A Laboratory Protocol for Routine Therapeutic Drug Monitoring of Beta-Lactams Antimicrobials in Horses and Dogs
Abstract
:1. Introduction
2. Results
2.1. Method Validation
2.2. Method Application
3. Discussion
4. Materials and Methods
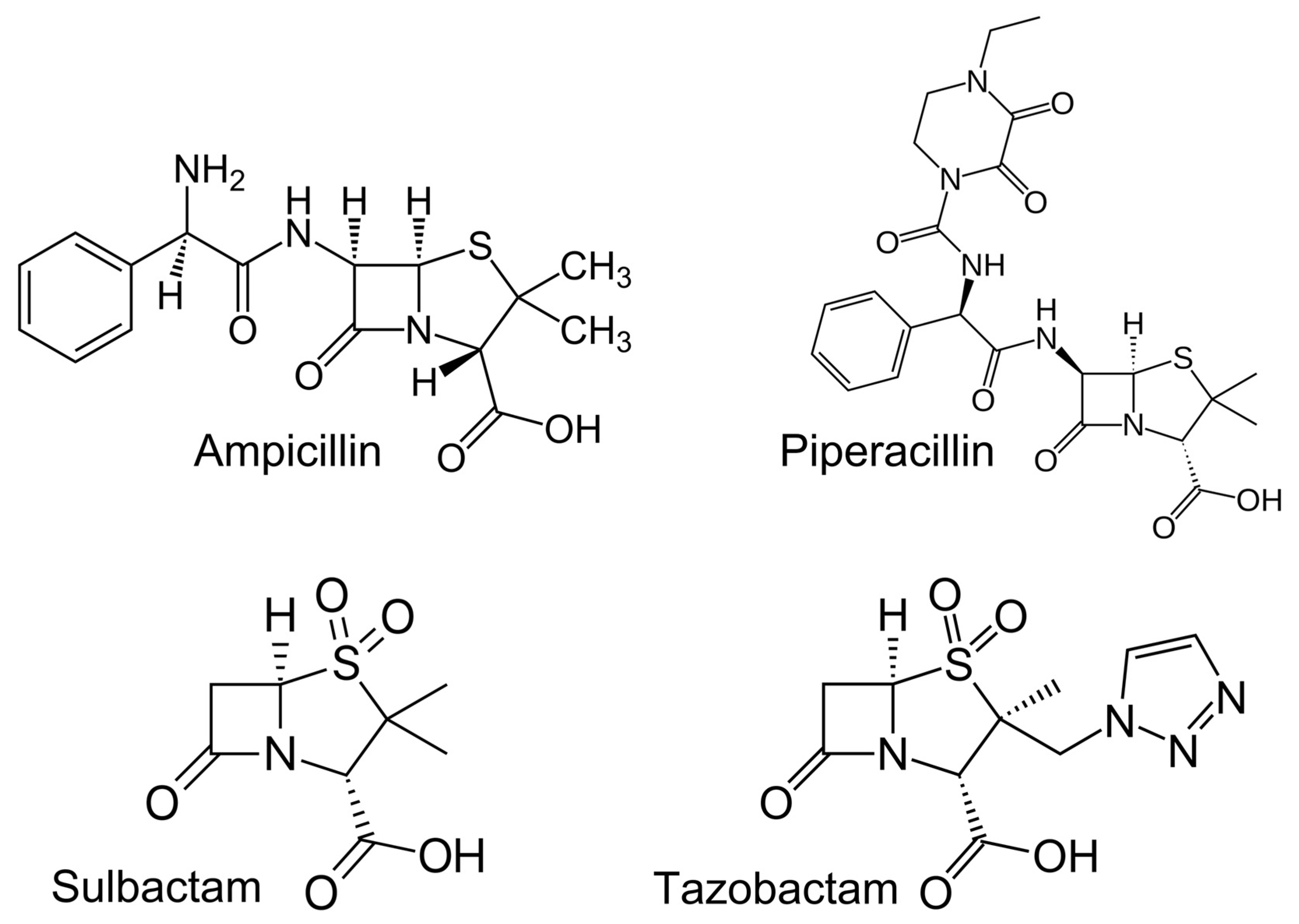
4.1. Materials
4.2. Stock and Working Solutions
4.3. Sample Preparation
4.3.1. Plasma/Serum
4.3.2. Dried Blood Spot
4.4. Liquid Chromatography–Tandem Mass Spectrometry (LC-MS/MS)
4.5. Method Validation
4.5.1. Specificity and Selectivity
4.5.2. Calibration Range
4.5.3. Accuracy and Precision
4.5.4. Matrix Effect, Recovery, Process Efficiency
4.5.5. Carry-Over
4.5.6. Stability
4.6. Method Application
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cattaneo, D.; Gervasoni, C.; Corona, A. The Issue of Pharmacokinetic-Driven Drug-Drug Interactions of Antibiotics: A Narrative Review. Antibiotics 2022, 11, 1410. [Google Scholar] [CrossRef] [PubMed]
- Calvo, M.; Stefani, S.; Migliorisi, G. Bacterial Infections in Intensive Care Units: Epidemiological and Microbiological Aspects. Antibiotics 2024, 13, 238. [Google Scholar] [CrossRef] [PubMed]
- Aslam, B.; Khurshid, M.; Arshad, M.I.; Muzammil, S.; Rasool, M.; Yasmeen, N.; Shah, T.; Chaudhry, T.H.; Rasool, M.H.; Shahid, A.; et al. Antibiotic Resistance: One Health One World Outlook. Front. Cell. Infect. Microbiol. 2021, 11, 771510. [Google Scholar] [CrossRef] [PubMed]
- Caneschi, A.; Bardhi, A.; Barbarossa, A.; Zaghini, A. The Use of Antibiotics and Antimicrobial Resistance in Veterinary Medicine, a Complex Phenomenon: A Narrative Review. Antibiotics 2023, 12, 487. [Google Scholar] [CrossRef]
- Lim, J.M.; Singh, S.R.; Duong, M.C.; Legido-Quigley, H.; Hsu, L.Y.; Tam, C.C. Impact of National Interventions to Promote Responsible Antibiotic Use: A Systematic Review. J. Antimicrob. Chemother. 2020, 75, 14–29. [Google Scholar] [CrossRef]
- Veringa, A.; Sturkenboom, M.G.G.; Dekkers, B.G.J.; Koster, R.A.; Roberts, J.A.; Peloquin, C.A.; Touw, D.J.; Alffenaar, J.-W.C. LC-MS/MS for Therapeutic Drug Monitoring of Anti-Infective Drugs. TrAC Trends Anal. Chem. 2016, 84, 34–40. [Google Scholar] [CrossRef]
- Kang, J.-S.; Lee, M.-H. Overview of Therapeutic Drug Monitoring. Korean J. Intern. Med. 2009, 24, 1–10. [Google Scholar] [CrossRef]
- Fang, Z.; Zhang, H.; Guo, J.; Guo, J. Overview of Therapeutic Drug Monitoring and Clinical Practice. Talanta 2024, 266, 124996. [Google Scholar] [CrossRef]
- Martin-Loeches, I. Therapeutic Drug Monitoring (TDM) in Real-Time: A Need for the Present Future. Expert Rev. Anti-Infect. Ther. 2022, 20, 1245–1247. [Google Scholar] [CrossRef]
- Voulgaridou, G.; Paraskeva, T.; Ragia, G.; Atzemian, N.; Portokallidou, K.; Kolios, G.; Arvanitidis, K.; Manolopoulos, V.G. Therapeutic Drug Monitoring (TDM) Implementation in Public Hospitals in Greece in 2003 and 2021: A Comparative Analysis of TDM Evolution over the Years. Pharmaceutics 2023, 15, 2181. [Google Scholar] [CrossRef]
- Junaid, T.; Wu, X.; Thanukrishnan, H.; Venkataramanan, R. Chapter 30-Therapeutic Drug Monitoring. In Clinical Pharmacy Education, Practice and Research; Thomas, D., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 425–436. ISBN 978-0-12-814276-9. [Google Scholar]
- Ranjan, G.; Jamal, F.; Das, S.; Gupta, V. Therapeutic Drug Monitoring: A Review. J. Drug Deliv. Ther. 2023, 13, 134–136. [Google Scholar] [CrossRef]
- Gross, A.S. Best Practice in Therapeutic Drug Monitoring. Br. J. Clin. Pharmacol. 2001, 52, 5S–10S. [Google Scholar] [CrossRef]
- Märtson, A.-G.; Sturkenboom, M.G.G.; Stojanova, J.; Cattaneo, D.; Hope, W.; Marriott, D.; Patanwala, A.E.; Peloquin, C.A.; Wicha, S.G.; van der Werf, T.S.; et al. How to Design a Study to Evaluate Therapeutic Drug Monitoring in Infectious Diseases? Clin. Microbiol. Infect. 2020, 26, 1008–1016. [Google Scholar] [CrossRef]
- EMA ICH M10 on Bioanalytical Method Validation-Scientific Guideline. Available online: https://www.ema.europa.eu/en/ich-m10-bioanalytical-method-validation-scientific-guideline (accessed on 24 October 2024).
- FDA Q2(R2) Guideline on Validation of Analytical Procedures Guidance for Industry. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/q2r2-validation-analytical-procedures (accessed on 24 October 2024).
- Wilhelm, A.J.; den Burger, J.C.G.; Swart, E.L. Therapeutic Drug Monitoring by Dried Blood Spot: Progress to Date and Future Directions. Clin. Pharmacokinet. 2014, 53, 961–973. [Google Scholar] [CrossRef]
- Avataneo, V.; D’Avolio, A.; Cusato, J.; Cantù, M.; De Nicolò, A. LC-MS Application for Therapeutic Drug Monitoring in Alternative Matrices. J. Pharm. Biomed. Anal. 2019, 166, 40–51. [Google Scholar] [CrossRef]
- Shipkova, M.; Svinarov, D. LC–MS/MS as a Tool for TDM Services: Where Are We? Clin. Biochem. 2016, 49, 1009–1023. [Google Scholar] [CrossRef]
- Seger, C.; Salzmann, L. After Another Decade: LC-MS/MS Became Routine in Clinical Diagnostics. Clin. Biochem. 2020, 82, 2–11. [Google Scholar] [CrossRef]
- Thomas, S.N.; French, D.; Jannetto, P.J.; Rappold, B.A.; Clarke, W.A. Liquid Chromatography–Tandem Mass Spectrometry for Clinical Diagnostics. Nat. Rev. Methods Primers 2022, 2, 96. [Google Scholar] [CrossRef]
- Gaspar, V.P.; Ibrahim, S.; Zahedi, R.P.; Borchers, C.H. Utility, Promise, and Limitations of Liquid Chromatography-Mass Spectrometry-Based Therapeutic Drug Monitoring in Precision Medicine. J. Mass Spectrom. 2021, 56, e4788. [Google Scholar] [CrossRef]
- Rahman, M.M.; Alam Tumpa, M.A.; Zehravi, M.; Sarker, M.T.; Yamin, M.; Islam, M.R.; Harun-Or-Rashid, M.; Ahmed, M.; Ramproshad, S.; Mondal, B.; et al. An Overview of Antimicrobial Stewardship Optimization: The Use of Antibiotics in Humans and Animals to Prevent Resistance. Antibiotics 2022, 11, 667. [Google Scholar] [CrossRef]
- Kondampati, K.D.; Saini, S.P.S.; Sidhu, P.K.; Anand, A.; Kumar, D.; Beesam, S.; Bedi, J.S.; Kaur, R.; Bhardwaj, R. Pharmacokinetic-Pharmacodynamic Study of Ampicillin-Cloxacillin Combination in Indian Thoroughbred Horses (Equus Caballus) and Safety Evaluation of the Computed Dosage Regimen. J. Equine Vet. Sci. 2022, 115, 104020. [Google Scholar] [CrossRef] [PubMed]
- Monaghan, K.; Labato, M.; Papich, M. Ampicillin Pharmacokinetics in Azotemic and Healthy Dogs. J. Vet. Intern. Med. 2021, 35, 987–992. [Google Scholar] [CrossRef] [PubMed]
- Bardhi, A.; Gazzotti, T.; Pagliuca, G.; Mari, G.; Barbarossa, A. Validation of a Single Liquid Chromatography-tandem Mass Spectrometry Approach for Oxytetracycline Determination in Bull Plasma, Seminal Plasma and Urine. Drug Test. Anal. 2022, 14, 1338–1342. [Google Scholar] [CrossRef]
- Barbarossa, A.; Bardhi, A.; Gazzotti, T.; Mari, G.; Pagliuca, G. A Single LC-MS/MS Validated Method for Tulathromycin Quantification in Plasma, Seminal Plasma, and Urine to Be Applied in a Pharmacokinetic Study in Bull. Drug Test. Anal. 2022, 14, 1525–1531. [Google Scholar] [CrossRef]
- Bardhi, A.; Romano, J.E.; Pagliuca, G.; Caneschi, A.; Barbarossa, A. Florfenicol and Florfenicol Amine Quantification in Bull Serum and Seminal Plasma by a Single Validated UHPLC-MS/MS Method. Vet. Med. Int. 2023, 2023, 6692920. [Google Scholar] [CrossRef]
- Van den Hoven, R.; Hierweck, B.; Dobretsberger, M.; Ensink, J.M.; Meijer, L.A. Intramuscular Dosing Strategy for Ampicillin Sodium in Horses, Based on Its Distribution into Tissue Chambers before and after Induction of Inflammation. J. Vet. Pharmacol. Ther. 2003, 26, 405–411. [Google Scholar] [CrossRef]
- Robbins, S.N.; Goggs, R.; Lhermie, G.; Lalonde-Paul, D.F.; Menard, J. Antimicrobial Prescribing Practices in Small Animal Emergency and Critical Care. Front. Vet. Sci. 2020, 7, 110. [Google Scholar] [CrossRef]
- Escher, M.; Vanni, M.; Intorre, L.; Caprioli, A.; Tognetti, R.; Scavia, G. Use of Antimicrobials in Companion Animal Practice: A Retrospective Study in a Veterinary Teaching Hospital in Italy. J. Antimicrob. Chemother. 2011, 66, 920–927. [Google Scholar] [CrossRef]
- Black, D.M.; Rankin, S.C.; King, L.G. Antimicrobial Therapy and Aerobic Bacteriologic Culture Patterns in Canine Intensive Care Unit Patients: 74 Dogs (January–June 2006). J. Vet. Emerg. Crit. Care 2009, 19, 489–495. [Google Scholar] [CrossRef]
- Buckland, E.L.; O’Neill, D.; Summers, J.; Mateus, A.; Church, D.; Redmond, L.; Brodbelt, D. Characterisation of Antimicrobial Usage in Cats and Dogs Attending UK Primary Care Companion Animal Veterinary Practices. Vet. Rec. 2016, 179, 489. [Google Scholar] [CrossRef]
- Schmitt, K.; Lehner, C.; Schuller, S.; Schüpbach-Regula, G.; Mevissen, M.; Peter, R.; Müntener, C.R.; Naegeli, H.; Willi, B. Antimicrobial Use for Selected Diseases in Cats in Switzerland. BMC Vet. Res. 2019, 15, 94. [Google Scholar] [CrossRef] [PubMed]
- Chinemerem Nwobodo, D.; Ugwu, M.C.; Oliseloke Anie, C.; Al-Ouqaili, M.T.S.; Chinedu Ikem, J.; Victor Chigozie, U.; Saki, M. Antibiotic Resistance: The Challenges and Some Emerging Strategies for Tackling a Global Menace. J. Clin. Lab. Anal. 2022, 36, e24655. [Google Scholar] [CrossRef] [PubMed]
- Odenholt, I.; Gustafsson, I.; Löwdin, E.; Cars, O. Suboptimal Antibiotic Dosage as a Risk Factor for Selection of Penicillin-Resistant Streptococcus Pneumoniae: In Vitro Kinetic Model. Antimicrob. Agents Chemother. 2003, 47, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Póvoa, P.; Moniz, P.; Pereira, J.G.; Coelho, L. Optimizing Antimicrobial Drug Dosing in Critically Ill Patients. Microorganisms 2021, 9, 1401. [Google Scholar] [CrossRef]
- Mabilat, C.; Gros, M.F.; Nicolau, D.; Mouton, J.W.; Textoris, J.; Roberts, J.A.; Cotta, M.O.; van Belkum, A.; Caniaux, I. Diagnostic and Medical Needs for Therapeutic Drug Monitoring of Antibiotics. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 791–797. [Google Scholar] [CrossRef]
- Nair, A.B.; Jacob, S. A Simple Practice Guide for Dose Conversion between Animals and Human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef]
- Nahata, M.C.; Vashi, V.I.; Swanson, R.N.; Messig, M.A.; Chung, M. Pharmacokinetics of Ampicillin and Sulbactam in Pediatric Patients. Antimicrob. Agents Chemother. 1999, 43, 1225–1229. [Google Scholar] [CrossRef]
- Lee, H.J.; Ryu, P.D.; Lee, H.; Cho, M.H.; Lee, M.H. Screening for Penicillin Plasma Residues in Cattle by Enzyme-Linked Immunosorbent Assay. Acta Vet. Brno 2001, 70, 353–358. [Google Scholar] [CrossRef]
- McWhinney, B.C.; Wallis, S.C.; Hillister, T.; Roberts, J.A.; Lipman, J.; Ungerer, J.P.J. Analysis of 12 Beta-Lactam Antibiotics in Human Plasma by HPLC with Ultraviolet Detection. J. Chromatogr. B-Anal. Technol. Biomed. Life Sci. 2010, 878, 2039–2043. [Google Scholar] [CrossRef]
- Carlier, M.; Stove, V.; De Waele, J.J.; Verstraete, A.G. Ultrafast Quantification of β-Lactam Antibiotics in Human Plasma Using UPLC-MS/MS. J. Chromatogr. B-Anal. Technol. Biomed. Life Sci. 2015, 978–979, 89–94. [Google Scholar] [CrossRef]
- Sturgeon, C.M.; Viljoen, A. Analytical Error and Interference in Immunoassay: Minimizing Risk. Ann. Clin. Biochem. 2011, 48, 418–432. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, G.; Keating, A.E. Protein Binding Specificity versus Promiscuity. Curr. Opin. Struct. Biol. 2011, 21, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Strathmann, F.G.; Hoofnagle, A.N. Current and Future Applications of Mass Spectrometry to the Clinical Laboratory. Am. J. Clin. Pathol. 2011, 136, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Swezey, R.R.; Green, C.E.; Mirsalis, J.C. Enhancement of Sensitivity and Quantification Quality in the LC-MS/MS Measurement of Large Biomolecules with Sum of MRM (SMRM). Anal. Bioanal. Chem. 2022, 414, 1933–1947. [Google Scholar] [CrossRef]
- Rankin-Turner, S.; Heaney, L.M. Mass Spectrometry in the Clinical Laboratory. A Short Journey through the Contribution to the Scientific Literature by CCLM. Clin. Chem. Lab. Med. (CCLM) 2023, 61, 873–879. [Google Scholar] [CrossRef]
- Bardhi, A.; Zaghini, A.; Levionnois, O.; Barbarossa, A. A Quick Approach for Medetomidine Enantiomer Determination in Dog Plasma by Chiral Liquid Chromatography-Tandem Mass Spectrometry and Application to a Pharmacokinetic Study. Drug Test. Anal. 2021, 13, 1249–1255. [Google Scholar] [CrossRef]
- Romano, J.E.; Bardhi, A.; Pagliuca, G.; Villadόniga, G.B.; Barbarossa, A. Pharmacokinetics of Florfenicol in Serum and Seminal Plasma in Beef Bulls. Theriogenology 2024, 218, 276–281. [Google Scholar] [CrossRef]
- Bardhi, A.; Vecchiato, C.G.; Sabetti, M.C.; Tardo, A.M.; Vasylyeva, K.; Biagi, G.; Pietra, M.; Barbarossa, A. A Novel UHPLC–MS/MS Method for the Measurement of 25-Hydroxyvitamin D3 in Canine Serum and Its Application to Healthy Dogs. Animals 2024, 14, 62. [Google Scholar] [CrossRef]
- Schmerold, I.; van Geijlswijk, I.; Gehring, R. European Regulations on the Use of Antibiotics in Veterinary Medicine. Eur. J. Pharm. Sci. 2023, 189, 106473. [Google Scholar] [CrossRef]
- Zakaria, R.; Allen, K.J.; Koplin, J.J.; Roche, P.; Greaves, R.F. Advantages and Challenges of Dried Blood Spot Analysis by Mass Spectrometry across the Total Testing Process. J. Int. Fed. Clin. Chem. Lab. Med. 2016, 27, 288–317. [Google Scholar]
- Samsonova, J.V.; Saushkin, N.Y.; Osipov, A.P. Dried Blood Spots Technology for Veterinary Applications and Biological Investigations: Technical Aspects, Retrospective Analysis, Ongoing Status and Future Perspectives. Vet. Res. Commun. 2022, 46, 655–698. [Google Scholar] [CrossRef] [PubMed]
- Allaway, D.; Alexander, J.E.; Carvell-Miller, L.J.; Reynolds, R.M.; Winder, C.L.; Weber, R.J.M.; Lloyd, G.R.; Southam, A.D.; Dunn, W.B. Suitability of Dried Blood Spots for Accelerating Veterinary Biobank Collections and Identifying Metabolomics Biomarkers With Minimal Resources. Front. Vet. Sci. 2022, 9, 887163. [Google Scholar] [CrossRef] [PubMed]
- Wickremsinhe, E.R.; Perkins, E.J. Using Dried Blood Spot Sampling to Improve Data Quality and Reduce Animal Use in Mouse Pharmacokinetic Studies. J. Am. Assoc. Lab. Anim. Sci. 2015, 54, 139–144. [Google Scholar] [PubMed]
- Dunkel, B.; Johns, I.C. Antimicrobial Use in Critically Ill Horses. J. Vet. Emerg. Crit. Care 2015, 25, 89–100. [Google Scholar] [CrossRef]
- EMA/CVMP/CHMP/682198/2017. Categorisation of Antibiotics for Use in Animals: Answer to the Request from the European Commission for Updating the Scientific Advice on the Impact on Public Health and Animal Health of the Use of Antibiotics in Animals. December 2019. Available online: https://www.ema.europa.eu/en/documents/report/categorisation-antibiotics-european-union-answer-request-european-commission-updating-scientific-advice-impact-public-health-and-animal-health-use-antibiotics-animals_en.pdf (accessed on 30 September 2024).
- Taylor, S. A Review of Equine Sepsis. Equine Vet. Educ. 2015, 27, 99–109. [Google Scholar] [CrossRef]
- Theelen, M.J.; David Wilson, W.; Dacvim, H.; Byrne, B.A.; Edman, J.M.; Gary Magdesian, K. Cumulative Antimicrobial Susceptibility of Bacteria Isolated From Foals With Sepsis: 1990–2015; CABI Digital Library: Wallingford, UK, 2016. [Google Scholar]
- Matuszewski, B.K. Standard Line Slopes as a Measure of a Relative Matrix Effect in Quantitative HPLC–MS Bioanalysis. J. Chromatogr. B 2006, 830, 293–300. [Google Scholar] [CrossRef]
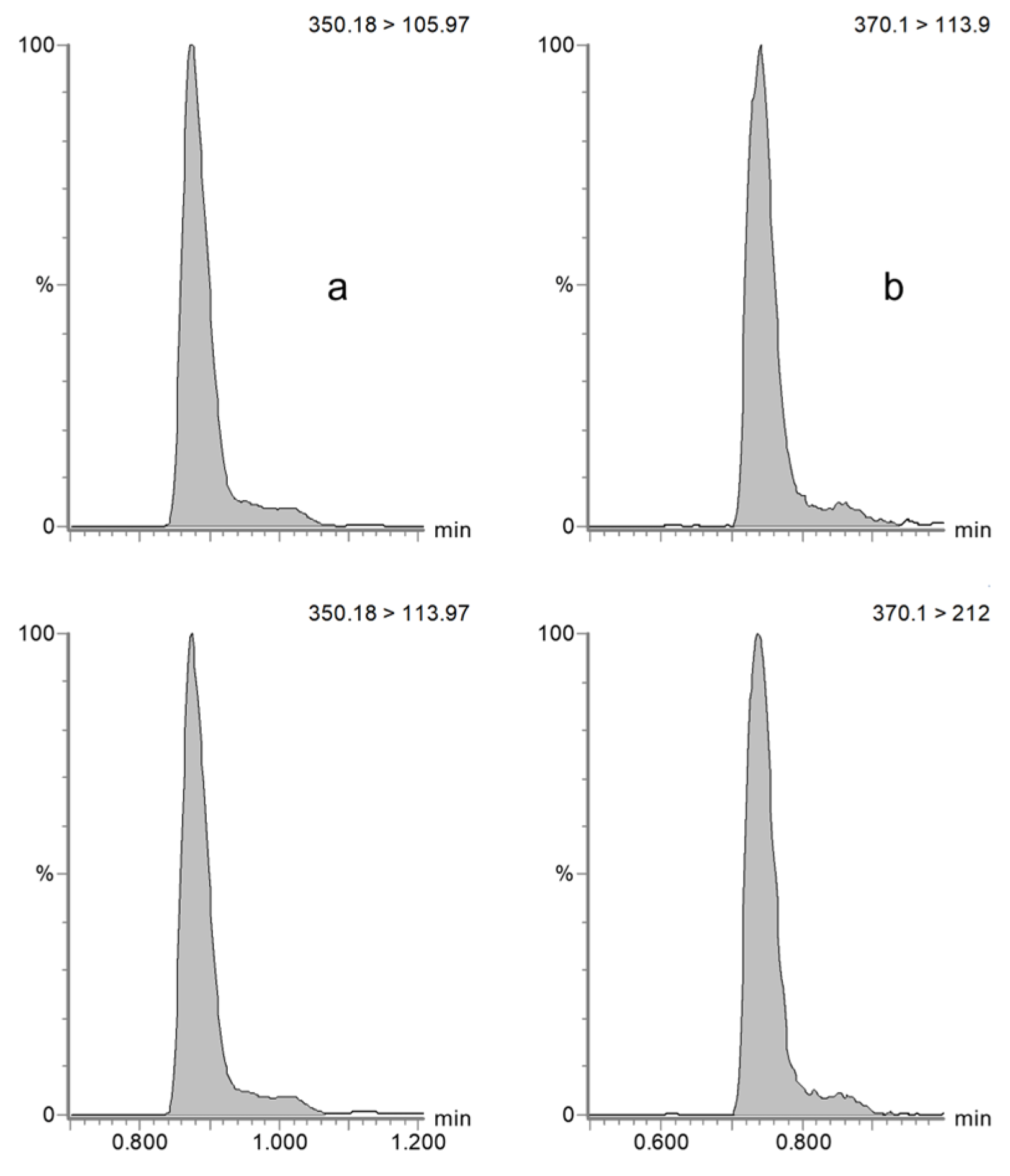
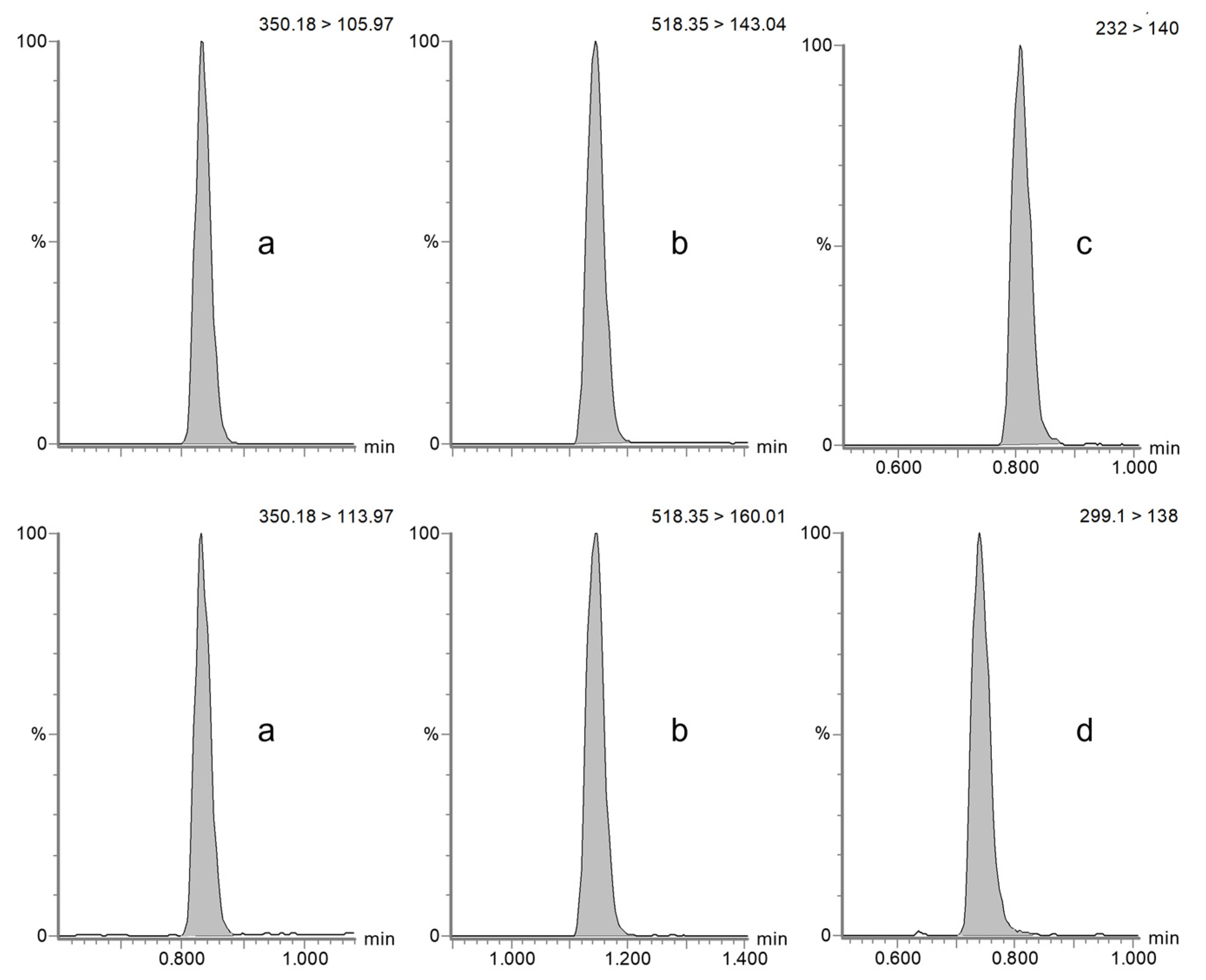
| Horse | Dog | |||
|---|---|---|---|---|
| Accuracy (%) | Precision (%) | Accuracy (%) | Precision (%) | |
| LLOQ (0.1 µg/mL) | LLOQ (0.1 µg/mL) | |||
| Day 1 (n = 5) | 0.3 | 0.6 | 1.0 | 1.7 |
| Day 2 (n = 5) | 0.5 | 1.2 | 2.0 | 3.4 |
| Day 3 (n = 5) | 1.5 | 2.1 | 0.3 | 0.6 |
| Interday (n = 15) | 0.8 | 1.2 | 1.1 | 2.1 |
| QCL (0.5 µg/mL) | QCL (0.5 µg/mL) | |||
| Day 1 (n = 5) | 3.9 | 4.8 | −0.7 | 1.2 |
| Day 2 (n = 5) | 9.3 | 12.9 | 0.0 | 2.0 |
| Day 3 (n = 5) | −0.9 | 8.8 | −1.3 | 2.3 |
| Interday (n = 15) | 4.1 | 9.3 | −0.7 | 1.7 |
| QCM (5 µg/mL) | QCM (5 µg/mL) | |||
| Day 1 (n = 5) | 3.2 | 6.9 | 1.7 | 2.8 |
| Day 2 (n = 5) | 0.6 | 5.4 | −1.7 | 2.9 |
| Day 3 (n = 5) | 0.2 | 7.3 | 0.0 | 8.7 |
| Interday (n = 15) | 1.3 | 5.9 | 0.0 | 5.0 |
| QCH (50 µg/mL) | QCH (50 µg/mL) | |||
| Day 1 (n = 5) | −1.7 | 4.8 | −6.2 | 5.3 |
| Day 2 (n = 5) | 2.7 | 2.3 | −2.2 | −10.5 |
| Day 3 (n = 5) | 4.5 | 1.3 | 8.8 | 3.1 |
| Interday (n = 15) | 1.9 | 3.8 | 0.2 | 9.0 |
| Dog | ||
|---|---|---|
| Accuracy (%) | Precision (%) | |
| QCL (0.5 µg/mL) | ||
| Day 1 (n = 5) | 0.3 | 0.6 |
| Day 2 (n = 5) | 1.0 | 1.7 |
| Day 3 (n = 5) | 0.7 | 1.1 |
| Interday (n = 15) | 0.7 | 1.1 |
| QCM (5 µg/mL) | ||
| Day 1 (n = 5) | 0.0 | 0.0 |
| Day 2 (n = 5) | −5.0 | 0.0 |
| Day 3 (n = 5) | −1.7 | 2.9 |
| Interday (n = 15) | −2.2 | 2.7 |
| QCH (50 µg/mL) | ||
| Day 1 (n = 5) | −3.3 | 3.0 |
| Day 2 (n = 5) | −0.2 | 11.4 |
| Day 3 (n = 5) | 13.0 | 5.2 |
| Interday (n = 15) | 3.2 | 9.7 |
| Mean Peak Area (Arbitrary Units, ×103, n = 3) | ME (%) | RE (%) | PE (%) | |||
|---|---|---|---|---|---|---|
| A | B | C | ||||
| Ampicillin horse | ||||||
| 0.1 µg/mL | 3.3 | 3.2 | 2.7 | 97.7 | 84.0 | 82.6 |
| 2 µg/mL | 41.4 | 37.5 | 35.4 | 89.5 | 94.0 | 84.5 |
| 20 µg/mL | 423.7 | 392.4 | 407.5 | 92.6 | 104.0 | 96.2 |
| Ampicillin dog | ||||||
| 0.1 µg/mL | 3.3 | 2.8 | 2.7 | 84.3 | 99.0 | 83.8 |
| 2 µg/mL | 41.9 | 41.5 | 41.8 | 98.9 | 101.0 | 99.6 |
| 20 µg/mL | 423.6 | 381.3 | 373.2 | 90.0 | 98.0 | 88.1 |
| Sulbactam dog | ||||||
| 2 µg/mL | 2.6 | 2.6 | 2.6 | 101.0 | 99.7 | 100.9 |
| 20 µg/mL | 24.9 | 26.6 | 24.5 | 106.7 | 92.0 | 98.3 |
| 1 h | 3 h | 6 h | 24 h | 4 d | 28 d | 40 d | |
|---|---|---|---|---|---|---|---|
| 22 °C | 14.6% | −13.1% | −17.9 | −40.2% | - | - | - |
| 4 °C | 8.3% | −3.3% | −8.2% | −10.3% | - | - | - |
| −20 °C | - | - | - | −7.7% | −3.2% | −12.8% | −9.8% |
| −80 °C | - | - | - | - | - | - | −5.3% |
| Analyte | MRM Transition (m/z) | Cone Voltage (V) | Collision Energy (eV) |
|---|---|---|---|
| Ampicillin | 350.18 > 105.97 | 42 | 18 |
| 350.18 > 113.97 | 42 | 30 | |
| Sulbactam | 232.00 > 140.00 | 25 | 12 |
| Piperacillin | 518.35 > 143.04 | 40 | 18 |
| 518.35 > 160.01 | 40 | 9 | |
| Tazobactam | 299.10 > 138.00 | 25 | 13 |
| Amoxicillin-d4 | 370.10 > 113.90 | 30 | 20 |
| 370.10 > 212.00 | 30 | 12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bardhi, A.; Lanci, A.; Mannini, A.; Castagnetti, C.; Barbarossa, A. A Laboratory Protocol for Routine Therapeutic Drug Monitoring of Beta-Lactams Antimicrobials in Horses and Dogs. Antibiotics 2025, 14, 390. https://doi.org/10.3390/antibiotics14040390
Bardhi A, Lanci A, Mannini A, Castagnetti C, Barbarossa A. A Laboratory Protocol for Routine Therapeutic Drug Monitoring of Beta-Lactams Antimicrobials in Horses and Dogs. Antibiotics. 2025; 14(4):390. https://doi.org/10.3390/antibiotics14040390
Chicago/Turabian StyleBardhi, Anisa, Aliai Lanci, Aurora Mannini, Carolina Castagnetti, and Andrea Barbarossa. 2025. "A Laboratory Protocol for Routine Therapeutic Drug Monitoring of Beta-Lactams Antimicrobials in Horses and Dogs" Antibiotics 14, no. 4: 390. https://doi.org/10.3390/antibiotics14040390
APA StyleBardhi, A., Lanci, A., Mannini, A., Castagnetti, C., & Barbarossa, A. (2025). A Laboratory Protocol for Routine Therapeutic Drug Monitoring of Beta-Lactams Antimicrobials in Horses and Dogs. Antibiotics, 14(4), 390. https://doi.org/10.3390/antibiotics14040390








