In Vitro and In Vivo Antibacterial and Antibiofilm Activity of Zinc Sulfate (ZnSO4) and Carvacrol (CV) Alone and in Combination with Antibiotics Against Pseudomonas aeruginosa
Abstract
:1. Introduction
2. Results
2.1. Selected Agents for Experiments
2.2. Antimicrobial Synergy Investigation—Planktonic Cultures
2.3. Antimicrobial Synergy Investigation—Biofilms
2.4. In Vivo Respiratory Infection in a Murine Model
2.4.1. Pulmonary Bacterial Clearance
2.4.2. Host Immune Response
2.4.3. Histopathological Analysis
3. Discussion
4. Materials and Methods
4.1. Ethics Approval
4.2. Bacterial Strain and Culture Media
4.3. Stock and Working Metal(loid)-Based Antibiotic (MBA) Solutions
4.4. Planktonic Susceptibility
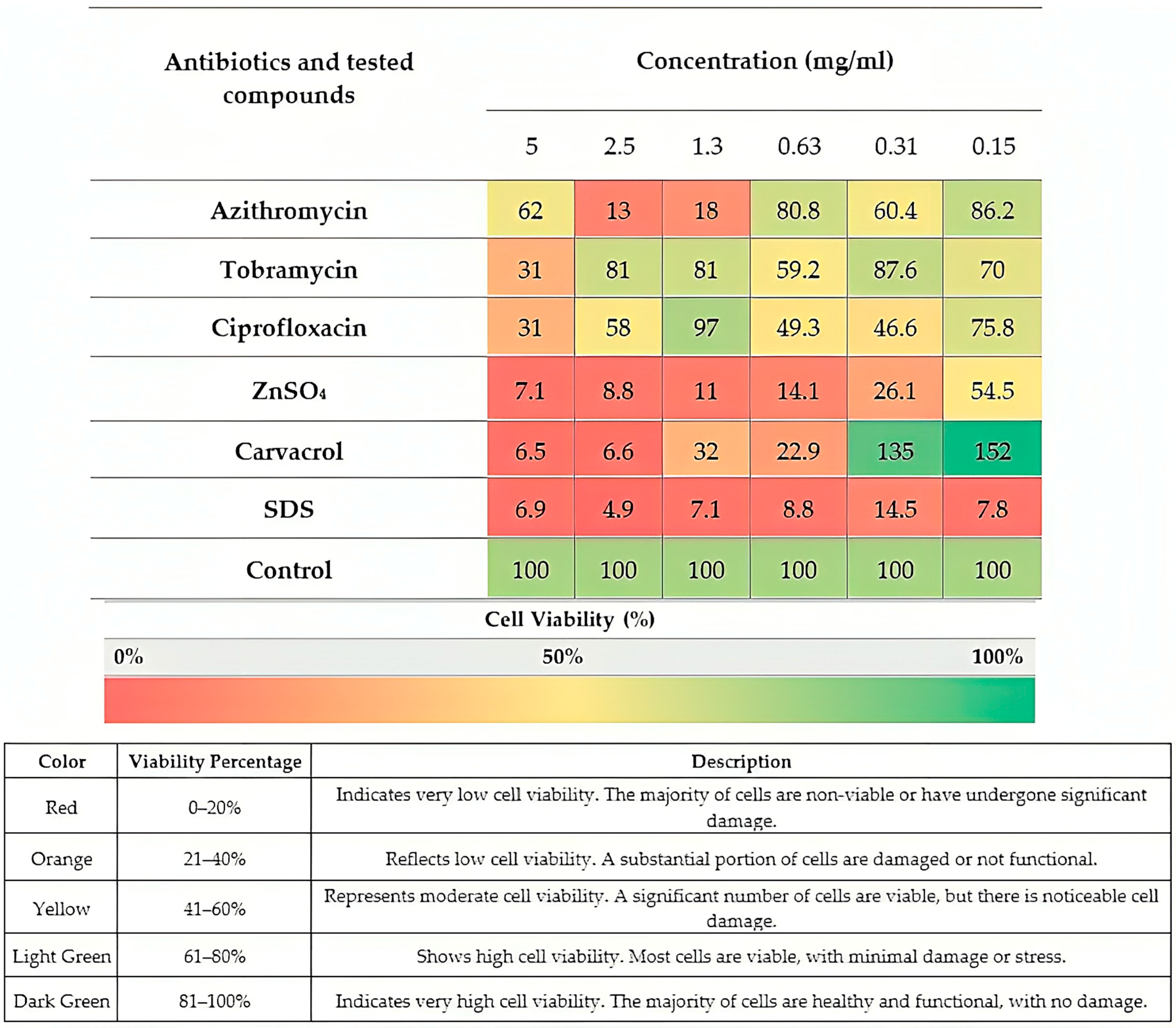
4.5. In Vitro Assay for Cell Viability and Cytotoxicity
Cell Culture
4.6. Synergism Susceptibility Testing of Microbial Planktonic Growth
Determination of FIC for the Detection of Synergism Effects
4.7. Biofilm Cultivation
4.8. Determining the Minimal Biofilm Inhibition Concentration (MBIC)
4.9. Minimum Biofilm Eradication Concentration (MBEC)
4.10. Synergism High-Throughput Susceptibility Testing of Microbial Biofilm Growth
Fractional Biofilm Inhibition and Eradication Concentration (FBIC/FBEC)
4.11. In Vivo Experiments (PAO1 Respiratory Infection in a Murine Model)
4.11.1. Blood and Lung Collection
4.11.2. Hematologic Assessment
4.11.3. Histopathological Studies
4.12. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ZnSO4 | zinc sulfate |
| CV | carvacrol |
| CIP | ciprofloxacin |
| TOB | tobramycin |
| AZM | azithromycin |
| MIC | minimum inhibitory concentration |
| MBC | minimum bactericidal concentration |
| FIC | fractional inhibitory concentration |
| FBC | fractional bactericidal concentration |
| MBIC | minimum biofilm inhibition concentration |
| MBEC | minimum biofilm eradication concentration |
| PBC | plant-based natural compounds |
| MBA(s) | metal(loid)-based antimicrobials |
| IT | intratracheal |
| IP | intraperitoneal |
| PO | oral |
| MRC-5 | human lung fibroblast cells |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| CFU | colony-forming unit |
| WBC | white blood cell |
| RBC | red blood cell |
| WHO | World Health Organization |
| COPD | chronic obstructive pulmonary disease |
| PBS | phosphate-buffered saline |
| FOXP3 | forkhead box P3 |
| IFNγ | interferon gamma |
| TGF-β | transforming growth factor beta |
| NF-κB | nuclear factor kappa B |
| IL | interleukin |
| MAPK | mitogen-activated protein kinase |
| ARDS | acute respiratory distress syndrome |
| CBD | Calgary biofilm device |
References
- Villeret, B.; Ghinnagow, R.; Kheir, S.; Born-Bony, M.; Kolls, J.K.; Garcia-Verdugo, I.; Sallenave, J.-M. Pseudomonas aeruginosa lung infection subverts lymphocytic responses through IL-23 and IL-22 post-transcriptional regulation. Int. J. Mol. Sci. 2022, 23, 8427. [Google Scholar] [CrossRef] [PubMed]
- O’Shaughnessy, M.; McCarron, P.; Viganor, L.; McCann, M.; Devereux, M.; Howe, O. The antibacterial and anti-biofilm activity of metal complexes incorporating 3, 6, 9-trioxaundecanedioate and 1, 10-phenanthroline ligands in clinical isolates of Pseudomonas aeruginosa from irish cystic fibrosis patients. Antibiotics 2020, 9, 674. [Google Scholar]
- Mosadegh, M.; Asadian, R.; Emamie, A.D.; Rajabpour, M.; Najafinasab, E.; Pourmand, M.R.; Azarsa, M. Impact of laboratory methods and gene targets on detection of Streptococcus pneumoniae in isolates and clinical specimens. Rep. Biochem. Mol. Biol. 2020, 9, 216. [Google Scholar]
- Varponi, I.; Ferro, S.; Menilli, L.; Grapputo, A.; Moret, F.; Mastrotto, F.; Marin, O.; Sandrelli, F. Fighting Pseudomonas aeruginosa infections: Antibacterial and antibiofilm activity of D-Q53 cecB, a synthetic analog of a silkworm natural cecropin B variant. Int. J. Mol. Sci. 2023, 24, 12496. [Google Scholar] [CrossRef]
- Garcia-Clemente, M.; de la Rosa, D.; Máiz, L.; Girón, R.; Blanco, M.; Olveira, C.; Canton, R.; Martinez-García, M.A. Impact of Pseudomonas aeruginosa infection on patients with chronic inflammatory airway diseases. J. Clin. Med. 2020, 9, 3800. [Google Scholar] [CrossRef]
- Faure, E.; Kwong, K.; Nguyen, D. Pseudomonas aeruginosa in chronic lung infections: How to adapt within the host? Front. Immunol. 2018, 9, 2416. [Google Scholar]
- Lyu, J.; Chen, H.; Bao, J.; Liu, S.; Chen, Y.; Cui, X.; Guo, C.; Gu, B.; Li, L. Clinical distribution and drug resistance of Pseudomonas aeruginosa in Guangzhou, China from 2017 to 2021. J. Clin. Med. 2023, 12, 1189. [Google Scholar] [CrossRef]
- Jangra, V.; Sharma, N.; Chhillar, A.K. Therapeutic approaches for combating Pseudomonas aeruginosa infections. Microbes Infect. 2022, 24, 104950. [Google Scholar]
- Emerson, J.; Rosenfeld, M.; McNamara, S.; Ramsey, B.; Gibson, R.L. Pseudomonas aeruginosa and other predictors of mortality and morbidity in young children with cystic fibrosis. Pediatr. Pulmonol. 2002, 34, 91–100. [Google Scholar] [CrossRef]
- Wood, D.M.; Smyth, A.R. Antibiotic strategies for eradicating Pseudomonas aeruginosa in people with cystic fibrosis. In Cochrane Database of Systematic Reviews; John Wiley & Sons: Hoboken, NJ, USA, 2006; p. Cd004197. [Google Scholar] [CrossRef]
- UK Cystic Fibrosis TrustWorking Group. Antibiotic Treatment for Cystic Fibrosis, 3rd ed.; Cystic Fibrosis TrustWorking Group: London, UK, 2009. [Google Scholar]
- Smith, S.; Rowbotham, N.J.; Regan, K.H. Inhaled anti-pseudomonal antibiotics for long-term therapy in cystic fibrosis. In Cochrane Database of Systematic Reviews; John Wiley & Sons: Hoboken, NJ, USA, 2018; Volume 3, p. Cd001021. [Google Scholar] [CrossRef]
- Torres, A.; Bauer, T.T.; León-Gil, C.; Castillo, F.; Alvarez-Lerma, F.; Martínez-Pellús, A.; Leal-Noval, S.R.; Nadal, P.; Palomar, M.; Blanquer, J.; et al. Treatment of severe nosocomial pneumonia: A prospective randomised comparison of intravenous ciprofloxacin with imipenem/cilastatin. Thorax 2000, 55, 1033–1039. [Google Scholar] [CrossRef]
- Peterson, J.W. Bacterial Pathogenesis. In Medical Microbiology, 4th ed.; Baron, S., Ed.; University of Texas Medical Branch: Galveston, TX, USA, 1996; Chapter 7. [Google Scholar]
- Bulska, M.; Orszulak-Michalak, D. Immunomodulatory and anti-inflammatory properties of macrolides. Curr. Issues Pharm. Med. Sci. 2014, 27, 61–64. [Google Scholar] [CrossRef]
- Nguyen, D.; Singh, P.K. Evolving stealth: Genetic adaptation of Pseudomonas aeruginosa during cystic fibrosis infections. Proc. Natl. Acad. Sci. USA 2006, 103, 8305–8306. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.E.; Buckley, D.G.; Wu, Z.; Saenphimmachak, C.; Hoffman, L.R.; D’Argenio, D.A.; Miller, S.I.; Ramsey, B.W.; Speert, D.P.; Moskowitz, S.M.; et al. Genetic adaptation by Pseudomonas aeruginosa to the airways of cystic fibrosis patients. Proc. Natl. Acad. Sci. USA 2006, 103, 8487–8492. [Google Scholar] [CrossRef]
- Pormohammad, A.; Hansen, D.; Turner, R.J. Antibacterial, antibiofilm, and antioxidant activity of 15 different plant-based natural compounds in comparison with ciprofloxacin and gentamicin. Antibiotics 2022, 11, 1099. [Google Scholar] [CrossRef]
- Chen, X.; Su, S.; Yan, Y.; Yin, L.; Liu, L. Anti-Pseudomonas aeruginosa activity of natural antimicrobial peptides when used alone or in combination with antibiotics. Front. Microbiol. 2023, 14, 1239540. [Google Scholar]
- Mosadegh, M.; Habibi Ghahfarokhi, S.; Ahmadi, A.; Pourmand, M.R.; Erfani, Y.; Mashhadi, R. Identification and molecular characterization of penicillin-nonsusceptible Streptococcus pneumoniae isolates recovered from invasive infections in a pre-pneumococcal vaccine era. J. Clin. Lab. Anal. 2022, 36, e24566. [Google Scholar]
- Christophersen, L.; Schwartz, F.A.; Lerche, C.J.; Svanekjær, T.; Kragh, K.N.; Laulund, A.S.; Thomsen, K.; Henneberg, K.-Å.; Sams, T.; Høiby, N. In vivo demonstration of Pseudomonas aeruginosa biofilms as independent pharmacological microcompartments. J. Cyst. Fibros. 2020, 19, 996–1003. [Google Scholar]
- Roilides, E.; Simitsopoulou, M.; Katragkou, A.; Walsh, T.J. How biofilms evade host defenses. Microb. Biofilms 2015, 3, 287–300. [Google Scholar]
- Masihzadeh, S.; Amin, M.; Farshadzadeh, Z. In vitro and in vivo antibiofilm activity of the synthetic antimicrobial peptide WLBU2 against multiple drug resistant Pseudomonas aeruginosa strains. BMC Microbiol. 2023, 23, 131. [Google Scholar]
- Kolpen, M.; Kragh, K.N.; Enciso, J.B.; Faurholt-Jepsen, D.; Lindegaard, B.; Egelund, G.B.; Jensen, A.V.; Ravn, P.; Mathiesen, I.H.M.; Gheorge, A.G. Bacterial biofilms predominate in both acute and chronic human lung infections. Thorax 2022, 77, 1015–1022. [Google Scholar]
- Vestby, L.K.; Grønseth, T.; Simm, R.; Nesse, L.L. Bacterial biofilm and its role in the pathogenesis of disease. Antibiotics 2020, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Mosadegh, M.; Khalkhali, A.; Erfani, Y.; Nezamdoost, M. The effect of Nutrition Bio-shield superfood (NBS) on disease severity and laboratory biomarkers in patients with COVID-19: A randomized clinical trial. Microb. Pathog. 2022, 172, 105792. [Google Scholar] [PubMed]
- Pormohammad, A.; Turner, R.J. Silver antibacterial synergism activities with eight other metal (loid)-based antimicrobials against Escherichia coli, Pseudomonas aeruginosa, and Staphylococcus aureus. Antibiotics 2020, 9, 853. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.J. Metal-Based antimicrobial strategies. Microb. Biotechnol. 2017, 10, 1062–1065. [Google Scholar]
- Turner, R.J. The good, the bad, and the ugly of metals as antimicrobials. Biometals 2024, 37, 545–559. [Google Scholar] [CrossRef]
- Pormohammad, A.; Firrincieli, A.; Salazar-Alemán, D.A.; Mohammadi, M.; Hansen, D.; Cappelletti, M.; Zannoni, D.; Zarei, M.; Turner, R.J. Insights into the synergistic antibacterial activity of silver nitrate with potassium tellurite against Pseudomonas aeruginosa. Microbiol. Spectr. 2023, 11, e0062823. [Google Scholar]
- Cuajungco, M.P.; Ramirez, M.S.; Tolmasky, M.E. Zinc: Multidimensional Effects on Living Organisms. Biomedicines 2021, 9, 208. [Google Scholar] [CrossRef]
- Abdelraheem, W.M.; Kamel, H.S.; Gamil, A.N. Evaluation of anti-biofilm and anti-virulence effect of zinc sulfate on Staphylococcus aureus isolates. Sci. Rep. 2024, 14, 25747. [Google Scholar] [CrossRef]
- Shebl, R.I.; Elkhatib, W.F.; Badawy, M.S.E.M. Modulating the transcriptomic profile of multidrug-resistant Klebsiella pneumoniae biofilm formation by antibiotics in combination with zinc sulfate. Ann. Clin. Microbiol. Antimicrob. 2023, 22, 84. [Google Scholar] [CrossRef]
- Skalny, A.V.; Rink, L.; Ajsuvakova, O.P.; Aschner, M.; Gritsenko, V.A.; Alekseenko, S.I.; Svistunov, A.A.; Petrakis, D.; Spandidos, D.A.; Aaseth, J.; et al. Zinc and respiratory tract infections: Perspectives for COVID-19 (Review). Int. J. Mol. Med. 2020, 46, 17–26. [Google Scholar] [CrossRef]
- Lila-Krasniqi, Z.D.; Bajrami Halili, R.; Hamza, V.; Krasniqi, S. Evaluation of Antimicrobial Effectiveness of Dental Cement Materials on Growth of Different Bacterial Strains. Med. Sci. Monit. Basic Res. 2022, 28, e937893. [Google Scholar] [CrossRef] [PubMed]
- Elkhatib, W.; Noreddin, A. In Vitro Antibiofilm Efficacies of Different Antibiotic Combinations with Zinc Sulfate against Pseudomonas aeruginosa Recovered from Hospitalized Patients with Urinary Tract Infection. Antibiotics 2014, 3, 64–84. [Google Scholar] [CrossRef] [PubMed]
- Guzzo, F.; Scognamiglio, M.; Fiorentino, A.; Buommino, E.; D’Abrosca, B. Plant derived natural products against Pseudomonas aeruginosa and Staphylococcus aureus: Antibiofilm activity and molecular mechanisms. Molecules 2020, 25, 5024. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Hernández, G.B.; Taboada-Rodríguez, A.; Marin-Iniesta, F. Plant Bioactive Compounds in Foods and Food Packages. Foods 2024, 13, 1419. [Google Scholar] [CrossRef]
- Baser, K.H.C. Biological and Pharmacological Activities of Carvacrol and Carvacrol Bearing Essential Oils. Curr. Pharm. Des. 2008, 14, 3106–3119. [Google Scholar] [CrossRef]
- Deepak, V.; Kasonga, A.; Kruger, M.C.; Coetzee, M. Carvacrol inhibits osteoclastogenesis and negatively regulates the survival of mature osteoclasts. Biol. Pharm. Bull. 2016, 39, 1150–1158. [Google Scholar]
- Friedman, M. Chemistry and multibeneficial bioactivities of carvacrol (4-isopropyl-2-methylphenol), a component of essential oils produced by aromatic plants and spices. J. Agric. Food Chem. 2014, 62, 7652–7670. [Google Scholar]
- Cicalău, G.I.P.; Babes, P.A.; Calniceanu, H.; Popa, A.; Ciavoi, G.; Iova, G.M.; Ganea, M.; Scrobotă, I. Anti-Inflammatory and Antioxidant Properties of Carvacrol and Magnolol, in Periodontal Disease and Diabetes Mellitus. Molecules 2021, 26, 6899. [Google Scholar] [CrossRef]
- Force, M.; Sparks, W.S.; Ronzio, R.A. Inhibition of enteric parasites by emulsified oil of oregano in vivo. Phytother. Res. 2000, 14, 213–214. [Google Scholar]
- Mousa, S.; El-Hamid, A.; Haggran, A.; El-Kawokgy, M.; El-Kheir, Z.; Sabry, S.; Rashad, S. Assessment of Cytotoxicity and Genotoxicity Response of Zinc Sulphate on Eukaryotic Cells. Egypt. J. Chem. 2022, 65, 707–725. [Google Scholar]
- Krawczyk, S.J.; Leśniczak-Staszak, M.; Gowin, E.; Szaflarski, W. Mechanistic Insights into Clinically Relevant Ribosome-Targeting Antibiotics. Biomolecules 2024, 14, 1263. [Google Scholar] [CrossRef] [PubMed]
- Heidary, M.; Ebrahimi Samangani, A.; Kargari, A.; Kiani Nejad, A.; Yashmi, I.; Motahar, M.; Taki, E.; Khoshnood, S. Mechanism of action, resistance, synergism, and clinical implications of azithromycin. J. Clin. Lab. Anal. 2022, 36, e24427. [Google Scholar] [CrossRef] [PubMed]
- Dalhoff, A. Pharmacokinetics and pharmacodynamics of aerosolized antibacterial agents in chronically infected cystic fibrosis patients. Clin. Microbiol. Rev. 2014, 27, 753–782. [Google Scholar] [CrossRef]
- Hasanvand, T.; Mohammadi, M.; Abdollahpour, F.; Kamarehie, B.; Jafari, A.; Ghaderpoori, A.; Karami, M.A. A comparative study on antibacterial activity of carvacrol and glutaraldehyde on Pseudomonas aeruginosa and Staphylococcus aureus isolates: An in vitro study. J. Environ. Health Sci. Eng. 2021, 19, 475–482. [Google Scholar]
- Sharifi-Rad, M.; Varoni, E.M.; Iriti, M.; Martorell, M.; Setzer, W.N.; del Mar Contreras, M.; Salehi, B.; Soltani-Nejad, A.; Rajabi, S.; Tajbakhsh, M. Carvacrol and human health: A comprehensive review. Phytother. Res. 2018, 32, 1675–1687. [Google Scholar] [CrossRef]
- Walczak, M.; Michalska-Sionkowska, M.; Olkiewicz, D.; Tarnawska, P.; Warżyńska, O. Potential of carvacrol and thymol in reducing biofilm formation on technical surfaces. Molecules 2021, 26, 2723. [Google Scholar] [CrossRef]
- Mechmechani, S.; Gharsallaoui, A.; Fadel, A.; El Omari, K.; Khelissa, S.; Hamze, M.; Chihib, N.-E. Microencapsulation of carvacrol as an efficient tool to fight Pseudomonas aeruginosa and Enterococcus faecalis biofilms. PLoS ONE 2022, 17, e0270200. [Google Scholar] [CrossRef]
- Gobin, M.; Proust, R.; Lack, S.; Duciel, L.; Des Courtils, C.; Pauthe, E.; Gand, A.; Seyer, D. A combination of the natural molecules gallic acid and carvacrol eradicates P. aeruginosa and S. aureus mature biofilms. Int. J. Mol. Sci. 2022, 23, 7118. [Google Scholar] [CrossRef]
- Nong, W.; Guan, W.; Yin, Y.; Lu, C.; Wang, Q.; Luo, Y.; Zhang, B.; Xu, Z.; Wu, J.; Guan, Y. Photo-Triggered on-demand carvacrol vapor release from nano-generators for non-contact bacterial inactivation between nanomaterials and bacteria. Chem. Eng. J. 2021, 420, 129874. [Google Scholar] [CrossRef]
- Sampaio, L.A.; Pina, L.T.S.; Serafini, M.R.; Tavares, D.D.S.; Guimarães, A.G. Antitumor Effects of Carvacrol and Thymol: A Systematic Review. Front. Pharmacol. 2021, 12, 702487. [Google Scholar] [CrossRef]
- Bakhtiari, R.; Shiri, M.; Reza Mohammadi, M.; Reza Pourmand, M.; Mirzaie, A.; Taghiabadi, Z. Enhanced antimicrobial effects of carvacrol against methicillin-resistant Staphylococcus aureus strains using niosome formulations. Rev. Argent. de Microbiol. 2025, 57, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Khazdair, M.R.; Boskabady, M.H. The effect of carvacrol on inflammatory mediators and respiratory symptoms in veterans exposed to sulfur mustard, a randomized, placebo-controlled trial. Respir. Med. 2019, 150, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Ghorani, V.; Alavinezhad, A.; Rajabi, O.; Boskabady, M.H. Carvacrol improves pulmonary function tests, oxidant/antioxidant parameters and cytokine levels in asthmatic patients: A randomized, double-blind, clinical trial. Phytomedicine 2021, 85, 153539. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, F.O.; Silva, É.R.; Gomes, I.A.; Santana, H.S.R.; do Nascimento Santos, D.; de Oliveira Souza, G.P.; de Jesus Silva, D.; Monteiro, J.C.M.; de Albuquerque Júnior, R.L.C.; de Souza Araújo, A.A. Anti-Inflammatory and antioxidant activity of carvacrol in the respiratory system: A systematic review and meta-analysis. Phytother. Res. 2020, 34, 2214–2229. [Google Scholar]
- Luan, R.; Ding, D.; Xue, Q.; Li, H.; Wang, Y.; Yang, J. Protective role of zinc in the pathogenesis of respiratory diseases. Eur. J. Clin. Nutr. 2023, 77, 427–435. [Google Scholar] [CrossRef]
- Sadeghsoltani, F.; Mohammadzadeh, I.; Safari, M.-M.; Hassanpour, P.; Izadpanah, M.; Qujeq, D.; Moein, S.; Vaghari-Tabari, M. Zinc and respiratory viral infections: Important trace element in anti-viral response and immune regulation. Biol. Trace Elem. Res. 2021, 200, 2556–2571. [Google Scholar] [CrossRef]
- Ramdas, I.; Devi, C.S. Inhibitory Effect of Zinc Sulfate on Clinical Isolates of Pseudomonas aeruginosa and Acinetobacter baumannii. J. Glob. Infect. Dis. 2020, 12, 217–218. [Google Scholar]
- Wessels, I.; Pupke, J.T.; von Trotha, K.-T.; Gombert, A.; Himmelsbach, A.; Fischer, H.J.; Jacobs, M.J.; Rink, L.; Grommes, J. Zinc supplementation ameliorates lung injury by reducing neutrophil recruitment and activity. Thorax 2020, 75, 253–261. [Google Scholar] [CrossRef]
- Liu, X.; Ali, M.K.; Dua, K.; Xu, R. The Role of Zinc in the Pathogenesis of Lung Disease. Nutrients 2022, 14, 2115. [Google Scholar] [CrossRef]
- Pormohammad, A.; Monych, N.K.; Turner, R.J. Zinc and SARS-CoV-2: A molecular modeling study of Zn interactions with RNA-dependent RNA-polymerase and 3C-like proteinase enzymes. Int. J. Mol. Med. 2021, 47, 326–334. [Google Scholar] [CrossRef]
- Asadi, S.; Nayeri-Fasaei, B.; Zahraei-Salehi, T.; Yahya-Rayat, R.; Shams, N.; Sharifi, A. Antibacterial and anti-biofilm properties of carvacrol alone and in combination with cefixime against Escherichia coli. BMC Microbiol. 2023, 23, 55. [Google Scholar]
- Puca, V.; Turacchio, G.; Marinacci, B.; Supuran, C.T.; Capasso, C.; Di Giovanni, P.; D’Agostino, I.; Carradori, S.; Grande, R. Antimicrobial and antibiofilm activities of carvacrol, amoxicillin and salicylhydroxamic acid alone and in combination vs. Helicobacter pylori: Towards a new multi-targeted therapy. Int. J. Mol. Sci. 2023, 24, 4455. [Google Scholar] [CrossRef] [PubMed]
- Ashrafudoulla, M.; Mizan, M.F.R.; Park, S.H.; Ha, S.-D. Antibiofilm activity of carvacrol against Listeria monocytogenes and Pseudomonas aeruginosa biofilm on MBEC™ biofilm device and polypropylene surface. LWT 2021, 147, 111575. [Google Scholar]
- Ghizlane, Z.; Hind, M.; Adnane, R. Carvacrol and thymol components inhibiting Pseudomonas aeruginosa adherence and biofilm formation. Afr. J. Microbiol. Res. 2011, 5, 3229–3232. [Google Scholar]
- Duplessis, C.; Warawa, J.M.; Lawrenz, M.B.; Henry, M.; Biswas, B. Successful intratracheal treatment of phage and antibiotic combination therapy of a multi-drug resistant Pseudomonas aeruginosa murine model. Antibiotics 2021, 10, 946. [Google Scholar] [CrossRef]
- He, S.; Gui, J.; Xiong, K.; Chen, M.; Gao, H.; Fu, Y. A roadmap to pulmonary delivery strategies for the treatment of infectious lung diseases. J. Nanobiotechnol. 2022, 20, 101. [Google Scholar] [CrossRef]
- Cresti, L.; Cappello, G.; Vailati, S.; Melloni, E.; Brunetti, J.; Falciani, C.; Bracci, L.; Pini, A. In Vivo Efficacy and Toxicity of an Antimicrobial Peptide in a Model of Endotoxin-Induced Pulmonary Inflammation. Int. J. Mol. Sci. 2023, 24, 7967. [Google Scholar] [CrossRef]
- Marzaman, A.N.F.; Roska, T.P.; Sartini, S.; Utami, R.N.; Sulistiawati, S.; Enggi, C.K.; Manggau, M.A.; Rahman, L.; Shastri, V.P.; Permana, A.D. Recent Advances in Pharmaceutical Approaches of Antimicrobial Agents for Selective Delivery in Various Administration Routes. Antibiotics 2023, 12, 822. [Google Scholar] [CrossRef]
- Mayer-Hamblett, N.; Retsch-Bogart, G.; Kloster, M.; Accurso, F.; Rosenfeld, M.; Albers, G.; Black, P.; Brown, P.; Cairns, A.; Davis, S.D.; et al. Azithromycin for Early Pseudomonas Infection in Cystic Fibrosis. The OPTIMIZE Randomized Trial. Am. J. Respir. Crit. Care Med. 2018, 198, 1177–1187. [Google Scholar] [CrossRef]
- Zhang, Y.-B.; Xu, D.; Bai, L.; Zhou, Y.-M.; Zhang, H.; Cui, Y.-L. A Review of Non-Invasive Drug Delivery through Respiratory Routes. Pharmaceutics 2022, 14, 1974. [Google Scholar] [CrossRef]
- Dugernier, J.; Ehrmann, S.; Sottiaux, T.; Roeseler, J.; Wittebole, X.; Dugernier, T.; Jamar, F.; Laterre, P.F.; Reychler, G. Aerosol delivery during invasive mechanical ventilation: A systematic review. Crit. Care 2017, 21, 264. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Rao, Y.; Liu, X.; Sun, L.; Gong, J.; Zhang, H.; Shen, L.; Bao, A.; Yang, H. Administration route governs the therapeutic efficacy, biodistribution and macrophage targeting of anti-inflammatory nanoparticles in the lung. J. Nanobiotechnol. 2021, 19, 56. [Google Scholar] [CrossRef]
- Szychowiak, P.; Desgrouas, M.; Ehrmann, S. Inhaled antibiotics in critical care: State of the art and future perspectives. Infect. Dis. Now 2022, 52, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Quon, B.S.; Goss, C.H.; Ramsey, B.W. Inhaled antibiotics for lower airway infections. Ann. Am. Thorac Soc. 2014, 11, 425–434. [Google Scholar] [CrossRef]
- Clark, A.R. Half a Century of Technological Advances in Pulmonary Drug Delivery: A Personal Perspective. Front. Drug Deliv. 2022, 2, 871147. [Google Scholar] [CrossRef]
- Wenzler, E.; Fraidenburg, D.R.; Scardina, T.; Danziger, L.H. Inhaled Antibiotics for Gram-Negative Respiratory Infections. Clin. Microbiol. Rev. 2016, 29, 581–632. [Google Scholar] [CrossRef]
- Spencer, S.; Felix, L.M.; Milan, S.J.; Normansell, R.; Goeminne, P.C.; Chalmers, J.D.; Donovan, T. Oral versus inhaled antibiotics for bronchiectasis. In Cochrane Database System Review; John Wiley & Sons: Hoboken, NJ, USA, 2018; Volume 3, p. Cd012579. [Google Scholar] [CrossRef]
- Rodvold, K.A.; George, J.M.; Yoo, L. Penetration of anti-infective agents into pulmonary epithelial lining fluid: Focus on antibacterial agents. Clin. Pharmacokinet. 2011, 50, 637–664. [Google Scholar] [CrossRef]
- Birchall, J. Pulmonary delivery of nucleic acids. Expert Opin. Drug Deliv. 2007, 4, 575–578. [Google Scholar] [CrossRef]
- Schanker, L.S.; Mitchell, E.W.; Brown, R.A., Jr. Species comparison of drug absorption from the lung after aerosol inhalation or intratracheal injection. Drug Metab. Dispos. 1986, 14, 79–88. [Google Scholar]
- Gunes-Bayir, A.; Guler, E.M.; Bilgin, M.G.; Ergun, I.S.; Kocyigit, A.; Dadak, A. Anti-Inflammatory and antioxidant effects of carvacrol on N-methyl-N′-nitro-N-Nitrosoguanidine (MNNG) induced gastric carcinogenesis in Wistar rats. Nutrients 2022, 14, 2848. [Google Scholar] [CrossRef]
- Albus, U. Guide for the Care and Use of Laboratory Animals (8th edn). (Book Review). Lab. Anim. 2012, 46, 267–268. [Google Scholar] [CrossRef]
- Lemire, J.A.; Kalan, L.; Bradu, A.; Turner, R.J. Silver oxynitrate, an unexplored silver compound with antimicrobial and antibiofilm activity. Antimicrob. Agents Chemother. 2015, 59, 4031–4039. [Google Scholar] [PubMed]
- Monych, N.K.; Turner, R.J. Multiple compounds secreted by Pseudomonas aeruginosa increase the tolerance of Staphylococcus aureus to the antimicrobial metals copper and silver. mSystems 2020, 5, e00746-20. [Google Scholar] [CrossRef] [PubMed]
- Sabet, M.; Miller, C.E.; Nolan, T.G.; Senekeo-Effenberger, K.; Dudley, M.N.; Griffith, D.C. Efficacy of aerosol MP-376, a levofloxacin inhalation solution, in models of mouse lung infection due to Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2009, 53, 3923–3928. [Google Scholar] [CrossRef]
- Hoffmann, N.; Lee, B.; Hentzer, M.; Rasmussen, T.B.; Song, Z.; Johansen, H.K.; Givskov, M.; Høiby, N. Azithromycin blocks quorum sensing and alginate polymer formation and increases the sensitivity to serum and stationary-growth-phase killing of Pseudomonas aeruginosa and attenuates chronic P. aeruginosa lung infection in Cftr−/− mice. Antimicrob. Agents Chemother. 2007, 51, 3677–3687. [Google Scholar]
- Pormohammad, A.; Moradi, M.; Hommes, J.W.; Pujol, E.; Naesens, L.; Vázquez, S.; Surewaard, B.G.J.; Zarei, M.; Vazquez-Carrera, M.; Turner, R.J. Novel pentafluorosulfanyl-containing triclocarban analogs selectively kill Gram-positive bacteria. Microbiol. Spectr. 2024, 12, e00071-24. [Google Scholar] [CrossRef]
- Calderón-Montaño, J.M.; Martínez-Sánchez, S.M.; Jiménez-González, V.; Burgos-Morón, E.; Guillén-Mancina, E.; Jiménez-Alonso, J.J.; Díaz-Ortega, P.; García, F.; Aparicio, A.; López-Lázaro, M. Screening for Selective Anticancer Activity of 65 Extracts of Plants Collected in Western Andalusia, Spain. Plants 2021, 10, 2193. [Google Scholar] [CrossRef]
- Ahmad, R.; Ahmad, N.; Naqvi, A.A. “Ziziphus oxyphylla”: Ethnobotanical, ethnopharmacological and phytochemical review. Biomed. Pharmacother. 2017, 91, 970–998. [Google Scholar] [CrossRef]
- Alotaibi, B.; Negm, W.A.; Elekhnawy, E.; El-Masry, T.A.; Elseady, W.S.; Saleh, A.; Alotaibi, K.N.; El-Sherbeni, S.A. Antibacterial, immunomodulatory, and lung protective effects of Boswellia dalzielii oleoresin ethanol extract in pulmonary diseases: In vitro and in vivo studies. Antibiotics 2021, 10, 1444. [Google Scholar]
- Isber, C.; Stockman, D.L.; Daoud, Z. Quadruple-Checkerboard: A Modification of the Three-Dimensional Checkerboard for Studying Drug Combinations. J. Vis. Exp. 2021, 173, e62311. [Google Scholar] [CrossRef]
- Moody, J. Synergism testing: Broth microdilution checkerboard and broth macrodilution method. In Clinical Microbiology Procedures Handbook; ASM Press: Washington, DC, USA, 2004; pp. 1–28. [Google Scholar]
- Bonapace, C.R.; Bosso, J.A.; Friedrich, L.V.; White, R.L. Comparison of methods of interpretation of checkerboard synergy testing. Diagn. Microbiol. Infect. Dis. 2002, 44, 363–366. [Google Scholar] [CrossRef] [PubMed]
- Ceri, H.; Olson, M.E.; Stremick, C.; Read, R.; Morck, D.; Buret, A. The Calgary Biofilm Device: New technology for rapid determination of antibiotic susceptibilities of bacterial biofilms. J. Clin. Microbiol. 1999, 37, 1771–1776. [Google Scholar] [CrossRef] [PubMed]
- Allkja, J.; Bjarnsholt, T.; Coenye, T.; Cos, P.; Fallarero, A.; Harrison, J.J.; Lopes, S.P.; Oliver, A.; Pereira, M.O.; Ramage, G. Minimum information guideline for spectrophotometric and fluorometric methods to assess biofilm formation in microplates. Biofilm 2020, 2, 100010. [Google Scholar]
- Aslam, B.; Wang, W.; Arshad, M.I.; Khurshid, M.; Muzammil, S.; Rasool, M.H.; Nisar, M.A.; Alvi, R.F.; Aslam, M.A.; Qamar, M.U. Antibiotic resistance: A rundown of a global crisis. Infect. Drug Resist. 2018, 11, 1645–1658. [Google Scholar]
- Harrison, J.J.; Turner, R.J.; Ceri, H. High-Throughput metal susceptibility testing of microbial biofilms. BMC Microbiol. 2005, 5, 53. [Google Scholar]
- Harrison, J.J.; Turner, R.J.; Joo, D.A.; Stan, M.A.; Chan, C.S.; Allan, N.D.; Vrionis, H.A.; Olson, M.E.; Ceri, H. Copper and quaternary ammonium cations exert synergistic bactericidal and antibiofilm activity against Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2008, 52, 2870–2881. [Google Scholar] [CrossRef]
- Pormohammad, A.; Greening, D.; Turner, R.J. Synergism inhibition and eradication activity of silver nitrate/potassium tellurite combination against Pseudomonas aeruginosa biofilm. J. Antimicrob. Chemother. 2022, 77, 1635–1644. [Google Scholar] [CrossRef]
- Doern, C.D. When does 2 plus 2 equal 5? A review of antimicrobial synergy testing. J. Clin. Microbiol. 2014, 52, 4124–4128. [Google Scholar]
- Chen, C.; Deslouches, B.; Montelaro, R.C.; Di, Y.P. Enhanced efficacy of the engineered antimicrobial peptide WLBU2 via direct airway delivery in a murine model of Pseudomonas aeruginosa pneumonia. Clin. Microbiol. Infect. 2018, 24, e1–e8. [Google Scholar]
- Oiso, Y.; Akita, T.; Kato, D.; Yamashita, C. Method for Pulmonary Administration Using Negative Pressure Generated by Inspiration in Mice. Pharmaceutics 2020, 12, 200. [Google Scholar] [CrossRef]
- Ortiz-Muñoz, G.; Looney, M.R. Non-invasive Intratracheal Instillation in Mice. Bio-Protocol 2015, 5, e1504. [Google Scholar] [CrossRef]
- Bell, R.R.; Nonavinakere, V.K.; Soliman, M.R.I. Intratracheal exposure of the guinea pig lung to cadmium and/or selenium: A histological evaluation. Toxicol. Lett. 2000, 114, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Beaulac, C.; Clément-Major, S.; Hawari, J.; Lagacé, J. Eradication of mucoid Pseudomonas aeruginosa with fluid liposome-encapsulated tobramycin in an animal model of chronic pulmonary infection. Antimicrob. Agents Chemother. 1996, 40, 665–669. [Google Scholar] [CrossRef] [PubMed]



| Agents | MIC (mg/mL) | MBC (mg/mL) |
|---|---|---|
| Carvacrol (CV) | 10 | 20 |
| Zinc sulfate (ZnSO4) | 2.5 | 5 |
| Ciprofloxacin (CIP) | 0.001 | 0.007 |
| Azithromycin (AZM) | 0.007 | 0.5 |
| Tobramycin (TOB) | 0.00025 | 0.003 |
| Agent A | Agent B | MIC/MBC (mg/mL) * | FIC/FBC Index ** | Outcome | |||
|---|---|---|---|---|---|---|---|
| Alone A | Alone B | Combination A | Combination B | ||||
| Carvacrol (CV) | Ciprofloxacin (CIP) | 10/20 | 0.001/0.007 | 0.009/0.078 | 0.0002/0.003 | 0.2/0.4 | Synergistic/synergistic |
| Zinc sulfate (ZnSO4) | Ciprofloxacin (CIP) | 2.5/5 | 0.001/0.007 | 0.01/0.156 | 0.0005/0.007 | 0.5/1 | Synergistic/partially synergistic |
| Ciprofloxacin (CIP) | CV + Zn | 0.001/0.007 | 1.25/2.5 | 0.0002/0.003 | 0.009/0.078 | 0.2/0.4 | Synergistic/synergistic |
| Carvacrol (CV) | Azithromycin (AZM) | 10/20 | 0.007/0.5 | 0.078/5 | 0.003/0.25 | 0.4/0.7 | Synergistic/synergistic |
| Zinc sulfate (ZnSO4) | Azithromycin (AZM) | 2.5/5 | 0.007/0.5 | 0.78/2.5 | 0.003/0.125 | 0.4/2 | Synergistic/antagonistic |
| Azithromycin (AZM) | CV + Zn | 0.007/0.5 | 1.25/5 | 0.015/0.0625 | 0.313/1.25 | 2.2/1.8 | Antagonistic |
| Carvacrol (CV) | Tobramycin (TOB) | 10/20 | 0.00025/0.003 | 0.009/0.009 | 0.00025/0.00025 | 1/0.08 | Partially synergistic/synergistic |
| Zinc sulfate (ZnSO4) | Tobramycin (TOB) | 1.25/5 | 0.00025/0.003 | 0.009/0.01 | 0.00025/0.0005 | 1/0.1 | Partially synergistic/synergistic |
| Tobramycin (TOB) | CV + Zn | 0.00025/0.003 | 1.25/2.5 | 0.0006/0.00025 | 0.004/0.009 | 0.2/0.08 | Synergistic/synergistic |
| Agent A | Agent B | Biofilm Eradication (mg/mL) * | FBEC Index ** | Outcome | |||
|---|---|---|---|---|---|---|---|
| Alone A | Alone B | Combination A | Combination B | ||||
| Carvacrol (CV) | Ciprofloxacin (CIP) | 20 | 0.0312 | 0.625 | 0.0312 | 1.03 | Partially synergistic |
| Zinc sulfate (ZnSO4) | Ciprofloxacin (CIP) | 5 | 0.0312 | 0.625 | 0.0312 | 1.1 | Partially synergistic |
| Ciprofloxacin (CIP) | CV + Zn | 0.0312 | 10 | 0.007 | 0.156 | 0.2 | Synergistic |
| Carvacrol (CV) | Azithromycin (AZM) | 20 | 2 | 5 | 0.25 | 0.3 | Synergistic |
| Zinc sulfate (ZnSO4) | Azithromycin (AZM) | 10 | 2 | 5 | 0.25 | 0.6 | Synergistic |
| Azithromycin (AZM) | CV + Zn | 2 | 10 | 0.125 | 2.5 | 0.4 | Synergistic |
| Carvacrol (CV) | Tobramycin (TOB) | 20 | 0.125 | 1.25 | 0.625 | 0.5 | Synergistic |
| Zinc sulfate (ZnSO4) | Tobramycin (TOB) | 2.5 | 0.125 | 0.625 | 0.0312 | 0.4 | Synergistic |
| Tobramycin (TOB) | CV + Zn | 0.125 | 2.5 | 0.0312 | 0.625 | 0.5 | Synergistic |
| Groups | Percentage of Alveolar Air Area (%) | Pneumonitis Score (Alveolar/Interstitial) | Periluminal Infiltrates Score (Around Airways/Vessels) | Percentage of Lung Tissue Involved (%) |
|---|---|---|---|---|
| Control | 80 | 0 | 0 | 0 |
| Bacteria | 10 | 3 | 3 | 80 |
| CV + Zn (IP *) | 55 | 2 | 2 | 45 |
| Ciprofloxacin + CV + Zn (IP) | 65 | 1 | 0.8 | 35 |
| Ciprofloxacin (IP) | 55 | 2 | 0.9 | 45 |
| Tobramycin (IT) | 50 | 2 | 2 | 60 |
| CV + Zn (IT *) | 65 | 1 | 0.5 | 8 |
| Tobramycin + CV + Zn (IT) | 70 | 1 | 0.2 | 5 |
| Azithromycin (PO *) | 60 | 2 | 1 | 55 |
| Azithromycin + CV + Zn (PO) | 65 | 1 | 0.7 | 30 |
| CV + Zn (PO) | 65 | 1 | 0.7 | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moradi, M.; Montazeri, E.A.; Rafiei Asl, S.; Pormohammad, A.; Farshadzadeh, Z.; Dayer, D.; Turner, R.J. In Vitro and In Vivo Antibacterial and Antibiofilm Activity of Zinc Sulfate (ZnSO4) and Carvacrol (CV) Alone and in Combination with Antibiotics Against Pseudomonas aeruginosa. Antibiotics 2025, 14, 367. https://doi.org/10.3390/antibiotics14040367
Moradi M, Montazeri EA, Rafiei Asl S, Pormohammad A, Farshadzadeh Z, Dayer D, Turner RJ. In Vitro and In Vivo Antibacterial and Antibiofilm Activity of Zinc Sulfate (ZnSO4) and Carvacrol (CV) Alone and in Combination with Antibiotics Against Pseudomonas aeruginosa. Antibiotics. 2025; 14(4):367. https://doi.org/10.3390/antibiotics14040367
Chicago/Turabian StyleMoradi, Melika, Effat Abbasi Montazeri, Sirous Rafiei Asl, Ali Pormohammad, Zahra Farshadzadeh, Dian Dayer, and Raymond J. Turner. 2025. "In Vitro and In Vivo Antibacterial and Antibiofilm Activity of Zinc Sulfate (ZnSO4) and Carvacrol (CV) Alone and in Combination with Antibiotics Against Pseudomonas aeruginosa" Antibiotics 14, no. 4: 367. https://doi.org/10.3390/antibiotics14040367
APA StyleMoradi, M., Montazeri, E. A., Rafiei Asl, S., Pormohammad, A., Farshadzadeh, Z., Dayer, D., & Turner, R. J. (2025). In Vitro and In Vivo Antibacterial and Antibiofilm Activity of Zinc Sulfate (ZnSO4) and Carvacrol (CV) Alone and in Combination with Antibiotics Against Pseudomonas aeruginosa. Antibiotics, 14(4), 367. https://doi.org/10.3390/antibiotics14040367








