1. Introduction
With the increasing resistance of pathogens to common antibiotics, the development of new antimicrobial drugs and their analogues has become an urgent need for current research in the treatment of human and animal diseases. Antimicrobial peptides (AMPs) with unique antimicrobial mechanisms are currently attracting increasing interest as therapeutic candidates. However, most AMPs have the disadvantages of low oral bioavailability, high risk of proteolytic degradation, and high excretion rate, which greatly influence their development in therapeutic fields, especially when administered intravenously or orally [
1,
2,
3,
4]. Therefore, improving the stability and prolonging the in vitro and in vivo half-life of AMPs have become the focus of research and development of new AMP drugs.
There are many methods to improve the stability and prolong the half-life of peptides in the literature, including chemical modification (cyclization [
5], halogenation [
6], terminal modification [
7,
8,
9], etc.), unnatural amino acid modification [
10,
11,
12], and the design of self-assembled nanomaterials [
13,
14]. Cyclization of a peptide refers to a modification method that uses chemical methods to cyclize the head and tail of a linear peptide or partially cyclize it to make it into a more stable ring structure and extend its half-life. Compared with linear peptides, cyclic peptides have stronger biological activity, protease (including exopeptidase and endopeptidase) stability, and cell selectivity [
15,
16,
17,
18]. Therefore, cyclization of linear AMPs is expected to be an effective method to improve the stability of AMPs, prolong the half-life of AMPs, and improve their absorption and utilization in vivo.
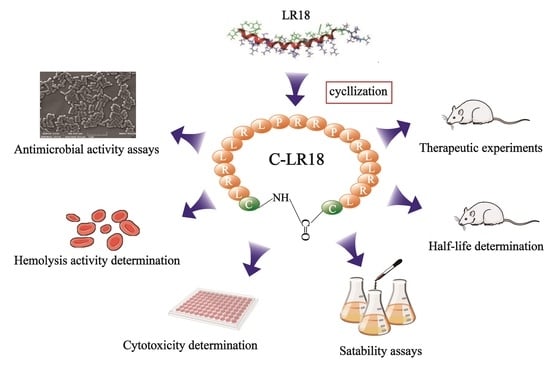
Cyclization of peptides can be achieved chemically via techniques such as head to side chain, side chain to side chain, head to tail, and side chain to tail, which are determined by the functional groups [
16,
19,
20,
21]. LR18 is an α-helical AMP designed by our research team that is mainly composed of leucine and arginine. Our research results have shown that LR18 has high antimicrobial activity, low hemolytic activity, and low cytotoxicity [
22]. However, the susceptibility to degradation of the peptidase enzyme and a short half-life hinder its application as a therapeutic agent. In order to improve the antibacterial activity and stability of the antimicrobial peptide LR18, prolong its half-life, and promote its clinical application, in this study, cysteine was added to the N-terminal and C-terminal of the linear peptide LR18, and a new cyclic antimicrobial peptide, named C-LR18, was synthesized by means of end-to-end cyclization via disulfide bonds. The biological activity, half-life, and therapeutic effect of C-LR18 on
E. coli-infected mouse models were studied. This provides a basis for the study of obtaining new antimicrobial drugs via the structural modification of existing antimicrobial peptides.
3. Discussion
Antimicrobial peptides have attracted much attention from researchers for many years due to their broad spectrum and high antibacterial activity in vitro. More than 3000 AMPs have been found so far. However, only a few AMPs have been used as therapeutic agents. Poor stability, short half-life, and easy removal by enzymes in plasma are the main reasons that hinder their clinical application as therapeutic agents. Therefore, improving the stability and prolonging the half-life of antimicrobial peptides have become the focus of the research and development of new antimicrobial peptide preparations. Cyclization of linear peptides is a modification method to improve the stability of peptides, prolong their half-life, and increase the bioavailability of peptides. Compared with linear peptides, the side chains of cyclic peptides are more tightly arranged, which makes the peptide conformation more stable. Due to the lack of amino and carboxyl termini, cyclic peptides are more resistant to peptidases and endopeptidases, thus showing better in vivo stability. Gunasekera S et al. [
17] synthesized skeletally looped KR-12 dimers and studied their antimicrobial activity and proteolytic stability. Their study showed that dimerization and main chain looping were effective strategies to improve the antimicrobial activity and stability of linear antimicrobial peptides. Lee CH et al. [
18] used the coupling reagent DMTMM to generate succinimide in LL37-derived peptide KR12 for cyclizing, and compared with unmodified KR12, the serum stability of KR12 containing succinimide increased. Etayash et al. [
5] used head–tail cyclization, side chain and tail cyclization, and added two cysteines to form disulfide bonds to cyclize IDR 1018, respectively, and found that the three cyclic peptides had a tolerance time of 120 min to trypsin, while the template peptide was completely degraded in less than 30 min. In this study, cysteine was added to the N-terminal and C-terminal of linear peptide LR18, and a new cycloantimicrobial peptide, named C-LR18, was designed and synthesized by means of end-to-end cyclization via disulfide bonds. The stability of cyclic peptide C-LR18 in protease was significantly improved. The antibacterial activity of C-LR18 against
E. coli ATCC25922 remained unchanged after treatment with 1 mg/mL papain for 1 h, while that of the original peptide LR18 was reduced by at least 54 times. The half-life of cyclic peptide C-LR18 in rat plasma was prolonged by 147.33 min, which was 3.37 times that of the original peptide. And the half-life of C-LR18 in SD rats was also prolonged by 4.66 times that of the original peptide. Our results indicated that cyclization modification helped improve the anti-enzymatic hydrolysis ability and enhance the stability of the peptide.
Antimicrobial peptides have a variety of ways to play their role in the body. Intravenous or subcutaneous drug delivery has low requirements for the stability of the peptide itself, but oral administration undoubtedly exposes antimicrobial peptides to proteases secreted by serum, the digestive tract, and even bacteria [
23]. In order to evaluate the possibility of the oral administration of antimicrobial peptide C-LR18, artificial gastric fluid and artificial intestinal fluid were used to simulate the gastrointestinal environment of animals in this study, and the MIC of C-LR18 to
E. coli ATCC25922 was determined after treatment with artificial gastric fluid and artificial intestinal fluid. The MIC of C-LR18 increased by 4 times and 16 times after treatment with artificial intestinal fluid for 30 min and 60 min, respectively. But the MIC increased by 8 times and 32 times after treatment with artificial gastric fluid for 30 min and 60 min, respectively. These results indicated that C-LR18 is more stable in the intestine than in the stomach. It is suggested that C-LR18 can be encapsulated into an oral preparation using a non-toxic material that is stable to the gastric environment but sensitive to the intestinal environment, but this idea needs to be further verified by experiments.
An in vivo toxicity test is an essential step in the safety evaluation of exogenous drugs. The purpose is to observe the systemic toxicity of drugs on animals, including whether there are adverse physiological reactions, pathological changes in organs and tissues of the whole body, and the mortality of animals. Therefore, before the treatment of C-LR18 on
E. coli-infected mice, we tested the acute toxicity of C-LR18 in mice. The LD50 of C-LR18 in mice was 37.8 mg/kg after a single injection of drug into the tail vein. This toxicity is significantly lower than that of many antimicrobial peptides reported so far, such as melittin (LD50 = 4.98 mg/kg) [
24] and polymyxin B (LD50 = 6.52 mg/kg) [
25], which was consistent with the results of in vitro hemolysis and cytotoxicity tests of C-LR18. The result of the systemic toxicity test provided a dose reference for the pharmacodynamic study of antimicrobial peptide C-LR18 in animals.
The in vitro antibacterial activity study showed that C-LR18 had a strong inhibitory effect on a variety of Gram-positive and Gram-negative bacteria. We expected that C-LR18 would also have high antibacterial activity in animals, but the in vivo environment is more complex than in vitro, and the activity and stability of antimicrobial peptides are affected by PH, salt ionizers, serum, and various proteases in vivo. Therefore, in this study, a mouse model of E. coli infection was constructed, and then the mice were treated with different doses of C-LR18. The therapeutic effect of C-LR18 was evaluated by measuring the survival rate of the mice, the number of colonies in the abdominal cavity, and the bacterial load in the liver and spleen. The results showed that the survival rate of mice treated with the 7.5 mg/kg C-LR18 group was 60%, slightly higher than those treated with 5.0 mg/kg of polymyxin B (50%). The number of colonies in the abdominal cavity of mice treated with 7.5 mg/kg C-LR18 was reduced to 104 CFU/mL, which was significantly lower than that in the control group (1 × 109 CFU/mL). The bacteria carrying capacity in the liver and spleen of mice in the C-LR18 treatment group decreased significantly, which was less than that in the polymyxin B group. These results indicated that C-LR18 had a therapeutic effect on E. coli-infected mice.
4. Materials and Methods
4.1. Materials
E. coli K88, E. coli ATCC 25922, Staphylococcus aureus ATCC 25923, Staphylococcus aureus ATCC 29213, Klebsiella pneumoniae CMCC 46117, Streptococcus faecalis ATCC 29212, and RAW264.7 cells were obtained from the pharmacology and toxicology laboratory of the College of Veterinary Medicine, Jilin Agricultural University (Changchun, China). TransDetect Cell Counting Kit-8 (CCK-8) and Dulbecco’s modified Eagle’s medium (DMEM) were purchased from TransGen Biotech (Beijing, China). Fetal bovine serum (FBS), fetal bovine serum, papain, trypsin, pepsin, carboxypeptidase, Triton X-100, Trifluoroethanol (TFE), and phosphate-buffered saline (PBS) solution were purchased from Solarbio (Beijing, China). Artificial gastric fluid and artificial intestinal fluid were purchased from Saint-bio (Shanghai, China). Mueller-Hinton broth (MHB), Mueller-Hinton agar (MHA), and bovine serum albumin (BSA) were purchased from GL Biochem (Shanghai, China). Specific Pathogen Free (SPF) SD rats (200 ± 20 g) were purchased from Liaoning Changsheng Biotechnology Co., Ltd. (Shenyang, China). Specific Pathogen Free (SPF) KM mice were purchased from the Laboratory Animal Center of Jilin University, (Changchun, China) The animals were maintained at constant room temperature [(25 ± 2) °C] with free access to food and water for 24 h or more before experiments. All animal experiments comply with the 3R principle and comply with animal ethics standards. The ethical review board reference number is 20210610001, and the approval date is 18 June 2021.
4.2. Design, Synthesis and Sequence Analysis of Cyclic Peptide C-LR18
The cyclic peptide C-LR18 and the original peptide LR18 were synthesized and purified by GL Biochem (Shanghai, China). The purity of peptides was over 98%, determined using high-performance liquid chromatography purification. The molecular mass of peptides was analyzed using LC-MS.
Peptide structure drawing software: MarvinSketchV20.8, ChemDraw3DV16.0, MercuryV6.0.0.
Physical and chemical parameters of peptides were analyzed online:
http://web.expasy.org/ (accessed on 21 March 2021).
4.3. CD Spectroscopy of Cyclic Peptide C-LR18
The CD Spectroscopy of cyclic peptide C-LR18 was detected on a Bio-Logic MOS-500 CD spectropolarimeter (Bio-Logic, Seyssinet-Pariset, France) at 25 °C according to the method described in [
22]. The final concentration of C-LR18 in 10 mM PBS (pH 7.4), 30 mM SDS, and 50% TFE was 150 µM. The test conditions were as follows: wavelength range of 190–250 nm, speed of 10 nm/min, and light diameter of 1 nm. C-LR18 was scanned three times and averaged to generate the final spectra. Circular dichroism data were analyzed according to the following formula to obtain the mean residue ellipticity (θM) (deg·cm
2·dmol
−1):
In the formula, θobs represents the measured ellipticity (mdeg) obtained by scanning in the wavelength range, l represents the light diameter length (cm), c represents the peptide concentration (mM), and n represents the amino acid number of the peptide C-LR18.
4.4. Antimicrobial Activity Assay
4.4.1. Minimum Bacteriostatic Concentration (MIC) Determination
The MIC test was performed using the method recommended by the National Committee for Clinical Laboratory Standards (NCCLS) Standards and Guidelines, and the method was modified according to the characteristics of antimicrobial peptides [
26]. The bacteria were cultured at 37 °C for 16 h, and the microbial suspension was diluted to a final concentration of 5 × 10
5 CFU/mL. Then, 50 µL of bacterial solution was mixed with 50 µL of MHB containing different concentrations peptides (0.5–256 µM) in a 96-well plate. After incubation at 37 °C for 16 h, MIC was examined by measuring the OD value at 492 nm (Microplate reader, TECAN GENios F129004, Tecan, Salzburg, Austria). The assays were performed three times. Geometric mean MIC represents the average MIC of the peptide against all the tested strains.
4.4.2. Minimum Bactericidal Concentration (MBC) Determination
MBC detection was performed according to the method of Taiyari et al. [
27]. According to the MIC results, 50 µL of bacterial cultures was absorbed from the minimum inhibitory concentration hole and the three previous holes, respectively; transferred to a new MH solid medium; and incubated in an incubator at 37 °C overnight, and the bacteria on the plate were counted. The concentration of antibacterial peptide that kills 99.99% of the tested bacteria is defined as the minimum bactericidal concentration (MBC) of the peptide. The experiment was repeated three times. Geometric mean MBC represents the average MIC of the peptide against all the tested strains. Therapeutic index (TI) represents the ratio of the minimum concentration of peptide that causes hemolysis of 10% of erythrocytes to the GM MIC (added in the Methods section).
4.4.3. Bactericidal Kinetics Assay
Bactericidal kinetics assay was performed according to the method outlined by Zhang et al. [
28].
E. coli ATCC25922 of 1 × 10
6 CFU/mL was mixed with MHB containing different concentrations of AMPs; the final concentrations of AMPs were set to 1 × MIC and 2 × MIC, respectively. The mixed solutions were incubated in a shaker at 37 °C. A total of 100 μL of the above mixed solution was absorbed every 20 min and diluted with sterile MH liquid medium, and then 50 μL of bacterial solution was aspirated and evenly coated on the solid MH plate. After incubation at 37 °C for 16–18 h, the bacteria colonies on the plate were counted. The bactericidal kinetics curves of LR18 and C-LR18 against
E. coli ATCC25922 were plotted. The experiment was repeated three times.
4.5. Antimicrobial Mechanism Study
4.5.1. Membrane Permeability Assay
The effect of C-LR18 on bacterial membrane permeability was measured using the propidium iodide (PI) uptake assay described in [
22].
E. coli ATCC25922 in logarithmic growth phase were adjusted to 1 × 10
6 CFU/mL. C-LR18 was added with the final concentration of 4 μM. The mixtures were incubated for 30 min at 37 °C. Then, PI was added at a concentration of 10 mg/mL, incubated for 10 min at room temperature in the dark, and compared with the bacteria treated with PBS (negative control) and bacteria treated with melittin (25 μg/mL) (positive control). After eliminating unbound dye by washing twice with PBS, the bacterial cells were detected using a flow cytometer (Ex = 535 nm/Em = 615 nm). The results were analyzed using FlowJo 6.2.1 and GraphPad Prism 5 software.
4.5.2. Scanning Electron Microscopy
E. coli ATCC25922 cells were washed three times in 10 mM PBS, diluted to 107 CFU/mL, and treated with 4 μg/mL C-LR18, and bacteria without peptide treatment were used as negative controls. After incubation at 37 °C for 1 h and centrifuging at 3000 r/min for 10 min, the super serum was discarded. Then, 2.5% glutaraldehyde solution was added to fix for 6 h. After fixation, it was washed with PBS for 2–3 times; washed with 30%, 50%, 70%, and 90% ethanol solution; and 100% ethanol dehydration. It was then scanned using an Extreme-resolution Analytical Field Emission SEM (Mira 3 XH, Tescan, Brno, Czech) after coating with gold–palladium.
4.5.3. DNA Binding Assay
The DNA binding test was performed according to gel retardation experiments described previously [
22]. Firstly, genomic DNA was extracted from
E. coli ATCC25922 using a DNA extraction kit. A total of 100 ng of genomic DNA was mixed with 1–512 µM peptide in a binding buffer (50 µg/mL of BSA, 1 mM of ethylene diamine tetra-acetic acid, 20 mM of KCl, 10 mM of Tris-HCl (pH = 8.0), 5% glycerol, and 1 mM of dithiothreitol) and incubated at 37 °C for 60 min. Subsequently, the samples were examined using 0.8% agarose gel electrophoresis. Observation and photography were performed in the UVP gel imaging system, and the electrophoresis mobility was analyzed.
4.6. Hemolysis Activity Determination
The hemolytic activity was examined according to the literature [
29]. Briefly, the collected rabbit red blood cells [purchased from Oumarsi (Shanghai, China) Biotechnology Co., Ltd.] were washed three times with PBS (pH 7.4) and diluted to 2% (
v/
v). A total of 100 µL of rabbit red blood cells suspension were mixed with 100 µL of PBS (pH 7.4) containing different concentrations of peptides (1–256 µM) in a 96-well plate. The positive control was a mixture of 100 µL of red blood cell suspension and 100 µL of 0.2% TritonX-100 solution, and the negative control was a mixture of 100 µL of red blood cell suspension and 100 µL of PBS solution. The mixtures were incubated at 37 °C for 1 h, then centrifuged at 1000×
g for 10 min. The OD value at 570 nm of the supernatant was measured using a microplate reader (TECAN GENios F129004, Tecan, Salzburg, Austria). The hemolytic index was calculated according to the following formula:
The concentration of the antimicrobial peptide causing 10% erythrocyte hemolysis was defined as the minimum hemolytic concentration of the antimicrobial peptide on rabbit red blood cells. The experiment was repeated three times.
4.7. Cytotoxicity Determination
The cytotoxicity of LR18 and C-LR18 to eukaryotic
RAW264.7 was determined using CCK-8 assay described by Jia et al. [
26]. Briefly, RAW264.7 cells were placed into 96-well plates at a density of 2.0 × 10
4 and then cultured at 37 °C under conditions of 5% CO
2 for 12–16 h. After the cells adhered to the wall, 50 μL of different concentrations (0.5–256 µM) of AMPs in DMEM were added to Wells 1–10. A 100 μL cell suspension was added to well 11 as positive control, and 100 μL of DMEM was added to well 12 as a negative control. The 96-well plates were cultured in 37 °C 5% CO
2 incubator for 12–16 h; then, CCK-8 (10%,
v/
v) was put into each well and incubated at 37 °C for 4 h. The cytotoxicity assay was examined by measuring the OD value of the mixtures at 450 nm. The assays were performed three times.
4.8. Stability Assays
4.8.1. Impact of Salts, pH, and Temperature on Antimicrobial Activity
The impact of salts, pH, and temperature on antimicrobial activity of peptides was determined according to the literature [
26]. The impact of different salts on peptides’ antimicrobial activity was tested by determining the effect on their MIC. Peptides were diluted in the MHB medium containing 2.5 mM of CaCl
2, 150 mM of NaCl, 4.5 mM of KCl, 6 mM of NH
4Cl, 1 mM of MgCl
2, 8 mM of ZnCl
2, and 4 mM of FeCl
3, respectively. Then, the MIC of peptides treated with different salts for
E. coli ATCC 25922 was detected; subsequent steps were the same as for the MIC assay.
The impact of temperature on peptides’ antimicrobial activity was also tested. Peptides were incubated at 0 °C, 37 °C, and 100 °C for 30 min, respectively, and then the MIC of peptides to E. coli ATCC 25922 was detected; subsequent steps were the same as for the MIC assay.
To detect the impact of pH on peptides’ antimicrobial activity, peptides were diluted in different pH (4.0, 6.0, 8.0, and 10.0) solutions and cultured for 1 h, and then the MIC of peptides to E. coli ATCC 25922 was detected; subsequent steps were the same as for the MIC assay.
4.8.2. The Stability of Peptide in Protease, Serum, and GastroIntestinal Conditions
The stability of LR18 and C-LR18 in protease, serum, and gastrointestinal conditions was determined using MIC assay, as described previously [
19,
26].
To detect the stability of peptides in protease, the peptides were diluted in 1 mg/mL of trypsin, pepsin, papain, and protease K, respectively, and incubated at 37 °C for 1 h. Then, the protease in the peptide solutions was inactivated in a water bath at 60 °C for 30 min. Subsequent steps were the same as for the MIC assay. The assays were performed three times.
To detect the stability of peptides in serum, the peptides were mixed with various concentrations of fetal bovine serum and mouse serum solutions, and the final concentrations of serum were 6.25%, 12.5%, 25%, and 50%. The MIC of peptides against E. coli ATCC25922 in the presence of different concentrations of serum was determined. Subsequent steps were the same as for the MIC assay. The assays were performed three times.
The stability in gastrointestinal conditions was also determined. The peptides were diluted in artificial gastric fluid and artificial intestinal fluid, digested in water bath at 37 °C for 0.5 and 1 h, and then inactivated in water bath at 60 °C for 30 min. Then, the PH of the solution was adjusted to 7.0 with 10% Na2CO3. Subsequent steps were the same as for the MIC assay. The assays were performed three times.
4.9. Half-Life Determination
4.9.1. Half-Life Determination of Peptides in Plasma In Vitro
Half-life of peptides in vitro was determined using HPLC according to the method described in [
30]. Blood from SPF SD rats was collected in a centrifuge tube containing 0.5% heparin sodium via cardiac sampling. Plasma was separated and stored at −80 °C until analyzed. The plasma samples with final concentrations of 25 μg/mL, 50 μg/mL, 100 μg/mL, 200 μg/mL, 400 μg/mL, and 750 μg/mL were prepared by mixing 1500 μg/mL of antimicrobial peptides with rat plasma, then adding acetonitrile containing 0.1% TFA at 1/1 volume. After vortexing for 30 s, the samples were centrifuged at 14,000 r/min for 20 min, the supernatant was aspirated, sterilized using a 0.22 μm sterilization filter, and 30 μL of the sample was used for HPLC determination. After HPLC detection, the value of the chromatographic peak area was counted, the mathematical relationship between the sample concentration and the chromatographic peak area was calculated, and the standard curve was drawn. The standard curve was regressio-calculated by linear fitting Y = aX + b and weighted least square method (w = 1/c2).
The 1 mg/mL peptide solution was mixed with SD rat plasma and incubated in a water bath at 37 °C. Samples were taken at different time points of 0, 20, 30, 40, 60, 80, 100, 120, 140, 160, 180, 200, 210, 240, 300, 400, 500, and 600 min. After vortexing for 30 s, the samples were centrifuged at 14,000 r/min for 20 min, the supernatant was aspirated and sterilized using a 0.22 μm sterilization filter, and 30 μL of the sample was used for HPLC determination. The experiment was repeated three times. The peak area measured by HPLC was substituted into the standard curve obtained by the above experiment, the peptide concentration at different time points was calculated, and the half-life of peptides in plasma was calculated.
4.9.2. Half-Life Determination of Peptides in SD Rats
Half-life of peptides in SD rats was determined via HPLC according to the method described in [
31]. The SD rats were maintained at constant room temperature [(25 ± 2) °C] with free access to food and water for 48 h. Each SD rat was weighed to an accuracy of 0.10 g, numbered, and grouped randomly, with 6 rats in each group. LR18 and C-LR18 were injected into SD rats via tail vein at a dose of 5 mg/kg body weight. A total of 200 μL of blood was collected from the orbital venous plexus at different time points of 0, 0.5, 0.8, 1.0, 1.5, 2.5, 3.5, 5, 10, 30, and 60 min, and placed into a centrifuge tube containing 0.5% heparin sodium. After centrifugation at 3000 r/min for 15 min, the supernatant was absorbed into a new centrifuge tube, and then acetonitrile containing 0.1% TFA was added at 1/1 volume. Vortexing for 30 s, the samples were centrifuged at 14,000 r/min for 20 min, the supernatant was aspirated and sterilized using a 0.22 μm sterilization filter, and 30 μL of the sample was used for HPLC determination. The pharmacokinetic parameters were calculated using Phoenix Winnonlin 7.0 software and analyzed with non-atrioventricular model. Drug–time curves were plotted using Graphpad Prim, and Mean ± SD was used to interpolate each point during drug–time curve fitting.
4.10. Therapeutic Experiments in Mouse Models Infected with E. coli
4.10.1. Acute Toxicity of Antimicrobial Peptide C-LR18 in Mice
To obtain the reasonable dose range of C-LR18 on mouse LD50, KM mice (18–21 g) were randomly divided into 5 groups with 8 mice in each group. Different doses of C-LR18 (100 mg/kg, 75 mg/kg, 50 mg/kg, 30 mg/kg, and 10 mg/kg) were injected into the tail vein of mice in different groups. The health status of the mice was observed, dose data leading to 100% (10/10) and 0% (0/10) death of the mice was recorded, and the reasonable dose range of C-LR18 on mouse LD50 was determined.
To determine the median lethal dose (LD50) of C-LR18 in mice, KM mice (18–21 g) were randomly divided into 5 groups with 10 mice in each group; different doses of C-LR18 (77 mg/kg, 64 mg/kg, 53 mg/kg, 44 mg/kg, and 36 mg/kg) were injected into the tail vein of mice in different groups. The health status of mice was observed, and the mortality data of 5 groups of mice were recorded. The LD50 and 95% confidence limit of C-LR18 for mice were calculated according to the following formula:
Xm: dose pair value of maximum dose group, i: the difference between the dose pairs of two adjacent groups, P: animal mortality in each group, ΣP: the sum of the mortality of each group of animals, n: number of animals per group, q: survival rate per dose group, q = p − 1.
SX50: Standard error of logLD50.
4.10.2. Establishment of a Mouse Model Infected with E. coli
The mice (female, 18–21 g) were randomly divided into 6 groups, with PBS dilution as the control group. A total of 1–5 groups were injected intraperitoneally with 0.5 mL of E. coli solution containing 1010, 109, 108, 107, and 106 CFU/mL, respectively. After inoculation, the mice were observed continuously for 5 days, and the mortality rate was recorded. The minimum dose (LD 100) at which all mice die within 5 days was used as the optimal dose for the bacterial attack in this experiment.
4.10.3. Therapeutic Experiments in Mouse Models
In order to further evaluate the therapeutic potential of antimicrobial peptide C-LR18, a mouse model infected with E. coli was constructed in this study. The Km mice (female, 18–21 g) were maintained at constant room temperature [(25 ± 2) °C] with free access to food and water for 48 h. Each Km mouse was weighed to an accuracy of 0.10 g and numbered. According to the principles of safe pharmacology research (generally no less than 10 small animals per group and no less than 6 large animals per group), Km mice were randomly divided into 5 groups with 10 mice in each group. A total of 0.5 mL of E. coli Y1 suspension (109 CFU/m L) was intraperitoneally injected into each group of mice. One hour after challenge, different doses of C-LR18 (2.5 mg/kg, 5 mg/kg, and 7.5 mg/kg) and polymyxin B (5 mg/kg) were intraperitoneally injected into mice. The group injected with the same dose of physiological water was the control group. The health status of the mice was observed, and the mice that did not reach the criteria for euthanasia within 48 h were defined as “survival” in this experiment.
4.10.4. Determination of Bacterial Colony Number in Mouse Abdominal Cavity
One hour after the mice were intraperitoneally injected with 0.5 mL of E. coli Y1 suspension (109 CFU/mL), different doses of C-LR18 (2.5 mg/kg, 5 mg/kg, and 7.5 mg/kg) were injected into the mice through the tail vein, respectively. After 24 h, 2 mL sterile saline was injected into the abdominal cavity of the mice, and the mice were killed via cervical dislocation. The peritoneal cavity of mice was opened, the peritoneal fluid was absorbed, and the peritoneal fluid was diluted with sterilized MH medium in a double ratio. After coating the plate, the peritoneal fluid was cultured at 37 °C for 16–20 h, and the colony count was performed. Three mice were taken from each group.
4.10.5. Determination of Bacterial Load in Liver and Spleen of Mice
One hour after the mice were intraperitoneally injected with 0.5 mL of E. coli Y1 suspension (109 CFU/mL), C-LR18 was injected into the mice at a dose of 7.5 mg/kg through the tail vein. The normal saline group was used as negative control, and the polymyxin B group (1 mg/kg) was used as positive control. After that, one mouse was taken every 24 h, sacrificed via cervical dislocation, and the liver and spleen were removed, weighed, and added to PBS buffer for grinding at 3000 r/min for 2 min. The abrasive solution was diluted with sterile PBS (1:10,000), and 0.1 mL diluent was evenly coated in solid MH medium and cultured at 37 °C overnight. Colony counting was performed on the samples, and the bacterial colonization amount in liver and spleen was calculated.