Efficacy of Ceftobiprole and Daptomycin at Bone Concentrations Against Methicillin-Resistant Staphylococcus aureus Biofilm: Results of a Dynamic In Vitro PK/PD Model
Abstract
1. Introduction
2. Results
2.1. Pharmacokinetics
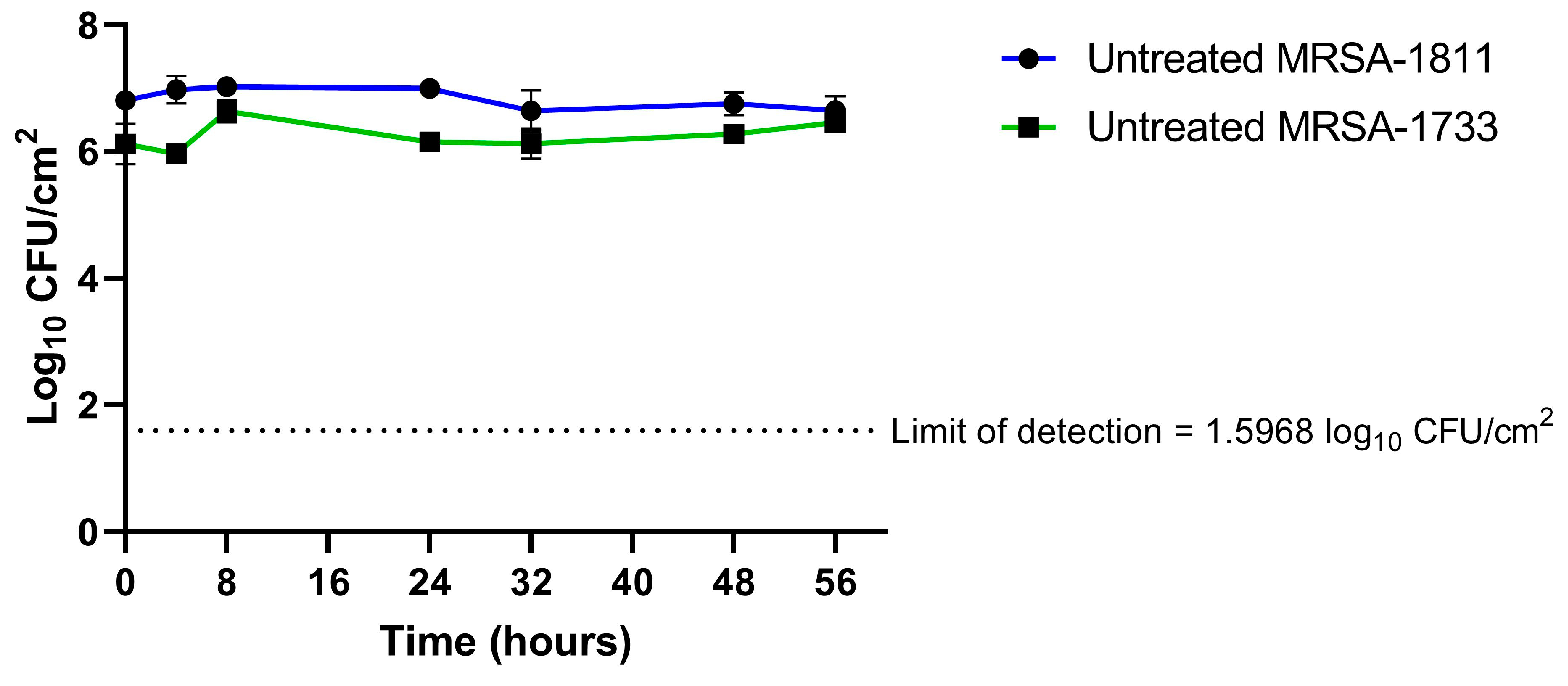
2.2. Biofilm Time–Kill Analyses
2.3. Emergence of Resistance
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains
4.2. Ethical Approval
4.3. Antimicrobials and Susceptibility Testing
4.4. Dynamic In Vitro Biofilm Model
4.5. Pharmacokinetic Analysis
4.6. Pharmacodynamic Analysis
4.7. Detection of Resistance Development
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brauner, A.; Fridman, O.; Gefen, O.; Balaban, N.Q. Distinguishing Between Resistance, Tolerance and Persistence to Antibiotic Treatment. Nat. Rev. Microbiol. 2016, 14, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Sendi, P.; Zimmerli, W. Antimicrobial Treatment Concepts for Orthopaedic Device-Related Infection. Clin. Microbiol. Infect. 2012, 18, 1176–1184. [Google Scholar] [CrossRef] [PubMed]
- Wouthuyzen-Bakker, M.; Sebillotte, M.; Lomas, J.; Taylor, A.; Palomares, E.B.; Murillo, O.; Parvizi, J.; Shohat, N.; Reinoso, J.C.; Sánchez, R.E.; et al. Clinical Outcome and Risk Factors for Failure in Late Acute Prosthetic Joint Infections Treated with Debridement and Implant Retention. J. Infect. 2019, 78, 40–47. [Google Scholar] [CrossRef]
- Shohat, N.; Goswami, K.; Tan, T.L.; Yayac, M.; Soriano, A.; Sousa, R.; Wouthuyzen-Bakker, M.; Parvizi, J.; On behalf of the ESCMID Study Group of Implant Associated Infections (ESGIAI) and the Northern Infection Network of Joint Arthroplasty (NINJA). 2020 Frank Stinchfield Award: Identifying Who Will Fail Following Irrigation and Debridement for Prosthetic Joint Infection: A Machine Learning-Based Validated Tool. Bone Jt. J. 2020, 102-B, 11–19. [Google Scholar] [CrossRef]
- Ariza, J.; Cobo, J.; Baraia-Etxaburu, J.; Benito, N.; Bori, G.; Cabo, J.; Corona, P.; Esteban, J.; Horcajada, J.P.; Lora-Tamayo, J.; et al. Executive Summary of Management of Prosthetic Joint Infections. Clinical Practice Guidelines by the Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC). Enfermedades Infecc. Microbiol. Clínica 2017, 35, 189–195. [Google Scholar] [CrossRef]
- Gould, I.M.; Miró, J.M.; Rybak, M.J. Daptomycin: The Role of High-Dose and Combination Therapy for Gram-Positive Infections. Int. J. Antimicrob. Agents 2013, 42, 202–210. [Google Scholar] [CrossRef]
- Garrigós, C.; Murillo, O.; Lora-Tamayo, J.; Verdaguer, R.; Tubau, F.; Cabellos, C.; Cabo, J.; Ariza, J. Efficacy of Daptomycin-Cloxacillin Combination in Experimental Foreign-Body Infection Due to Methicillin-Resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2012, 56, 3806–3811. [Google Scholar] [CrossRef]
- Abbanat, D.; Shang, W.; Amsler, K.; Santoro, C.; Baum, E.; Crespo-Carbone, S.; Lynch, A.S. Evaluation of the In Vitro Activities of Ceftobiprole and Comparators in Staphylococcal Colony or Microtitre Plate Biofilm Assays. Int. J. Antimicrob. Agents 2014, 43, 32–39. [Google Scholar] [CrossRef]
- Vaudaux, P.; Gjinovci, A.; Bento, M.; Li, D.; Schrenzel, J.; Lew, D.P. Intensive Therapy with Ceftobiprole Medocaril of Experimental Foreign-Body Infection by Methicillin-Resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2005, 49, 3789–3793. [Google Scholar] [CrossRef]
- Yin, L.-Y.; Calhoun, J.H.; Thomas, J.K.; Shapiro, S.; Schmitt-Hoffmann, A. Efficacies of Ceftobiprole Medocaril and Comparators in a Rabbit Model of Osteomyelitis Due to Methicillin-Resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2008, 52, 1618–1622. [Google Scholar] [CrossRef]
- Barber, K.E.; Werth, B.J.; Ireland, C.E.; Stone, N.E.; Nonejuie, P.; Sakoulas, G.; Pogliano, J.; Rybak, M.J. Potent Synergy of Ceftobiprole plus Daptomycin against Multiple Strains of Staphylococcus aureus with Various Resistance Phenotypes. J. Antimicrob. Chemother. 2014, 69, 3006–3010. [Google Scholar] [CrossRef]
- Grayson, M.L.; Cosgrove, S.E.; Crowe, S.M.; Hope, W.; McCarthy, J.S.; Mills, J.; Mouton, J.W.; Paterson, D.L. Kucers’ the Use of Antibiotics: A Clinical Review of Antibacterial, Antifungal, Antiparasitic and Antiviral Drugs, 7th ed.; Taylor & Francis Group, LLC: Boca Raton, FL, USA, 2018; ISBN 978-1-4987-4795-0. [Google Scholar]
- Landersdorfer, C.B. Penetration of Antibacterials into Bone. Clin. Pharmacokinet. 2009, 48, 89–124. [Google Scholar] [PubMed]
- Hall Snyder, A.D.; Vidaillac, C.; Rose, W.; McRoberts, J.P.; Rybak, M.J. Evaluation of High-Dose Daptomycin Versus Vancomycin Alone or Combined with Clarithromycin or Rifampin Against Staphylococcus aureus and S. epidermidis in a Novel In Vitro PK/PD Model of Bacterial Biofilm. Infect. Dis. Ther. 2015, 4, 51–65. [Google Scholar] [CrossRef]
- Hidalgo-Tenorio, C.; Pitto-Robles, I.; Arnés García, D.; De Novales, F.; Morata, L.; Mendez, R.; De Pablo, O.; López De Medrano, V.; Lleti, M.; Vizcarra, P.; et al. Cefto Real-Life Study: Real-World Data on the Use of Ceftobiprole in a Multicenter Spanish Cohort. Antibiotics 2023, 12, 1218. [Google Scholar] [CrossRef] [PubMed]
- El Haj, C.; Murillo, O.; Ribera, A.; Vivas, M.; Garcia-Somoza, D.; Tubau, F.; Cabo, J.; Ariza, J. Comparative Efficacies of Cloxacillin-Daptomycin and the Standard Cloxacillin-Rifampin Therapies against an Experimental Foreign-Body Infection by Methicillin-Susceptible Staphylococcus aureus. Antimicrob. Agents Chemother. 2014, 58, 5576–5580. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sharma, A.D.; Gutheil, W.G. Synergistic Combinations of FDA-Approved Drugs with Ceftobiprole against Methicillin-Resistant Staphylococcus aureus. Microbiol. Spectr. 2023, 11, e03726-22. [Google Scholar] [CrossRef]
- Barber, K.E.; Smith, J.R.; Ireland, C.E.; Boles, B.R.; Rose, W.E.; Rybak, M.J. Evaluation of Ceftaroline Alone and in Combination against Biofilm-Producing Methicillin-Resistant Staphylococcus aureus with Reduced Susceptibility to Daptomycin and Vancomycin in an In Vitro Pharmacokinetic/Pharmacodynamic Model. Antimicrob. Agents Chemother. 2015, 59, 4497–4503. [Google Scholar] [CrossRef]
- Reinisch, K.; Schläppi, M.; Meier, C.; Wahl, P. Local Antibiotic Treatment with Calcium Sulfate as Carrier Material Improves the Outcome of Debridement, Antibiotics, and Implant Retention Procedures for Periprosthetic Joint Infections after Hip Arthroplasty—A Retrospective Study. J. Bone Jt. Infect. 2022, 7, 11–21. [Google Scholar] [CrossRef]
- Ferguson, J.; Diefenbeck, M.; McNally, M. Ceramic Biocomposites as Biodegradable Antibiotic Carriers in the Treatment of Bone Infections. J. Bone Jt. Infect. 2017, 2, 38–51. [Google Scholar] [CrossRef]
- Totten, K.M.C.; Patel, R. Phage Activity against Planktonic and Biofilm Staphylococcus aureus Periprosthetic Joint Infection Isolates. Antimicrob. Agents Chemother. 2022, 66, e01879-21. [Google Scholar] [CrossRef]
- Cano, E.J.; Caflisch, K.M.; Bollyky, P.L.; Van Belleghem, J.D.; Patel, R.; Fackler, J.; Brownstein, M.J.; Horne, B.; Biswas, B.; Henry, M.; et al. Phage Therapy for Limb-Threatening Prosthetic Knee Klebsiella pneumoniae Infection: Case Report and In Vitro Characterization of Anti-Biofilm Activity. Clin. Infect. Dis. 2021, 73, e144–e151. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing, 32nd ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2022; ISBN 978-1-68440-134-5. [Google Scholar]
- Ceri, H.; Olson, M.E.; Stremick, C.; Read, R.R.; Morck, D.; Buret, A. The Calgary Biofilm Device: New Technology for Rapid Determination of Antibiotic Susceptibilities of Bacterial Biofilms. J. Clin. Microbiol. 1999, 37, 1771–1776. [Google Scholar] [CrossRef] [PubMed]
- Lora-Tamayo, J.; Murillo, O.; Bergen, P.J.; Nation, R.L.; Poudyal, A.; Luo, X.; Yu, H.Y.; Ariza, J.; Li, J. Activity of Colistin Combined with Doripenem at Clinically Relevant Concentrations Against Multidrug-Resistant Pseudomonas Aeruginosa in an In Vitro Dynamic Biofilm Model. J. Antimicrob. Chemother. 2014, 69, 2434–2442. [Google Scholar] [CrossRef] [PubMed]
- Blaser, J. In-Vitro Model for Simultaneous Simulation of the Serum Kinetics of Two Drugs with Different Half-Lives. J. Antimicrob. Chemother. 1985, 15, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Onaghise, O. Pharmaceutical Microbiology Manual; Food and Drug Administration Office of Regulatory Affairs: Rockville, MD, USA, 2020. [Google Scholar]

| Strain | MIC (mg/L) | MBEC (mg/L) | |||
|---|---|---|---|---|---|
| Ceftobiprole | Daptomycin | Cefazolin | Ceftobiprole | Daptomycin | |
| MRSA-1811 | 1.5 | 0.5 | >32 | >512 | >256 |
| MRSA-1733 | 0.5 | 1 | >32 | >256 | >256 |
| Antibiotic (Usual Human Dosage) | fCmax Plasma a (mg/L) | Bone Penetration b (%) | fCmax Model (mg/L) | Half-Life (t1/2) (h) | fAUC0–24 h (mg·h/L) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Theoretical | Experimental | Error (%) | Theoretical | Experimental | Error (%) | ||||
| Ceftobiprole (500 mg/8 h) | 42.5 | 21.4 | 9.1 | 8.9 | −1.7 | 3 | 2.89 | −3.5 | 40.42 |
| Daptomycin (10 mg/kg/24 h) | 141.1 | 8.6 | 12.1 | 11.2 | −7.4 | 8 | 7.66 | −4.2 | 114.7 |
| Cefazolin (2 g/24 h) | 404 | 17.9 | 72.3 | 77.0 | 6.6 | 1.8 | 1.86 | 3.1 | 224.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mancheño-Losa, M.; Meléndez-Carmona, M.Á.; Lumbreras, C.; Lora-Tamayo, J. Efficacy of Ceftobiprole and Daptomycin at Bone Concentrations Against Methicillin-Resistant Staphylococcus aureus Biofilm: Results of a Dynamic In Vitro PK/PD Model. Antibiotics 2025, 14, 386. https://doi.org/10.3390/antibiotics14040386
Mancheño-Losa M, Meléndez-Carmona MÁ, Lumbreras C, Lora-Tamayo J. Efficacy of Ceftobiprole and Daptomycin at Bone Concentrations Against Methicillin-Resistant Staphylococcus aureus Biofilm: Results of a Dynamic In Vitro PK/PD Model. Antibiotics. 2025; 14(4):386. https://doi.org/10.3390/antibiotics14040386
Chicago/Turabian StyleMancheño-Losa, Mikel, María Ángeles Meléndez-Carmona, Carlos Lumbreras, and Jaime Lora-Tamayo. 2025. "Efficacy of Ceftobiprole and Daptomycin at Bone Concentrations Against Methicillin-Resistant Staphylococcus aureus Biofilm: Results of a Dynamic In Vitro PK/PD Model" Antibiotics 14, no. 4: 386. https://doi.org/10.3390/antibiotics14040386
APA StyleMancheño-Losa, M., Meléndez-Carmona, M. Á., Lumbreras, C., & Lora-Tamayo, J. (2025). Efficacy of Ceftobiprole and Daptomycin at Bone Concentrations Against Methicillin-Resistant Staphylococcus aureus Biofilm: Results of a Dynamic In Vitro PK/PD Model. Antibiotics, 14(4), 386. https://doi.org/10.3390/antibiotics14040386






