In Vitro Evaluation of the Antibacterial Effect and Influence on the Bacterial Biofilm Formation of Glutamic Acid and Some Structural Analogues
Abstract
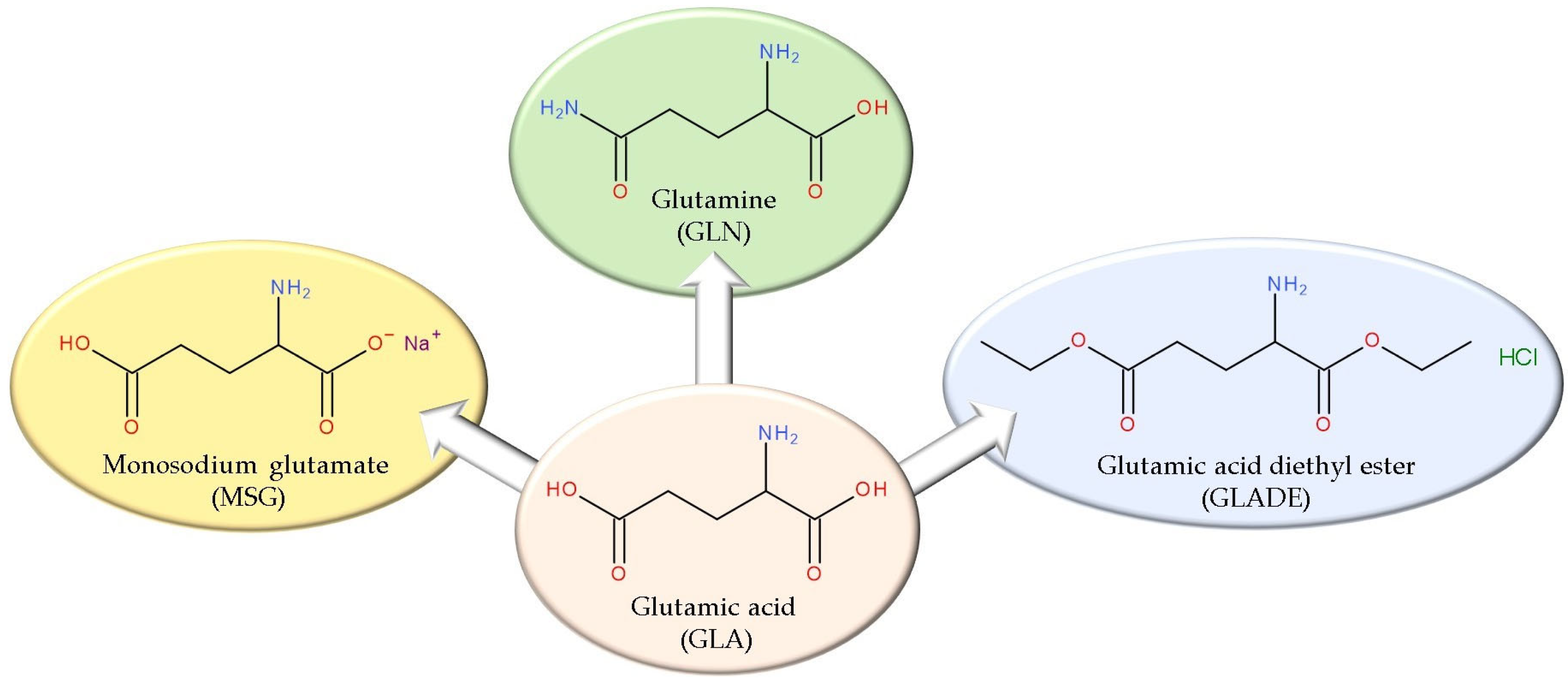
:1. Introduction
2. Results
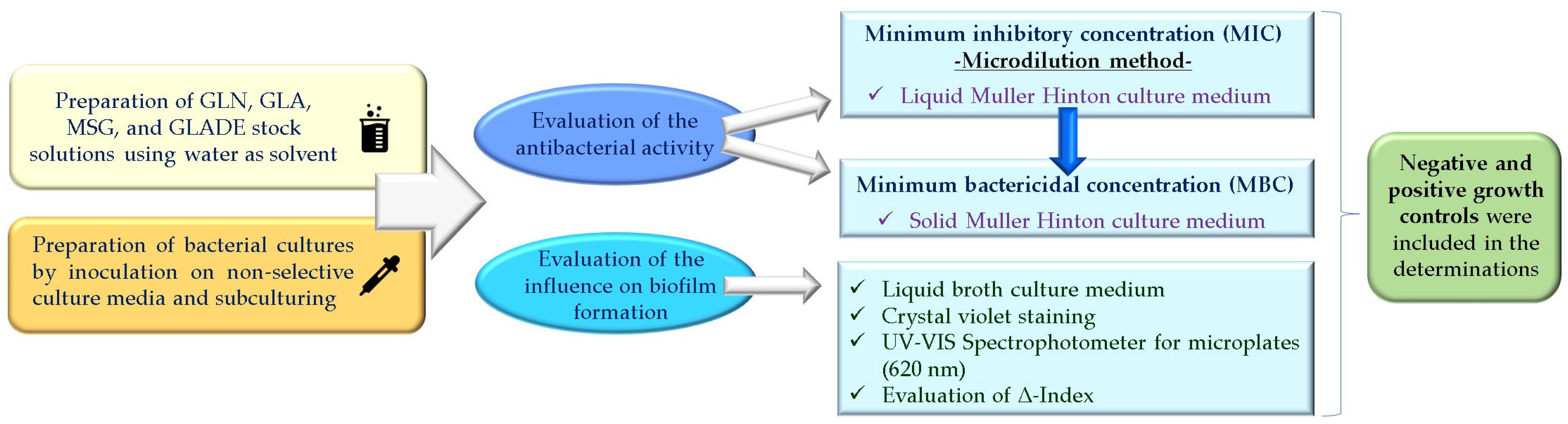
2.1. Evaluation of the Antibacterial Effect (Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) Determination)
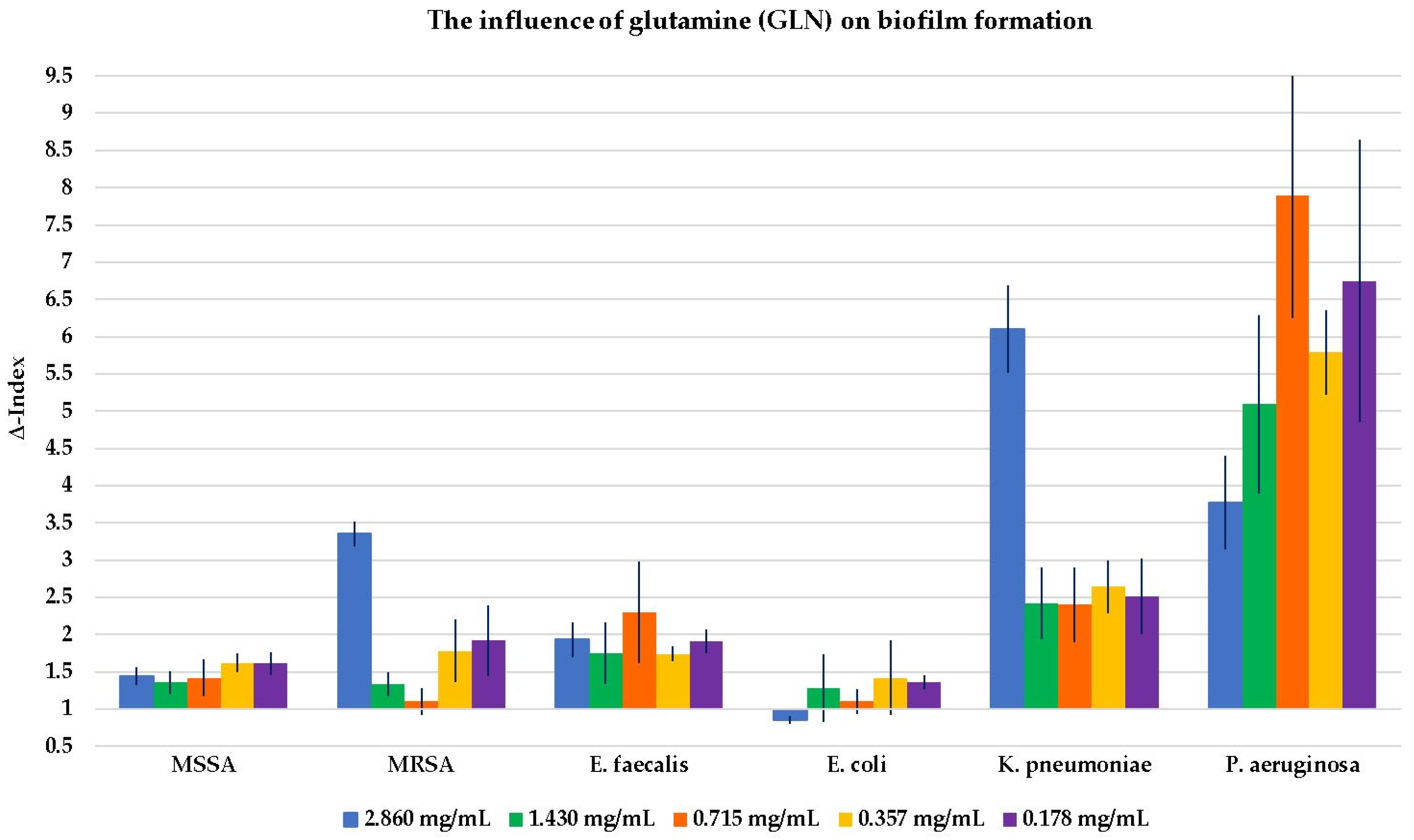
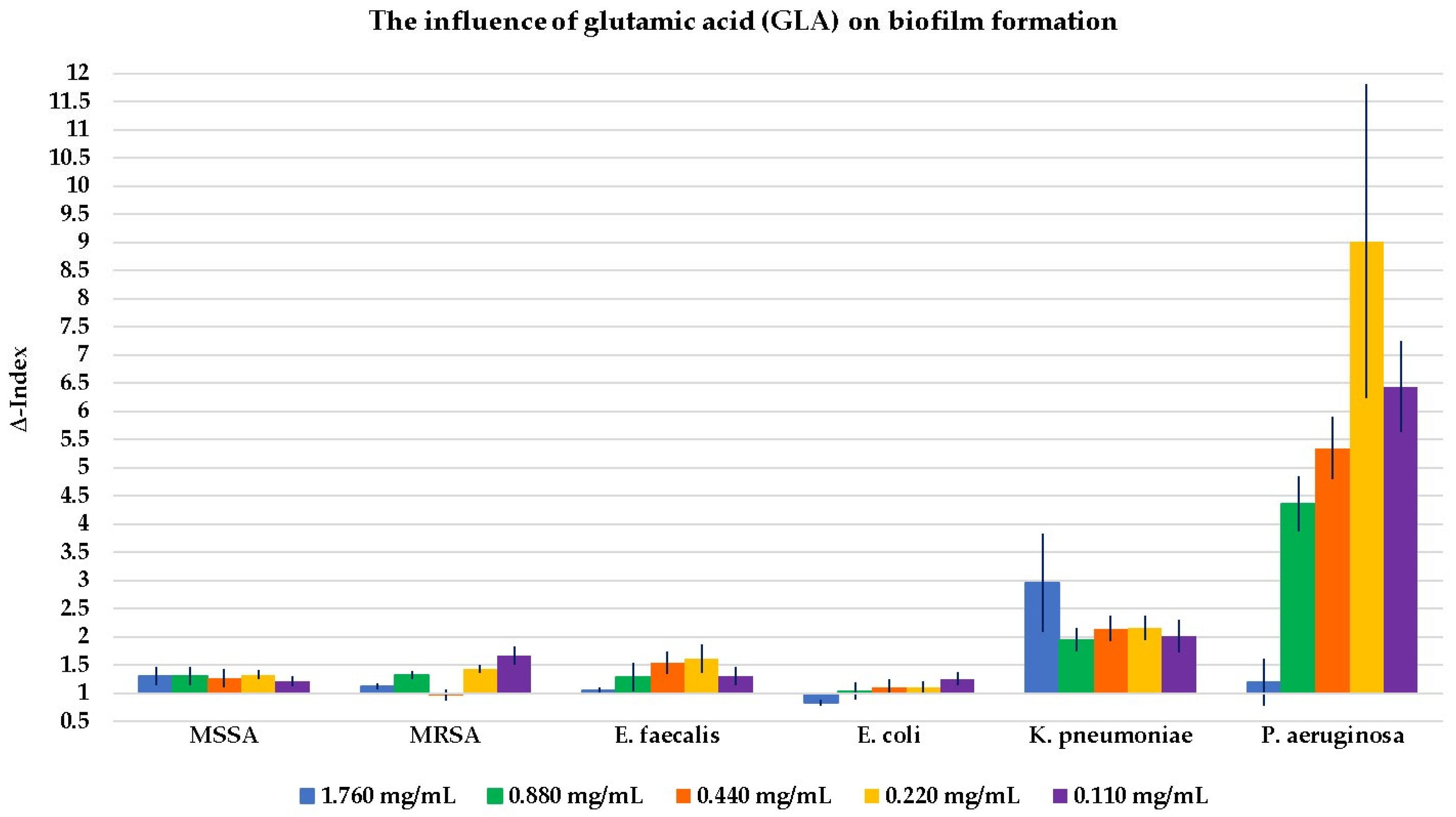
2.2. Evaluation of the Influence of the Four Compounds on Biofilm Formation
2.3. In Silico Evaluation of Glutamic Acid Diethyl Ester’s (GLADE’s) Properties as a Potential Drug Candidate
3. Discussion
3.1. The Antibacterial Effect (Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) Determination)
3.2. The Influence on Biofilm Formation
3.3. Key Aspects of Glutamic Acid (GLA) and the Glutamine (GLN) Effect on Bacterial Growth and Biofilm Formation
3.4. In Silico Evaluation of Glutamic Acid Diethyl Ester’s (GLADE’s) Properties as a Potential Drug Candidate
- Based on the theoretical predictions targeted in this sub-chapter, GLADE is a hydrophilic compound, but it presents good gastrointestinal absorption and BD (SwissADME platform);
- Several antibacterial effects were identified, although not presenting a very high probability; some of them could be further assessed by in vitro or in vivo studies (Pass online platform);
- The toxicity profile defined by several determinations (Toxtree software, version 3.1.0.1851) indicates that GLADE is theoretically safe.
4. Materials and Methods
4.1. In Vitro Evaluation of the Antibacterial Activity (Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC))
4.1.1. In Vitro Determination of Minimum Inhibitory Concentration (MIC) by the Microdilution Method
4.1.2. In Vitro Determination of the Minimum Bactericidal Concentration (MBC)
4.2. In Vitro Evaluation of the Influence on Biofilm Formation
4.3. In Silico Evaluation of Glutamic Acid Diethyl Ester’s (GLADE’s) Properties as a Potential Drug Candidate
5. Conclusions
6. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AOA | Aminooxy acetic acid |
| BBB | Blood–brain barrier |
| BD | Bioavailability |
| D-Asp | D-Aspartic acid |
| GLA | Glutamic acid |
| GLADE | Glutamic acid diethyl ester |
| GLN | Glutamine |
| Gln | Glutamine transport system permease protein |
| GS | Glutamine synthetase |
| HA | Heavy atoms |
| HAcc | Hydrogen bond acceptors |
| HD | Hydrogen bond donors |
| IC50 | Half-maximal inhibitory concentration |
| L-Asp | L-Aspartic acid |
| MBC | Minimum bactericidal concentration |
| MIC | Minimum inhibitory concentration |
| MRSA | Methicillin-resistant Staphylococcus aureus |
| MSSA | Methicillin-susceptible Staphylococcus aureus |
| MSG | Monosodium glutamate |
| MW | Molecular weight |
| OD | Optical densities |
| PG | Polygamma-glutamic acid |
| P-gp | P-glycoprotein |
| RB | Rotatable bonds |
| TPSA | Topological polar surface area |
| TTC | Threshold of toxicological concern |
References
- Moldovan, O.-L.; Vari, C.-E.; Tero-Vescan, A.; Cotoi, O.S.; Cocuz, I.G.; Tabaran, F.A.; Pop, R.; Fülöp, I.; Chis, R.F.; Lungu, I.-A.; et al. Potential Defence Mechanisms Triggered by Monosodium Glutamate Sub-Chronic Consumption in Two-Year-Old Wistar Rats. Nutrients 2023, 15, 4436. [Google Scholar] [CrossRef] [PubMed]
- Moldovan, O.-L.; Rusu, A.; Tanase, C.; Vari, C.-E. Glutamate—A Multifaceted Molecule: Endogenous Neurotransmitter, Controversial Food Additive, Design Compound for Anti-Cancer Drugs. A Critical Appraisal. Food Chem. Toxicol. 2021, 153, 112290. [Google Scholar] [CrossRef] [PubMed]
- Oancea, O.-L.; Gâz, Ș.A.; Marc, G.; Lungu, I.-A.; Rusu, A. In Silico Evaluation of Some Computer-Designed Fluoroquinolone–Glutamic Acid Hybrids as Potential Topoisomerase II Inhibitors with Anti-Cancer Effect. Pharmaceuticals 2024, 17, 1593. [Google Scholar] [CrossRef]
- Moldovan, O.-L.; Sandulea, A.; Lungu, I.-A.; Gâz, Ș.A.; Rusu, A. Identification of Some Glutamic Acid Derivatives with Biological Potential by Computational Methods. Molecules 2023, 28, 4123. [Google Scholar] [CrossRef] [PubMed]
- Bhuvaneswari, S.; Nannu Shankar, S.; Nyayiru Kannaian, U.P. Ajinomoto: Antibacterial Impact. Indian J. Appl. Microbiol. 2015, 18, 28–33. [Google Scholar]
- Tong, Z.; Zhang, L.; Ling, J.; Jian, Y.; Huang, L.; Deng, D. An In Vitro Study on the Effect of Free Amino Acids Alone or in Combination with Nisin on Biofilms as Well as on Planktonic Bacteria of Streptococcus Mutans. PLoS ONE 2014, 9, e99513. [Google Scholar] [CrossRef]
- Yang, J.; Ran, Y.; Liu, S.; Ren, C.; Lou, Y.; Ju, P.; Li, G.; Li, X.; Zhang, D. Synergistic D-Amino Acids Based Antimicrobial Cocktails Formulated via High-Throughput Screening and Machine Learning. Adv. Sci. 2024, 11, 2307173. [Google Scholar] [CrossRef]
- Fan, L.; Pan, Z.; Zhong, Y.; Guo, J.; Liao, X.; Pang, R.; Xu, Q.; Ye, G.; Su, Y. L-Glutamine Sensitizes Gram-Positive-Resistant Bacteria to Gentamicin Killing. Microbiol. Spectr. 2023, 11, e01619-23. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, Z.; Yang, T.; Jiang, M.; Wang, J.; Cheng, Z.; Yang, M.; Zhu, J.; Zhang, T.; Li, H.; et al. Glutamine Promotes Antibiotic Uptake to Kill Multidrug-Resistant Uropathogenic Bacteria. Sci. Transl. Med. 2021, 13, eabj0716. [Google Scholar] [CrossRef]
- Kimura, T.; Kobayashi, K. Role of Glutamate Synthase in Biofilm Formation by Bacillus Subtilis. J. Bacteriol. 2020, 202, e00120-20. [Google Scholar] [CrossRef]
- Mazloomi, E.; Jazani, N.H.; Sohrabpour, M.; Ilkhanizadeh, B.; Shahabi, S. Synergistic Effects of Glutamine and Ciprofloxacin in Reduction of Pseudomonas Aeruginosa-Induced Septic Shock Severity. Int. Immunopharmacol. 2011, 11, 2214–2219. [Google Scholar] [CrossRef]
- Tomašić, T.; Šink, R.; Zidar, N.; Fic, A.; Contreras-Martel, C.; Dessen, A.; Patin, D.; Blanot, D.; Müller-Premru, M.; Gobec, S.; et al. Dual Inhibitor of MurD and MurE Ligases from Escherichia coli and Staphylococcus aureus. ACS Med. Chem. Lett. 2012, 3, 626–630. [Google Scholar] [CrossRef] [PubMed]
- Nowak, M.G.; Skwarecki, A.S.; Milewska, M.J. Amino Acid Based Antimicrobial Agents—Synthesis and Properties. ChemMedChem 2021, 16, 3513–3544. [Google Scholar] [CrossRef]
- Potapnev, M.P.; Andreyev, S.V.; Goncharova, N.V.; Viatkina, O.I.; Berdina, E.L.; Gapanovich, V.N. Dual Effect of Amino Acid Compositions on Antibacterial Activity of Human Neutrophilic Granulocytes. Biochem. Mosc. Suppl. Ser. B 2023, 17, 17–25. [Google Scholar] [CrossRef]
- Warraich, A.A.; Mohammed, A.R.; Perrie, Y.; Hussain, M.; Gibson, H.; Rahman, A. Evaluation of Anti-Biofilm Activity of Acidic Amino Acids and Synergy with Ciprofloxacin on Staphylococcus Aureus Biofilms. Sci. Rep. 2020, 10, 9021. [Google Scholar] [CrossRef] [PubMed]
- Ajayeoba, T.A.; Dula, S.; Ijabadeniyi, O.A. Properties of Poly-γ-Glutamic Acid Producing-Bacillus Species Isolated From Ogi Liquor and Lemon-Ogi Liquor. Front. Microbiol. 2019, 10, 771. [Google Scholar] [CrossRef]
- Yu, Z.; Wei, Y.; Fu, C.; Sablani, S.S.; Huang, Z.; Han, C.; Li, D.; Sun, Z.; Qin, H. Antimicrobial Activity of Gamma-Poly (Glutamic Acid), a Preservative Coating for Cherries. Colloids Surf. B Biointerfaces 2023, 225, 113272. [Google Scholar] [CrossRef]
- Ijadi Bajestani, M.; Mousavi, S.M.; Mousavi, S.B.; Jafari, A.; Shojaosadati, S.A. Purification of Extra Cellular Poly-γ-Glutamic Acid as an Antibacterial Agent Using Anion Exchange Chromatography. Int. J. Biol. Macromol. 2018, 113, 142–149. [Google Scholar] [CrossRef]
- Laverty, G.; Gorman, S.P.; Gilmore, B.F. The Potential of Antimicrobial Peptides as Biocides. Int. J. Mol. Sci. 2011, 12, 6566–6596. [Google Scholar] [CrossRef]
- Huan, Y.; Kong, Q.; Mou, H.; Yi, H. Antimicrobial Peptides: Classification, Design, Application and Research Progress in Multiple Fields. Front. Microbiol. 2020, 11, 582779. [Google Scholar] [CrossRef]
- Lalonde, R.; Joyal, C.C. Effects of Ketamine and 1-Glutamic Acid Diethyl Ester on Concept Learning in Rats. Pharmacol. Biochem. Behav. 1991, 39, 829–833. [Google Scholar] [CrossRef] [PubMed]
- Freed, W.J.; Wyatt, R.J. Impairment of Instrumental Learning in Rats by Glutamic Acid Diethyl Ester. Pharmacol. Biochem. Behav. 1981, 14, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Zhao, A.; Sun, J.; Liu, Y. Understanding Bacterial Biofilms: From Definition to Treatment Strategies. Front. Cell. Infect. Microbiol. 2023, 13, 1137947. [Google Scholar] [CrossRef]
- Sharma, S.; Mohler, J.; Mahajan, S.D.; Schwartz, S.A.; Bruggemann, L.; Aalinkeel, R. Microbial Biofilm: A Review on Formation, Infection, Antibiotic Resistance, Control Measures, and Innovative Treatment. Microorganisms 2023, 11, 1614. [Google Scholar] [CrossRef] [PubMed]
- Aliashkevich, A.; Alvarez, L.; Cava, F. New Insights Into the Mechanisms and Biological Roles of D-Amino Acids in Complex Eco-Systems. Front. Microbiol. 2018, 9, 683. [Google Scholar] [CrossRef]
- Shibamura-Fujiogi, M.; Wang, X.; Maisat, W.; Koutsogiannaki, S.; Li, Y.; Chen, Y.; Lee, J.C.; Yuki, K. GltS Regulates Biofilm Formation in Methicillin-Resistant Staphylococcus Aureus. Commun. Biol. 2022, 5, 1284. [Google Scholar] [CrossRef]
- Hassanov, T.; Karunker, I.; Steinberg, N.; Erez, A.; Kolodkin-Gal, I. Novel Antibiofilm Chemotherapies Target Nitrogen from Glutamate and Glutamine. Sci. Rep. 2018, 8, 7097. [Google Scholar] [CrossRef]
- Bessembayeva, L.; Kirkimbayeva, Z.; Biyashev, B.; Zholdasbekova, A.; Kuzembekova, G.; Sarybayeva, D.; Zhylkaidar, A.; Oryntaev, K.; Bakiyeva, F. Investigation of the Antibiotic Resistance and Biofilm-Forming Ability of Staphylococcus Species from Bovine Mastitis Cases in the Almaty Region, Kazakhstan. Int. J. Vet. Sci. 2024, 13, 853–861. [Google Scholar]
- Eman, S.I.; Abdalhamed, A.M.; Arafa, A.A.; Eid, R.H.; Khalil, H.M.; Hedia, R.H.; Dorgham, S.M.; Hozyen, H.F. In Vitro and in Vivo Antibacterial and Antibiofilm Efficacy of Selenium Nanoparticles against Staphylococcus Aureus Supported with Toxicopathological and Behavioral Studies in Rats. Int. J. Vet. Sci. 2024, 13, 490–500. [Google Scholar]
- Cui, W.-Q.; Qu, Q.-W.; Wang, J.-P.; Bai, J.-W.; Bello-Onaghise, G.; Li, Y.-A.; Zhou, Y.-H.; Chen, X.-R.; Liu, X.; Zheng, S.-D.; et al. Discovery of Potential Anti-Infective Therapy Targeting Glutamine Synthetase in Staphylococcus Xylosus. Front. Chem. 2019, 7, 381. [Google Scholar] [CrossRef]
- Ali, A.; Zahra, A.; Kamthan, M.; Husain, F.M.; Albalawi, T.; Zubair, M.; Alatawy, R.; Abid, M.; Noorani, M.S. Microbial Biofilms: Applications, Clinical Consequences, and Alternative Therapies. Microorganisms 2023, 11, 1934. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Cheng, X.; Wang, Y.; Luo, J. Effect of Different D-Amino Acids on Biofilm Formation of Mixed Microorganisms. Water Sci. Technol. 2021, 85, 116–124. [Google Scholar] [CrossRef]
- Erez, A.; Kolodkin-Gal, I. From Prokaryotes to Cancer: Glutamine Flux in Multicellular Units. Trends Endocrinol. Metab. 2017, 28, 637–644. [Google Scholar] [CrossRef]
- Caro-Astorga, J.; Frenzel, E.; Perkins, J.R.; Álvarez-Mena, A.; de Vicente, A.; Ranea, J.A.G.; Kuipers, O.P.; Romero, D. Biofilm Formation Displays Intrinsic Offensive and Defensive Features of Bacillus Cereus. NPJ Biofilms Microbiomes 2020, 6, 3. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, Y.; Morimoto, S.; Yoshida, E.; Naka, S.; Inaba, H.; Matsumoto-Nakano, M. Identification and Functional Analysis of Glutamine Transporter in Streptococcus Mutans. J. Oral Microbiol. 2020, 12, 1797320. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Weiss, E.C.; Otto, M.; Fey, P.D.; Smeltzer, M.S.; Somerville, G.A. Staphylococcus Aureus Biofilm Metabolism and the Influence of Arginine on Polysaccharide Intercellular Adhesin Synthesis, Biofilm Formation, and Pathogenesis. Infect. Immun. 2007, 75, 4219–4226. [Google Scholar] [CrossRef]
- Vudhya Gowrisankar, Y.; Manne Mudhu, S.; Pasupuleti, S.K.; Suthi, S.; Chaudhury, A.; Sarma, P.V.G.K. Staphylococcus Aureus Grown in Anaerobic Conditions Exhibits Elevated Glutamine Biosynthesis and Biofilm Units. Can. J. Microbiol. 2021, 67, 323–331. [Google Scholar] [CrossRef]
- Morichi, T.; Irie, R.; Yano, N.; Kembo, H. Protective Effect of Glutamic Acid and Related Compounds on Bacterial Cells Subjected to Freeze-Drying. J. Gen. Appl. Microbiol. 1963, 9, 149–161. [Google Scholar] [CrossRef]
- Yu, Y.; Yan, F.; Chen, Y.; Jin, C.; Guo, J.-H.; Chai, Y. Poly-γ-Glutamic Acids Contribute to Biofilm Formation and Plant Root Colonization in Selected Environmental Isolates of Bacillus Subtilis. Front. Microbiol. 2016, 7, 1811. [Google Scholar] [CrossRef]
- Kocianova, S.; Vuong, C.; Yao, Y.; Voyich, J.M.; Fischer, E.R.; DeLeo, F.R.; Otto, M. Key Role of Poly-γ-Dl-Glutamic Acid in Immune Evasion and Virulence of Staphylococcus Epidermidis. J. Clin. Investig. 2005, 115, 688–694. [Google Scholar] [CrossRef]
- Idrees, M.; Mohammad, A.R.; Karodia, N.; Rahman, A. Multimodal Role of Amino Acids in Microbial Control and Drug Development. Antibiotics 2020, 9, 330. [Google Scholar] [CrossRef]
- Huang, X.; Duan, X.; Li, J.; Niu, J.; Yuan, S.; Wang, X.; Lambert, N.; Li, X.; Xu, J.; Gong, Z.; et al. The Synergistic Effect of Exogenous Glutamine and Rifampicin Against Mycobacterium Persisters. Front. Microbiol. 2018, 9, 1625. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Prindle, A.; Humphries, J.; Gabalda-Sagarra, M.; Asally, M.; Lee, D.D.; Ly, S.; Garcia-Ojalvo, J.; Süel, G.M. Metabolic Codependence Gives Rise to Collective Oscillations within Biofilms. Nature 2015, 523, 550–554. [Google Scholar] [CrossRef]
- Ondrey, J.M. The Role of Central Metabolism and Electron Transport in Biofilm Formation by Vibrio Fischeri. Master’s Thesis, Loyola University Chicago, Chicago, IL, USA, 2015; pp. 33–34. [Google Scholar]
- Damyanova, T.; Dimitrova, P.D.; Borisova, D.; Topouzova-Hristova, T.; Haladjova, E.; Paunova-Krasteva, T. An Overview of Biofilm-Associated Infections and the Role of Phytochemicals and Nanomaterials in Their Control and Prevention. Pharmaceutics 2024, 16, 162. [Google Scholar] [CrossRef]
- Folliero, V.; Franci, G.; Dell’Annunziata, F.; Giugliano, R.; Foglia, F.; Sperlongano, R.; De Filippis, A.; Finamore, E.; Galdiero, M. Evaluation of Antibiotic Resistance and Biofilm Production among Clinical Strain Isolated from Medical Devices. Int. J. Microbiol. 2021, 2021, 9033278. [Google Scholar] [CrossRef] [PubMed]
- Prasanna, S.; Doerksen, R.J. Topological Polar Surface Area: A Useful Descriptor in 2D-QSAR. Curr. Med. Chem. 2009, 16, 21. [Google Scholar] [CrossRef] [PubMed]
- Delaney, J.S. ESOL: Estimating Aqueous Solubility Directly from Molecular Structure. J. Chem. Inf. Comput. Sci. 2004, 44, 1000–1005. [Google Scholar] [CrossRef]
- Ali, J.; Camilleri, P.; Brown, M.B.; Hutt, A.J.; Kirton, S.B. Revisiting the General Solubility Equation: In Silico Prediction of Aqueous Solubility Incorporating the Effect of Topographical Polar Surface Area. J. Chem. Inf. Model. 2012, 52, 420–428. [Google Scholar] [CrossRef]
- Winter, H.D. Your Partner in Computational Drug Design. Available online: https://www.silicos-it.be/ (accessed on 5 February 2025).
- SwissADME. Available online: http://www.swissadme.ch/ (accessed on 8 December 2024).
- Islam, M.S.; Mitra, S. Effect of Nano Graphene Oxide (NGO). Incorporation on the Lipophilicity of Hydrophobic Drugs. Hybrid Adv. 2023, 3, 100074. [Google Scholar] [CrossRef]
- Chauhan, H.H.; Chavan, M.D.; Choudhary, R.R.; Madkaikar, H.M.; Dalvi, T.S.; Shah, N.J. Screening of Phytochemicals from Couroupita Guianensis as Drug Candidates against Lethal Diseases Using Insilico Analysis. Int. J. Appl. Chem. Biol. Sci. 2022, 3, 9–19. [Google Scholar]
- Rameshbabu, S.; Alehaideb, Z.; Alghamdi, S.S.; Suliman, R.S.; Almourfi, F.; Yacoob, S.A.M.; Venkataraman, A.; Messaoudi, S.; Matou-Nasri, S. Identification of Anastatica hierochuntica L. Methanolic Leaves Extract-Derived Metabolites Exhibiting Xanthine Oxidase Inhibitory Activities: In Vitro and in Silico Approaches. Metabolites 2024, 14, 368. [Google Scholar] [CrossRef] [PubMed]
- Elbouzidi, A.; Taibi, M.; Laaraj, S.; Loukili, E.H.; Haddou, M.; Hachlafi, N.E.; Mrabti, H.N.; Baraich, A.; Bellaouchi, R.; Asehraou, A.; et al. Chemical Profiling of Volatile Compounds of the Essential Oil of Grey-Leaved Rockrose (Cistus albidus L.) and Its Antioxidant, Anti-Inflammatory, Antibacterial, Antifungal, and Anticancer Activity in Vitro and in Silico. Front. Chem. 2024, 12, 1334028. [Google Scholar] [CrossRef]
- Martin, Y.C. A Bioavailability Score. J. Med. Chem. 2005, 48, 3164–3170. [Google Scholar] [CrossRef] [PubMed]
- Price, G.; Patel, D.A. Drug Bioavailability. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Rajan, R.; Karthikeyan, S.; Desikan, R. Synthesis, Structural Elucidation, In Silico and In Vitro Studies of New Class of Methylenedioxyphenyl-Based Amide Derivatives as Potential Myeloperoxidase Inhibitors for Cardiovascular Protection. ACS Omega 2024, 9, 7850. [Google Scholar] [CrossRef]
- SMARTCyp (Version 3.0). Available online: https://smartcyp.sund.ku.dk/mol_to_som (accessed on 8 December 2024).
- Athar, M.; Sona, A.N.; Bekono, B.D.; Ntie-Kang, F. Fundamental Physical and Chemical Concepts behind “Drug-Likeness” and “Natural Product-Likeness”. Phys. Sci. Rev. 2019, 4, 101. [Google Scholar] [CrossRef]
- Polinsky, A. Lead-Likeness and Drug-Likeness. In The Practice of Medicinal Chemistry; Elsevier: Amsterdam, The Netherlands, 2008; pp. 244–254. [Google Scholar]
- Hann, M.M.; Oprea, T.I. Pursuing the Leadlikeness Concept in Pharmaceutical Research. Curr. Opin. Chem. Biol. 2004, 8, 255–263. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
- Teague, S.J.; Davis, A.M.; Leeson, P.D.; Oprea, T. The Design of Leadlike Combinatorial Libraries. Angew. Chem. Int. Ed. 1999, 38, 3743–3748. [Google Scholar] [CrossRef]
- Way2Drug—Main (Version 2.0). Available online: http://www.way2drug.com/PASSOnline/index.php (accessed on 8 December 2024).
- Cramer, G.M.; Ford, R.A.; Hall, R.L. Estimation of Toxic Hazard—A Decision Tree Approach. Food Cosmet. Toxicol. 1976, 16, 255–276. [Google Scholar] [CrossRef]
- Kroes, R.; Renwick, A.G.; Cheeseman, M.; Kleiner, J.; Mangelsdorf, I.; Piersma, A.; Schilter, B.; Schlatter, J.; Van Schothorst, F.; Vos, J.G.; et al. Structure-Based Thresholds of Toxicological Concern (TTC): Guidance for Application to Substances Present at Low Levels in the Diet. Food Chem. Toxicol. 2004, 42, 65–83. [Google Scholar] [CrossRef]
- Eucast: MIC Determination. Available online: https://www.eucast.org/ast_of_bacteria/mic_determination (accessed on 8 August 2024).
- Ciurea, C.N.; Mare, A.D.; Mareș, M.; Toma, F.; Kosovski, I.-B.; Cighir, A.; Man, A. The Influence of Farnesol and Tyrosol on Candida Spp. Virulence Traits. Germs 2024, 14, 344–351. [Google Scholar]
- Potts, R.O.; Guy, R.H. Predicting Skin Permeability. Pharm. Res. 1992, 9, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Jeliazkova, N.; Martinov, M.; Tcheremenskaia, O.J.K.; Networks, M.; Rydberg, P.; Avramova, S.; Kochev, N.; Jeliazkov, V.; Iliev, L. Toxtree—Toxtree—Toxic Hazard Estimation by Decision Tree Approach (Version 3.1.0.1851). Available online: https://toxtree.sourceforge.net/ (accessed on 8 December 2024).
- Olsen, L.; Montefiori, M.; Tran, K.P.; Jørgensen, F.S. SMARTCyp 3.0: Enhanced Cytochrome P450 Site-of-Metabolism Prediction Server. Bioinformatics 2019, 35, 3174–3175. [Google Scholar] [CrossRef]
- Muegge, I.; Heald, S.L.; Brittelli, D. Simple Selection Criteria for Drug-like Chemical Matter. J. Med. Chem. 2001, 44, 1841–1846. [Google Scholar] [CrossRef] [PubMed]
- Egan, W.J.; Merz, K.M.; Baldwin, J.J. Prediction of Drug Absorption Using Multivariate Statistics. J. Med. Chem. 2000, 43, 3867–3877. [Google Scholar] [CrossRef]
- Ghose, A.K.; Viswanadhan, V.N.; John, J. Wendoloski A Knowledge-Based Approach in Designing Combinatorial or Medicinal Chemistry Libraries for Drug Discovery. 1. A Qualitative and Quantitative Characterization of Known Drug Databases. J. Comb. Chem. 1999, 1, 55–68. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.-Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular Properties That Influence the Oral Bioavailability of Drug Candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef]
- Benigni, R.; Bossa, C. Mechanisms of Chemical Carcinogenicity and Mutagenicity: A Review with Implications for Predictive Toxicology. Chem. Rev. 2011, 111, 2507–2536. [Google Scholar] [CrossRef]
- Benigni, R.; Bossa, C.; Netzeva, T.; Rodomonte, A.; Tsakovska, I. Mechanistic QSAR of Aromatic Amines: New Models for Discriminating between Homocyclic Mutagens and Nonmutagens, and Validation of Models for Carcinogens. Environ. Mol. Mutagen. 2007, 48, 754–771. [Google Scholar] [CrossRef]
- Benigni, R.; Bossa, C.; Tcheremenskaia, O. Nongenotoxic Carcinogenicity of Chemicals: Mechanisms of Action and Early Recognition through a New Set of Structural Alerts. Chem. Rev. 2013, 113, 2940–2957. [Google Scholar] [CrossRef]


| Bacterial Strain | MIC (mg/mL) | |||
|---|---|---|---|---|
| GLN | GLA | MSG | GLADE | |
| Gram-positive | ||||
| Staphylococcus aureus ATCC 29213 (MSSA) | >2.86 | 1.76 | 112 | 12.75 |
| Staphylococcus aureus ATCC 43300 (MRSA) | >2.86 | 0.88 | 112 | 12.75 |
| Enterococcus faecalis ATCC 700609 | >2.86 | 1.76 | 112 | 12.75 |
| Gram-negative | ||||
| Klebsiella pneumoniae ATCC 25922 | >2.86 | >1.76 | 112 | 25.5 |
| Escherichia coli ATCC 29213 | >2.86 | >1.76 | 112 | 25.5 |
| Pseudomonas aeruginosa ATCC 27853 | >2.86 | 1.76 | 112 | 12.75 |
| Bacterial Strain | MBC (mg/mL) | ||
|---|---|---|---|
| GLA | MSG | GLADE | |
| Gram-positive | |||
| S. aureus ATCC 29213 (MSSA) | >1.76 | >112 | 51 |
| S. aureus ATCC 43300 (MRSA) | >1.76 | >112 | 25.5 |
| E. faecalis ATCC 700609 | >1.76 | >112 | 25.5 |
| Gram-negative | |||
| K. pneumoniae ATCC 25922 | - | >112 | 25.5 |
| E. coli ATCC 29213 | - | >112 | 25.5 |
| P. aeruginosa ATCC 27853 | >1.76 | >112 | 25.5 |
| Evaluated Property | Predicted Results | Platform/Software |
|---|---|---|
| Physicochemical properties | ||
| Molecular weight (MW) | 203.24 g/mol | SwissADME |
| Heavy atoms (HA) | 14 | |
| Csp3 fraction (the ratio between the number of sp3-hybridized carbon atoms and the total number of carbon atoms in the molecule) | 0.78 | |
| Rotatable bonds (RBs) | 8 | |
| Hydrogen bond acceptors (HAccs) | 5 | |
| Hydrogen bond donors (HDs) | 1 | |
| Topological polar surface area (TPSA) | 78.62 Å2 | |
| Water solubility | Very soluble (ESOL) Very soluble (Ali) Soluble (SILICOS-IT) | |
| Log P | 0.77 (Consensus Log P) | |
| Pharmacokinetic properties | ||
| Gastrointestinal absorption | High | SwissADME |
| Blood–brain barrier (BBB) permeation | Negative | |
| Substrate for P-glycoprotein (P-gp) | Negative | |
| Inhibitor of CYP450 isoforms (CYP1A2; CYP2C19; CYP2C9; CYP2D6; CYP3A4) | Negative | |
| Skin permeation (Log Kp) | −7.43 cm/s | |
| Bioavailability (BD) score | 0.55 | |
| Atom reactivity (sites involved in metabolism via CYP3A4, CYP2D6, and CYP2C9 isoforms) | Cyp3A4: C8 (the carbon atom linked to the amino group) Cyp2D6: C1 (the marginal carbon atom from the ethyl group part of the ester formed with the participation of the carboxyl from the gamma position) Cyp2C9: C8 | SmartCyp (version 3.0) |
| Cytochrome P450-mediated drug metabolism |
| Toxtree (version 3.1.0.1851); the sites of metabolism are predicted by SmartCyp and used by Toxtree |
| Drug-likeness and lead-likeness | ||
| Drug-likeness | GLADE respects the rules of Lipinsky, Muegge, Eagan, Ghose, and Veber | SwissADME |
| Lead-likeness | 2 broken rules: MW < 250 and RB > 7 | |
| Antibacterial activity | ||
| Antibiotic glycopeptide-like | Probability to be active (Pa) of 0.127 | Pass online (version 2.0) |
| Antibiotic | Pa of 0.099 | |
| Aureolysin inhibitor | Pa of 0.279 | |
| Antimycobacterial | Pa of 0.344 | |
| Antirickettsial | Pa of 0.291 | |
| Anti-Helicobacter pylori | Pa of 0.267 | |
| Bacterial leucyl aminopeptidase inhibitor | Pa of 0.196 | |
| Aerobactin synthase inhibitor | Pa of 0.195 | |
| Antibacterial (oftalmic) | Pa of 0.159 | |
| Antibacterial | Pa of 0.214 | |
| Antituberculosic | Pa of 0.366 | |
| Antispirochetal | Pa of 0.190 | |
| Cell wall synthesis inhibitor | Pa of 0.139 | |
| Toxic and adverse effects | ||
| Cramer rules | Class I (low toxicity) | Toxtree |
| Kroes TTC | The substance would not be expected to be a safety concern | |
| Carcinogenicity | Negative for genotoxic and non-genotoxic carcinogenicity | |
| In vitro mutagenicity (Ames test) | No alerts for Salmonella Typhimurium mutagenicity |
| Amino Acid | Tested Bacteria | Observations | Ref. |
|---|---|---|---|
| GLN | S. mutans | GLN is a vital nitrogen source provided by its specific transporter (glutamine transport system permease protein—GlnP). The membrane transport function allows the biofilm to survive stress and benefit from favourable conditions for development. | [35] |
| S. aureus |
| [36,37] | |
| GLA | B. subtilis | The biofilm-forming cells use a mechanism that directs excessive Krebs cycle metabolites to nitrogen metabolism, forming storage products. The level of some amino acids or nucleotides generated starting from GLA increases during biofilm formation. | [10] |
| Gram-positive and Gram-negative bacteria |
| [38] | |
| GLA and GLN | E. faecalis and P. aeruginosa | Following the administration of aminooxy acetic acid (AOA) as an aminotransferase inhibitor (inhibitor of glutamate oxaloacetate transaminase and aspartate aminotransferase, resulting in glutamate and its metabolite levels’ restriction) and 6-diazo-5-oxo-L norleucine as a GLN analogue, high sensitivity, especially to AOA, was observed, which affected biofilm growth; the biofilm formed at subinhibitory doses of the two compounds underwent morphological changes, also being more sensitive to AOA. | [27] |
| PG | B. subtilis | PG contributes to the robustness and complexity of the biofilms’ morphology. | [39] |
| Staphylococcus epidermidis | The bacterial strain produces PG that binds to antimicrobial peptides, having a protective effect against neutrophil phagocytosis. | [40] |
| No. Well | Percentage of the Maximum Concentration | Concentration/Well (mg/mL) | |||
|---|---|---|---|---|---|
| GLN | GLA | MSG | GLADE | ||
| 1 | 100% | 2.860 mg/mL | 1.760 mg/mL | 112.000 mg/mL | 102.000 mg/mL |
| 2 | 50% | 1.430 mg/mL | 0.880 mg/mL | 56.000 mg/mL | 51.000 mg/mL |
| 3 | 25% | 0.715 mg/mL | 0.440 mg/mL | 28.000 mg/mL | 25.500 mg/mL |
| 4 | 12.5% | 0.357 mg/mL | 0.220 mg/mL | 14.000 mg/mL | 12.750 mg/mL |
| 5 | 6.25% | 0.178 mg/mL | 0.110 mg/mL | 7.000 mg/mL | 6.375 mg/mL |
| 6 | 3.125% | 0.089 mg/mL | 0.055 mg/mL | 3.500 mg/mL | 3.187 mg/mL |
| 7 | 1.562% | 0.044 mg/mL | 0.027 mg/mL | 1.750 mg/mL | 1.593 mg/mL |
| 8 | 0.781% | 0.022 mg/mL | 0.013 mg/mL | 0.875 mg/mL | 0.796 mg/mL |
| 9 | 0.390% | 0.011 mg/mL | 0.006 mg/mL | 0.437 mg/mL | 0.398 mg/mL |
| 10 | 0.195% | 0.005 mg/mL | 0.003 mg/mL | 0.218 mg/mL | 0.199 mg/mL |
| 11 | 0.097% | 0.002 mg/mL | 0.001 mg/mL | 0.109 mg/mL | 0.099 mg/mL |
| 12 | 0.048% | 0.001 mg/mL | 0.00075 mg/mL | 0.054 mg/mL | 0.049 mg/mL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oancea, O.-L.; Ciurea, C.N.; Mare, A.D.; Man, A.; Stefanescu, R.; Rusu, A. In Vitro Evaluation of the Antibacterial Effect and Influence on the Bacterial Biofilm Formation of Glutamic Acid and Some Structural Analogues. Antibiotics 2025, 14, 415. https://doi.org/10.3390/antibiotics14040415
Oancea O-L, Ciurea CN, Mare AD, Man A, Stefanescu R, Rusu A. In Vitro Evaluation of the Antibacterial Effect and Influence on the Bacterial Biofilm Formation of Glutamic Acid and Some Structural Analogues. Antibiotics. 2025; 14(4):415. https://doi.org/10.3390/antibiotics14040415
Chicago/Turabian StyleOancea, Octavia-Laura, Cristina Nicoleta Ciurea, Anca Delia Mare, Adrian Man, Ruxandra Stefanescu, and Aura Rusu. 2025. "In Vitro Evaluation of the Antibacterial Effect and Influence on the Bacterial Biofilm Formation of Glutamic Acid and Some Structural Analogues" Antibiotics 14, no. 4: 415. https://doi.org/10.3390/antibiotics14040415
APA StyleOancea, O.-L., Ciurea, C. N., Mare, A. D., Man, A., Stefanescu, R., & Rusu, A. (2025). In Vitro Evaluation of the Antibacterial Effect and Influence on the Bacterial Biofilm Formation of Glutamic Acid and Some Structural Analogues. Antibiotics, 14(4), 415. https://doi.org/10.3390/antibiotics14040415












