Antiparasitic and Antifungal Activities of Cetyl-Maritima, a New N-Cetyl-Modified Maritima Derivative
Abstract
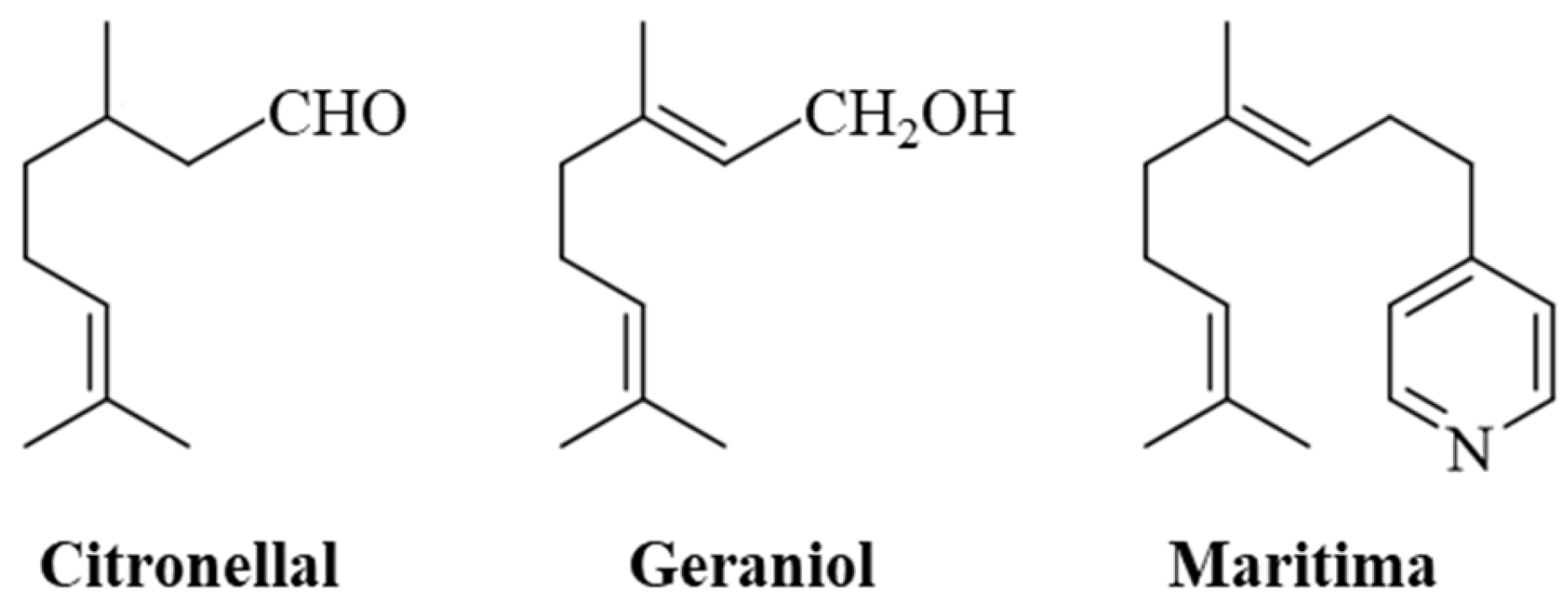
1. Introduction
2. Results
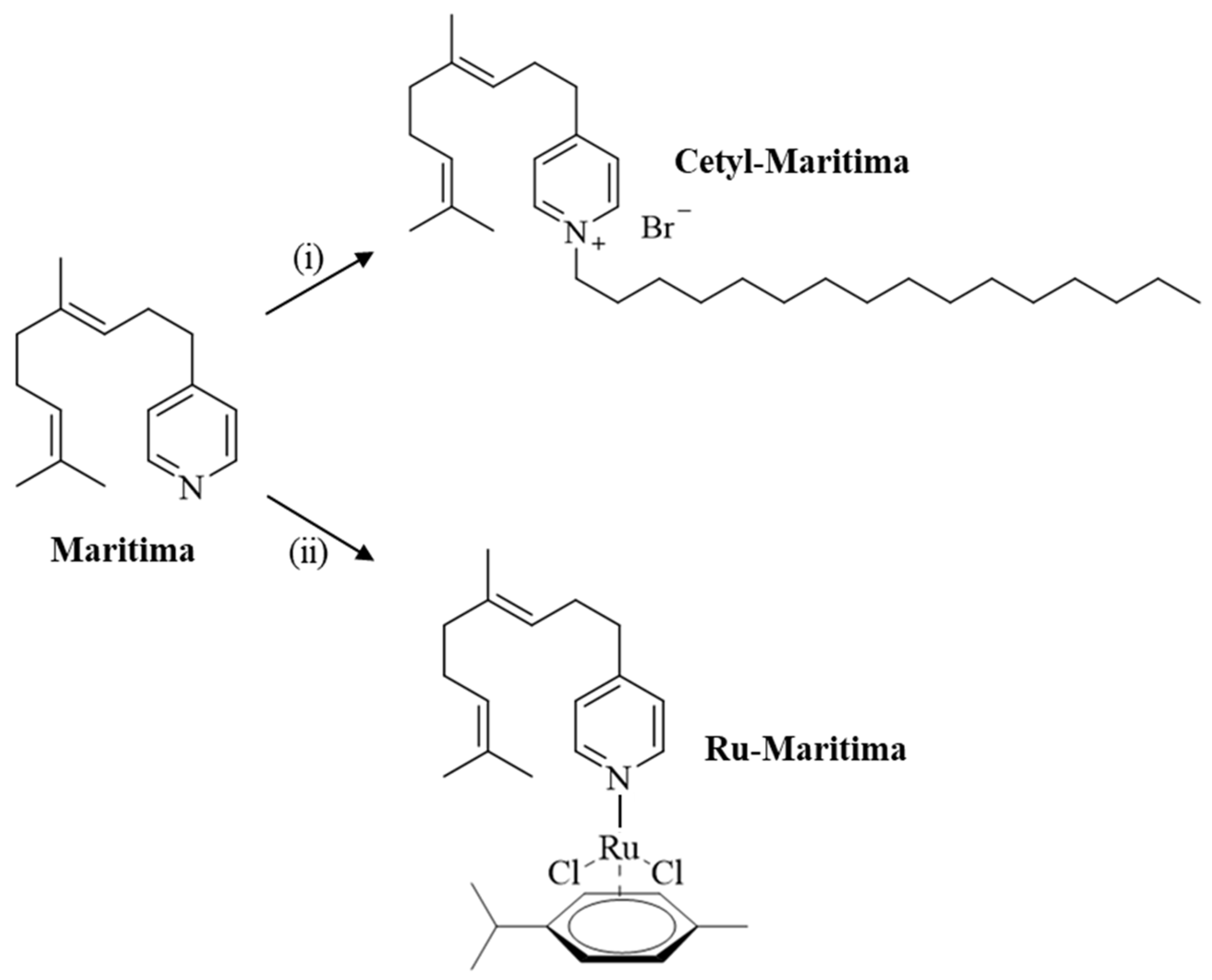
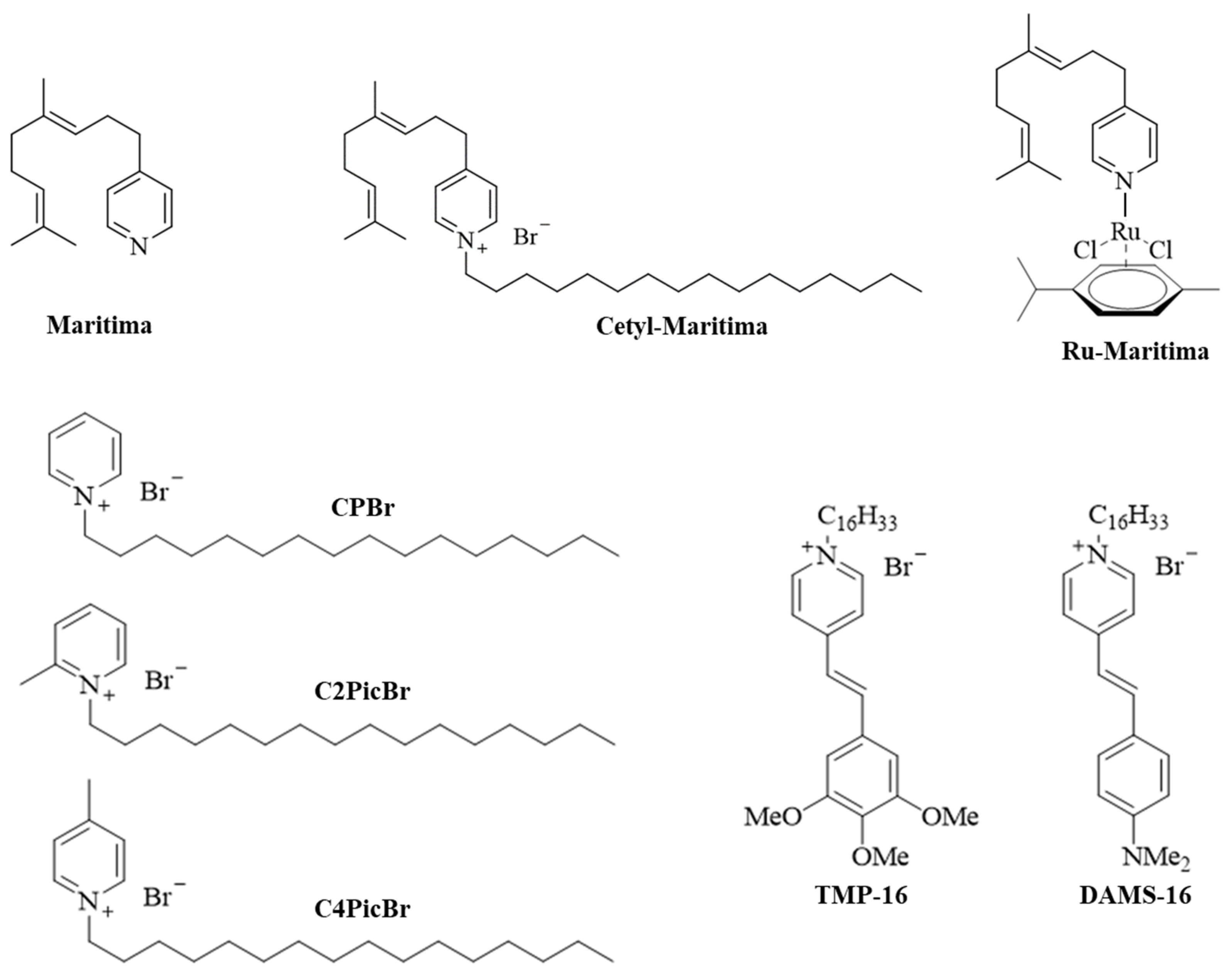
2.1. Chemistry
2.2. Antifungal Activity
2.3. Antiparasitic Activity
2.4. Toxicity Profile Prediction for Cetyl-Maritima
3. Discussion
4. Materials and Methods
4.1. Chemistry
4.1.1. 1-Hexadecyl-4-(4,8-dimethyl-3,7-nonadienyl)-pyridinium Bromide (Cetyl-Maritima)
4.1.2. Dichlorido(η6-p-cymene)[4-(4,8-dimethyl-3,7-nonadienyl)-pyridine]ruthenium(II) (Ru-Maritima)
4.2. Madurella mycetomatis Cells Drug Testing Assay
4.3. Toxoplasma gondii Cells Drug Testing Assay
4.4. Leishmania major Drug Testing Assays
4.5. Vero Cell and Macrophage Cytotoxicity
4.6. Toxicity Profile Prediction
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weld, E.D.; Waitt, C.; Barnes, K.; Garcia Bournissen, F. Twice neglected? Neglected diseases in neglected populations. Br. J. Clin. Pharmacol. 2022, 88, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Mitra, A.K.; Mawson, A.R. Neglected tropical diseases: Epidemiology and global burden. Trop. Med. Infect. Dis. 2017, 2, 36. [Google Scholar] [CrossRef] [PubMed]
- Hudu, S.A.; Jimoh, A.O.; Adeshina, K.A.; Otalike, E.G.; Tahir, A.; Hegazy, A.A. An insight into the success, challenges, and future perspectives of eliminating neglected tropical disease. Sci. Afr. 2024, 24, e02165. [Google Scholar] [CrossRef]
- Casulli, A. New global targets for NTDs in the WHO roadmap 2021–2030. PLoS Negl. Trop. Dis. 2021, 15, e0009373. [Google Scholar] [CrossRef]
- Fahal, A.H.; Bakhiet, S.M. Mycetoma and the environment. PLoS Negl. Trop. Dis. 2023, 17, e0011736. [Google Scholar] [CrossRef]
- Reis, C.M.S.; Reis-Filho, E.G.M. Mycetomas: An epidemiological, etiological, clinical, laboratory and therapeutic review. An. Bras. Dermatol. 2018, 93, 8–18. [Google Scholar] [CrossRef]
- Nyuykonge, B.; Siddig, E.E.; Nyaoke, B.A.; Zijlstra, E.E.; Verbon, A.; Bakhiet, S.M.; Fahal, A.H.; van de Sande, W.W.J. Using (1,3)-β-D-glucan concentrations in serum to monitor the response of azole therapy in patients with eumycetoma caused by Madurella mycetomatis. Mycoses 2024, 67, e13664. [Google Scholar] [CrossRef]
- van de Sande, W.W.J.; Fahal, A.H. An updated list of eumycetoma causative agents and their differences in grain formation and treatment response. Clin. Microbiol. Rev. 2024, 37, e0003423. [Google Scholar] [CrossRef]
- Clark, J.E.; Kim, H.Y.; van de Sande, W.W.J.; McMullan, B.; Verweij, P.; Alastruey-Izquierdo, A.; Chakrabarti, A.; Harrison, T.S.; Bongomin, F.; Hay, R.J.; et al. Eumycetoma causative agents: A systematic review to inform the World Health Organization priority list of fungal pathogens. Med. Mycol. 2024, 62, myae044. [Google Scholar] [CrossRef]
- Fahal, A.H.; Ahmed, E.S.; Bakhiet, S.M.; Bakhiet, O.E.; Fahal, L.A.; Mohamed, A.A.; Mohamedelamin, E.S.W.; Bahar, M.E.N.; Attalla, H.Y.; Siddig, E.E.; et al. Two dose levels of once-weekly fosravuconazole versus daily itraconazole in combination with surgery in patients with eumycetoma in Sudan: A randomized, double-blind, phase 2, proof-of-concept superiority trial. Lancet Infect. Dis. 2024, 24, 1254–1265. [Google Scholar] [CrossRef]
- Mann, S.; Frasca, K.; Scherrer, S.; Hernao-Martínez, A.F.; Newman, S.; Ramanan, P.; Suarez, J.A. A review of leishmaniasis: Current knowledge and future directions. Curr. Trop. Med. Rep. 2021, 8, 121–132. [Google Scholar] [CrossRef] [PubMed]
- WHO-Factsheets. Available online: http://www.who.int/mediacentre/factsheets/fs375/en/ (accessed on 9 August 2024).
- De Vries, H.J.C.; Schallig, H.D. Cutaneous leishmaniasis: A 2022 updated narrative review into diagnosis and management developments. Am. J. Clin. Dermatol. 2022, 23, 823–840. [Google Scholar] [CrossRef] [PubMed]
- Mathison, B.A.; Bradley, B.T. Review of the clinical presentation, pathology, diagnosis, and treatment of leishmaniasis. Lab. Med. 2023, 54, 363–371. [Google Scholar] [CrossRef]
- Al-Salem, W.S.; Solórzano, C.; Weedall, G.D.; Dyer, N.A.; Kelly-Hope, L.; Casas-Sánchez, A.; Alraey, Y.; Alyamani, E.J.; Halliday, A.; Balghonaim, S.M.; et al. Old World cutaneous leishmaniasis treatment response varies depending on parasite species, geographical location and development of secondary infection. Parasit. Vectors 2019, 12, 195. [Google Scholar] [CrossRef]
- Pinheiro, A.C.; de Souza, M.V.N. Current leishmaniasis drug discovery. RSC Med. Chem. 2022, 13, 1029–1043. [Google Scholar] [CrossRef]
- Wesołowski, R.; Pawłowska, M.; Smoguła, M.; Szewczyk-Golec, K. Advances and challenges in diagnostics of toxoplasmosis in HIV-infected patients. Pathogens 2023, 12, 110. [Google Scholar] [CrossRef]
- Wesołowski, R.; Pawłowska, M.; Mila-Kierzenkowska, C. The medical relevance of Toxoplasma infections in terms of the safety of blood recipients under immunosuppression—A meta-analysis. Microorganisms 2023, 11, 1980. [Google Scholar] [CrossRef]
- Araujo Coelho, D.R.; Oliveira da Luz, R.; Soares Melegario, C.; Vieira, W.F.; Bahia-Oliveira, L.M.G. Knowledge gaps and educational opportunities in congenital toxoplasmosis: A narrative review of Brazilian and global perspectives. Trop. Med. Infect. Dis. 2024, 9, 137. [Google Scholar] [CrossRef]
- Dunay, I.R.; Gajurel, K.; Dhakal, R.; Liesenfeld, O.; Montoya, J.G. Treatment of toxoplasmosis: Historical perspective, animal models, and current clinical practice. Clin. Microbiol. Rev. 2018, 31, e00057-17. [Google Scholar] [CrossRef]
- Crespo, M.; Quereda, C.; Pascual, J.; Rivera, M.; Clemente, L.; Cano, T. Patterns of sulfadiazine acute nephrotoxicity. Clin. Nephrol. 2000, 54, 68–72. [Google Scholar]
- Montazeri, M.; Mehrzadi, S.; Sharif, M.; Sarvi, S.; Tanzifi, A.; Aghayan, S.A.; Daryani, A. Drug resistance in Toxoplasma gondii. Front. Microbiol. 2018, 9, 2587. [Google Scholar] [CrossRef] [PubMed]
- Venancio, A.N.; Silva, M.J.; Parreira, L.A.; Júlio, A.A.; Souza, G.R.; Santos, M.F.C.; Menini, L. Citronellal: A natural aldehyde with important properties. Nat. Prod. Res. 2024, 39, 1199–1212. [Google Scholar] [CrossRef] [PubMed]
- Rokonuzzman, M.; Bhuja, M.S.; Al-Qaaneh, A.M.; El-Nashar, H.A.S.; Islam, T.; Chowdhury, R.; Shanto, H.H.; Al Hasan, M.S.; El-Shazly, M.; Islam, M.T. Biomedical perspectives of citronellal: Biological activities, toxicological profile and molecular mechanisms. Chem. Biodivers. 2024, 22, e202401973. [Google Scholar] [CrossRef]
- Gouveia, R.G.; Oliveira, N.R.; Andrade-Júnior, F.P.; Ferreira, R.C.; Amorim, G.M.W.; Silva, D.K.F.; Duarte, S.S.; Medeiros, C.I.S.; Oliveira-Filho, A.A.; Lima, E.O. Antifungal effect of (R) and (S)-citronellal enantiomers and their predictive mechanism of action on Candida albicans from voriconazole-resistant onychomycoses. Braz. J. Biol. 2023, 83, e271530. [Google Scholar] [CrossRef]
- Pereira, P.S.; Oliveira, C.V.B.; Maia, A.J.; Vega-Gomez, M.C.; Rolón, M.; Coronel, C.; Duarte, A.E.; Coutinho, H.D.M.; Siyadatpanah, A.; Norouzi, R.; et al. Evaluation of the in vitro antiparasitic effect of the essential oil of Cymbopogon winterianus and its chemical composition analysis. Molecules 2022, 27, 2753. [Google Scholar] [CrossRef]
- Riad, N.; Zahi, M.R.; Bouzidi, N.; Daghbouche, Y.; Touafek, O.; El Hattab, M. Occurrence of marine ingredients in fragrance: Update on the state of knowledge. Chemistry 2021, 3, 1437–1463. [Google Scholar] [CrossRef]
- Api, A.M.; Bartlett, A.; Belsito, D.; Botelho, D.; Bruze, M.; Bryant-Friedrich, A.; Burton, G.A., Jr.; Cancellieri, M.A.; Chon, H.; Cronin, M.; et al. RIFM fragrance ingredient safety assessment, 4-(4,8-dimethylnona-3,7-dienyl)pyridine, CAS registry number 38462-23-6. Food Chem. Toxicol. 2024, 194, 115109. [Google Scholar] [CrossRef]
- Sahu, D.; Sreekanth, P.S.R.; Behera, P.K.; Pradhan, M.K.; Patnaik, A.; Salunkhe, S.; Cep, R. Advances in synthesis, medicinal properties and biomedical applications of pyridine derivatives: A comprehensive review. Eur. J. Med. Chem. Rep. 2024, 12, 100210. [Google Scholar] [CrossRef]
- Islam, M.B.; Islam, M.I.; Nath, N.; Bin Emran, T.; Rahman, M.R.; Sharma, R.; Matin, M.M. Recent advances in pyridine scaffold: Focus on chemistry, synthesis, and antibacterial activity. BioMed Res. Int. 2023, 2023, 9967591. [Google Scholar] [CrossRef]
- Mao, X.; Auer, D.L.; Buchalla, W.; Hiller, K.-A.; Maisch, T.; Hellwig, E.; Al-Ahmad, A.; Cieplik, F. Cetylpyridinium chloride: Mechanism of action, antimicrobial efficacy in biofilms, and potential risk of resistance. Antimicrob. Agents Chemother. 2020, 64, e00576-20. [Google Scholar] [CrossRef]
- Otarbayeva, S.; Berillo, D. Poly(vinyl alcohol) drug and PVA–drug–surfactant complex organogel with dimethyl sulfoxide as a drug delivery system. Gels 2024, 10, 753. [Google Scholar] [CrossRef] [PubMed]
- Biersack, B. Anticancer activity and modes of action of (arene)ruthenium(II) complexes coordinated to C-, N-, and O- ligands. Mini-Rev. Med. Chem. 2016, 16, 804–814. [Google Scholar] [CrossRef] [PubMed]
- Briš, A.; Jašík, J.; Turel, I.; Roithová, J. Anti-cancer organoruthenium(II) complexes and their interactions with cysteine and its analogues. A mass-spectrometric study. Dalton Trans. 2019, 48, 2626–2634. [Google Scholar] [CrossRef]
- Al Nasr, I.S.; Daoud, I.; Koko, W.S.; Khan, T.A.; Schobert, R.; Ben Said, R.; Amdouni, N.; Rahali, S.; Al-Ghamdi, A.O.; Biersack, B. Activity of (η6-arene)dichloridoruthenium(II) complexes with antifungal imidazolyl-based ligands against Toxoplasma gondii and Leishmania major. Inorg. Chim. Acta 2024, 565, 122005. [Google Scholar] [CrossRef]
- Ma, J.; Todd, M.; van de Sande, W.W.J.; Biersack, B. Antifungal activity of natural naphthoquinones and anthraquinones against Madurella mycetomatis. Chem. Biodivers. 2023, 20, e202300151. [Google Scholar] [CrossRef]
- Al Nasr, I.S.; Hanachi, R.; Said, R.B.; Rahali, S.; Tangour, B.; Abdelwahab, S.I.; Farasani, A.; Taha, M.M.E.; Bidwai, A.; Koko, W.S.; et al. p-Trifluoromethyl- and p-pentafluorothio-substituted curcuminoids of the 2,6-di[(E)-benzylidene)]cycloalkanone type: Syntheses and activities against Leishmania major and Toxoplasma gondii parasites. Bioorg. Chem. 2021, 114, 105099. [Google Scholar] [CrossRef]
- Banerjee, P.; Kemmler, E.; Dunkel, M.; Preissner, R. ProTox 3.0: A webserver for the prediction of toxicity of chemicals. Nucleic Acids Res. 2024, 52, W513–W520. [Google Scholar] [CrossRef]
- González-Minero, F.J.; Bravo-Díaz, L.; Moreno-Toral, E. Pharmacy and fragrances: Traditional and current use of plants and their extracts. Cosmetics 2023, 10, 157. [Google Scholar] [CrossRef]
- Al Nasr, I.S.; Koko, W.S.; Khan, T.A.; Schobert, R.; Biersack, B. Antiparasitic activities of acyl hydrazones from cinnamaldehydes and structurally related fragrances. Antibiotics 2024, 13, 1114. [Google Scholar] [CrossRef]
- Konings, M.; Eadie, K.; Lim, W.; Fahal, A.H.; Mouton, J.; Tesse, N.; van de Sande, W.W.J. The synthetic cinnamon oil CIN-102 is active against Madurella mycetomatis, the most common causative agent of mycetoma. PLoS Negl. Trop. Dis. 2021, 15, e0009488. [Google Scholar] [CrossRef]
- Maziere, M.; Rompante, P.; Andrade, J.C.; Rodrigues, C.F. Are mouthwashes really effective against Candida spp.? J. Fungi 2024, 10, 528. [Google Scholar] [CrossRef] [PubMed]
- Kloezen, W.; Parel, F.; Brüggemann, R.; Asouit, K.; Helvert-van Poppel, M.; Fahal, A.; Mouton, J.; van de Sande, W. Amphotericin B and terbinafine but not the azoles prolong survival in Galleria mellonella larvae infected with Madurella mycetomatis. Med. Mycol. 2018, 56, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Fedorowicz, J.; Saczewski, J. Advances in the synthesis of biologically active quaternary ammonium compounds. Int. J. Mol. Sci. 2024, 25, 4649. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Casanova, Y.; Bravo-Chaucanés, C.P.; Martínez, A.X.H.; Costa, G.M.; Contreras-Herrera, J.L.; Medina, R.F.; Rivera-Monroy, Z.J.; García-Castañeda, J.E.; Parra-Giraldo, C.M. Combining the peptide RWQWRWQWR and an ethanolic extract of Bidens pilosa enhances the activity against sensitive and resistant Candida albicans and C. auris strains. J. Fungi 2023, 9, 817. [Google Scholar] [CrossRef]
- Vargas-Casanova, Y.; Bravo-Chaucanés, C.P.; Fuentes, S.d.l.C.; Martinez-Lopez, R.; Monteoliva, L.; Gil, C.; Rivera-Monroy, Z.J.; Costa, G.M.; Castañeda, J.E.G.; Parra-Giraldo, C.M. Antifungal synergy: Mechanistic insights into the R-1-R peptide and Bidens pilosa extract as potent therapeutics against Candida spp. through proteomics. Int. J. Mol. Sci. 2024, 25, 8938. [Google Scholar] [CrossRef]
- do Nascimento Dias, J.; Hurtado Erazo, F.A.; Bessa, L.J.; Eaton, P.; Leite, J.R.d.S.d.A.; Paes, H.C.; Nicola, A.M.; Silva-Pereira, I.; Albuquerque, P. Synergic effect of the antimicrobial peptide ToAP2 and fluconazole on Candida albicans biofilms. Int. J. Mol. Sci. 2024, 25, 7769. [Google Scholar] [CrossRef]
- Nahar, D.; Mohite, P.; Lonkar, A.; Chidrawar, V.R.; Dodiya, R.; Uddin, M.J.; Singh, S.; Prajapati, B.G. An insight into new strategies and targets to combat antifungal resistance: A comprehensive review. Eur. J. Med. Chem. Rep. 2024, 10, 100120. [Google Scholar] [CrossRef]
- Berg, D.; Regel, E.; Harenberg, H.E.; Plempel, M. Bifonazole and clotrimazole. Their mode of action and the possible reason for the fungicidal behaviour of bifonazole. Arzneimittel-Forschung 1984, 34, 139–146. [Google Scholar]
- Fromtling, R.A. Overview of medically important antifungal azole derivatives. Clin. Microbiol. Rev. 1988, 1, 187–217. [Google Scholar] [CrossRef]
- Teixeira, M.M.; Carvalho, D.T.; Sousa, E.; Pinto, E. New antifungal agents with azole moieties. Pharmaceuticals 2022, 15, 1427. [Google Scholar] [CrossRef]
- Fahal, A.H.; Ahmed, K.O.; Saeed, A.A.; Elkhawad, A.O.; Bakhiet, S.M. Why the mycetoma patients are still neglected. PLoS Negl. Trop. Dis. 2022, 16, e0010945. [Google Scholar] [CrossRef] [PubMed]
- Salajkova, S.; Benkova, M.; Marek, J.; Sleha, R.; Prchal, L.; Malinak, D.; Dolezal, R.; Sepcic, K.; Gunde-Cimerman, N.; Kuca, K.; et al. Wide-antimicrobial spectrum of picolinium salts. Molecules 2020, 25, 2254. [Google Scholar] [CrossRef] [PubMed]
- Elsheikha, H.M.; Marra, C.M.; Zhu, X.-Q. Epidemiology, pathophysiology, diagnosis, and management of cerebral toxoplasmosis. Clin. Microbiol. Rev. 2021, 34, e00115-19. [Google Scholar] [CrossRef]
- Ortiz-Martínez, Y.; Kouamé, M.G.; Bongomin, F.; Lakoh, S.; Henao-Martínez, A.F. Human African trypanosomiasis (sleeping sickness)–epidemiology, clinical manifestations, diagnosis, treatment, and prevention. Curr. Trop. Med. Rep. 2023, 10, 222–234. [Google Scholar] [CrossRef]
- Grace, E.; Asbill, S.; Virga, K. Naegleria fowleri: Pathogenesis, diagnosis, and treatment options. Antimicrob. Agents Chemother. 2015, 59, 6677–6681. [Google Scholar] [CrossRef]
- Qureshi, Z.A.; Ghazanfar, H.; Altaf, F.; Ghazanfar, A.; Hasan, K.Z.; Kandhi, S.; Fortuzi, K.; Dileep, A.; Shrivastava, S. Cryptococcosis and cryptococcal meningitis: A narrative review and the up-to-date management approach. Cureus 2024, 16, e55498. [Google Scholar] [CrossRef]
- Jooste, J.; Legoabe, L.J.; Ilbeigi, K.; Caljon, G.; Beteck, R.M. Hydrazinated geraniol derivatives as potential broad-spectrum antiprotozoal agents. Arch. Pharm. 2024, 357, e2400430. [Google Scholar] [CrossRef]
- Tan, S.; Tong, W.H.; Vyas, A. Impact of plant-based foods and nutraceuticals on Toxoplasma gondii cysts: Nutritional therapy as a viable approach for managing chronic brain toxoplasmosis. Front. Nutr. 2022, 9, 827286. [Google Scholar] [CrossRef]
- Vermeersch, M.; da Luz, R.I.; Toté, K.; Timmermans, J.-P.; Cos, P.; Maes, L. In vitro susceptibilities of Leishmania donovani promastigote and amastigote stages to antileishmanial reference drugs: Practical relevance of stage-specific differences. Antimicrob. Agents Chemother. 2009, 53, 3855–3859. [Google Scholar] [CrossRef]
- de Mulder, G.; Ang, K.K.H.; Chen, S.; Arkin, M.R.; Engel, J.C.; McKerrow, J.H. A screen against Leishmania intracellular amastigotes: Comparison to a promastigote screen and identification of a host cell-specific hit. PLoS Negl. Trop. Dis. 2011, 5, e1253. [Google Scholar] [CrossRef]
- Griewank, K.; Gazeau, C.; Eichhorn, A.; von Strebut, E. Miltefosine efficiently eliminates Leishmania major amastigotes from infected murine dendritic cells without altering their immune functions. Antimicrob. Agents Chemother. 2010, 54, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Sarouey, L.A.; Khanaliha, K.; Rahimi-Moghaddam, P.; Khorrami, S.; Dayer, M.S.; Tabataie, F. In vitro effects of ketotifen and cromolyn sodium on promastigotes and amastigotes of Leishmania major. Jundishapur J. Microbiol. 2019, 12, e82389. [Google Scholar]
- Ma, J.; Eadie, K.; Fahal, A.; Verbon, A.; van de Sande, W.W.J. The performance and costs of XTT, resazurin, MTS and luciferin as viability dyes in in vitro susceptibility testing of Madurella mycetomatis. Trans. R. Soc. Trop. Med. Hyg. 2024, 118, 729–735. [Google Scholar] [CrossRef]
- Palic, S.; Beijen, J.H.; Dorlo, T.P.C. An update on the clinical pharmacology of miltefosine in the treatment of leishmaniasis. Int. J. Antimicrob. Agents 2022, 59, 106459. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yan, R.; Liu, Y.; Yu, M.; He, Z.; Xiao, J.; Li, K.; Liu, G.; Ning, Q.; Li, Y. Progress in antileishmanial drugs: Mechanisms, challenges, and prospects. PLoS Negl. Trop. Dis. 2025, 19, e0012735. [Google Scholar] [CrossRef]
- Shahinas, D.; Debnath, A.; Benedict, C.; McKerrow, J.H.; Pillai, D.R. Heat shock protein 90 inhibitors repurposed against Entamoeba histolytica. Front. Microbiol. 2015, 6, 368. [Google Scholar] [CrossRef]
- Li, L.; Yang, M.; Li, C.; Liu, Y. Virtual screening based identification of miltefosine and octenidine as inhibitors of heat shock protein 90. Naunyn Schmiedebergs Arch. Pharmacol. 2021, 394, 2223–2232. [Google Scholar] [CrossRef]
- Porta, E.O.; Gao, L.; Denny, P.W.; Steel, P.G.; Kalesh, K. Inhibition of HSP90 distinctively modulates the global phosphoproteome of Leishmania mexicana developmental stages. Microbiol. Spectr. 2023, 11, e0296023. [Google Scholar] [CrossRef]
- Petersen, A.L.d.O.A.; Cull, B.; Dias, B.R.S.; Palma, L.C.; Luz, Y.d.S.; de Menezes, J.P.B.; Mottram, J.C.; Veras, P.S.T. 17-AAG-induced activation of the autophagic pathway in Leishmania is associated with parasite death. Microorganisms 2021, 9, 1089. [Google Scholar] [CrossRef]
- Santos, D.M.; Petersen, A.L.O.A.; Celes, F.S.; Borges, V.M.; Veras, P.S.T.; de Oliveira, C.I. Chemotherapeutic potential of 17-AAG against cutaneous leishmaniasis caused by Leishmania (Viannia) braziliensis. PLoS Negl. Trop. Dis. 2014, 8, e3275. [Google Scholar] [CrossRef]
- Cruz, K.P.; Petersen, A.L.O.A.; Amorim, M.F.; Pinho, A.G.S.F.; Palma, L.C.; Dantas, D.A.S.; Silveira, M.R.G.; Silva, C.S.A.; Cordeiro, A.L.J.; Oliveira, I.G.; et al. Intraperitoneal administration of 17-DMAG as an effective treatment against Leishmania braziliensis infection in BALB/c mice: A preclinical study. Pathogens 2024, 13, 630. [Google Scholar] [CrossRef] [PubMed]
- Angel, S.O.; Matrajt, M.; Echeverria, P.V. A review of recent patents on the protozoan parasite HSP90 as a drug target. Recent Pat. Biotechnol. 2013, 7, 2–8. [Google Scholar] [CrossRef]
- Zhen, C.; Lu, H.; Jiang, Y. Novel promising antifungal target proteins for conquering invasive fungal infections. Front. Microbiol. 2022, 13, 911322. [Google Scholar] [CrossRef]
- Palma, L.C.; Ferreira, L.F.G.R.; Petersen, A.L.O.A.; Dias, B.R.S.; de Menezes, J.P.B.; Moreira, D.R.M.; Hernandes, M.Z.; Veras, P.S.T. A docking-based structural analysis of geldanamycin-derived inhibitor binding to human or Leishmania Hsp90. Sci. Rep. 2019, 9, 14756. [Google Scholar] [CrossRef]
- Catalán, M.; Olmedo, I.; Faúndez, J.; Jara, J.A. Medicinal chemistry targeting mitochondria: From new vehicles and pharmacophore groups to old drugs with mitochondrial activity. Int. J. Mol. Sci. 2020, 21, 8684. [Google Scholar] [CrossRef]
- Benaim, G.; Garcia, C.R.S. Targeting calcium homeostasis as the therapy of Chagas’ disease and leishmaniasis—A review. Trop. Biomed. 2011, 28, 471–481. [Google Scholar]
- Brindha, J.; Balamurali, M.M.; Chanda, K. An overview on the therapeutics of neglected infectious diseases—Leishmaniasis and Chagas diseases. Front. Chem. 2021, 9, 622286. [Google Scholar]
- Abdualmjid, R.J.; Sergi, C.M. Mitochondrial dysfunction and induction of apoptosis in hepatocellular carcinoma and cholangiocarcinoma cell lines by thymoquinone. Int. J. Mol. Sci. 2022, 23, 14669. [Google Scholar] [CrossRef]
- Qureshi, K.A.; Imtiaz, M.; Al Nasr, I.; Koko, W.S.; Khan, T.A.; Jaremko, M.; Mahmood, S.; Fatmi, M.Q. Antiprotozoal activity of thymoquinone (2-isopropyl-5- methyl-1,4-benzoquinone) for the treatment of Leishmania major-induced leishmaniasis: In silico and in vitro studies. Antibiotics 2022, 11, 1206. [Google Scholar] [CrossRef]
- Islamuddin, M.; Ali, A.; Afzal, O.; Ali, A.; Ali, I.; Altamimi, A.S.A.; Alamri, M.A.; Kato, K.; Parveen, S. Thymoquinone induced leishmanicidal effect via programmed cell death in Leishmania donovani. ACS Omega 2022, 7, 10718–10728. [Google Scholar] [CrossRef]
- Mahmoudvand, H.; Tavakoli, R.; Sharififar, F.; Minaie, K.; Ezatpour, B.; Jahanbakhsh, S.; Sharifi, I. Leishmanicidal and cytotoxic activities of Nigella sativa and its active principle, thymoquinone. Pharm. Biol. 2015, 53, 1052–1057. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.C.J.; Carloto, A.C.M.; Goncalves, M.D.; Concato, V.M.; Detoni, M.B.; dos Santos, Y.M.; Cruz, E.M.S.; Madureira, M.B.; Nunes, A.P.; Pires, M.F.M.K.; et al. Exploring the leishmanicidal potential of terpenoids: A comprehensive review on mechanisms of cell death. Front. Cell. Infect. Microbiol. 2023, 13, 1260448. [Google Scholar] [CrossRef] [PubMed]
- Katsuno, K.; Burrows, J.N.; Duncam, K.; van Huijsduijnen, R.H.; Kaneko, T.; Kita, K.; Mowbray, C.E.; Schmatz, D.; Warner, P.; Slingsby, B.T. Hit and lead criteria in drug discovery for infectious diseases of the developing world. Nat. Rev. Drug Discov. 2015, 14, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Kumar, V.; Tiwari, R.K.; Ravidas, V.; Pandey, K.; Kumar, A. Amphotericin B: A drug of choice for visceral leishmaniasis. Acta Trop. 2022, 235, 106661. [Google Scholar] [CrossRef]
- Araujo, H.C.; Arias, L.S.; Caldeirao, A.C.M.; de Freitas Assumpcao, L.C.; Morceli, M.G.; de Souza Neto, F.N.; de Camargo, E.R.; Oliveira, S.H.P.; Pessan, J.P.; Monteiro, D.R. Novel colloidal nanocarrier of cetylpyridinium chloride: Antifungal activities on Candida species and cytotoxic potential on murine fibroblasts. J. Fungi 2020, 6, 218. [Google Scholar] [CrossRef]
- Hartmann, D.O.; Shimizu, K.; Rothkegel, M.; Petkovic, M.; Ferraz, R.; Petrovski, Z.; Branco, L.C.; Lopes, J.N.C.; Pereira, C.S. Tailoring amphotericin B as an ionic liquid: An upfront strategy to potentiate the biological activity of antifungal drugs. RSC Adv. 2021, 11, 14441. [Google Scholar] [CrossRef]
- Park, J.-B.; Kang, J.-H.; Song, K.B. Antibacterial activities of a cinnamon essential oil with cetylpyridinium chloride emulsion against Escherichia coli O157:H7 and Salmonella Typhimurium in basil leaves. Food Sci. Biotechnol. 2018, 27, 47–55. [Google Scholar] [CrossRef]
- Fang, J.-Y.; Lin, Y.-K.; Wang, P.-W.; Alalaiwe, A.; Yang, Y.-C.; Yang, S.-C. The droplet-size effect of squalene@cetylpyridinium chloride nanoemulsions on antimicrobial potency against planktonic and biofilm MRSA. Int. J. Nanomed. 2019, 14, 8133–8147. [Google Scholar] [CrossRef]
- Hwang, Y.Y.; Ramalingam, K.; Bienek, D.R.; Lee, V.; You, T.; Alvarez, R. Antimicrobial activity of nanoemulsion in combination with cetylpyridinium chloride in multidrug-resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 2013, 57, 3568–3575. [Google Scholar] [CrossRef]
- Marek, J.; Stodulka, P.; Cabal, J.; Soukup, O.; Pohanka, M.; Korabecny, J.; Musilek, K.; Kuca, K. Preparation of the pyridinium salts differing in the length of the N-alkyl substituent. Molecules 2010, 15, 1967–1972. [Google Scholar] [CrossRef]
- Stöter, M.; Biersack, B.; Reimer, N.; Herling, M.; Stock, N.; Schobert, R.; Breu, J. Ordered heterostructures of two strictly alternating types of nanoreactors. Chem. Mater. 2014, 26, 5412–5419. [Google Scholar] [CrossRef]
- van Deun, R.; Nockemann, P.; Parac-Vogt, T.N.; van Hecke, K.; van Meervelt, L.; Görller-Walrand, C.; Binnemans, K. Near-infrared photoluminescence of lanthanide complexes containing the hemicyanine chromophore. Polyhedron 2007, 26, 5441–5447. [Google Scholar] [CrossRef]
- Lim, W.; Nyuykonge, B.; Eadie, K.; Konings, M.; Smeets, J.; Fahal, A.; Bonifaz, A.; Todd, M.; Perry, B.; Samby, K.; et al. Screening the pandemic response box identified benzimidazole carbamates, olorofim and ravuconazole as promising drug candidates for the treatment of eumycetoma. PLoS Negl. Trop. Dis. 2022, 16, e0010159. [Google Scholar] [CrossRef] [PubMed]


| Compounds | 100 μM 1 | 25 μM 1 | IC50 [μM] |
|---|---|---|---|
| Maritima | 13.8 ± 33.8 | 54.3 ± 7.5 | - |
| Cetyl-Maritima | 13.1 ± 2.0 | 12.2 ± 6.9 | 8.2 |
| Ru-Maritima | −6.6 ± 12.2 | 155.7 ± 22.2 | - |
| CPBr | 4.0 ± 0.8 | 7.9 ± 1.5 | 16.0 |
| C4PicBr | 5.0 ± 0.8 | 18.4 ± 17.7 | 16.1 |
| C2PicBr | 4.5 ± 0.8 | 8.7 ± 0.8 | 16.1 |
| TMP-16 | 19.1 ± 1.1 | 13.3 ± 1.5 | 18.2 |
| DAMS-16 | 25.4 ± 4.3 | 56.9 ± 69.3 | 16.0 |
| Itraconazole 2 | 2.34 ± 4.49 | −1.04 ± 2.37 | 0.09 |
| Compounds | T. gondii (μM) | Vero (μM) | SI 1 |
|---|---|---|---|
| Maritima | >314 | >314 | - |
| Cetyl-Maritima | 8.6 ± 1.2 | 11.4 ± 1.3 | 1.3 |
| ATO 2 | 0.07 ± 0.01 | 9.5 ± 0.8 | 135 |
| Compounds | Promastigotes (μM) | Amastigotes (μM) | Macrophages (μM) | SI 1 |
|---|---|---|---|---|
| Maritima | >314 | >314 | >314 | - |
| Cetyl-Maritima | 1.5 ± 0.2 | 0.60 ± 0.2 | 4.3 ± 0.5 | 7.2 |
| AmB 2 | 0.83 ± 0.1 | 0.47 ± 0.2 | 8.1 ± 0.9 | 17.2 |
| Compounds | Maritima | Cetyl-Maritima | CPBr |
|---|---|---|---|
| Mol. Weight (g/mol) | 229.36 | 454.79 (without Br−) | 304.53 (without Br−) |
| H-Bond Acceptors | 1 | 0 | 0 |
| H-Bond Donors | 0 | 0 | 0 |
| No. of Atoms/Bonds (non-H) | 17 | 33 | 22 |
| Rotatable Bonds | 6 | 21 | 15 |
| Molecular Refractivity | 76.33 | 154.23 | 102.14 |
| Topological Polar Surface Area (Å2) | 12.89 | 3.88 | 3.88 |
| logP 1 | 4.71 | 10.08 | 6.46 |
| Oral Toxicity (LD50, mg/kg) 2 | 1750 | 525 | 946 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Nasr, I.S.; Ma, J.; Khan, T.A.; Koko, W.S.; Ben Abdelmalek, I.; Schobert, R.; van de Sande, W.; Biersack, B. Antiparasitic and Antifungal Activities of Cetyl-Maritima, a New N-Cetyl-Modified Maritima Derivative. Antibiotics 2025, 14, 321. https://doi.org/10.3390/antibiotics14030321
Al Nasr IS, Ma J, Khan TA, Koko WS, Ben Abdelmalek I, Schobert R, van de Sande W, Biersack B. Antiparasitic and Antifungal Activities of Cetyl-Maritima, a New N-Cetyl-Modified Maritima Derivative. Antibiotics. 2025; 14(3):321. https://doi.org/10.3390/antibiotics14030321
Chicago/Turabian StyleAl Nasr, Ibrahim S., Jingyi Ma, Tariq A. Khan, Waleed S. Koko, Imen Ben Abdelmalek, Rainer Schobert, Wendy van de Sande, and Bernhard Biersack. 2025. "Antiparasitic and Antifungal Activities of Cetyl-Maritima, a New N-Cetyl-Modified Maritima Derivative" Antibiotics 14, no. 3: 321. https://doi.org/10.3390/antibiotics14030321
APA StyleAl Nasr, I. S., Ma, J., Khan, T. A., Koko, W. S., Ben Abdelmalek, I., Schobert, R., van de Sande, W., & Biersack, B. (2025). Antiparasitic and Antifungal Activities of Cetyl-Maritima, a New N-Cetyl-Modified Maritima Derivative. Antibiotics, 14(3), 321. https://doi.org/10.3390/antibiotics14030321






