Abstract
Antimicrobial resistance is already a major global health threat, contributing to nearly 5 million deaths annually. The rise of multidrug-resistant pathogens has made many infections increasingly difficult to treat. This growing threat has driven the search for alternative therapeutic approaches. Among the most promising candidates are bacterial extracellular vesicles (BEVs) and antimicrobial peptides (AMPs), which offer unique mechanisms of action, potential synergistic effects, and the ability to bypass conventional resistance pathways. This review summarizes the current research on synergistic effects of BEVs and AMPs to overcome antimicrobial resistance.
1. Introduction
Antimicrobial resistance represents one of the most pressing global health challenges, significantly limiting the efficacy of conventional treatments for bacterial infections. The overuse and misuse of antibiotics in healthcare, agriculture, and animal husbandry have accelerated the development of resistance, allowing bacteria to evolve defense mechanisms against multiple drug classes [1].
Antimicrobial resistance (AMR) occurs when microorganisms, including bacteria, fungi, viruses, and parasites, evolve mechanisms to withstand the effects of antimicrobial agents. In general, AMR in bacteria is generally classified into two main types: inherent (intrinsic) resistance and acquired resistance [2,3]. Intrinsic resistance refers to the natural, pre-existing ability of certain bacterial species to resist the effects of specific antibiotics without prior exposure or genetic changes. This type of resistance is a fundamental property of the bacterial species and is usually due to structural or functional characteristics that prevent antibiotics from working effectively [4]. Acquired resistance occurs when previously susceptible bacteria develop resistance due to genetic changes. This type of resistance arises through mutations or horizontal gene transfer (HGT) from other resistant bacteria [1].
The rise of multidrug-resistant (MDR) and extensively drug-resistant (XDR) pathogens, such as Methicillin-resistant Staphylococcus aureus (MRSA), Carbapenem-resistant Enterobacteriaceae (CRE), and Vancomycin-resistant Enterococci (VRE), has rendered many infections nearly untreatable. The emergence of such multidrug-resistant pathogens has necessitated alternative therapeutic strategies. Among these, bacterial extracellular vesicles (BEVs) and antimicrobial peptides (AMPs) have emerged as promising candidates due to their unique mechanisms of action, potential for synergy, and ability to circumvent traditional resistance pathways. This review discusses relevant biological aspects of BEVs including biogenesis mechanisms, composition, methods of extraction and purification, and their role in antibiotic resistance. Also, AMPs are described as solutions to overcome conventional resistance mechanisms, especially in combination with BEVs. This synergistic activity is an innovative, still-developing topic but it has a high potential in regard to the antimicrobial resistance problem.
Mechanisms of Antimicrobial Resistance
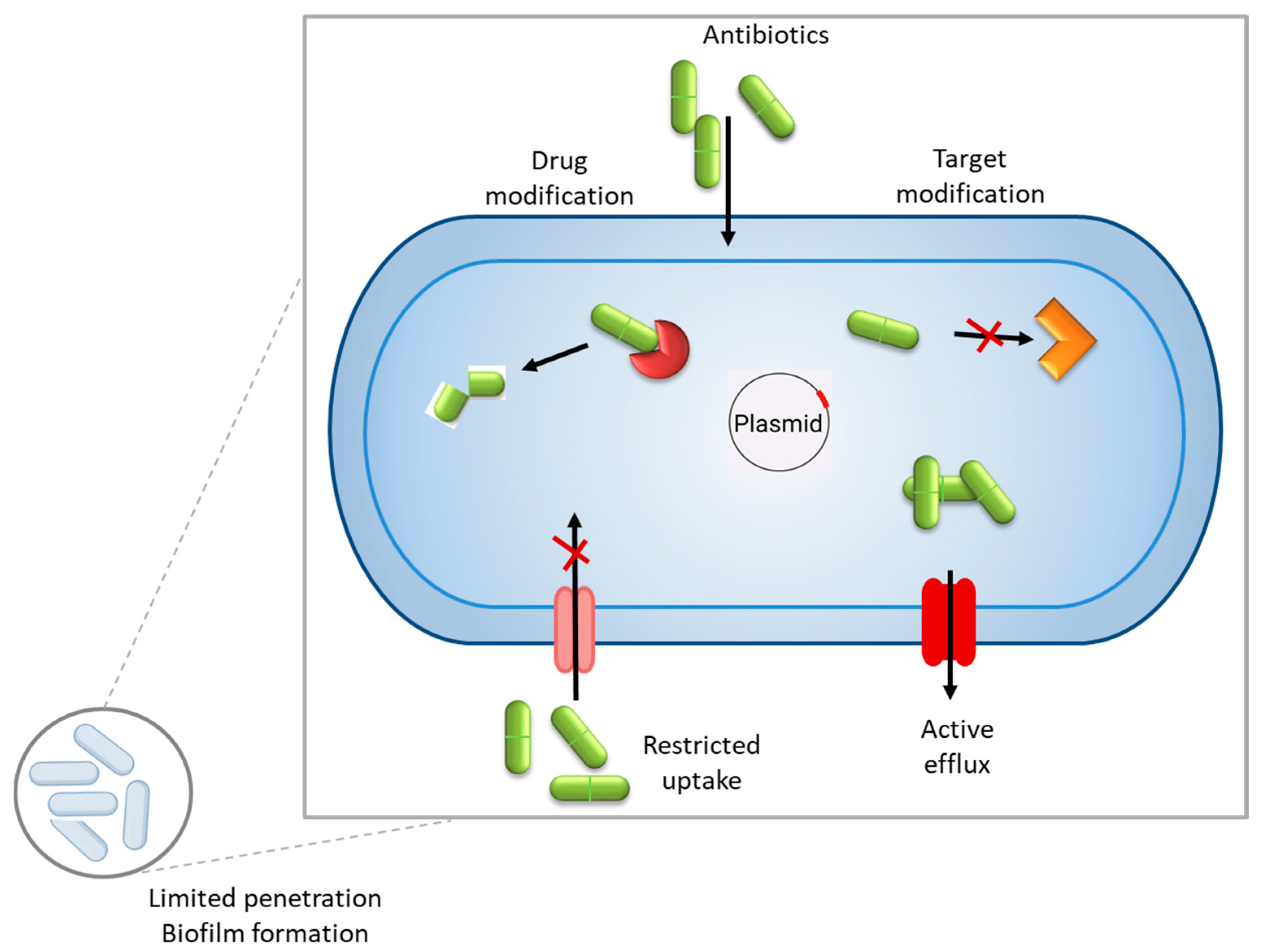
In general, antimicrobial resistance mechanisms may be divided into four main categories: limiting the uptake of a drug, the inactivation of the drug, the modification of the drug target, and active drug efflux (see Figure 1).
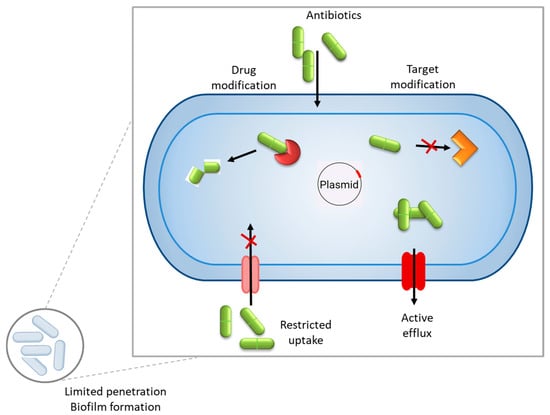
Figure 1.
General mechanisms of antibiotic resistance in bacteria include inactivation of the drug, modifying the drug target, limiting the uptake of a drug, and active drug efflux. Within a biofilm, bacterial cells are embedded in a self-produced extracellular polymeric substance (EPS) matrix that acts as a physical and chemical barrier, impeding the penetration of antimicrobial agents.
Enzymatic degradation or modification of antimicrobial drugs is one of the most common and effective resistance mechanisms by which bacteria produce specific enzymes that degrade or modify antibiotics, rendering them ineffective. β-Lactam antibiotics (e.g., penicillins, cephalosporins, and carbapenems) target bacterial cell wall synthesis. Some bacteria produce β-lactamase enzymes that hydrolyze the β-lactam ring, inactivating the antibiotic [5]. Aminoglycosides (e.g., gentamicin and tobramycin) inhibit bacterial protein synthesis by binding to the 30S ribosomal subunit. Bacteria can produce aminoglycoside-modifying enzymes (AMEs) that modify aminoglycosides via acetylation, phosphorylation, or adenylation, preventing drug binding [6]. Also, some bacteria produce chloramphenicol acetyltransferase (CAT), which inactivates chloramphenicol through acetylation, blocking its ability to bind the ribosome [7]. Macrolide resistance can occur due to esterases or phosphotransferases that modify drugs like erythromycin [8]. Rifamycin resistance is often mediated by ADP-ribosyltransferases, which modify the drug’s structure [9]. Some bacteria evade antibiotics through the alteration of their target sites, preventing drug binding while maintaining essential cellular functions. Macrolides, tetracyclines, aminoglycosides, and oxazolidinones (e.g., linezolid) target ribosomal subunits (30S or 50S). Some bacteria, like Streptococcus pneumoniae, can mutate ribosomal proteins or methylate RNA to prevent antibiotic binding [10].
Biofilms are a survival strategy of bacteria that enhances survival against antibiotics and host defenses, leading to antibiotic resistance. Bacteria develop biofilms through a complex multistep process that allows them to form structured, resilient communities. The process begins when free-floating (planktonic) bacteria encounter a surface. This initial attachment is reversible and weak, driven by physical forces like van der Waals interactions, hydrophobic effects, or electrostatic charges. Bacteria use appendages such as flagella or pili to approach the surface [11]. Once in contact, specific adhesins (proteins on the bacterial surface) may bind to the substrate, making the attachment more secure and irreversible. Environmental factors, like nutrient availability or surface conditioning (e.g., a layer of organic molecules), can influence this step. After attaching, bacteria begin to proliferate and form microcolonies. They divide and produce extracellular polymeric substances (EPSs), a sticky matrix of polysaccharides, proteins, and DNA that anchors them to the surface and to each other. This EPS layer not only provides structural support but also traps nutrients and protects the growing community from external threats like antibiotics or immune responses. As the biofilm grows, it develops into a complex, three-dimensional structure. This stage involves the formation of water channels within the EPS matrix, which facilitate nutrient and oxygen distribution while removing waste. Genetic regulation fine-tunes the community, with some cells differentiating into distinct roles (e.g., persister cells that resist stress). At this point, the biofilm becomes highly resistant to antibiotics, up to 1000 times more so than planktonic cells, due to the protective EPS and slower metabolic rates of deeper layers [12]. Eventually, some bacteria leave the biofilm to colonize new areas.
Numerous bacterial species possess efflux pumps, which are specialized membrane proteins that actively expel a wide range of antimicrobial agents from the cell, significantly contributing to drug resistance. These pumps reduce the intracellular concentration of antibiotics, causing them to be less effective and allowing bacteria to survive in hostile environments. For example, Enterobacteriaceae, a family of Gram-negative bacteria commonly found in clinical settings, frequently exhibits the presence of efflux pumps. Notable members, such as Escherichia coli and Klebsiella pneumoniae, utilize these systems to extrude multiple classes of drugs, including β-lactams, fluoroquinolones, and tetracyclines, thereby enhancing their multidrug resistance profiles [13]. The expression and activity of efflux pumps in Enterobacteriaceae are often regulated by complex genetic mechanisms, making them a critical target for overcoming resistance in therapeutic strategies [14].
2. Bacterial Extracellular Vesicles
Bacterial extracellular vesicles (BEVs) are membrane-lipid bilayer structures released by bacteria into their surrounding environment. These vesicles are typically spherical and range in size from about 20 to 400 nanometers in diameter, depending on the bacterial species [15]. BEVs are produced by both Gram-positive and Gram-negative bacteria and play diverse roles in bacterial physiology, communication, and interaction with host organisms.
The composition of bacterial extracellular vesicles (BEVs) is highly dynamic and varies significantly based on multiple factors, including the bacterial species, the environmental conditions in which they are produced, and their biological function. Different bacterial species secrete BEVs with distinct molecular compositions, including variations in proteins, lipids, nucleic acids, and metabolites, reflecting their unique genetic and metabolic characteristics [16,17]. Environmental factors such as nutrient availability, stress conditions, pH, and temperature further influence BEV composition, leading to adaptations that optimize bacterial survival and communication. Additionally, the functional role of BEVs, whether in pathogenesis, intercellular communication, antibiotic resistance, or host immune modulation, dictates the selective packaging of specific bioactive molecules [18,19].
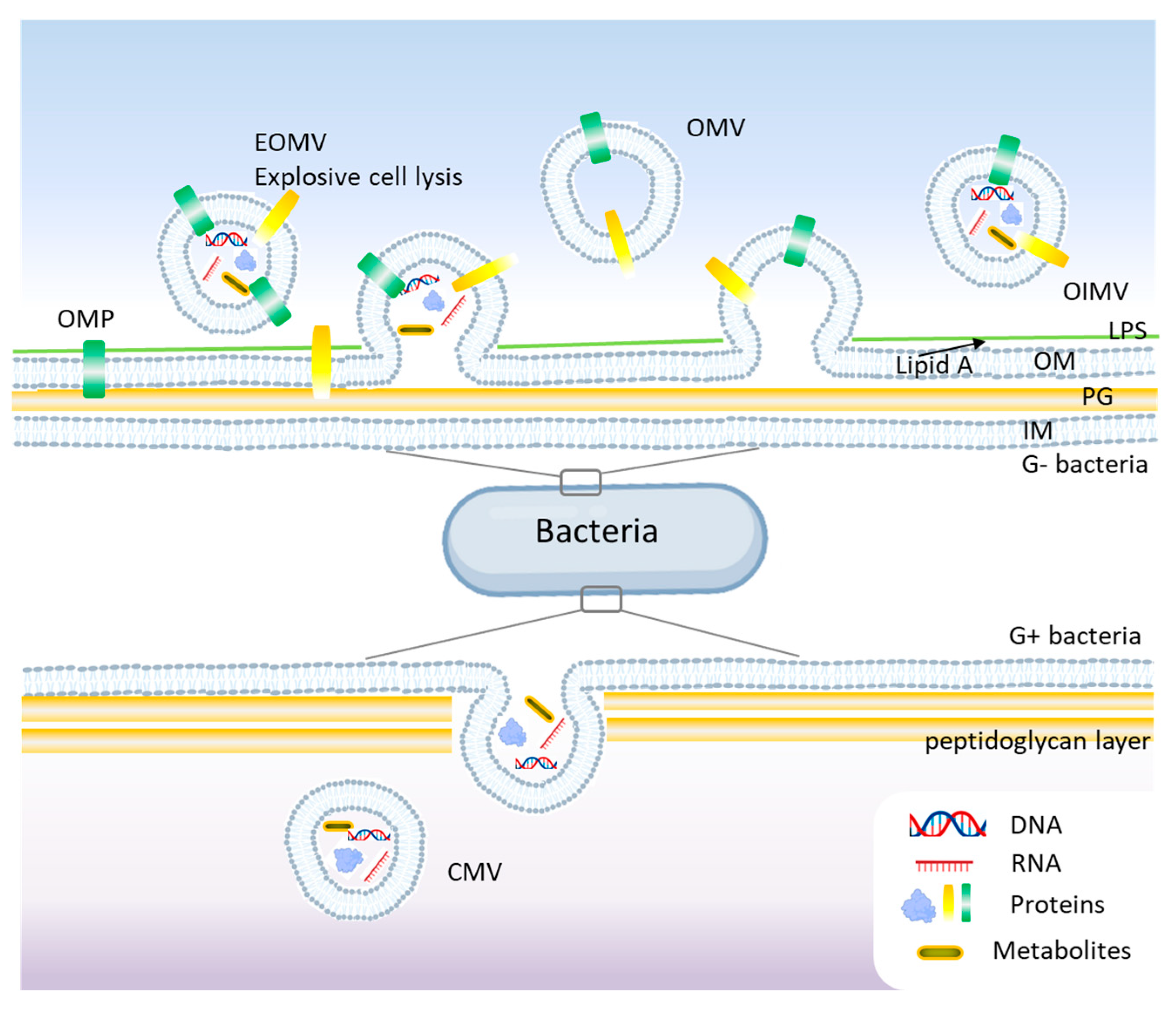
Gram-negative bacteria possess an outer membrane (OM), a periplasmic space, and an inner membrane (IM), which influences the mechanisms of BEV formation. They primarily release two types of vesicles: outer membrane vesicles (OMVs) and outer-inner membrane vesicles (OIMVs) [20]. OMVs are derived solely from the outer membrane and contain lipopolysaccharides (LPS), outer membrane proteins, phospholipids, and periplasmic components [21]. OIMVs contain both outer and inner membrane components, allowing the packaging of cytoplasmic proteins and nucleic acids [22]. See Figure 2 for a schematic representation of BEVs’ biogenesis.
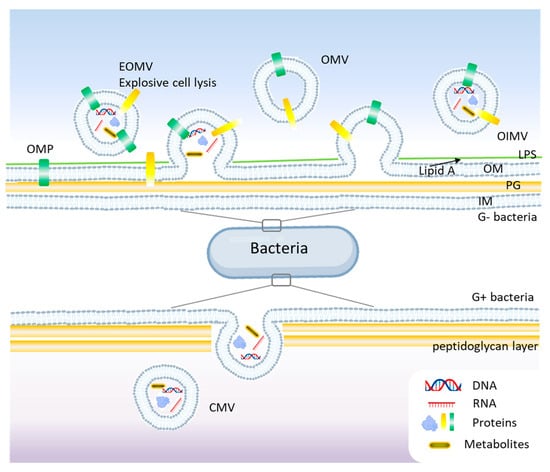
Figure 2.
Biogenesis mechanisms of bacterial extracellular vesicles (BEVs) models. In Gram-negative bacteria, blebbing and explosive cell lysis are among the main mechanisms for BEVs, while budding is common in Gram-positive bacteria. OMV, outer membrane vesicle; OIMV, outer-inner membrane vesicle; EOMV, explosive outer membrane vesicle; OM, outer membrane; IM, inner membrane; Lipid A, LPS, lipopolysaccharides; PG, peptidoglycan; OMP, outer membrane proteins.
Unlike Gram-negative bacteria, Gram-positive bacteria lack an outer membrane and have a thick peptidoglycan (PG) layer, which was initially thought to prevent vesicle formation. The formation of membrane vesicles in Gram-positive bacteria is primarily initiated by the expansion of lipid-rich domains within the cytoplasmic membrane [19]. This expansion, combined with the activity of endolysins that degrade the PG, compromises the integrity of the thick peptidoglycan layer, facilitating the release of these vesicles (Figure 2). However, studies showed that Gram-positive bacteria produce BEVs, albeit via different mechanisms [23]. Membrane vesicles (MVs) and cytoplasmic membrane vesicles (CMVs) are found in both Gram-positive and Gram-negative bacteria and are derived from cytoplasmic or plasma membranes. These vesicles carry bacterial proteins, peptidoglycan fragments, and toxins. Some bacteria, like Pseudomonas aeruginosa produce tubular or explosive vesicles (explosive outer membrane vesicles, EOMVs) as a defensive or communication strategy [24]. Currently, different mechanisms are reported to understand the generation of BEVs like the blebbing or budding of the cell membrane (CM), explosive cell lysis or bubbling cell death, and the formation of nanotubes [25,26]. Also, the membrane differences between Gram-positive and Gram-negative bacteria impact the biological functions, immunogenicity, and physiological roles of bacterial extracellular vesicles [23]. Since LPS is profoundly immunogenic, its absence in Gram-positive membranes represents a crucial distinction, affecting how bacterial vesicles interact with the host immune system and their potential biomedical applications.
Bacterial extracellular vesicles are highly diverse, with different roles in bacterial communication, pathogenesis, and antibiotic resistance and vary depending on the bacterial strain, environment, and function. Table 1 summarizes the composition, role in antibiotic resistance, and pathogenicity of BEVs from different bacterial strains.

Table 1.
Composition, resistance, and pathogenicity of bacterial extracellular vesicles (BEVs) by strain.
2.1. Role of BEVs in Antibiotic Resistance
BEVs play a multifaceted role in promoting antibiotic resistance among bacterial populations through several mechanisms. Firstly, BEVs serve as vehicles for horizontal gene transfer, encapsulating and disseminating resistance genes between bacterial cells, even across species. This transfer enhances the spread of resistance traits within microbial communities, amplifying the resilience of populations against antibiotics. For example, studies have shown that BEVs from Escherichia coli and Pseudomonas aeruginosa can transfer β-lactam resistance genes to recipient cells, enhancing their survival in the presence of antibiotics like ampicillin or imipenem [38,39]. The degradation or sequestration of antibiotics is another way for BEVs to induce antibiotic resistance. BEVs can contain enzymes, such as β-lactamases, that degrade antibiotics extracellularly before they reach the bacterial cell. For instance, BEVs from methicillin-resistant Staphylococcus aureus (MRSA) have been shown to degrade β-lactam antibiotics when exposed to sub-lethal concentrations of ampicillin. Alternatively, BEVs can act as traps, binding antibiotics (e.g., membrane-targeting peptides like colistin) and reducing the effective concentration that reaches the bacterial cell [40]. Also, antibiotic exposure often increases BEV production. Research on MRSA has demonstrated a 22.4-fold increase in BEV secretion when stressed with sub-minimum inhibitory concentrations (MICs) of ampicillin. These vesicles can protect susceptible bacteria in a population by neutralizing antibiotics or exporting harmful substances, like misfolded proteins or excess drugs, out of the cell [41]. Thirdly, BEVs contribute to biofilm formation, a key resistance strategy, by delivering structural components or signaling molecules that fortify the extracellular matrix, making the penetration of antibiotics more difficult. BEVs contribute to biofilm formation by delivering extracellular polymeric substances (e.g., polysaccharides, proteins, and DNA). Biofilms are notoriously resistant to antibiotics due to their physical barrier and altered metabolic state. BEVs from Pseudomonas aeruginosa, for example, enhance biofilm growth during the exponential phase, making infections harder to treat [42]. Additionally, BEVs released by resistant bacteria can protect nearby susceptible bacteria. For instance, vesicles from resistant Klebsiella pneumoniae have been shown to expel antibiotics via efflux pumps, creating a local environment where susceptible strains can survive longer [43].
2.2. Methods for BEV Extraction and Purification
Bacterial extracellular vesicles (BEVs) are valuable tools for various applications, from antimicrobial strategies to vaccine development and gene therapy. To tackle BEVs effectively, it is crucial to isolate them with high purity and yield. BEVs can be extracted using a variety of methods. Differential centrifugation is the most commonly used, while density gradient ultracentrifugation and size-exclusion chromatography (SEC) provide higher purity. The best method depends on the bacterial strain, vesicle properties, and research goals. See Table 2 for a comparison of BEV extraction methods. Differential centrifugation is a commonly used, cost-effective, and an easy to perform method which separates vesicles based on size and density using a stepwise centrifugation process [44]. Density gradient ultracentrifugation uses a sucrose or iodixanol (OptiPrep) gradient to separate vesicles by density [45]. SEC separates vesicles based on size using a gel filtration column [46]. This method may require multiple runs to obtain high yields and is time consuming. Ultrafiltration is a fast method that uses membranes of different pore sizes to retain vesicles while removing small contaminants [47]. Immunoaffinity capture uses antibodies targeting specific BEV markers (e.g., LPS, outer membrane proteins) to pull out vesicles [48]. To achieve both a high yield and high purity, many protocols combine differential centrifugation with density gradient ultracentrifugation or SEC.

Table 2.
Comparison of BEV isolation methods.
3. Antimicrobial Peptides
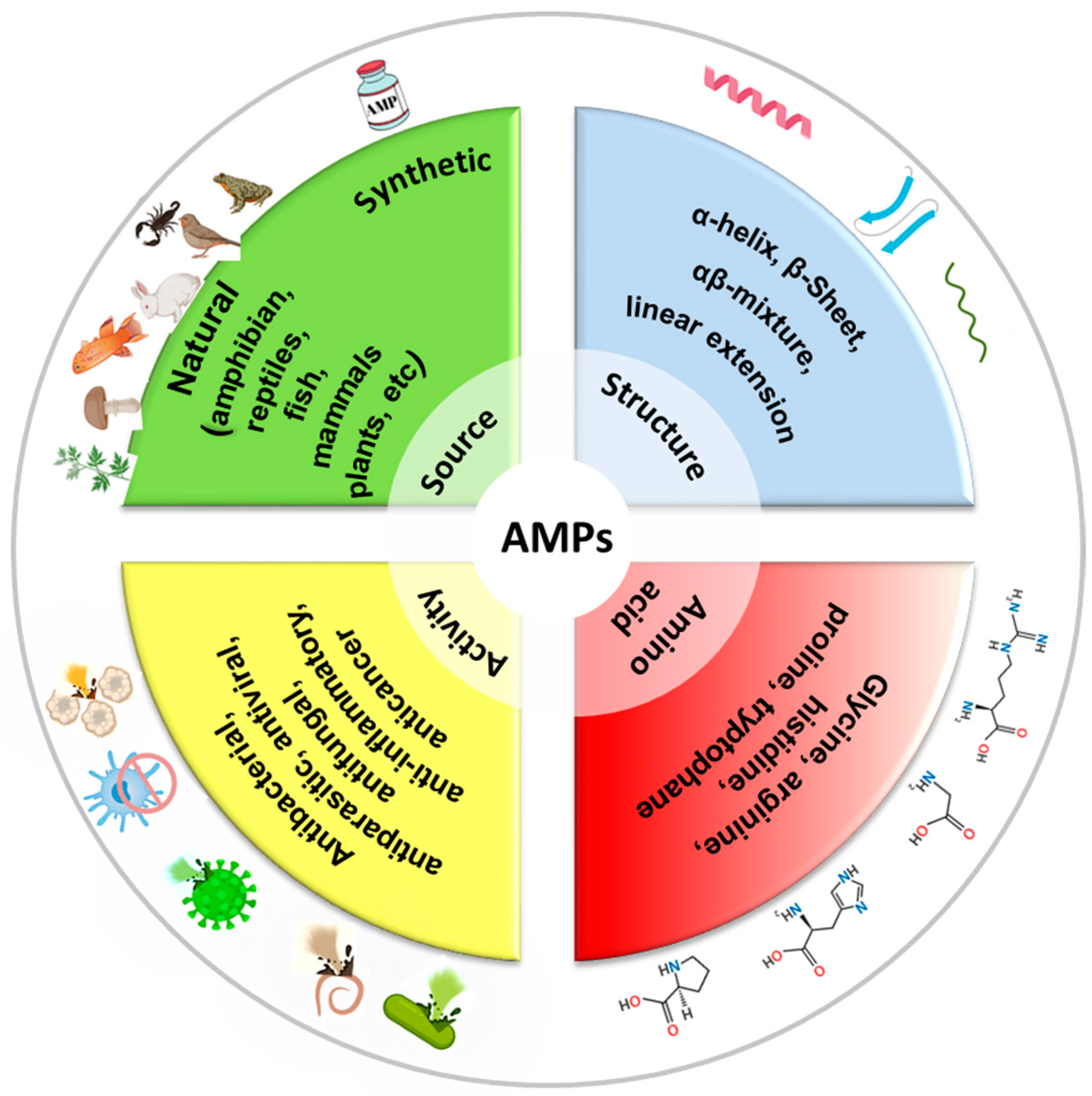
Antimicrobial peptides (AMPs) are short chains of amino acids, typically ranging from 10 to 50 residues, which exhibit broad-spectrum antimicrobial activity. They are naturally produced by a wide range of organisms, including humans, animals, plants, and even microorganisms, as part of their innate immune defense systems. AMPs are often cationic (positively charged) and amphipathic (having both hydrophilic and hydrophobic regions), which allows them to interact with and disrupt microbial membranes [3,50]. See Figure 3 for a classification of AMPs. AMPs play a critical role in combating infections, as they have a broad spectrum activity against bacteria (both Gram-positive and Gram-negative), fungi, viruses, and even some parasites [51,52,53]. Also, AMPs kill microbes quickly by physically disrupting their membranes, unlike many traditional antibiotics that target specific metabolic processes. Several models describe how AMPs disrupt membranes, including the barrel-stave, carpet, or toroidal pore models. In the barrel-stave model, AMPs like alamethicin or ceratotoxins insert into the membrane perpendicularly, forming a pore by aligning like staves of a barrel. This allows the leakage of cellular contents, leading to cell death [54]. In the carpet model, AMPs (cecropin for example) coat the membrane surface like a carpet, eventually causing it to disintegrate or form micelles at high concentrations, disrupting membrane integrity [55]. In the toroidal pore model, AMPs like magainin or LL-37 induce the membrane to bend, forming pores lined with both peptides and lipid headgroups, which destabilizes the membrane and causes leakage [56]. Because they attack the microbial membranes rather than specific molecular targets, bacteria have a harder time developing resistance to AMPs compared to conventional antibiotics [57]. Beyond direct antimicrobial effects, some AMPs enhance the immune response by recruiting immune cells or neutralizing inflammatory toxins like lipopolysaccharides (LPSs). In addition to membrane disruption, some AMPs can enter bacterial cells and target critical intracellular components, such as inhibiting DNA and RNA synthesis, blocking protein synthesis and disrupting enzymatic activity [58]. Indolicidin, a peptide derived from the cytoplasmic granules of bovine neutrophils binds bacterial DNA and prevents transcription [59]. Bac7 disrupts ribosome function and inhibits protein synthesis [60]. Other peptides such as protegrins inhibit metabolic enzymes [61]. AMPs can also affect cellular organelles, such as mitochondria, by causing structural damage and finally leading to cell death [62]. More importantly, AMPs can prevent or break down biofilms by interfering with quorum sensing (bacterial communication) or directly degrading the extracellular matrix, making pathogens more vulnerable to immune responses or other treatments [63].
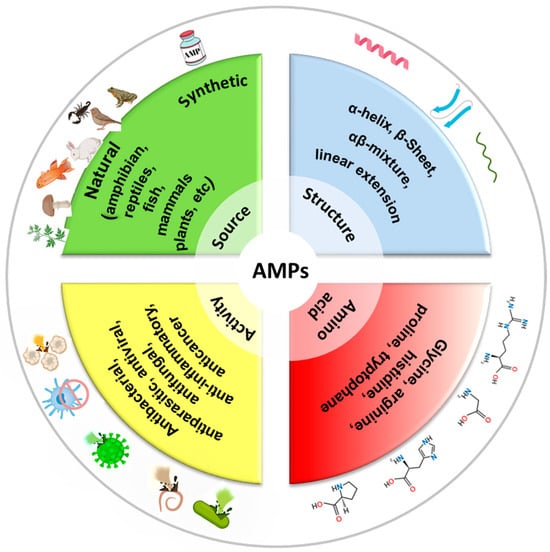
Figure 3.
Classification of AMPs by source, structure, activity, and amino acid composition.
AMPs have emerged as powerful alternatives to traditional antibiotics and due to their unique mechanisms of action and broad-spectrum antimicrobial activity, they also have the ability to overcome conventional resistance mechanisms. These peptides can be used in combination with traditional antibiotics to reduce antibiotic resistance development by preventing bacterial adaptation, and to enhance antibiotic efficacy by making bacteria more susceptible to treatment and lower antibiotic dosages to minimize toxicity and side effects. AMPs are already in use or in development to treat drug-resistant bacterial infections, particularly those caused by MDR pathogens. For example, daptomycin is effective against MRSA and VRE infections [64], polymyxins (colistin, polymyxin B) are last-resort drugs for carbapenem-resistant Gram-negative bacteria [65], and gramicidin is used in topical antibiotics (e.g., eye drops, skin infections) [66]. The combination of colistin and rifampin is more effective than either drug alone against carbapenem-resistant Klebsiella pneumoniae [67].
AMPs show potential for the treatment of antibiotic-resistant infections but they still need to be optimized through chemical modifications, nanotechnology-based delivery systems, and bioengineering to enhance their safety, efficacy, and practicality for medical use [19,68]. Table 3 presents some AMPs with challenges and potential solutions.

Table 3.
AMP applications, challenges, and solutions.
4. Synergistic Effects of AMPs and BEVs Against Antibiotic Resistance
Initially considered cellular debris, BEVs have emerged as highly versatile tools with transformative applications across medicine, biotechnology, vaccine development, drug delivery, and diagnostics [16]. Their natural ability to transport and protect bioactive cargo, transfer information at distal sites, interact with host cells, and modulate biological processes supports their growing significance in scientific research and industry [17]. BEVs play a dual role in medicine, both as mediators of disease and as therapeutic agents. Pathogenic bacteria, such as Escherichia coli, Pseudomonas aeruginosa, and Mycobacterium tuberculosis, release BEVs that deliver virulence factors, contributing to infection and immune evasion [83]. Understanding these mechanisms of delivery provides new solutions into combating bacterial diseases. BEVs have a dual role. For pathogenic bacteria, they serve as delivery vehicles for virulence factors such as toxins, enzymes, or adhesion molecules, that can damage host tissues, manipulate immune responses, or promote infection and antibiotic resistance (detailed at Section 2.1). In bacterial communities, BEVs facilitate communication and resource sharing by transferring genetic material (like antibiotic resistance genes) or signaling molecules between cells. This helps bacteria adapt to stressors, such as antibiotics or nutrient scarcity. For some host-associated bacteria, especially beneficial ones like certain gut microbiota, BEVs can even modulate the immune system in a positive way, promoting tolerance or aiding in gut health [43,84]. Conversely, BEVs from non-pathogenic or engineered bacteria can be utilized for beneficial purposes. For instance, they can stimulate immune responses or deliver therapeutic molecules to target tissues, offering a novel approach to treating infections, inflammation, or even cancer [85]. The combination of BEVs and AMPs represents a relatively new and emerging concept in antimicrobial research. Although both components have been extensively studied individually in the context of bacterial resistance, their combined application is still developing but promising to offer new opportunities for next-generation antimicrobial strategies.
BEVs have the ability to encapsulate and protect the cargo from degradation and function as efficient drug delivery vehicles for bioactive molecules. Their small size enables penetration into tissues and cells, while their natural origin offers biocompatibility advantages over synthetic nanoparticles. BEVs are being studied for delivering antibiotics, anticancer drugs, or RNA-based therapeutics [86,87]. It has been shown that BEVs have innate antimicrobial properties and can be used as natural antibiotics. In a study of the lytic ability of OMVs of Gram-negative bacteria against 17 strains of Gram-negative and gram-pozitive bacteria, it has been shown that OMVs contain PG hydrolases and can destroy other bacteria [88]. In another study, OMVs from Cystobacter velatus Cbv34 were found to inhibit the growth of E. coli similarly to the antibiotic gentamicin, with low acute inflammatory potential and this underlined the potential for the treatment of these bacterial infections [89]. Another way for OMVs to exert their antimicrobial activity is to induce the production of antimicrobial peptides. For example, OMVs from Nontypeable Haemophilus influenzae (NTHI) initiated the release of immunomodulatory cytokine interleukin-8 (IL-8) and also the production of antimicrobial peptide LL-37 by host epithelial cells [90]. Similarly, BEVs from Gram-negative mucosal pathogens Helicobacter pylori were shown to induce the expression of human β-defensins HBD2 and HBD3 in epithelial cells [91] and BEVs from Campylobacter jejuni and nontypeable H. influenza induced the expression of the antimicrobial peptides HBD3 [92].
The distinctive ability of BEVs to transport molecules to the outer membrane of Gram-negative bacteria suggests that they could serve as natural antibiotic carriers, potentially overcoming the challenges associated with delivering antibiotics to these resistant pathogens. Various loading techniques, including electroporation, high-pressure treatment, and sonication, have been explored as effective methods for incorporating diverse therapeutic cargo into BEVs. These techniques enhance the potential of BEVs as drug delivery systems, particularly in improving the encapsulation efficiency of antibiotics [93,94]. A recent study explored extracellular vesicles from lipopolysaccharide-induced hepatocellular carcinoma (HepG2) cells (iEVs) coated with a cationic AMP to combat bacterial sepsis. This approach demonstrated the feasibility of surface-coating EVs with AMPs, improving antibacterial activity (e.g., a 2-fold reduction in minimum bactericidal concentration) against pathogens like Escherichia coli and Staphylococcus aureus [95]. In another study, antibiotic-loaded BEVs exhibited remarkable stability and biocompatibility, making them highly effective as drug delivery vehicles. Their dual functionality enables them to directly invade bacterial cells, enhancing drug penetration, and also release antibiotics in a controlled manner, maximizing their bactericidal effects. Additionally, when administered orally, these vesicles can protect against intestinal bacterial infections, offering a promising strategy for targeted antibiotic therapy while minimizing systemic side effects [96].
Among the various strategies aimed at reducing antimicrobial use, certain interventions can help prevent the development of multidrug resistance. These include enhanced personal and environmental hygiene, improved management and husbandry practices, vaccination, and emerging technologies such as phage therapy and monoclonal antibody therapy. Of these options, vaccination stands out as one of the most effective approaches to decreasing the reliance on antimicrobial treatments. Surface plasmon resonance (SPR) studies using BEVs from Klebsiella pneumoniae, Acinetobacter baumannii, and Pseudomonas aeruginosa have probed interactions with AMPs, showing that BEVs can serve as realistic models of bacterial membranes. These experiments, while not directly loading AMPs into BEVs, indicate that AMPs can bind to or interact with BEV surfaces, supporting the idea of functionalizing BEVs with AMPs as cargo or coatings [97].
One application of BEVs has been their use in combating Neisseria meningitidis, the pathogen that causes meningococcal disease. Meningococcal outbreaks, particularly those involving Neisseria meningitidis serogroup B, posed significant public health threats, especially in closed populations such as university campuses and military barracks. In these situations, BEV-based vaccines emerged as a promising solution. These vaccines harness the bacterial vesicles, which contain key antigens that stimulate the immune system to mount a defense against the pathogen, and three BEV-based vaccines, MenBvac, MeNZB, and VA-MENGOCOC-BC, were developed and successfully used in response to meningococcal outbreaks [98]. BEVs can also be used as a delivery vehicle for antigens and adjuvants, facilitating the direct delivery of these components to host cells. In models of H1N1 influenza infection, BEV-based vaccines have been shown to effectively stimulate immune responses, providing protection against the virus. The use of BEVs as a delivery platform for influenza antigens ensures that the immune system can recognize and combat the virus, potentially offering an alternative to traditional vaccine platforms [99]. BEV-based vaccines targeting A. baumannii and S. pneumonia have shown promise in preclinical models, demonstrating their ability to induce immune protection against these challenging pathogens [100]. OMVs, a subset of BEVs, have been successfully used in vaccines like Bexsero against Neisseria meningitides [101,102].
The ability of BEVs to deliver AMPs efficiently to biofilms holds great promise for the treatment of biofilm-associated infections, which are notoriously difficult to manage with traditional antibiotics. BEVs possess membrane-disrupting properties due to their lipid and protein composition, which can weaken biofilm integrity. When combined with AMPs, BEVs enhance the ability to break down the biofilm matrix, making bacterial cells more susceptible to antimicrobial attack [86,103,104]. AMPs like LL-37 and hepcidin have been studied for their ability to disrupt biofilms. Loading these into BEVs could amplify their delivery to bacterial communities [105]. The concept of loading AMPs like LL-37 into BEVs to enhance delivery to bacterial communities is a promising avenue for research. While direct studies on this combination are limited, the individual properties of AMPs and BEVs suggest potential synergistic effects in targeting biofilms and persistent infections.
The combination of BEVs and AMPs represents an exciting avenue for next-generation antibacterial therapies, particularly against antibiotic-resistant bacteria. Their synergistic effects, including enhanced stability, targeted delivery, and biofilm penetration, offer a novel strategy to improve antimicrobial efficacy. Table 4 summarize these synergistic effects of AMPs and BEVs.

Table 4.
The hypothetical synergistic effects of AMPs and BEVs.
5. Conclusions and Outlook
Antibiotic resistance is a critical global health threat that severely reduces the effectiveness of standard treatments for bacterial infections. The widespread overuse and improper use of antibiotics in healthcare, agriculture, and animal husbandry have driven the rapid emergence of resistant bacteria, enabling them to develop defense mechanisms against multiple classes of drugs.
Bacterial extracellular vesicles are lipid bilayer structures released by bacteria into their environment. They have different functional roles, ranging from pathogenesis and intercellular communication to antibiotic resistance and host immune modulation. While BEVs contribute to resistance, they also hold promise as tools to fight it. Antimicrobial peptides are short amino acid chains, naturally produced by humans, animals, plants, and microorganisms as part of their innate immune defense. AMPs have emerged as promising alternatives to conventional antibiotics. Their unique mechanisms of action not only enable them to combat a wide range of pathogens but also help bypass traditional resistance mechanisms. Recent research suggests that BEVs and AMPs can synergistically enhance antibacterial efficacy. BEVs can serve as delivery vehicles for AMPs, increasing their stability, bioavailability, and targeted delivery to bacterial cells. Additionally, BEVs may modulate bacterial membrane integrity, making bacteria more susceptible to AMPs. This synergy presents a promising strategy to combat antibiotic-resistant pathogens, but the field is still developing. The studies suggesting BEVs can serve as delivery platforms for AMPs or enhance AMP activity are not so numerous but there is strong theoretical support and early-stage experimental evidence that combining AMPs with vesicle-mediated delivery could enhance antimicrobial effects and potentially bypass antibiotic resistance.
Funding
The present study was financially supported by grant PN-IV-P1-PCE-2023-0678 (UEFISCDI-Romania).
Conflicts of Interest
The author declares no conflicts of interest.
References
- Huemer, M.; Shambat, S.M.; Brugger, S.D.; Zinkernagel, A.S. Antibiotic Resistance and Persistence—Implications for Human Health and Treatment Perspectives. EMBO Rep. 2020, 21, e51034. [Google Scholar] [CrossRef] [PubMed]
- Xuan, J.; Feng, W.; Wang, J.; Wang, R.; Zhang, B.; Bo, L.; Chen, Z.-S.; Yang, H.; Sun, L. Antimicrobial Peptides for Combating Drug-Resistant Bacterial Infections. Drug Resist. Updat. 2023, 68, 100954. [Google Scholar] [CrossRef]
- Bucataru, C.; Ciobanasu, C. Antimicrobial Peptides: Opportunities and Challenges in Overcoming Resistance. Microbiol. Res. 2024, 286, 127822. [Google Scholar] [CrossRef] [PubMed]
- Reygaert, W.C. An Overview of the Antimicrobial Resistance Mechanisms of Bacteria. AIMS Microbiol. 2018, 4, 482–501. [Google Scholar] [CrossRef] [PubMed]
- Kitano, Y.; Hsu, H.-C.; Saito, H.; Wakatake, H.; Yoshida, H.; Umekawa, S.; Tsutsumi, K.; Yoshida, T.; Masui, Y.; Taira, Y.; et al. CTX-M Group Distribution and Positivity of Extended-Spectrum β-Lactamase (ESBL)-Producing Enterobacteriaceae in Urinary Tract Infections in a Tertiary Metropolitan Hospital in Japan. J. St. Marian. Univ. 2020, 11, 133–141. [Google Scholar] [CrossRef]
- Doi, Y.; Arakawa, Y. 16S Ribosomal RNA Methylation: Emerging Resistance Mechanism against Aminoglycosides. Clin. Infect. Dis. 2007, 45, 88–94. [Google Scholar] [CrossRef]
- Kleanthous, C.; Shaw, W.V. Analysis of the Mechanism of Chloramphenicol Acetyltransferase by Steady-State Kinetics. Evidence for a Ternary-Complex Mechanism. Biochem. J. 1984, 223, 211–220. [Google Scholar] [CrossRef]
- Zieliński, M.; Park, J.; Sleno, B.; Berghuis, A.M. Structural and Functional Insights into Esterase-Mediated Macrolide Resistance. Nat. Commun. 2021, 12, 1732. [Google Scholar] [CrossRef]
- Baysarowich, J.; Koteva, K.; Hughes, D.W.; Ejim, L.; Griffiths, E.; Zhang, K.; Junop, M.; Wright, G.D. Rifamycin Antibiotic Resistance by Adp-Ribosylation: Structure and Diversity of Arr. Proc. Natl. Acad. Sci. USA 2008, 105, 4886–4891. [Google Scholar] [CrossRef]
- Cillóniz, C.; Garcia-Vidal, C.; Ceccato, A.; Torres, A. Antimicrobial Resistance among Streptococcus pneumoniae. In Antimicrobial Resistance in the 21st Century; Fong, I.W., Shlaes, D., Drlica, K., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 13–38. [Google Scholar]
- Kostakioti, M.; Hadjifrangiskou, M.; Hultgren, S.J. Bacterial Biofilms: Development, Dispersal, and Therapeutic Strategies in the Dawn of the Postantibiotic Era. Cold Spring Harb. Perspect. Med. 2013, 3, a010306. [Google Scholar] [CrossRef]
- Venkatesan, N.; Perumal, G.; Doble, M. Bacterial Resistance in Biofilm-Associated Bacteria. Futur. Microbiol. 2015, 10, 1743–1750. [Google Scholar] [CrossRef] [PubMed]
- Swick, M.C.; Morgan-Linnell, S.K.; Carlson, K.M.; Zechiedrich, L. Expression of Multidrug Efflux Pump Genes Acrab-Tolc, Mdfa, and Nore in Escherichia Coli Clinical Isolates as a Function of Fluoroquinolone and Multidrug Resistance. Antimicrob. Agents Chemother. 2011, 55, 921–924. [Google Scholar] [CrossRef]
- Du, D.; Wang-Kan, X.; Neuberger, A.; van Veen, H.W.; Pos, K.M.; Piddock, L.J.V.; Luisi, B.F. Multidrug Efflux Pumps: Structure, Function and Regulation. Nat. Rev. Microbiol. 2018, 16, 523–539. [Google Scholar] [CrossRef] [PubMed]
- Schwechheimer, C.; Kuehn, M.J. Outer-Membrane Vesicles from Gram-Negative Bacteria: Biogenesis and Functions. Nat. Rev. Microbiol. 2015, 13, 605–619. [Google Scholar] [CrossRef]
- Toyofuku, M.; Nomura, N.; Eberl, L. Types and Origins of Bacterial Membrane Vesicles. Nat. Rev. Microbiol. 2019, 17, 13–24. [Google Scholar] [CrossRef]
- Guo, J.; Huang, Z.; Wang, Q.; Wang, M.; Ming, Y.; Chen, W.; Huang, Y.; Tang, Z.; Huang, M.; Liu, H.; et al. Opportunities and Challenges of Bacterial Extracellular Vesicles in Regenerative Medicine. J. Nanobiotechnol. 2025, 23, 4. [Google Scholar] [CrossRef]
- Muñoz-Echeverri, L.M.; Benavides-López, S.; Geiger, O.; Trujillo-Roldán, M.A.; Valdez-Cruz, N.A. Bacterial Extracellular Vesicles: Biotechnological Perspective for Enhanced Productivity. World J. Microbiol. Biotechnol. 2024, 40, 174. [Google Scholar] [CrossRef]
- Rima, M.; Dakramanji, M.; El Hayek, E.; El Khoury, T.; Fajloun, Z.; Rima, M. Unveiling the Wonders of Bacteria-Derived Extracellular Vesicles: From Fundamental Functions to Beneficial Applications. Heliyon 2025, 11, e42509. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.Y.; Choi, D.Y.; Kim, D.K.; Kim, J.W.; Park, J.O.; Kim, S.; Kim, S.H.; Desiderio, D.M.; Kim, Y.K.; Kim, K.P.; et al. Gram-Positive Bacteria Produce Membrane Vesicles: Proteomics-Based Characterization of Staphylococcus Aureus-Derived Membrane Vesicles. Proteomics 2009, 9, 5425–5436. [Google Scholar] [CrossRef]
- Villageliu, D.N.; Samuelson, D.R. The Role of Bacterial Membrane Vesicles in Human Health and Disease. Front. Microbiol. 2022, 13, 828704. [Google Scholar] [CrossRef]
- Bitto, N.J.; Chapman, R.; Pidot, S.; Costin, A.; Lo, C.; Choi, J.; D’cruze, T.; Reynolds, E.C.; Dashper, S.G.; Turnbull, L.; et al. Bacterial Membrane Vesicles Transport Their DNA Cargo into Host Cells. Sci. Rep. 2017, 7, 7072. [Google Scholar] [CrossRef] [PubMed]
- Palomino, R.A.Ñ.; Vanpouille, C.; Costantini, P.E.; Margolis, L. Microbiota–Host Communications: Bacterial Extracellular Vesicles as a Common Language. PLoS Pathog. 2021, 17, e1009508. [Google Scholar] [CrossRef]
- Henriquez, T.; Falciani, C. Extracellular Vesicles of Pseudomonas: Friends and Foes. Antibiotics 2023, 12, 703. [Google Scholar] [CrossRef]
- Jeong, D.; Kim, M.J.; Park, Y.; Chung, J.; Kweon, H.-S.; Kang, N.-G.; Hwang, S.J.; Youn, S.H.; Hwang, B.K.; Kim, D. Visualizing Extracellular Vesicle Biogenesis in Gram-Positive Bacteria Using Super-Resolution Microscopy. BMC Biol. 2022, 20, 270. [Google Scholar] [CrossRef]
- Pathirana, R.D.; Kaparakis-Liaskos, M. Bacterial Membrane Vesicles: Biogenesis, Immune Regulation and Pathogenesis. Cell Microbiol 2016, 18, 1518–1524. [Google Scholar] [CrossRef] [PubMed]
- Ciofu, O.; Beveridge, T.J.; Kadurugamuwa, J.; Walther-Rasmussen, J.; Høiby, N. Chromosomal Beta-Lactamase is Packaged into Membrane Vesicles and Secreted from Pseudomonas aeruginosa. J. Antimicrob. Chemother. 2000, 45, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Najar Peerayeh, S.; Mahabadi, R.P.; Toupkanlou, S.P.; Siadat, S.D. Diversity of Beta-Lactamases Produced by Imipenem Resistant, Pseudomonas Aeruginosa Isolates from the Bloodstream. Burns 2014, 40, 1360–1364. [Google Scholar] [CrossRef]
- Jiang, B.; Lai, Y.; Xiao, W.; Zhong, T.; Liu, F.; Gong, J.; Huang, J. Microbial Extracellular Vesicles Contribute to Antimicrobial Resistance. PLoS Pathog. 2024, 20, e1012143. [Google Scholar] [CrossRef]
- Kim, S.W.; Seo, J.-S.; Bin Park, S.; Lee, A.R.; Lee, J.S.; Jung, J.W.; Chun, J.H.; Lazarte, J.M.S.; Kim, J.; Kim, J.-H.; et al. Significant Increase in the Secretion of Extracellular Vesicles and Antibiotics Resistance from Methicillin-Resistant Staphylococcus aureus Induced by ampicillin stress. Sci. Rep. 2020, 10, 21066. [Google Scholar] [CrossRef]
- Saad, M.G.; Beyenal, H.; Dong, W.-J. Dual Roles of the Conditional Extracellular Vesicles Derived from Pseudomonas aeruginosa Biofilms: Promoting and Inhibiting Bacterial Biofilm Growth. Biofilm 2024, 7, 100183. [Google Scholar] [CrossRef]
- Kraus, S.; Fletcher, M.L.; Łapińska, U.; Chawla, K.; Baker, E.; Attrill, E.L.; O’neill, P.; Farbos, A.; Jeffries, A.; Galyov, E.E.; et al. Phage-Induced Efflux Down-Regulation Boosts Antibiotic Efficacy. PLoS Pathog. 2024, 20, e1012361. [Google Scholar] [CrossRef] [PubMed]
- Nasrollahian, S.; Graham, J.P.; Halaji, M. A Review of the Mechanisms That Confer Antibiotic Resistance in Pathotypes of E. coli. Front. Cell. Infect. Microbiol. 2024, 14, 1387497. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Billón, M.; Llambías-Cabot, A.E.; Jordana-Lluch, E.; Oliver, A.; Macià, M.D. Mechanisms of Antibiotic Resistance in Pseudomonas aeruginosa biofilms. Biofilm 2023, 5, 100129. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, S.J.; Wylam, M.E. Methicillin-Resistant Staphylococcus Aureus Infection and Treatment Options. Methods Mol. Biol. 2020, 2069, 229–251. [Google Scholar]
- Acevedo, R.; Zayas, C.; Norheim, G.; Fernández, S.; Cedré, B.; Aranguren, Y.; Cuello, M.; Rodriguez, Y.; González, H.; Mandiarote, A.; et al. Outer Membrane Vesicles Extracted from Neisseria Meningitidis Serogroup X for Prevention of Meningococcal Disease in Africa. Pharmacol. Res. 2017, 121, 194–201. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.; Luo, H.; Xie, Y.; Cao, H.; Mao, L.; Liu, T.; Yue, Y.; Qian, H. Extracellular Vesicles in Helicobacter Pylori-Mediated Diseases: Mechanisms and Therapeutic Potential. Cell Commun. Signal. 2025, 23, 79. [Google Scholar] [CrossRef]
- Bawali, P.; Brahma, A.; Rana, S.R.; Pal, A.; Bhattacharyya, A. Helicobacter Pylori Infection and Inflammatory Events: The Extracellular Vesicle-Connect in Driving Gastrointestinal Tract Cancers. Front. Med. 2024, 11, 1444242. [Google Scholar] [CrossRef]
- Goman, A.; Ize, B.; Jeannot, K.; Pin, C.; Payros, D.; Goursat, C.; Ravon-Katossky, L.; Murase, K.; Chagneau, C.V.; Revillet, H.; et al. Uncovering a New Family of Conserved Virulence Factors That Promote the Production of Host-Damaging Outer Membrane Vesicles in Gram-Negative Bacteria. J. Extracell. Vesicles 2025, 14, e270032. [Google Scholar] [CrossRef]
- Abbasnia, S.; Asnaashari, A.M.H.; Sharebiani, H.; Soleimanpour, S.; Mosavat, A.; Rezaee, S.A. Mycobacterium Tuberculosis and Host Interactions in the Manifestation of Tuberculosis. J. Clin. Tuberc. Other Mycobact. Dis. 2024, 36, 100458. [Google Scholar] [CrossRef]
- Chatterjee, D.; Chaudhuri, K. Association of Cholera Toxin with Vibrio cholerae Outer Membrane Vesicles Which are Internalized by Human Intestinal Epithelial Cells. FEBS Lett. 2011, 585, 1357–1362. [Google Scholar] [CrossRef] [PubMed]
- Minarini, L.A.d.R. Exploring Bacterial Extracellular Vesicles: Focus on Who Critical Priority Pathogens. Curr. Top. Membr. 2024, 94, 225–246. [Google Scholar]
- Vicente-Gil, S.; Nuñez-Ortiz, N.; Morel, E.; Serra, C.R.; Docando, F.; Díaz-Rosales, P.; Tafalla, C. Immunomodulatory Properties of Bacillus Subtilis Extracellular Vesicles on Rainbow Trout Intestinal Cells and Splenic Leukocytes. Front. Immunol. 2024, 15, 1394501. [Google Scholar] [CrossRef]
- Singorenko, P.D.; Chang, V.; Whitcombe, A.; Simonov, D.; Hong, J.; Phillips, A.; Swift, S.; Blenkiron, C. Isolation of Membrane Vesicles from Prokaryotes: A Technical and Biological Comparison Reveals Heterogeneity. J. Extracell. Vesicles 2017, 6, 1324731. [Google Scholar] [CrossRef]
- Linares, R.; Tan, S.; Gounou, C.; Arraud, N.; Brisson, A.R. High-Speed Centrifugation Induces Aggregation of Extracellular Vesicles. J. Extracell. Vesicles 2015, 4, 29509. [Google Scholar] [CrossRef] [PubMed]
- Le, L.H.M.; Steele, J.R.; Ying, L.; Schittenhelm, R.B.; Ferrero, R.L. A New Isolation Method for Bacterial Extracellular Vesicles Providing Greater Purity and Improved Proteomic Detection of Vesicle Proteins. J. Extracell. Biol. 2023, 2, e84. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Dauros-Singorenko, P.; Whitcombe, A.; Payne, L.; Blenkiron, C.; Phillips, A.; Swift, S. Analysis of the Escherichia coli Extracellular Vesicle Proteome Identifies Markers of Purity and Culture Conditions. J. Extracell. Vesicles 2019, 8, 1632099. [Google Scholar] [CrossRef]
- Reimer, S.L.; Beniac, D.R.; Hiebert, S.L.; Booth, T.F.; Chong, P.M.; Westmacott, G.R.; Zhanel, G.G.; Bay, D.C. Comparative Analysis of Outer Membrane Vesicle Isolation Methods With an Escherichia coli Tola Mutant Reveals a Hypervesiculating Phenotype With Outer-Inner Membrane Vesicle Content. Front. Microbiol. 2021, 12, 628801. [Google Scholar] [CrossRef]
- Alves, N.J.; Turner, K.B.; DiVito, K.A.; Daniele, M.A.; Walper, S.A. Affinity Purification of Bacterial Outer Membrane Vesicles (Omvs) Utilizing a His-Tag Mutant. Res. Microbiol. 2017, 168, 139–146. [Google Scholar] [CrossRef]
- Ciobanasu, C.; Rzeszutek, A.; Kubitscheck, U.; Willumeit, R. Nkcs, a Mutant of the Nk-2 Peptide, Causes Severe Distortions and Perforations in Bacterial, but Not Human Model Lipid Membranes. Molecules 2015, 20, 6941–6958. [Google Scholar] [CrossRef]
- Michira, B.B.; Wang, Y.; Mwangi, J.; Wang, K.; Asmamaw, D.; Tadese, D.A.; Gao, J.; Khalid, M.; Lu, Q.-M.; Lai, R.; et al. A Tachyplesin Antimicrobial Peptide from Theraphosidae Spiders with Potent Antifungal Activity against Cryptococcus neoformans. Microorganisms 2024, 12, 2648. [Google Scholar] [CrossRef]
- Narula, P.; Kiruthika, S.; Chowdhari, S.; Vivekanandan, P.; Chugh, A. Inhibition of Hepatitis B Virus (Hbv) by Tachyplesin, a Marine Antimicrobial Cell-Penetrating Peptide. Pharmaceutics 2023, 15, 672. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Chugh, A. Peptide-Mediated Leishmaniasis Management Strategy: Tachyplesin Emerges as an Effective Anti-leishmanial Peptide against Leishmania Donovani. Biochim. Biophys. Acta (BBA)-Biomembr. 2021, 1863, 183629. [Google Scholar] [CrossRef]
- Huang, H.W. Molecular Mechanism of Antimicrobial Peptides: The Origin of Cooperativity. Biochim. Biophys. Acta (BBA)-Biomembr. 2006, 1758, 1292–1302. [Google Scholar] [CrossRef] [PubMed]
- Gazit, E.; Miller, I.R.; Biggin, P.C.; Sansom, M.S.; Shai, Y. Structure and Orientation of the Mammalian Antibacterial Peptide Cecropin P1 within Phospholipid Membranes. J. Mol. Biol. 1996, 258, 860–870. [Google Scholar] [CrossRef]
- Raheem, N.; Straus, S.K. Mechanisms of Action for Antimicrobial Peptides With Antibacterial and Antibiofilm Functions. Front. Microbiol. 2019, 10, 2866. [Google Scholar] [CrossRef]
- Luo, Y.; Song, Y. Mechanism of Antimicrobial Peptides: Antimicrobial, Anti-Inflammatory and Antibiofilm Activities. Int. J. Mol. Sci. 2021, 22, 11401. [Google Scholar] [CrossRef]
- Scocchi, M.; Mardirossian, M.; Runti, G.; Benincasa, M. Non-Membrane Permeabilizing Modes of Action of Antimicrobial Peptides on Bacteria. Curr. Top. Med. Chem. 2016, 16, 76–88. [Google Scholar] [CrossRef]
- Subbalakshmi, C.; Sitaram, N. Mechanism of Antimicrobial Action of Indolicidin. FEMS Microbiol. Lett. 1998, 160, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Mardirossian, M.; Grzela, R.; Giglione, C.; Meinnel, T.; Gennaro, R.; Mergaert, P.; Scocchi, M. The Host Antimicrobial Peptide Bac71-35 Binds to Bacterial Ribosomal Proteins and Inhibits Protein Synthesis. Chem. Biol. 2014, 21, 1639–1647. [Google Scholar] [CrossRef]
- Shi, J.; Ganz, T. The Role of Protegrins and Other Elastase-Activated Polypeptides in the Bactericidal Properties of Porcine Inflammatory Fluids. Infect. Immun. 1998, 66, 3611–3617. [Google Scholar] [CrossRef]
- Lee, H.; Hwang, J.S.; Lee, D.G. Periplanetasin-4, a Novel Antimicrobial Peptide from the Cockroach, Inhibits Communications between Mitochondria and Vacuoles. Biochem. J. 2019, 476, 1267–1284. [Google Scholar] [CrossRef] [PubMed]
- Segev-Zarko, L.-A.; Saar-Dover, R.; Brumfeld, V.; Mangoni, M.L.; Shai, Y. Mechanisms of Biofilm Inhibition and Degradation by Antimicrobial Peptides. Biochem. J. 2015, 468, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Jaber, R.H.; Beahm, N.P. Daptomycin for the Treatment of Acute Bacterial Meningitis: A Narrative Review. Int. J. Antimicrob. Agents 2023, 61, 106770. [Google Scholar] [CrossRef] [PubMed]
- Ledger, E.V.K.; Sabnis, A.; Edwards, A.M. Polymyxin and Lipopeptide Antibiotics: Membrane-Targeting Drugs of Last Resort. Microbiology 2022, 168, 001136. [Google Scholar] [CrossRef]
- Swierstra, J.; Kapoerchan, V.; Knijnenburg, A.; van Belkum, A.; Overhand, M. Structure, Toxicity and Antibiotic Activity of Gramicidin S and Derivatives. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 763–769. [Google Scholar] [CrossRef]
- Aydemir, H.; Akduman, D.; Piskin, N.; Comert, F.; Horuz, E.; Terzi, A.; Kokturk, F.; Ornek, T.; Celebi, G. Colistin vs. The Combination of Colistin and Rifampicin for the Treatment of Carbapenem-Resistantacinetobacter Baumanniiventilator-Associated Pneumonia. Epidemiol. Infect. 2012, 141, 1214–1222. [Google Scholar] [CrossRef]
- Campos, J.V.; de Pontes, J.T.C.; Canales, C.S.C.; Roque-Borda, C.A.; Pavan, F.R. Advancing Nanotechnology: Targeting Biofilm-Forming Bacteria with Antimicrobial Peptides. BME Front. 2025, 6, 0104. [Google Scholar] [CrossRef]
- Yu, S.; Pan, J.; Xu, M.; Chen, Y.; Li, P.; Hu, H. Antibacterial Activity and Mechanism of Colistin-Loaded Polymeric Nanoparticles for Combating Multidrug-Resistant Pseudomonas aeruginosa biofilms: A synergistic approach. Int. J. Biol. Macromol. 2024, 282, 136757. [Google Scholar] [CrossRef]
- Pogue, J.; Ortwine, J.; Kaye, K. Clinical Considerations for Optimal Use of the Polymyxins: A Focus on Agent Selection and Dosing. Clin. Microbiol. Infect. 2017, 23, 229–233. [Google Scholar] [CrossRef]
- Lange, A.; Thunberg, U.; Söderquist, B. Ototoxicity Associated with Extended Dalbavancin Treatment for a Shoulder Prosthetic Joint Infection. BMC Infect. Dis. 2023, 23, 706. [Google Scholar] [CrossRef]
- Tegethoff, J.I.; Teitelbaum, I.; Kiser, T.H. Rapid and Effective Treatment of Peritonitis in Peritoneal Dialysis Patients with Intravenous Dalbavancin. Am. J. Case Rep. 2023, 25, e942755. [Google Scholar] [CrossRef] [PubMed]
- Demirci, M.; Yigin, A.; Demir, C. Efficacy of Antimicrobial Peptide Ll-37 against Biofilm Forming Staphylococcus Aureus Strains Obtained from Chronic Wound Infections. Microb. Pathog. 2021, 162, 105368. [Google Scholar] [CrossRef]
- Araujo, J.B.; de Souza, G.S.; Lorenzon, E.N. Indolicidin Revisited: Biological Activity, Potential Applications and Perspectives of an Antimicrobial Peptide not yet Fully Explored. World J. Microbiol. Biotechnol. 2022, 38, 39. [Google Scholar]
- Takada, Y.; Itoh, H.; Paudel, A.; Panthee, S.; Hamamoto, H.; Sekimizu, K.; Inoue, M. Discovery of Gramicidin a Analogues with Altered Activities by Multidimensional Screening of a One-Bead-One-Compound Library. Nat. Commun. 2020, 11, 4935. [Google Scholar] [CrossRef] [PubMed]
- Gottler, L.M.; Ramamoorthy, A. Structure, Membrane Orientation, Mechanism, and Function of Pexiganan--a Highly Potent Antimicrobial Peptide Designed from Magainin. Biochim Biophys Acta 2009, 1788, 1680–1686. [Google Scholar] [CrossRef]
- Schäfer-Korting, M.; Rolff, J. Chapter 2—Skin Delivery of Antimicrobial Peptides. In Emerging Nanotechnologies in Immunology; Shegokar, R., Souto, E.B., Eds.; Elsevier: Boston, MA, USA, 2018; pp. 23–45. [Google Scholar]
- Deshayes, C.; Arafath, N.; Apaire-Marchais, V.; Roger, E. Drug Delivery Systems for the Oral Administration of Antimicrobial Peptides: Promising Tools to Treat Infectious Diseases. Front. Med. Technol. 2022, 3, 778645. [Google Scholar] [CrossRef]
- Ganz, T. Defensins: Antimicrobial Peptides of Innate Immunity. Nat. Rev. Immunol. 2003, 3, 710–720. [Google Scholar] [CrossRef]
- Zanetti, M.; Litteri, L.; Gennaro, R.; Horstmann, H.; Romeo, D. Bactenecins, Defense Polypeptides of Bovine Neutrophils, are Generated from Precursor Molecules Stored in the Large Granules. J. Cell Biol. 1990, 111, 1363–1371. [Google Scholar] [CrossRef]
- Dutta, P.; Das, S. Mammalian Antimicrobial Peptides: Promising Therapeutic Targets against Infection and Chronic Inflammation. Curr. Top. Med. Chem. 2015, 16, 99–129. [Google Scholar] [CrossRef]
- Khurshid, Z.; Najeeb, S.; Mali, M.; Moin, S.F.; Raza, S.Q.; Zohaib, S.; Sefat, F.; Zafar, M.S. Histatin peptides: Pharmacological functions and their applications in dentistry. Saudi Pharm. J. 2017, 25, 25–31. [Google Scholar] [CrossRef]
- Kumar, M.A.; Baba, S.K.; Sadida, H.Q.; Marzooqi, S.A.; Jerobin, J.; Altemani, F.H.; Algehainy, N.; Alanazi, M.A.; Abou-Samra, A.-B.; Kumar, R.; et al. Extracellular Vesicles as Tools and Targets in Therapy for Diseases. Signal Transduct. Target. Ther. 2024, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Higginbotham, J.N.; Liu, L.; Zhao, G.; Acra, S.A.; Peek, R.M.; Polk, D.B.; Li, H.; Yan, F. Production of a Functional Factor, p40, by Lactobacillus rhamnosus GG Is Promoted by Intestinal Epithelial Cell-Secreted Extracellular Vesicles. Infect. Immun. 2019, 87, e00113-19. [Google Scholar] [CrossRef]
- Liu, C.; Yazdani, N.; Moran, C.S.; Salomon, C.; Seneviratne, C.J.; Ivanovski, S.; Han, P. Unveiling Clinical Applications of Bacterial Extracellular Vesicles as Natural Nanomaterials in Disease Diagnosis and Therapeutics. Acta Biomater. 2024, 180, 18–45. [Google Scholar] [CrossRef]
- Sonallya, T.; Juhasz, T.; Szigyarto, I.C.; Ilyes, K.; Singh, P.; Khamari, D.; Buzas, E.I.; Varga, Z.; Beke-Somfai, T. Categorizing Interaction Modes of Antimicrobial Peptides with Extracellular Vesicles: Disruption, Membrane Trespassing, and Clearance of the Protein Corona. J. Colloid Interface Sci. 2025, 679, 496–509. [Google Scholar] [CrossRef]
- Ciobanasu, C. Peptides-Based Therapy and Diagnosis. Strategies for Non-Invasive Therapies in Cancer. J. Drug Target. 2021, 29, 1063–1079. [Google Scholar] [PubMed]
- Li, Z.; Clarke, A.J.; Beveridge, T.J. Gram-Negative Bacteria Produce Membrane Vesicles Which Are Capable of Killing Other Bacteria. J. Bacteriol. 1998, 180, 5478–5483. [Google Scholar] [CrossRef] [PubMed]
- Schulz, E.; Goes, A.; Garcia, R.; Panter, F.; Koch, M.; Müller, R.; Fuhrmann, K.; Fuhrmann, G. Biocompatible Bacteria-Derived Vesicles Show Inherent Antimicrobial Activity. J. Control. Release 2018, 290, 46–55. [Google Scholar] [CrossRef]
- Sharpe, S.W.; Kuehn, M.J.; Mason, K.M. Elicitation of Epithelial Cell-Derived Immune Effectors by Outer Membrane Vesicles of Nontypeable Haemophilus Influenzae. Infect. Immun. 2011, 79, 4361–4369. [Google Scholar] [CrossRef]
- Kaparakis, M.; Turnbull, L.; Carneiro, L.; Firth, S.; Coleman, H.A.; Parkington, H.C.; Le Bourhis, L.; Karrar, A.; Viala, J.; Mak, J.; et al. Bacterial Membrane Vesicles Deliver Peptidoglycan to NOD1 in Epithelial cells. Cell. Microbiol. 2010, 12, 372–385. [Google Scholar] [CrossRef]
- Elmi, A.; Watson, E.; Sandu, P.; Gundogdu, O.; Mills, D.C.; Inglis, N.F.; Manson, E.; Imrie, L.; Bajaj-Elliott, M.; Wren, B.W.; et al. Campylobacter Jejuni Outer Membrane Vesicles Play an Important Role in Bacterial Interactions with Human Intestinal Epithelial Cells. Infect. Immun. 2012, 80, 4089–4098. [Google Scholar] [CrossRef]
- Kim, M.S.; Haney, M.J.; Zhao, Y.; Mahajan, V.; Deygen, I.; Klyachko, N.L.; Inskoe, E.; Piroyan, A.; Sokolsky, M.; Okolie, O.; et al. Development of Exosome-Encapsulated Paclitaxel to Overcome mdr in Cancer Cells. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Ayed, Z.; Cuvillier, L.; Dobhal, G.; Goreham, R.V. Electroporation of Outer Membrane Vesicles Derived from Pseudomonas aeruginosa with Gold Nanoparticles. SN Appl. Sci. 2019, 1, 1600. [Google Scholar] [CrossRef]
- Ibrahim, U.H.; Gafar, M.A.; Khan, R.; Tageldin, A.; Govender, T.; Mackraj, I. Engineered Extracellular Vesicles Coated with an Antimicrobial Peptide for Advanced Control of Bacterial Sepsis. J. Extracell. Biol. 2024, 3, e70000. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zhang, Q.; Li, W.; Yuan, M.; Zhou, J.; Hua, L.; Chen, Y.; Ye, C.; Ma, Y. Development of Novel Nanoantibiotics Using an Outer Membrane Vesicle-Based Drug Efflux Mechanism. J. Control. Release 2020, 317, 1–22. [Google Scholar] [CrossRef]
- Bril’kov, M.S.; Stenbakk, V.; Jakubec, M.; Vasskog, T.; Kristoffersen, T.; Cavanagh, J.P.; Ericson, J.U.; Isaksson, J.; Flaten, G.E. Bacterial Extracellular Vesicles: Towards Realistic Models for Bacterial Membranes in Molecular Interaction Studies by Surface Plasmon Resonance. Front. Mol. Biosci. 2023, 10, 1277963. [Google Scholar] [CrossRef]
- Masignani, V.; Pizza, M.; Moxon, E.R. The Development of a Vaccine against Meningococcus B Using Reverse Vaccinology. Front. Immunol. 2019, 10, 751. [Google Scholar] [CrossRef]
- Rappazzo, C.G.; Watkins, H.C.; Guarino, C.M.; Chau, A.; Lopez, J.L.; DeLisa, M.P.; Leifer, C.A.; Whittaker, G.R.; Putnam, D. Recombinant M2e Outer Membrane Vesicle Vaccines Protect against Lethal Influenza a Challenge in BALB/c Mice. Vaccine 2016, 34, 1252–1258. [Google Scholar] [CrossRef]
- Price, N.L.; Goyette-Desjardins, G.; Nothaft, H.; Valguarnera, E.; Szymanski, C.M.; Segura, M.; Feldman, M.F. Glycoengineered Outer Membrane Vesicles: A Novel Platform for Bacterial Vaccines. Sci. Rep. 2016, 6, 24931. [Google Scholar] [CrossRef]
- Velimirov, B.; Velimirov, B.A. Immune Responses Elicited by Outer Membrane Vesicles of Gram-Negative Bacteria: Important Players in Vaccine Development. Life 2024, 14, 1584. [Google Scholar] [CrossRef]
- Ladhani, S.N.; Giuliani, M.M.; Biolchi, A.; Pizza, M.; Beebeejaun, K.; Lucidarme, J.; Findlow, J.; Ramsay, M.E.; Borrow, R. Effectiveness of Meningococcal B Vaccine against Endemic Hypervirulent Neisseria Meningitidis W Strain, England. Emerg. Infect. Dis. 2016, 22, 309–311. [Google Scholar] [CrossRef]
- Chen, N.; Li, Y.; Liang, X.; Qin, K.; Zhang, Y.; Wang, J.; Wu, Q.; Gupta, T.B.; Ding, Y. Bacterial Extracellular Vesicle: A Non-Negligible Component in Biofilm Life Cycle and Challenges in Biofilm Treatments. Biofilm 2024, 8, 100216. [Google Scholar] [CrossRef] [PubMed]
- Jeong, G.-J.; Khan, F.; Tabassum, N.; Cho, K.-J.; Kim, Y.-M. Bacterial Extracellular Vesicles: Modulation of Biofilm and Virulence Properties. Acta Biomater. 2024, 178, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Overhage, J.; Campisano, A.; Bains, M.; Torfs, E.C.W.; Rehm, B.H.A.; Hancock, R.E.W. Human Host Defense Peptide Ll-37 Prevents Bacterial Biofilm Formation. Infect. Immun. 2008, 76, 4176–4182. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).