment and Human Beings
- Specificity: Traditional antimicrobials, while designed to disrupt specific bacterial processes, can also damage the beneficial microbiota in the body. This disruption can lead to the proliferation of already-present harmful bacteria, the overgrowth of pathogens with acquired resistance, and ultimately, a higher risk of infection [159]. AgNPs, while having broad-spectrum antimicrobial activity, can also induce oxidative stress and affect healthy cells [160].The off-target toxicity of AgNPs in biological systems has been extensively documented in recent studies [161,162,163,164]. It has been shown that AgNPs disrupt tight junction integrity (particularly occludin and zonulin proteins) in epithelial tissues, compromising epithelial barrier functionality [124]. AgNPs have been shown to alter the regulation of gene expression associated with motor neuron pathologies, neurodegenerative conditions, and immune cell functionality. For instance, they can be toxic to macrophages and monocytes [151,165]. Furthermore, AgNPs significantly modify cardiomyocytes’ contractility through interference with ion channel function and the disruption of key structural proteins [166].In some studies, the effect of repeated low-dose exposure to AgNPs has been studied. Other investigations have been conducted using high doses and short exposure durations. From an exposure perspective, acute protocols more closely simulate accidental and acute exposure scenarios, whereas chronic regimens largely correspond to occupational exposure scenarios [165,167,168].
- Therapeutic Monitoring: Therapeutic drug monitoring is less studied for AgNPs compared to traditional antimicrobials, making it harder to optimize AgNP dosage to minimize toxicity. The analysis of therapeutic drug monitoring of traditional antimicrobial agents helps to reduce their toxicity, although suboptimal concentrations may promote the development of resistance. In contrast, AgNPs demonstrate sustained antimicrobial activity at levels compatible with host cell viability [68]. The toxicity of AgNPs correlates with the particles’ size, agglomeration, and coating [172].
- Antimicrobial Activity: Antimicrobials are among the most prevalent agents responsible for drug-induced hepatic and renal injuries, many of which are dose-dependent, indicating that the toxicity is linked to the amount of administered drug. These agents frequently target specific bacterial pathways, although they also produce collateral damage to host cellular structures. For example, metronidazole causes the fragmentation of DNA and proteins. Some antimicrobial agents, such as aminoglycosides, are related to nephrotoxicity, while others, like fluoroquinolones, generate neurotoxicity, and some, like linezolid, even cause hematotoxicity [173]. On the contrary, AgNPs demonstrate sustained antimicrobial activity at levels compatible with host cell viability. For instance, serum-capped silver nanoparticles have shown high antimicrobial activity and a wide margin of safety for mammalian cells in a mice model [174].
7. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- González-Fernández, S.; Lozano-Iturbe, V.; García, B.; Andrés, L.J.; Menéndez, M.F.; Rodríguez, D.; Vazquez, F.; Martín, C.; Quirós, L.M. Antibacterial Effect of Silver Nanorings. BMC Microbiol. 2020, 20, 172. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, A.P.G.B.; Müller, N.; Sant’Anna, C. Applications of Silver Nanoparticles in Patent Research. Recent Pat. Nanotechnol. 2024, 18, 361–373. [Google Scholar] [CrossRef]
- Ma, C.; Liu, B.; Du, L.; Liu, W.; Zhu, Y.; Chen, T.; Wang, Z.; Chen, H.; Pang, Y. Green Preparation and Antibacterial Activity Evaluation of AgNPs-Blumea Balsamifera Oil Nanoemulsion. Molecules 2024, 29, 2009. [Google Scholar] [CrossRef]
- Abedini, S.; Pourseyedi, S.; Zolala, J.; Mohammadi, H.; Abdolshahi, R. Green Synthesis of Superparamagnetic Iron Oxide and Silver Nanoparticles in Satureja Hortensis Leave Extract: Evaluation of Antifungal Effects on Botryosphaeriaceae Species. Curr. Microbiol. 2024, 81, 149. [Google Scholar] [CrossRef]
- Padzik, M.; Chomicz, L.; Bluszcz, J.; Maleszewska, K.; Grobelny, J.; Conn, D.B.; Hendiger, E.B. Tannic Acid-Modified Silver Nanoparticles in Conjunction with Contact Lens Solutions Are Useful for Progress against the Adhesion of Acanthamoeba spp. to Contact Lenses. Microorganisms 2022, 10, 1076. [Google Scholar] [CrossRef] [PubMed]
- Saadh, M.J. Silver Nanoparticles Inhibit Goatpox Virus Replication. Arch. Virol. 2023, 168, 32. [Google Scholar] [CrossRef]
- Menichetti, A.; Mavridi-Printezi, A.; Mordini, D.; Montalti, M. Effect of Size, Shape and Surface Functionalization on the Antibacterial Activity of Silver Nanoparticles. J. Funct. Biomater. 2023, 14, 244. [Google Scholar] [CrossRef]
- Matras, E.; Gorczyca, A.; Przemieniecki, S.W.; Oćwieja, M. Surface Properties-Dependent Antifungal Activity of Silver Nanoparticles. Sci. Rep. 2022, 12, 18046. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Burmistrov, D.E.; Smirnova, V.V.; Semenova, A.A.; Lisitsyn, A.B. A Mini Review of Antibacterial Properties of Al2O3 Nanoparticles. Nanomaterials 2022, 12, 2635. [Google Scholar] [CrossRef] [PubMed]
- Garibo Ruiz, D.; Nefedova, E.; Shkil, N.N.; Shkil, N.A.; Vazquez-Gomez, R.L.; Pestryakov, A.; Bogdanchikova, N. Silver Nanoparticles Targeting the Drug Resistance Problem of Streptococcus Dysgalactiae: Susceptibility to Antibiotics and Efflux Effect. Int. J. Mol. Sci. 2022, 23, 6024. [Google Scholar] [CrossRef]
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver Nanoparticles and Their Antibacterial Applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef]
- Lange, A.; Grzenia, A.; Wierzbicki, M.; Strojny-Cieslak, B.; Kalińska, A.; Gołębiewski, M.; Radzikowski, D.; Sawosz, E.; Jaworski, S. Silver and Copper Nanoparticles Inhibit Biofilm Formation by Mastitis Pathogens. Animals 2021, 11, 1884. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Xu, X.; Han, H.; Dai, J.; Sun, R.; Yang, L.; Xie, J.; Wang, Y. Preparation of Triangular Silver Nanoparticles and Their Biological Effects in the Treatment of Ovarian Cancer. J. Ovarian Res. 2022, 15, 121. [Google Scholar] [CrossRef]
- El-Baz, Y.G.; Moustafa, A.; Ali, M.A.; El-Desoky, G.E.; Wabaidur, S.M.; Iqbal, A. Green Synthesized Silver Nanoparticles for the Treatment of Diabetes and the Related Complications of Hyperlipidemia and Oxidative Stress in Diabetic Rats. Exp. Biol. Med. 2023, 248, 2237–2248. [Google Scholar] [CrossRef] [PubMed]
- Camerlingo, C. Topical Silver Nanoparticles Reduced with Ethylcellulose Enhance Skin Wound Healing. Eur. Rev. 2023, 27, 744–754. [Google Scholar]
- Khan, S.; Zahoor, M.; Sher Khan, R.; Ikram, M.; Islam, N.U. The Impact of Silver Nanoparticles on the Growth of Plants: The Agriculture Applications. Heliyon 2023, 9, e16928. [Google Scholar] [CrossRef]
- Abbas, R.; Luo, J.; Qi, X.; Naz, A.; Khan, I.A.; Liu, H.; Yu, S.; Wei, J. Silver Nanoparticles: Synthesis, Structure, Properties and Applications. Nanomaterials 2024, 14, 1425. [Google Scholar] [CrossRef]
- dos Santos, M.A.; Paterno, L.G.; Moreira, S.G.C.; Sales, M.J.A. Original Photochemical Synthesis of Ag Nanoparticles Mediated by Potato Starch. SN Appl. Sci. 2019, 1, 554. [Google Scholar] [CrossRef]
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.V.; Zolfaghari, B. Synthesis of Silver Nanoparticles: Chemical, Physical and Biological Methods. Res. Pharm. Sci. 2014, 9, 385–406. [Google Scholar]
- Lavecchia, R.; García-Martínez, J.B.; Contreras-Ropero, J.E.; Barajas-Solano, A.F.; Zuorro, A. Antibacterial and Photocatalytic Applications of Silver Nanoparticles Synthesized from Lacticaseibacillus Rhamnosus. Int. J. Mol. Sci. 2024, 25, 11809. [Google Scholar] [CrossRef]
- Joshi, A.S.; Singh, P.; Mijakovic, I. Interactions of Gold and Silver Nanoparticles with Bacterial Biofilms: Molecular Interactions behind Inhibition and Resistance. Int. J. Mol. Sci. 2020, 21, 7658. [Google Scholar] [CrossRef]
- Tariq, M.; Mohammad, K.N.; Ahmed, B.; Siddiqui, M.A.; Lee, J. Biological Synthesis of Silver Nanoparticles and Prospects in Plant Disease Management. Molecules 2022, 27, 4754. [Google Scholar] [CrossRef] [PubMed]
- Arshad, F.; Naikoo, G.A.; Hassan, I.U.; Chava, S.R.; El-Tanani, M.; Aljabali, A.A.; Tambuwala, M.M. Bioinspired and Green Synthesis of Silver Nanoparticles for Medical Applications: A Green Perspective. Appl. Biochem. Biotechnol. 2024, 196, 3636–3669. [Google Scholar] [CrossRef] [PubMed]
- More, P.R.; Pandit, S.; Filippis, A.D.; Franci, G.; Mijakovic, I.; Galdiero, M. Silver Nanoparticles: Bactericidal and Mechanistic Approach against Drug Resistant Pathogens. Microorganisms 2023, 11, 369. [Google Scholar] [CrossRef]
- Vasiliev, G.; Kubo, A.-L.; Vija, H.; Kahru, A.; Bondar, D.; Karpichev, Y.; Bondarenko, O. Synergistic Antibacterial Effect of Copper and Silver Nanoparticles and Their Mechanism of Action. Sci. Rep. 2023, 13, 9202. [Google Scholar] [CrossRef]
- Davies, K.J. Oxidative Stress, Antioxidant Defenses, and Damage Removal, Repair, and Replacement Systems. IUBMB Life 2000, 50, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Mammari, N.; Lamouroux, E.; Boudier, A.; Duval, R.E. Current Knowledge on the Oxidative-Stress-Mediated Antimicrobial Properties of Metal-Based Nanoparticles. Microorganisms 2022, 10, 437. [Google Scholar] [CrossRef]
- Frippiat, T.; Art, T.; Delguste, C. Silver Nanoparticles as Antimicrobial Agents in Veterinary Medicine: Current Applications and Future Perspectives. Nanomaterials 2025, 15, 202. [Google Scholar] [CrossRef]
- Vahabirad, M.; Daei, S.; Abbasalipourkabir, R.; Ziamajidi, N. Anticancer Action of Silver Nanoparticles in SKBR3 Breast Cancer Cells through Promotion of Oxidative Stress and Apoptosis. BioMed Res. Int. 2024, 2024, 7145339. [Google Scholar] [CrossRef]
- Hsin, Y.-H.; Chen, C.-F.; Huang, S.; Shih, T.-S.; Lai, P.-S.; Chueh, P.J. The Apoptotic Effect of Nanosilver Is Mediated by a ROS- and JNK-Dependent Mechanism Involving the Mitochondrial Pathway in NIH3T3 Cells. Toxicol. Lett. 2008, 179, 130–139. [Google Scholar] [CrossRef]
- Hamida, R.S.; Albasher, G.; Bin-Meferij, M.M. Oxidative Stress and Apoptotic Responses Elicited by Nostoc-Synthesized Silver Nanoparticles against Different Cancer Cell Lines. Cancers 2020, 12, 2099. [Google Scholar] [CrossRef]
- Radhakrishnan, V.S.; Mudiam, M.K.R.; Kumar, M.; Dwivedi, S.P.; Singh, S.P.; Prasad, T. Silver Nanoparticles Induced Alterations in Multiple Cellular Targets, Which Are Critical for Drug Susceptibilities and Pathogenicity in Fungal Pathogen (Candida albicans). Int. J. Nanomedicine 2018, 13, 2647–2663. [Google Scholar] [CrossRef] [PubMed]
- Kumari, M.; Giri, V.P.; Pandey, S.; Kumar, M.; Katiyar, R.; Nautiyal, C.S.; Mishra, A. An Insight into the Mechanism of Antifungal Activity of Biogenic Nanoparticles than Their Chemical Counterparts. Pestic. Biochem. Physiol. 2019, 157, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Al-Otibi, F.; Perveen, K.; Al-Saif, N.A.; Alharbi, R.I.; Bokhari, N.A.; Albasher, G.; Al-Otaibi, R.M.; Al-Mosa, M.A. Biosynthesis of Silver Nanoparticles Using Malva Parviflora and Their Antifungal Activity. Saudi J. Biol. Sci. 2021, 28, 2229–2235. [Google Scholar] [CrossRef] [PubMed]
- Mussin, J.; Giusiano, G. Biogenic Silver Nanoparticles as Antifungal Agents. Front. Chem. 2022, 10, 1023542. [Google Scholar] [CrossRef]
- Barros, D.; Pradhan, A.; Pascoal, C.; Cássio, F. Transcriptomics Reveals the Action Mechanisms and Cellular Targets of Citrate-Coated Silver Nanoparticles in a Ubiquitous Aquatic Fungus. Environ. Pollut. 2021, 268, 115913. [Google Scholar] [CrossRef]
- Awad, M.A.; Al Olayan, E.M.; Siddiqui, M.I.; Merghani, N.M.; Alsaif, S.S.A.; Aloufi, A.S. Antileishmanial Effect of Silver Nanoparticles: Green Synthesis, Characterization, in Vivo and in Vitro Assessment. Biomed. Pharmacother. 2021, 137, 111294. [Google Scholar] [CrossRef]
- Pimentel-Acosta, C.A.; Ramírez-Salcedo, J.; Morales-Serna, F.N.; Fajer-Ávila, E.J.; Chávez-Sánchez, C.; Lara, H.H.; García-Gasca, A. Molecular Effects of Silver Nanoparticles on Monogenean Parasites: Lessons from Caenorhabditis Elegans. Int. J. Mol. Sci. 2020, 21, 5889. [Google Scholar] [CrossRef]
- González-Fernández, S.; Lozano-Iturbe, V.; Menéndez, M.F.; Ordiales, H.; Fernández-Vega, I.; Merayo, J.; Vazquez, F.; Quirós, L.M.; Martín, C. A Promising Antifungal and Antiamoebic Effect of Silver Nanorings, a Novel Type of AgNP. Antibiotics 2022, 11, 1054. [Google Scholar] [CrossRef]
- Luceri, A.; Francese, R.; Lembo, D.; Ferraris, M.; Balagna, C. Silver Nanoparticles: Review of Antiviral Properties, Mechanism of Action and Applications. Microorganisms 2023, 11, 629. [Google Scholar] [CrossRef]
- Orłowski, P.; Kowalczyk, A.; Tomaszewska, E.; Ranoszek-Soliwoda, K.; Węgrzyn, A.; Grzesiak, J.; Celichowski, G.; Grobelny, J.; Eriksson, K.; Krzyzowska, M. Antiviral Activity of Tannic Acid Modified Silver Nanoparticles: Potential to Activate Immune Response in Herpes Genitalis. Viruses 2018, 10, 524. [Google Scholar] [CrossRef]
- Galdiero, S.; Falanga, A.; Vitiello, M.; Cantisani, M.; Marra, V.; Galdiero, M. Silver Nanoparticles as Potential Antiviral Agents. Molecules 2011, 16, 8894–8918. [Google Scholar] [CrossRef] [PubMed]
- Rios-Ibarra, C.P.; Salinas-Santander, M.; Orozco-Nunnelly, D.A.; Bravo-Madrigal, J. Nanoparticle-based Antiviral Strategies to Combat the Influenza Virus (Review). Biomed. Rep. 2024, 20, 65. [Google Scholar] [CrossRef] [PubMed]
- Pilaquinga, F.; Morey, J.; Torres, M.; Seqqat, R.; Piña, M. de las N. Silver Nanoparticles as a Potential Treatment against SARS-CoV-2: A Review. WIREs Nanomed. Nanobiotechnology 2021, 13, e1707. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, U.; Sayef Ahammed, K.; Mishra, S.; Bhaumik, A. The Emerging Roles of Silver Nanoparticles to Target Viral Life Cycle and Detect Viral Pathogens. Chem. Asian J. 2022, 17, e202101149. [Google Scholar] [CrossRef]
- Alavi, M.; Kamarasu, P.; McClements, D.J.; Moore, M.D. Metal and Metal Oxide-Based Antiviral Nanoparticles: Properties, Mechanisms of Action, and Applications. Adv. Colloid Interface Sci. 2022, 306, 102726. [Google Scholar] [CrossRef]
- Janik, S.; Grela, E.; Stączek, S.; Zdybicka-Barabas, A.; Luchowski, R.; Gruszecki, W.I.; Grudzinski, W. Amphotericin B-Silver Hybrid Nanoparticles Help to Unveil the Mechanism of Biological Activity of the Antibiotic: Disintegration of Cell Membranes. Molecules 2023, 28, 4687. [Google Scholar] [CrossRef] [PubMed]
- Yassin, M.T.; Mostafa, A.A.-F.; Al-Askar, A.A.; Al-Otibi, F.O. Synergistic Antifungal Efficiency of Biogenic Silver Nanoparticles with Itraconazole against Multidrug-Resistant Candidal Strains. Crystals 2022, 12, 816. [Google Scholar] [CrossRef]
- Rashidi, S.; Mansouri, R.; Ali-Hassanzadeh, M.; Muro, A.; Nguewa, P.; Manzano-Román, R. The Defensive Interactions of Prominent Infectious Protozoan Parasites: The Host’s Complement System. Biomolecules 2022, 12, 1564. [Google Scholar] [CrossRef]
- Tarannum, A.; Rodríguez-Almonacid, C.C.; Salazar-Bravo, J.; Karamysheva, Z.N. Molecular Mechanisms of Persistence in Protozoan Parasites. Microorganisms 2023, 11, 2248. [Google Scholar] [CrossRef]
- Sinclair, T.R.; van den Hengel, S.K.; Raza, B.G.; Rutjes, S.A.; de Roda Husman, A.M.; Peijnenburg, W.J.G.M.; Roesink, H.D.W.; de Vos, W.M. Surface Chemistry-Dependent Antiviral Activity of Silver Nanoparticles. Nanotechnology 2021, 32, 365101. [Google Scholar] [CrossRef]
- Ciriminna, R.; Albo, Y.; Pagliaro, M. New Antivirals and Antibacterials Based on Silver Nanoparticles. ChemMedChem 2020, 15, 1619–1623. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Ko, Y.-S.; Lee, S.J.; Lee, C.; Woo, K.; Ko, G. Inactivation of Influenza A Virus via Exposure to Silver Nanoparticle-Decorated Silica Hybrid Composites. Environ. Sci. Pollut. Res. 2018, 25, 27021–27030. [Google Scholar] [CrossRef]
- Yoon, B.K.; Jeon, W.-Y.; Sut, T.N.; Cho, N.-J.; Jackman, J.A. Stopping Membrane-Enveloped Viruses with Nanotechnology Strategies: Toward Antiviral Drug Development and Pandemic Preparedness. ACS Nano 2021, 15, 125–148. [Google Scholar] [CrossRef]
- Rheinemann, L.; Sundquist, W.I. Virus Budding. Encycl. Virol. 2021, 1, 519–528. [Google Scholar] [CrossRef]
- Jeremiah, S.S.; Miyakawa, K.; Morita, T.; Yamaoka, Y.; Ryo, A. Potent Antiviral Effect of Silver Nanoparticles on SARS-CoV-2. Biochem. Biophys. Res. Commun. 2020, 533, 195–200. [Google Scholar] [CrossRef]
- Lara, H.H.; Garza-Treviño, E.N.; Ixtepan-Turrent, L.; Singh, D.K. Silver Nanoparticles Are Broad-Spectrum Bactericidal and Virucidal Compounds. J. Nanobiotechnol. 2011, 9, 30. [Google Scholar] [CrossRef] [PubMed]
- Nefedova, A.; Rausalu, K.; Zusinaite, E.; Vanetsev, A.; Rosenberg, M.; Koppel, K.; Lilla, S.; Visnapuu, M.; Smits, K.; Kisand, V.; et al. Antiviral Efficacy of Cerium Oxide Nanoparticles. Sci. Rep. 2022, 12, 18746. [Google Scholar] [CrossRef] [PubMed]
- Wong, F.; Zheng, E.J.; Valeri, J.A.; Donghia, N.M.; Anahtar, M.N.; Omori, S.; Li, A.; Cubillos-Ruiz, A.; Krishnan, A.; Jin, W.; et al. Discovery of a Structural Class of Antibiotics with Explainable Deep Learning. Nature 2024, 626, 177–185. [Google Scholar] [CrossRef]
- Baran, A.; Kwiatkowska, A.; Potocki, L. Antibiotics and Bacterial Resistance—A Short Story of an Endless Arms Race. Int. J. Mol. Sci. 2023, 24, 5777. [Google Scholar] [CrossRef]
- Shukla, R.; Peoples, A.J.; Ludwig, K.C.; Maity, S.; Derks, M.G.N.; Benedetti, S.D.; Krueger, A.M.; Vermeulen, B.J.A.; Harbig, T.; Lavore, F.; et al. An Antibiotic from an Uncultured Bacterium Binds to an Immutable Target. Cell 2023, 186, 4059–4073.e27. [Google Scholar] [CrossRef]
- Alhajjar, R.K.; Roche, K.M.; Techtmann, S.M. Comparative Analysis of the Mechanism of Resistance to Silver Nanoparticles and the Biocide 2,2-Dibromo-3-Nitrilopropionamide. Antimicrob. Agents Chemother. 2022, 66, e02031-21. [Google Scholar] [CrossRef]
- Gaurav, A.; Bakht, P.; Saini, M.; Pandey, S.; Pathania, R. Role of Bacterial Efflux Pumps in Antibiotic Resistance, Virulence, and Strategies to Discover Novel Efflux Pump Inhibitors. Microbiology 2023, 169, 001333. [Google Scholar] [CrossRef]
- Lei, Z.; Karim, A. The Challenges and Applications of Nanotechnology against Bacterial Resistance. J. Vet. Pharmacol. Ther. 2021, 44, 281–297. [Google Scholar] [CrossRef]
- Dove, A.S.; Dzurny, D.I.; Dees, W.R.; Qin, N.; Nunez Rodriguez, C.C.; Alt, L.A.; Ellward, G.L.; Best, J.A.; Rudawski, N.G.; Fujii, K.; et al. Silver Nanoparticles Enhance the Efficacy of Aminoglycosides against Antibiotic-Resistant Bacteria. Front. Microbiol. 2023, 13, 1064095. [Google Scholar] [CrossRef]
- Tiwari, S.; Jamal, S.B.; Hassan, S.S.; Carvalho, P.V.S.D.; Almeida, S.; Barh, D.; Ghosh, P.; Silva, A.; Castro, T.L.P.; Azevedo, V. Two-Component Signal Transduction Systems of Pathogenic Bacteria As Targets for Antimicrobial Therapy: An Overview. Front. Microbiol. 2017, 8, 1878. [Google Scholar] [CrossRef]
- Ozdal, M.; Gurkok, S. Recent Advances in Nanoparticles as Antibacterial Agent. ADMET DMPK 2022, 10, 115–129. [Google Scholar] [CrossRef]
- Rodrigues, A.S.; Batista, J.G.S.; Rodrigues, M.Á.V.; Thipe, V.C.; Minarini, L.A.R.; Lopes, P.S.; Lugão, A.B. Advances in Silver Nanoparticles: A Comprehensive Review on Their Potential as Antimicrobial Agents and Their Mechanisms of Action Elucidated by Proteomics. Front. Microbiol. 2024, 15, 1440065. [Google Scholar] [CrossRef]
- Alavi, M.; Hamblin, M.R. Antibacterial Silver Nanoparticles: Effects on Bacterial Nucleic Acids. Cell. Mol. Biomed. Rep. 2023, 3, 35–40. [Google Scholar] [CrossRef]
- Salas-Orozco, M.F.; Niño-Martínez, N.; Martínez-Castañón, G.-A.; Méndez, F.T.; Morán, G.M.M.; Bendaña-Piñeiro, A.E.; Ruiz, F.; Bach, H. Proteomic Analysis of an Enterococcus Faecalis Mutant Generated against the Exposure to Silver Nanoparticles. J. Appl. Microbiol. 2022, 132, 244–255. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Lin, F.; Ling, B. In Vitro Activity of Biofilm Inhibitors in Combination with Antibacterial Drugs against Extensively Drug-Resistant Acinetobacter Baumannii. Sci. Rep. 2020, 10, 18097. [Google Scholar] [CrossRef] [PubMed]
- Mann, R.; Holmes, A.; McNeilly, O.; Cavaliere, R.; Sotiriou, G.A.; Rice, S.A.; Gunawan, C. Evolution of Biofilm-Forming Pathogenic Bacteria in the Presence of Nanoparticles and Antibiotic: Adaptation Phenomena and Cross-Resistance. J. Nanobiotechnology 2021, 19, 291. [Google Scholar] [CrossRef]
- Zhao, A.; Sun, J.; Liu, Y. Understanding Bacterial Biofilms: From Definition to Treatment Strategies. Front. Cell. Infect. Microbiol. 2023, 13, 1137947. [Google Scholar] [CrossRef]
- Xu, R.-Z.; Cao, J.-S.; Feng, G.; Luo, J.-Y.; Wu, Y.; Ni, B.-J.; Fang, F. Modeling Molecular Structure and Behavior of Microbial Extracellular Polymeric Substances through Interacting-Particle Reaction Dynamics. Chem. Eng. J. Adv. 2021, 8, 100154. [Google Scholar] [CrossRef]
- Singh, S.; Datta, S.; Narayanan, K.B.; Rajnish, K.N. Bacterial Exo-Polysaccharides in Biofilms: Role in Antimicrobial Resistance and Treatments. J. Genet. Eng. Biotechnol. 2021, 19, 140. [Google Scholar] [CrossRef]
- Seebach, E.; Elschner, T.; Kraus, F.V.; Souto-Carneiro, M.; Kubatzky, K.F. Bacterial and Metabolic Factors of Staphylococcal Planktonic and Biofilm Environments Differentially Regulate Macrophage Immune Activation. Inflammation 2023, 46, 1512–1530. [Google Scholar] [CrossRef]
- Sahreen, S.; Mukhtar, H.; Imre, K.; Morar, A.; Herman, V.; Sharif, S. Exploring the Function of Quorum Sensing Regulated Biofilms in Biological Wastewater Treatment: A Review. Int. J. Mol. Sci. 2022, 23, 9751. [Google Scholar] [CrossRef]
- Rather, M.A.; Gupta, K.; Mandal, M. Microbial Biofilm: Formation, Architecture, Antibiotic Resistance, and Control Strategies. Braz. J. Microbiol. 2021, 52, 1701–1718. [Google Scholar] [CrossRef]
- Mačák, L.; Velgosova, O.; Múdra, E.; Vojtko, M.; Dolinská, S. Transfer of AgNPs’ Anti-Biofilm Activity into the Nontoxic Polymer Matrix. Polymers 2023, 15, 1238. [Google Scholar] [CrossRef]
- Estevez, M.B.; Raffaelli, S.; Mitchell, S.G.; Faccio, R.; Alborés, S. Biofilm Eradication Using Biogenic Silver Nanoparticles. Molecules 2020, 25, 2023. [Google Scholar] [CrossRef]
- Vashistha, A.; Sharma, N.; Nanaji, Y.; Kumar, D.; Singh, G.; Barnwal, R.P.; Yadav, A.K. Quorum Sensing Inhibitors as Therapeutics: Bacterial Biofilm Inhibition. Bioorganic Chem. 2023, 136, 106551. [Google Scholar] [CrossRef]
- Wang, Y.; Bian, Z.; Wang, Y. Biofilm Formation and Inhibition Mediated by Bacterial Quorum Sensing. Appl. Microbiol. Biotechnol. 2022, 106, 6365–6381. [Google Scholar] [CrossRef]
- Kumaraguru, G.; Moorthy, S.; Krishna Kumar, A.K.; Malaiyandi, J. Chapter 14-Lipidomics Profiling of Microbial Biofilm. In Microbial Biofilms; Sarma, H., Joshi, S., Lahiri, D., Ray, R.R., Davoodbasha, M., Eds.; Advances in Biotechnology and Bioengineering; Academic Press: Cambridge, MA, USA, 2023; pp. 225–233. ISBN 978-0-323-95715-1. [Google Scholar]
- Limoli, D.H.; Jones, C.J.; Wozniak, D.J. Bacterial Extracellular Polysaccharides in Biofilm Formation and Function. Microbiol. Spectr. 2015, 3, 223–247. [Google Scholar] [CrossRef]
- Castro-Valenzuela, B.E.; Franco-Molina, M.A.; Zárate-Triviño, D.G.; Villarreal-Treviño, L.; Kawas, J.R.; García-Coronado, P.L.; Sobrevilla-Hernández, G.; Rodríguez-Padilla, C. Antibacterial Efficacy of Novel Bismuth-Silver Nanoparticles Synthesis on Staphylococcus aureus and Escherichia coli Infection Models. Front. Microbiol. 2024, 15, 1376669. [Google Scholar] [CrossRef]
- Loustau, E.; Leflaive, J.; Boscus, C.; Amalric, Q.; Ferriol, J.; Oleinikova, O.; Pokrovsky, O.S.; Girbal-Neuhauser, E.; Rols, J.-L. The Response of Extracellular Polymeric Substances Production by Phototrophic Biofilms to a Sequential Disturbance Strongly Depends on Environmental Conditions. Front. Microbiol. 2021, 12, 742027. [Google Scholar] [CrossRef]
- Silva, V.; Pereira, J.E.; Maltez, L.; Poeta, P.; Igrejas, G. Influence of Environmental Factors on Biofilm Formation of Staphylococci Isolated from Wastewater and Surface Water. Pathogens 2022, 11, 1069. [Google Scholar] [CrossRef]
- Su, Y.; Yrastorza, J.T.; Matis, M.; Cusick, J.; Zhao, S.; Wang, G.; Xie, J. Biofilms: Formation, Research Models, Potential Targets, and Methods for Prevention and Treatment. Adv. Sci. Weinh. Baden-Wurtt. Ger. 2022, 9, e2203291. [Google Scholar] [CrossRef]
- Hu, L.; Yang, X.; Yin, J.; Rong, X.; Huang, X.; Yu, P.; He, Z.; Liu, Y. Combination of AgNPs and Domiphen Is Antimicrobial Against Biofilms of Common Pathogens. Int. J. Nanomed. 2021, 16, 7181–7194. [Google Scholar] [CrossRef]
- Manasherob, R.; Mooney, J.A.; Lowenberg, D.W.; Bollyky, P.L.; Amanatullah, D.F. Tolerant Small-Colony Variants Form Prior to Resistance Within a Staphylococcus Aureus Biofilm Based on Antibiotic Selective Pressure. Clin. Orthop. Relat. Res. 2021, 479, 1471. [Google Scholar] [CrossRef]
- Domingo, G.; Villa, F.; Vannini, C.; Garuglieri, E.; Onelli, E.; Bracale, M.; Cappitelli, F. Label-Free Proteomic Approach to Study the Non-Lethal Effects of Silver Nanoparticles on a Gut Bacterium. Front. Microbiol. 2019, 10, 2709. [Google Scholar] [CrossRef]
- Yang, Y.; Alvarez, P.J.J. Sublethal Concentrations of Silver Nanoparticles Stimulate Biofilm Development. Environ. Sci. Technol. Lett. 2015, 2, 221–226. [Google Scholar] [CrossRef]
- da Cunha, K.F.; Albernaz, D.T.F.; Garcia, M.d.O.; Allend, S.O.; Hartwig, D.D. Silver Nanoparticles (AgNPs) in the Control of Staphylococcus Spp. Lett. Appl. Microbiol. 2023, 76, ovac032. [Google Scholar] [CrossRef]
- Valentin, E.; Bottomley, A.L.; Chilambi, G.S.; Harry, E.J.; Amal, R.; Sotiriou, G.A.; Rice, S.A.; Gunawan, C. Heritable Nanosilver Resistance in Priority Pathogen: A Unique Genetic Adaptation and Comparison with Ionic Silver and Antibiotics. Nanoscale 2020, 12, 2384–2392. [Google Scholar] [CrossRef]
- Zheng, K.; Setyawati, M.I.; Leong, D.T.; Xie, J. Antimicrobial Silver Nanomaterials. Coord. Chem. Rev. 2018, 357, 1–17. [Google Scholar] [CrossRef]
- Li, H.; Xu, H. Mechanisms of Bacterial Resistance to Environmental Silver and Antimicrobial Strategies for Silver: A Review. Environ. Res. 2024, 248, 118313. [Google Scholar] [CrossRef] [PubMed]
- McNeilly, O.; Mann, R.; Hamidian, M.; Gunawan, C. Emerging Concern for Silver Nanoparticle Resistance in Acinetobacter Baumannii and Other Bacteria. Front. Microbiol. 2021, 12, 652863. [Google Scholar] [CrossRef]
- Kovács, D.; Igaz, N.; Gopisetty, M.K.; Kiricsi, M. Cancer Therapy by Silver Nanoparticles: Fiction or Reality? Int. J. Mol. Sci. 2022, 23, 839. [Google Scholar] [CrossRef]
- Liu, H.; Ding, M.; Wang, H.; Chen, Y.; Liu, Y.; Wei, L.; Cui, X.; Han, Y.; Zhang, B.; Zou, T.; et al. Silver Nanoparticles Modified hFGF2-Linking Camelina Oil Bodies Accelerate Infected Wound Healing. Colloids Surf. B Biointerfaces 2023, 222, 113089. [Google Scholar] [CrossRef]
- Perumalsamy, R.; Krishnadhas, L. Anti-Diabetic Activity of Silver Nanoparticles Synthesized from the Hydroethanolic Extract of Myristica Fragrans Seeds. Appl. Biochem. Biotechnol. 2022, 194, 1136–1148. [Google Scholar] [CrossRef]
- Jini, D.; Sharmila, S.; Anitha, A.; Pandian, M.; Rajapaksha, R.M.H. In Vitro and in Silico Studies of Silver Nanoparticles (AgNPs) from Allium Sativum against Diabetes. Sci. Rep. 2022, 12, 22109. [Google Scholar] [CrossRef] [PubMed]
- Torabian, F.; Akhavan Rezayat, A.; Ghasemi Nour, M.; Ghorbanzadeh, A.; Najafi, S.; Sahebkar, A.; Sabouri, Z.; Darroudi, M. Administration of Silver Nanoparticles in Diabetes Mellitus: A Systematic Review and Meta-Analysis on Animal Studies. Biol. Trace Elem. Res. 2022, 200, 1699–1709. [Google Scholar] [CrossRef] [PubMed]
- Ul Haq, M.N.; Shah, G.M.; Menaa, F.; Khan, R.A.; Althobaiti, N.A.; Albalawi, A.E.; Alkreathy, H.M. Green Silver Nanoparticles Synthesized from Taverniera Couneifolia Elicits Effective Anti-Diabetic Effect in Alloxan-Induced Diabetic Wistar Rats. Nanomaterials 2022, 12, 1035. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Esquivias, F.; Guzmán-Flores, J.M.; Pérez-Larios, A.; Rico, J.L.; Becerra-Ruiz, J.S. A Review of the Effects of Gold, Silver, Selenium, and Zinc Nanoparticles on Diabetes Mellitus in Murine Models. Mini-Rev. Med. Chem. 2021, 21, 1798–1812. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, A.S.; Hidayathulla, S.; Rehman, M.T.; ElGamal, A.A.; Al-Massarani, S.; Razmovski-Naumovski, V.; Alqahtani, M.S.; El Dib, R.A.; AlAjmi, M.F. Alpha-Amylase and Alpha-Glucosidase Enzyme Inhibition and Antioxidant Potential of 3-Oxolupenal and Katononic Acid Isolated from Nuxia Oppositifolia. Biomolecules 2020, 10, 61. [Google Scholar] [CrossRef] [PubMed]
- Edrispour, Z.; Homaei, A. Exploring in Vitro Effect of Silver Nanoparticles and Holothuria Parva Extracts on Kinetics and Stability of α-Amylase. Biotechnol. Appl. Biochem. 2023, 70, 885–894. [Google Scholar] [CrossRef]
- Thirumal, S.; Sivakumar, T. Synthesis of silver nanoparticles using Cassia auriculata leaves extracts and their potential antidiabetic activity. Int. J. Bot. Stud. 2021, 6, 35–38. [Google Scholar]
- Rani, P.; Kumar, N.; Perinmbam, K.; Devanesan, S.; AlSalhi, M.S.; Asemi, N.; Nicoletti, M. Synthesis of Silver Nanoparticles by Leaf Extract of Cucumis Melo L. and Their In Vitro Antidiabetic and Anticoccidial Activities. Molecules 2023, 28, 4995. [Google Scholar] [CrossRef]
- Wahab, M.; Bhatti, A.; John, P. Evaluation of Antidiabetic Activity of Biogenic Silver Nanoparticles Using Thymus Serpyllum on Streptozotocin-Induced Diabetic BALB/c Mice. Polymers 2022, 14, 3138. [Google Scholar] [CrossRef]
- Ruffo, M.; Parisi, O.I.; Dattilo, M.; Patitucci, F.; Malivindi, R.; Pezzi, V.; Tzanov, T.; Puoci, F. Synthesis and Evaluation of Wound Healing Properties of Hydro-Diab Hydrogel Loaded with Green-Synthetized AGNPS: In Vitro and in Ex Vivo Studies. Drug Deliv. Transl. Res. 2022, 12, 1881–1894. [Google Scholar] [CrossRef]
- Nqakala, Z.B.; Sibuyi, N.R.S.; Fadaka, A.O.; Meyer, M.; Onani, M.O.; Madiehe, A.M. Advances in Nanotechnology towards Development of Silver Nanoparticle-Based Wound-Healing Agents. Int. J. Mol. Sci. 2021, 22, 11272. [Google Scholar] [CrossRef]
- Tarhan, T.; Şen, Ö.; Ciofani, M.E.; Yılmaz, D.; Çulha, M. Synthesis and Characterization of Silver Nanoparticles Decorated Polydopamine Coated Hexagonal Boron Nitride and Its Effect on Wound Healing. J. Trace Elem. Med. Biol. 2021, 67, 126774. [Google Scholar] [CrossRef]
- González-Pedroza, M.G.; Benítez, A.R.T.; Navarro-Marchal, S.A.; Martínez-Martínez, E.; Marchal, J.A.; Boulaiz, H.; Morales-Luckie, R.A. Biogeneration of Silver Nanoparticles from Cuphea Procumbens for Biomedical and Environmental Applications. Sci. Rep. 2023, 13, 790. [Google Scholar] [CrossRef] [PubMed]
- Miranda, R.R.; Sampaio, I.; Zucolotto, V. Exploring Silver Nanoparticles for Cancer Therapy and Diagnosis. Colloids Surf. B Biointerfaces 2022, 210, 112254. [Google Scholar] [CrossRef] [PubMed]
- Kitimu, S.R.; Kirira, P.; Abdille, A.A.; Sokei, J.; Ochwang’i, D.; Mwitari, P.; Makanya, A.; Maina, N. Anti-Angiogenic and Anti-Metastatic Effects of Biogenic Silver Nanoparticles Synthesized Using Azadirachta Indica. Adv. Biosci. Biotechnol. 2022, 13, 188–206. [Google Scholar] [CrossRef]
- Wang, T.; Dong, Y.; Huang, Z.; Zhang, G.; Zhao, Y.; Yao, H.; Hu, J.; Tüksammel, E.; Cai, H.; Liang, N.; et al. Antioxidants Stimulate BACH1-Dependent Tumor Angiogenesis. J. Clin. Investig. 2023, 133, e169671. [Google Scholar] [CrossRef]
- Noorbazargan, H.; Amintehrani, S.; Dolatabadi, A.; Mashayekhi, A.; Khayam, N.; Moulavi, P.; Naghizadeh, M.; Mirzaie, A.; Mirzaei Rad, F.; Kavousi, M. Anti-Cancer & Anti-Metastasis Properties of Bioorganic-Capped Silver Nanoparticles Fabricated from Juniperus Chinensis Extract against Lung Cancer Cells. AMB Express 2021, 11, 61. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, S.; Qadri, S.; Akbar, S.; Parray, A.; Haik, Y. Biogenesis of Exosomes Laden with Metallic Silver-Copper Nanoparticles Liaised by Wheat Germ Agglutinin for Targeted Delivery of Therapeutics to Breast Cancer. Adv. Biol. 2022, 6, e2200005. [Google Scholar] [CrossRef]
- Taati, H.; Sangani, H.; Davoudi, A.; Safabakhsh Kouchesfahani, S.; Hedayati, M.; Tarashandeh Hemmati, S.; Ghasemipour, T.; Aghajani, S.; Farah Andooz, M.; Amanollahi, M.; et al. Silver Nanoparticle Functionalized by Glutamine and Conjugated with Thiosemicarbazide Induces Apoptosis in Colon Cancer Cell Line. Sci. Rep. 2024, 14, 3809. [Google Scholar] [CrossRef]
- Adamo, F.M.; Silva Barcelos, E.C.; De Falco, F.; Dorillo, E.; Rompietti, C.; Sorcini, D.; Stella, A.; Del Papa, B.; Baldoni, S.; Esposito, A.; et al. Therapeutic Targeting Potential of Novel Silver Nanoparticles Coated with Anti-CD20 Antibody against Chronic Lymphocytic Leukemia. Cancers 2023, 15, 3618. [Google Scholar] [CrossRef]
- Bielka, W.; Przezak, A.; Molęda, P.; Pius-Sadowska, E.; Machaliński, B. Double Diabetes—When Type 1 Diabetes Meets Type 2 Diabetes: Definition, Pathogenesis and Recognition. Cardiovasc. Diabetol. 2024, 23, 62. [Google Scholar] [CrossRef]
- Singh, M.; Thakur, V.; Kumar, V.; Raj, M.; Gupta, S.; Devi, N.; Upadhyay, S.K.; Macho, M.; Banerjee, A.; Ewe, D.; et al. Silver Nanoparticles and Its Mechanistic Insight for Chronic Wound Healing: Review on Recent Progress. Molecules 2022, 27, 5587. [Google Scholar] [CrossRef]
- Bordoni, V.; Sanna, L.; Lyu, W.; Avitabile, E.; Zoroddu, S.; Medici, S.; Kelvin, D.J.; Bagella, L. Silver Nanoparticles Derived by Artemisia Arborescens Reveal Anticancer and Apoptosis-Inducing Effects. Int. J. Mol. Sci. 2021, 22, 8621. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, F.; Yalamarty, S.S.K.; Filipczak, N.; Jin, Y.; Li, X. Nano Silver-Induced Toxicity and Associated Mechanisms. Int. J. Nanomed. 2022, 17, 1851–1864. [Google Scholar] [CrossRef] [PubMed]
- Lozano, V.; Martín, C.; Blanco, N.; Alcalde, I.; Fernández-Vega Cueto, L.; Merayo-Lloves, J.; Quirós, L.M. Exosomes Released by Corneal Stromal Cells Show Molecular Alterations in Keratoconus Patients and Induce Different Cellular Behavior. Biomedicines 2022, 10, 2348. [Google Scholar] [CrossRef]
- Vaghari-Tabari, M.; Jafari-Gharabaghlou, D.; Mohammadi, M.; Hashemzadeh, M.S. Zinc Oxide Nanoparticles and Cancer Chemotherapy: Helpful Tools for Enhancing Chemo-Sensitivity and Reducing Side Effects? Biol. Trace Elem. Res. 2024, 202, 1878–1900. [Google Scholar] [CrossRef] [PubMed]
- Castorina, P.; Martorana, E.; Forte, S. Dynamical Synergy of Drug Combinations during Cancer Chemotherapy. J. Pers. Med. 2022, 12, 1873. [Google Scholar] [CrossRef]
- Nieves, L.M.; Mossburg, K.; Hsu, J.C.; Maidment, A.D.A.; Cormode, D.P. Silver Chalcogenide Nanoparticles: A Review of Their Biomedical Applications. Nanoscale 2021, 13, 19306–19323. [Google Scholar] [CrossRef]
- Beck, F.; Loessl, M.; Baeumner, A.J. Signaling Strategies of Silver Nanoparticles in Optical and Electrochemical Biosensors: Considering Their Potential for the Point-of-Care. Mikrochim. Acta 2023, 190, 91. [Google Scholar] [CrossRef]
- Shahbazi, N.; Zare-Dorabei, R.; Naghib, S.M. Multifunctional Nanoparticles as Optical Biosensing Probe for Breast Cancer Detection: A Review. Mater. Sci. Eng. C 2021, 127, 112249. [Google Scholar] [CrossRef]
- Ibrahim, N.; Jamaluddin, N.D.; Tan, L.L.; Mohd Yusof, N.Y. A Review on the Development of Gold and Silver Nanoparticles-Based Biosensor as a Detection Strategy of Emerging and Pathogenic RNA Virus. Sensors 2021, 21, 5114. [Google Scholar] [CrossRef]
- Narita, F.; Wang, Z.; Kurita, H.; Li, Z.; Shi, Y.; Jia, Y.; Soutis, C. A Review of Piezoelectric and Magnetostrictive Biosensor Materials for Detection of COVID-19 and Other Viruses. Adv. Mater. Deerfield Beach Fla 2021, 33, e2005448. [Google Scholar] [CrossRef]
- Zhang, J.-H.; Shen, Q.; Zhou, Y.-G. Quantification of Tumor Protein Biomarkers from Lung Patient Serum Using Nanoimpact Electrochemistry. ACS Sens. 2021, 6, 2320–2329. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Fan, H.; Chen, Y.; Huang, J.; Liu, G.L.; Huang, L. Application of Nanoplasmonic Biosensors Based on Nanoarrays in Biological and Chemical Detection. Opt. Express 2023, 31, 21586–21613. [Google Scholar] [CrossRef]
- Endo, T.; Ikeda, R.; Yanagida, Y.; Hatsuzawa, T. Stimuli-Responsive Hydrogel–Silver Nanoparticles Composite for Development of Localized Surface Plasmon Resonance-Based Optical Biosensor. Anal. Chim. Acta 2008, 611, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Bilal, S.; Saleem, S.; Akhtar, N.; Sami, A.J.; Gokce, G.; Hayat, A. A novel cellulosic non-enzymatic nanosensor based on carbon 1151 shell silver (Ag@C) nanoparticles for colorimetric detection of Chlorpyrifos in agricultural products. JSFA Rep. 2022, 2, 511–523. [Google Scholar] [CrossRef]
- Sami, A.J.; Bilal, S.; Ahsan, N.-A.; Hameed, N.; Saleem, S. Rhodamine B Functionalized Silver Nanoparticles Paper Discs as Turn-on Fluorescence Sensor, Coupled with a Smartphone for the Detection of Microbial Contamination in Drinking Water. Environ. Monit. Assess. 2023, 195, 1442. [Google Scholar] [CrossRef]
- Wang, T.; Santos, J.P.; Slaveykova, V.I.; Stoll, S.; Liu, W. From Microalgae to Gastropods: Understanding the Kinetics and Toxicity of Silver Nanoparticles in Freshwater Aquatic Environment. Environ. Pollut. Barking Essex 1987 2025, 367, 125643. [Google Scholar] [CrossRef]
- Murthy, M.K.; Mohanty, C.S.; Swain, P.; Pattanayak, R. Assessment of Toxicity in the Freshwater Tadpole Polypedates Maculatus Exposed to Silver and Zinc Oxide Nanoparticles: A Multi-Biomarker Approach. Chemosphere 2022, 293, 133511. [Google Scholar] [CrossRef]
- Sibiya, A.; Jeyavani, J.; Santhanam, P.; Preetham, E.; Freitas, R.; Vaseeharan, B. Comparative Evaluation on the Toxic Effect of Silver (Ag) and Zinc Oxide (ZnO) Nanoparticles on Different Trophic Levels in Aquatic Ecosystems: A Review. J. Appl. Toxicol. JAT 2022, 42, 1890–1900. [Google Scholar] [CrossRef]
- Babaei, M.; Tayemeh, M.B.; Jo, M.S.; Yu, I.J.; Johari, S.A. Trophic Transfer and Toxicity of Silver Nanoparticles along a Phytoplankton-Zooplankton-Fish Food Chain. Sci. Total Environ. 2022, 842, 156807. [Google Scholar] [CrossRef]
- Mat Lazim, Z.; Salmiati, S.; Marpongahtun, M.; Arman, N.Z.; Mohd Haniffah, M.R.; Azman, S.; Yong, E.L.; Salim, M.R. Distribution of Silver (Ag) and Silver Nanoparticles (AgNPs) in Aquatic Environment. Water 2023, 15, 1349. [Google Scholar] [CrossRef]
- Yan, C.; Huang, J.; Cao, C.; Li, R.; Ma, Y.; Wang, Y. Effects of PVP-Coated Silver Nanoparticles on Enzyme Activity, Bacterial and Archaeal Community Structure and Function in a Yellow-Brown Loam Soil. Environ. Sci. Pollut. Res. Int. 2020, 27, 8058–8070. [Google Scholar] [CrossRef] [PubMed]
- Kolesnikov, S.; Tsepina, N.; Minnikova, T.; Kazeev, K.; Mandzhieva, S.; Sushkova, S.; Minkina, T.; Mazarji, M.; Singh, R.K.; Rajput, V.D. Influence of Silver Nanoparticles on the Biological Indicators of Haplic Chernozem. Plants 2021, 10, 1022. [Google Scholar] [CrossRef] [PubMed]
- Minnikova, T.; Kolesnikov, S.; Minkina, T.; Mandzhieva, S. Assessment of Ecological Condition of Haplic Chernozem Calcic Contaminated with Petroleum Hydrocarbons during Application of Bioremediation Agents of Various Natures. Land 2021, 10, 169. [Google Scholar] [CrossRef]
- Salleh, A.; Naomi, R.; Utami, N.D.; Mohammad, A.W.; Mahmoudi, E.; Mustafa, N.; Fauzi, M.B. The Potential of Silver Nanoparticles for Antiviral and Antibacterial Applications: A Mechanism of Action. Nanomaterials 2020, 10, 1566. [Google Scholar] [CrossRef] [PubMed]
- González-Vega, J.G.; García-Ramos, J.C.; Chavez-Santoscoy, R.A.; Castillo-Quiñones, J.E.; Arellano-Garcia, M.E.; Toledano-Magaña, Y. Lung Models to Evaluate Silver Nanoparticles’ Toxicity and Their Impact on Human Health. Nanomaterials 2022, 12, 2316. [Google Scholar] [CrossRef]
- Samal, D.; Khandayataray, P.; Sravani, M.; Murthy, M.K. Silver Nanoparticle Ecotoxicity and Phytoremediation: A Critical Review of Current Research and Future Prospects. Environ. Sci. Pollut. Res. 2024, 31, 8400–8428. [Google Scholar] [CrossRef]
- Muhamad, M.; Ab Rahim, N.; Wan Omar, W.A.; Nik Mohamed Kamal, N.N.S. Cytotoxicity and Genotoxicity of Biogenic Silver Nanoparticles in A549 and BEAS-2B Cell Lines. Bioinorg. Chem. Appl. 2022, 2022, 8546079. [Google Scholar] [CrossRef]
- Güzel, D.; Güneş, M.; Yalçın, B.; Akarsu, E.; Rencüzoğulları, E.; Kaya, B. Genotoxic Potential of Different Nano-Silver Halides in Cultured Human Lymphocyte Cells. Drug Chem. Toxicol. 2023, 46, 768–780. [Google Scholar] [CrossRef]
- Noga, M.; Milan, J.; Frydrych, A.; Jurowski, K. Toxicological Aspects, Safety Assessment, and Green Toxicology of Silver Nanoparticles (AgNPs)-Critical Review: State of the Art. Int. J. Mol. Sci. 2023, 24, 5133. [Google Scholar] [CrossRef]
- Wāng, Y.; Han, Y.; Xu, D.-X. Developmental Impacts and Toxicological Hallmarks of Silver Nanoparticles across Diverse Biological Models. Environ. Sci. Ecotechnology 2024, 19, 100325. [Google Scholar] [CrossRef]
- Olugbodi, J.O.; Lawal, B.; Bako, G.; Onikanni, A.S.; Abolenin, S.M.; Mohammud, S.S.; Ataya, F.S.; Batiha, G.E.-S. Author Correction: Effect of Sub-Dermal Exposure of Silver Nanoparticles on Hepatic, Renal and Cardiac Functions Accompanying Oxidative Damage in Male Wistar Rats. Sci. Rep. 2023, 13, 11274. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Ullah, K.; Bibi, N.; Ahmad, B.; Shah, K.; Qiang, T.Y. The Potential Toxicity of Chemically Fabricated Silver Nanomaterials Based on Accumulation and Histological Changes in Fish (Cyprinus Carpio). Microsc. Res. Tech. 2024, 87, 2292–2300. [Google Scholar] [CrossRef] [PubMed]
- Osborne, C.J.; Norton, A.E.; Whitworth, R.J.; Silver, K.S.; Cohnstaedt, L.W. Tiny Silver Bullets: Silver Nanoparticles Are Insecticidal to Culicoides Sonorensis (Diptera: Ceratopogonidae) Biting Midge Larvae. J. Med. Entomol. 2024, 61, 1427–1434. [Google Scholar] [CrossRef]
- Ong, W.T.J.; Nyam, K.L. Evaluation of Silver Nanoparticles in Cosmeceutical and Potential Biosafety Complications. Saudi J. Biol. Sci. 2022, 29, 2085–2094. [Google Scholar] [CrossRef]
- Wang, X.; Cui, X.; Wu, J.; Bao, L.; Chen, C. Oral Administration of Silver Nanomaterials Affects the Gut Microbiota and Metabolic Profile Altering the Secretion of 5-HT in Mice. J. Mater. Chem. B 2023, 11, 1904–1915. [Google Scholar] [CrossRef] [PubMed]
- Ferdous, Z.; Nemmar, A. Health Impact of Silver Nanoparticles: A Review of the Biodistribution and Toxicity Following Various Routes of Exposure. Int. J. Mol. Sci. 2020, 21, 2375. [Google Scholar] [CrossRef]
- de Nies, L.; Kobras, C.M.; Stracy, M. Antibiotic-Induced Collateral Damage to the Microbiota and Associated Infections. Nat. Rev. Microbiol. 2023, 21, 789–804. [Google Scholar] [CrossRef]
- Silva, T.; Pokhrel, L.R.; Dubey, B.; Tolaymat, T.M.; Maier, K.J.; Liu, X. Particle Size, Surface Charge and Concentration Dependent Ecotoxicity of Three Organo-Coated Silver Nanoparticles: Comparison between General Linear Model-Predicted and Observed Toxicity. Sci. Total Environ. 2014, 468–469, 968–976. [Google Scholar] [CrossRef]
- Mao, B.-H.; Chen, Z.-Y.; Wang, Y.-J.; Yan, S.-J. Silver Nanoparticles Have Lethal and Sublethal Adverse Effects on Development and Longevity by Inducing ROS-Mediated Stress Responses. Sci. Rep. 2018, 8, 2445. [Google Scholar] [CrossRef]
- Wu, K.; Li, H.; Wang, Y.; Liu, D.; Li, H.; Zhang, Y.; Lynch, M.; Long, H. Silver Nanoparticles Elevate Mutagenesis of Eukaryotic Genomes. G3 GenesGenomesGenetics 2023, 13, jkad008. [Google Scholar] [CrossRef]
- Stensberg, M.C.; Wei, Q.; McLamore, E.S.; Porterfield, D.M.; Wei, A.; Sepúlveda, M.S. Toxicological Studies on Silver Nanoparticles: Challenges and Opportunities in Assessment, Monitoring and Imaging. Nanomed. 2011, 6, 879–898. [Google Scholar] [CrossRef] [PubMed]
- Zharkova, M.S.; Golubeva, O.Y.; Orlov, D.S.; Vladimirova, E.V.; Dmitriev, A.V.; Tossi, A.; Shamova, O.V. Silver Nanoparticles Functionalized with Antimicrobial Polypeptides: Benefits and Possible Pitfalls of a Novel Anti-Infective Tool. Front. Microbiol. 2021, 12, 750556. [Google Scholar] [CrossRef] [PubMed]
- Dalzon, B.; Torres, A.; Diemer, H.; Ravanel, S.; Collin-Faure, V.; Pernet-Gallay, K.; Jouneau, P.-H.; Bourguignon, J.; Cianférani, S.; Carrière, M.; et al. How Reversible Are the Effects of Silver Nanoparticles on Macrophages? A Proteomic-Instructed View. Environ. Sci. Nano 2019, 6, 3133–3157. [Google Scholar] [CrossRef]
- Hu, B.; Yin, N.; Yang, R.; Liang, S.; Liang, S.; Faiola, F. Silver Nanoparticles (AgNPs) and AgNO3 Perturb the Specification of Human Hepatocyte-like Cells and Cardiomyocytes. Sci. Total Environ. 2020, 725, 138433. [Google Scholar] [CrossRef]
- Braeuning, A.; Oberemm, A.; Görte, J.; Böhmert, L.; Juling, S.; Lampen, A. Comparative Proteomic Analysis of Silver Nanoparticle Effects in Human Liver and Intestinal Cells. J. Appl. Toxicol. JAT 2018, 38, 638–648. [Google Scholar] [CrossRef]
- Park, E.-J.; Yi, J.; Kim, Y.; Choi, K.; Park, K. Silver Nanoparticles Induce Cytotoxicity by a Trojan-Horse Type Mechanism. Toxicol. Vitro Int. J. Publ. Assoc. BIBRA 2010, 24, 872–878. [Google Scholar] [CrossRef] [PubMed]
- Foldbjerg, R.; Autrup, H. Mechanisms of Silver Nanoparticle Toxicity. Arch. Basic Appl. Med. 2013, 1, 5–15. [Google Scholar]
- Zhou, Y.; Chen, L.; Wang, T. Nanoparticles in Gynecologic Cancers: A Bibliometric and Visualization Analysis. Front. Oncol. 2025, 14, 1465987. [Google Scholar] [CrossRef]
- Tian, H.; Zhang, T.; Qin, S.; Huang, Z.; Zhou, L.; Shi, J.; Nice, E.C.; Xie, N.; Huang, C.; Shen, Z. Enhancing the Therapeutic Efficacy of Nanoparticles for Cancer Treatment Using Versatile Targeted Strategies. J. Hematol. Oncol. J. Hematol. Oncol. 2022, 15, 132. [Google Scholar] [CrossRef]
- Suthar, J.K.; Vaidya, A.; Ravindran, S. Toxic Implications of Silver Nanoparticles on the Central Nervous System: A Systematic Literature Review. J. Appl. Toxicol. JAT 2023, 43, 4–21. [Google Scholar] [CrossRef]
- Downes, K.J.; Goldman, J.L. Too Much of a Good Thing: Defining Antimicrobial Therapeutic Targets to Minimize Toxicity. Clin. Pharmacol. Ther. 2021, 109, 905–917. [Google Scholar] [CrossRef] [PubMed]
- Parveen, R.; Maiti, P.K.; Murmu, N.; Datta, A. Preparation of Serum Capped Silver Nanoparticles for Selective Killing of Microbial Cells Sparing Host Cells. Sci. Rep. 2021, 11, 11610. [Google Scholar] [CrossRef] [PubMed]
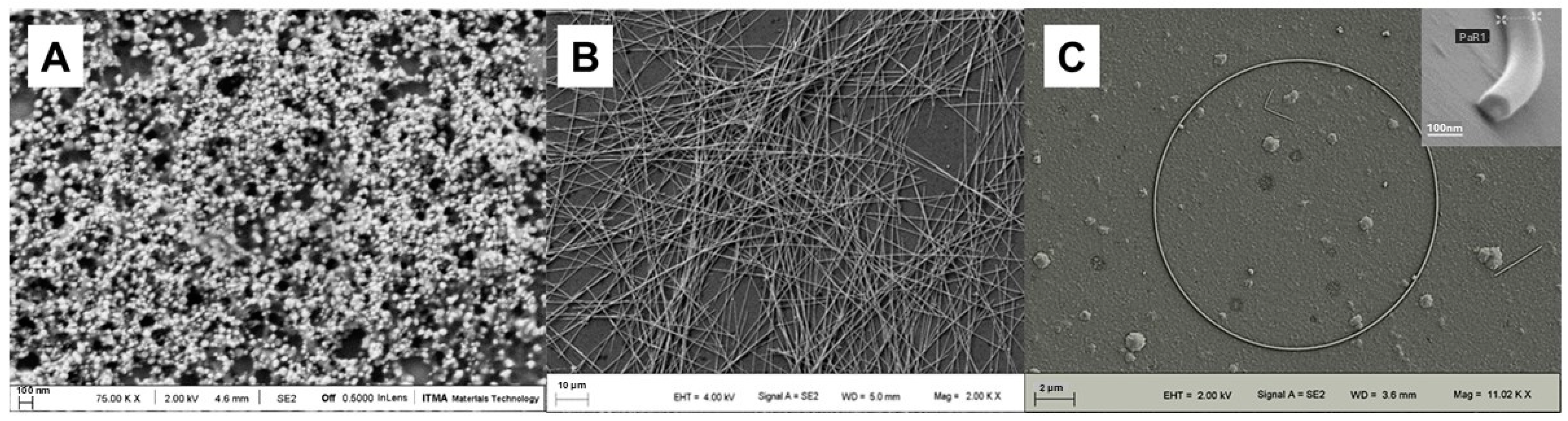

| Category | Subcategory | Description |
|---|---|---|
| Size | Ultrasmall AgNPs | <10 nm, characterized by enhanced surface reactivity and potentially increased cellular uptake |
| Small AgNPs | 10–30 nm, generally exhibit significant antimicrobial effects, with efficacy often inversely proportional to size | |
| Medium AgNPs | 30–60 nm, may display a balance between stability and biological activity | |
| Large AgNPs | 60–100 nm, may exhibit reduced antimicrobial efficacy compared to smaller counterparts | |
| Morphology | Nanospheres | Spherical morphology |
| Nanocubes | Cubic morphology, often associated with enhanced antibacterial effects due to closer membrane interaction | |
| Nanowires | One-dimensional structures, exhibiting variable antimicrobial activity depending on aspect ratio and surface properties | |
| Nanorings | Ring-shaped morphology, demonstrating potentially superior activity in certain applications over prolonged periods | |
| Other Morphologies | E.g., nanoprisms, nanoplates, etc., exhibiting distinct properties and requiring further characterization of their biological activities | |
| Surface modification/stabilization | Unmodified AgNPs | Lacking surface modifications or stabilizers |
| Stabilizer-Coated AgNPs | Possessing a surface layer of stabilizing agents to prevent aggregation and influence electrokinetic characteristics | |
| Functionalized AgNPs | Modified with biologically active compounds to enhance cell membrane penetration or target specific biological entities |
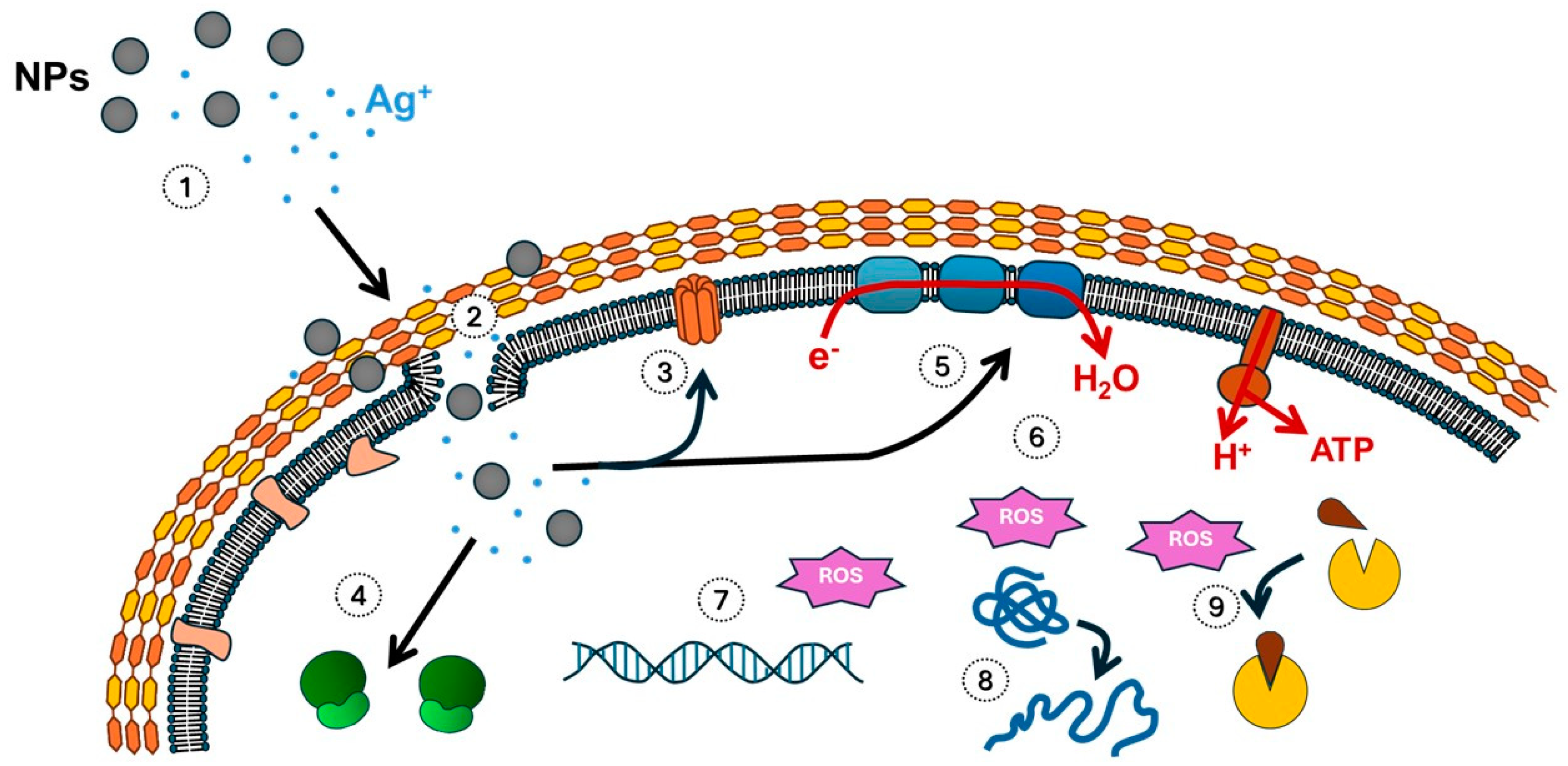
| Therapeutic Properties | Key Mechanisms of Action | References |
|---|---|---|
| Antibacterial properties | Disruption of cell wall and membrane integrity | [11,22] |
| Inhibition of ATP synthesis and respiratory enzymes | [22,23,24,25] | |
| Denaturation of ribosomes | [22,23,24,25] | |
| Interference with bacterial signal transduction | [22,23,24,25] | |
| Generation of ROS | [22,23,24,25,26,27,28,29,30,31] | |
| Antifungal properties | Increased intracellular ROS, leading to apoptosis | [28,32,33] |
| Inhibition of hyphal growth and spore germination | [34] | |
| Membrane disruption via Ag+ release | [32,35] | |
| Inhibition of H+ ATPase and cellular respiration | [35] | |
| Alteration of tricarboxylic acid cycle and ergosterol synthesis | [36] | |
| Antiprotozoa properties | Elevated ROS production, overwhelming parasite defenses | [37,38] |
| Inhibition of cyst germination in Acanthamoeba | [39] | |
| Antiviral properties | Prevention of viral penetration into host cells | [40,41] |
| Binding to sulfur-rich domains of glycoproteins | [40,42,43] | |
| Damage to viral nucleic acids | [44,45] | |
| Competitive binding of AgNPs to cellular receptor sites | [41] | |
| ROS production and reduction in possibility of aggregation | [46] |
| Therapeutic Properties | Key Mechanisms of Action | References |
|---|---|---|
| Anti-Diabetic | Insulin sensitization | [101] |
| GLUT2 membrane translocation enhancement | [102] | |
| Pancreatic protection against ROS | [103,104] | |
| Advanced glycation end-product inhibition | [105,106,107,108,109] | |
| Wound Healing | Prevent of microbial growth and improvement of oxygen delivery | [110] |
| Modulation of immune response | [111] | |
| Re-epithelialization and differentiation of fibroblasts | [112] | |
| Antitumoral properties | Mitochondrial apoptosis via ROS cascade | [113,114] |
| Dysregulation of angiogenesis | [115,116] | |
| Changes in cell cycle | [13,117] | |
| Theranostic drug delivery enhancement | [118,119,120] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Fernández, S.; Blanco-Agudín, N.; Rodríguez, D.; Fernández-Vega, I.; Merayo-Lloves, J.; Quirós, L.M. Silver Nanoparticles: A Versatile Tool Against Infectious and Non-Infectious Diseases. Antibiotics 2025, 14, 289. https://doi.org/10.3390/antibiotics14030289
González-Fernández S, Blanco-Agudín N, Rodríguez D, Fernández-Vega I, Merayo-Lloves J, Quirós LM. Silver Nanoparticles: A Versatile Tool Against Infectious and Non-Infectious Diseases. Antibiotics. 2025; 14(3):289. https://doi.org/10.3390/antibiotics14030289
Chicago/Turabian StyleGonzález-Fernández, Sara, Noelia Blanco-Agudín, David Rodríguez, Iván Fernández-Vega, Jesús Merayo-Lloves, and Luis M. Quirós. 2025. "Silver Nanoparticles: A Versatile Tool Against Infectious and Non-Infectious Diseases" Antibiotics 14, no. 3: 289. https://doi.org/10.3390/antibiotics14030289
APA StyleGonzález-Fernández, S., Blanco-Agudín, N., Rodríguez, D., Fernández-Vega, I., Merayo-Lloves, J., & Quirós, L. M. (2025). Silver Nanoparticles: A Versatile Tool Against Infectious and Non-Infectious Diseases. Antibiotics, 14(3), 289. https://doi.org/10.3390/antibiotics14030289








