Cranberry-Derived Phenolic Compounds Contribute to the Inhibition of FimH-Mediated Escherichia coli Hemagglutination
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cultivation of Bacteria
4.3. Blood
4.4. Hemagglutination Inhibition (HAI) Assay
4.5. Microscopy Examination of HAI
4.6. Biofilm Inhibition Assay
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guglietta, A. Recurrent Urinary Tract Infections in Women: Risk Factors, Etiology, Pathogenesis and Prophylaxis. Future Microbiol. 2017, 12, 239–246. [Google Scholar] [CrossRef]
- Naghavi, M.; Vollset, S.E.; Ikuta, K.S.; Swetschinski, L.R.; Gray, A.P.; Wool, E.E.; Aguilar, G.R.; Mestrovic, T.; Smith, G.; Han, C.; et al. Global Burden of Bacterial Antimicrobial Resistance 1990–2021: A Systematic Analysis with Forecasts to 2050. Lancet 2024, 404, 1199–1226. [Google Scholar] [CrossRef] [PubMed]
- Bernal-Mercado, A.T.; Vazquez-Armenta, F.J.; Tapia-Rodriguez, M.R.; Islas-Osuna, M.A.; Mata-Haro, V.; Gonzalez-Aguilar, G.A.; Lopez-Zavala, A.A.; Ayala-Zavala, J.F. Comparison of Single and Combined Use of Catechin, Protocatechuic, and Vanillic Acids as Antioxidant and Antibacterial Agents against Uropathogenic Escherichia coli at Planktonic and Biofilm Levels. Molecules 2018, 23, 2813. [Google Scholar] [CrossRef] [PubMed]
- Sarshar, M.; Behzadi, P.; Ambrosi, C.; Zagaglia, C.; Palamara, A.T.; Scribano, D. FimH and Anti-Adhesive Therapeutics: A Disarming Strategy Against Uropathogens. Antibiotics 2020, 9, 397. [Google Scholar] [CrossRef] [PubMed]
- Mydock-McGrane, L.K.; Hannan, T.J.; Janetka, J.W. Rational Design Strategies for FimH Antagonists: New Drugs on the Horizon for Urinary Tract Infection and Crohn’s Disease. Expert Opin. Drug Discov. 2017, 12, 711–731. [Google Scholar] [CrossRef]
- Damalanka, V.C.; Maddirala, A.R.; Janetka, J.W. Novel Approaches to Glycomimetic Design: Development of Small Molecular Weight Lectin Antagonists. Expert Opin. Drug Discov. 2020, 16, 513–536. [Google Scholar] [CrossRef]
- Hayward, G.; Mort, S.; Hay, A.D.; Moore, M.; Thomas, N.P.B.; Cook, J.; Robinson, J.; Williams, N.; Maeder, N.; Edeson, R.; et al. D-Mannose for Prevention of Recurrent Urinary Tract Infection Among Women: A Randomized Clinical Trial. JAMA Intern. Med. 2024, 184, 619–628. [Google Scholar] [CrossRef]
- Kranjčec, B.; Papeš, D.; Altarac, S. D-Mannose Powder for Prophylaxis of Recurrent Urinary Tract Infections in Women: A Randomized Clinical Trial. World J. Urol. 2014, 32, 79–84. [Google Scholar] [CrossRef]
- Barnich, N.; Carvalho, F.A.; Glasser, A.-L.; Darcha, C.; Jantscheff, P.; Allez, M.; Peeters, H.; Bommelaer, G.; Desreumaux, P.; Colombel, J.-F.; et al. CEACAM6 Acts as a Receptor for Adherent-Invasive E. Coli, Supporting Ileal Mucosa Colonization in Crohn Disease. J. Clin. Investig. 2007, 117, 1566–1574. [Google Scholar] [CrossRef]
- Ribić, R.; Meštrović, T.; Neuberg, M.; Kozina, G. Effective Anti-Adhesives of Uropathogenic Escherichia coli. Acta Pharm. 2018, 68, 1–18. [Google Scholar] [CrossRef]
- Gupta, K.; Chou, M.Y.; Howell, A.; Wobbe, C.; Grady, R.; Stapleton, A.E. Cranberry Products Inhibit Adherence of P-Fimbriated Escherichia coli to Primary Cultured Bladder and Vaginal Epithelial Cells. J. Urol. 2007, 177, 2357–2360. [Google Scholar] [CrossRef] [PubMed]
- Foo, L.Y.; Lu, Y.; Howell, A.B.; Vorsa, N. A-Type Proanthocyanidin Trimers from Cranberry That Inhibit Adherence of Uropathogenic P-Fimbriated Escherichia coli. J. Nat. Prod. 2000, 63, 1225–1228. [Google Scholar] [CrossRef] [PubMed]
- Lewis, A.J.; Richards, A.C.; Mendez, A.A.; Dhakal, B.K.; Jones, T.A.; Sundsbak, J.L.; Eto, D.S.; Rousek, A.A.; Mulvey, M.A. Plant Phenolics Inhibit Focal Adhesion Kinase and Suppress Host Cell Invasion by Uropathogenic Escherichia coli. Infect. Immun. 2024, 92, e00080-24. [Google Scholar] [CrossRef] [PubMed]
- Rafsanjany, N.; Senker, J.; Brandt, S.; Dobrindt, U.; Hensel, A. In Vivo Consumption of Cranberry Exerts Ex Vivo Antiadhesive Activity against FimH-Dominated Uropathogenic Escherichia coli: A Combined in Vivo, Ex Vivo, and in Vitro Study of an Extract from Vaccinium Macrocarpon. J. Agric. Food Chem. 2015, 63, 8804–8818. [Google Scholar] [CrossRef]
- González de Llano, D.; Moreno-Arribas, M.V.; Bartolomé, B. Cranberry Polyphenols and Prevention against Urinary Tract Infections: Relevant Considerations. Molecules 2020, 25, 3523. [Google Scholar] [CrossRef]
- Pappas, E.; Schaich, K.M. Phytochemicals of Cranberries and Cranberry Products: Characterization, Potential Health Effects, and Processing Stability. Crit. Rev. Food Sci. Nutr. 2009, 49, 741–781. [Google Scholar] [CrossRef]
- Jurikova, T.; Skrovankova, S.; Mlcek, J.; Balla, S.; Snopek, L. Bioactive Compounds, Antioxidant Activity, and Biological Effects of European Cranberry (Vaccinium oxycoccos). Molecules 2019, 24, 24. [Google Scholar] [CrossRef]
- Doyle, R.J. Contribution of the Hydrophobic Effect to Microbial Infection. Microbes Infect. 2000, 2, 391–400. [Google Scholar] [CrossRef]
- Hudson, K.L.; Bartlett, G.J.; Diehl, R.C.; Agirre, J.; Gallagher, T.; Kiessling, L.L.; Woolfson, D.N. Carbohydrate–Aromatic Interactions in Proteins. J. Am. Chem. Soc. 2015, 137, 15152–15160. [Google Scholar] [CrossRef]
- Ribić, R.; Meštrović, T.; Neuberg, M.; Kozina, G. Proposed Dual Antagonist Approach for the Prevention and Treatment of Urinary Tract Infections Caused by Uropathogenic Escherichia coli. Med. Hypotheses 2019, 124, 17–20. [Google Scholar] [CrossRef]
- Han, Z.; Yi, X.; Li, J.; Liao, D.; Ai, J. Nonantibiotic Prophylaxis for Urinary Tract Infections: A Network Meta-Analysis of Randomized Controlled Trials. Infection 2024, 53, 535–546. [Google Scholar] [CrossRef]
- Li, X.; Pang, Y.; Jiang, L.; Liu, L.; Zhou, J.; Jin, C.; Wang, Q.; Sun, H.; Li, Q.; Chen, Z.; et al. Two-Component System GrpP/GrpQ Promotes Pathogenicity of Uropathogenic Escherichia coli CFT073 by Upregulating Type 1 Fimbria. Nat. Commun. 2025, 16, 607. [Google Scholar] [CrossRef] [PubMed]
- Connell, I.; Agace, W.; Klemm, P.; Schembri, M.; Mărild, S.; Svanborg, C. Type 1 Fimbrial Expression Enhances Escherichia coli Virulence for the Urinary Tract. Proc. Natl. Acad. Sci. USA 1996, 93, 9827–9832. [Google Scholar] [CrossRef] [PubMed]
- Meena, J.; Thomas, C.C.; Kumar, J.; Raut, S.; Hari, P. Non-Antibiotic Interventions for Prevention of Urinary Tract Infections in Children: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Eur. J. Pediatr. 2021, 180, 3535–3545. [Google Scholar] [CrossRef]
- Lindhorst, T.K.; Kötter, S.; Kubisch, J.; Krallmann-Wenzel, U.; Ehlers, S.; Křen, V. Effect of P-Substitution of Aryl α-D-Mannosides on Inhibiting Mannose-Sensitive Adhesion of Escherichia coli—Syntheses and Testing. Eur. J. Org. Chem. 1998, 1998, 1669–1674. [Google Scholar] [CrossRef]
- Kötter, S.; Krallmann-Wenzel, U.; Ehlers, S.; Lindhorst, T.K. Multivalent Ligands for the Mannose-Specific Lectin on Type 1 Fimbriae of Escherichia coli: Syntheses and Testing of Trivalent α-D-Mannoside Clusters. J. Chem. Soc. Perkin Trans. 1 1998, 2193–2200. [Google Scholar] [CrossRef]
- Lindhorst, T.K.; Kieburg, C.; Krallmann-Wenzel, U. Inhibition of the Type 1 Fimbriae-Mediated Adhesion of Escherichia coli to Erythrocytes by Multiantennary α-Mannosyl Clusters: The Effect of Multivalency. Glycoconj. J. 1998, 15, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, S.; Jiang, X.; Schwardt, O.; Ernst, B. Expression of the Carbohydrate Recognition Domain of FimH and Development of a Competitive Binding Assay. Anal. Biochem. 2010, 407, 188–195. [Google Scholar] [CrossRef]
- Abgottspon, D.; Rölli, G.; Hosch, L.; Steinhuber, A.; Jiang, X.; Schwardt, O.; Cutting, B.; Smiesko, M.; Jenal, U.; Ernst, B.; et al. Development of an Aggregation Assay to Screen FimH Antagonists. J. Microbiol. Methods 2010, 82, 249–255. [Google Scholar] [CrossRef]
- González de Llano, D.; Esteban-Fernández, A.; Sánchez-Patán, F.; Martínlvarez, P.J.; Moreno-Arribas, M.V.; Bartolomé, B. Anti-Adhesive Activity of Cranberry Phenolic Compounds and Their Microbial-Derived Metabolites against Uropathogenic Escherichia coli in Bladder Epithelial Cell Cultures. Int. J. Mol. Sci. 2015, 16, 12119–12130. [Google Scholar] [CrossRef]
- González de Llano, D.; Liu, H.; Khoo, C.; Moreno-Arribas, M.V.; Bartolomé, B. Some New Findings Regarding the Antiadhesive Activity of Cranberry Phenolic Compounds and Their Microbial-Derived Metabolites against Uropathogenic Bacteria. J. Agric. Food Chem. 2019, 67, 2166–2174. [Google Scholar] [CrossRef] [PubMed]
- Synowiec, A.; Żyła, K.; Gniewosz, M.; Kieliszek, M. An Effect of Positional Isomerism of Benzoic Acid Derivatives on Antibacterial Activity against Escherichia coli. Open Life Sci. 2021, 16, 594–601. [Google Scholar] [CrossRef]
- Franck, T.; Mouithys-Mickalad, A.; Robert, T.; Ghitti, G.; Deby-Dupont, G.; Neven, P.; Serteyn, D. Differentiation between Stoichiometric and Anticatalytic Antioxidant Properties of Benzoic Acid Analogues: A Structure/Redox Potential Relationship Study. Chem. Biol. Interact. 2013, 206, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Miao, M.; Xiang, L. Chapter Three—Pharmacological Action and Potential Targets of Chlorogenic Acid. In Advances in Pharmacology; Du, G., Ed.; Pharmacological Advances in Natural Product Drug Discovery; Academic Press: Cambridge, MA, USA, 2020; Volume 87, pp. 71–88. [Google Scholar]
- Nunes, S.; Danesi, F.; Rio, D.D.; Silva, P. Resveratrol and Inflammatory Bowel Disease: The Evidence so Far. Nutr. Res. Rev. 2018, 31, 85–97. [Google Scholar] [CrossRef]
- Kundu, J.K.; Shin, Y.K.; Kim, S.H.; Surh, Y.-J. Resveratrol Inhibits Phorbol Ester-Induced Expression of COX-2 and Activation of NF-κB in Mouse Skin by Blocking IκB Kinase Activity. Carcinogenesis 2006, 27, 1465–1474. [Google Scholar] [CrossRef]
- Bernal-Mercado, A.T.; Gutierrez-Pacheco, M.M.; Encinas-Basurto, D.; Mata-Haro, V.; Lopez-Zavala, A.A.; Islas-Osuna, M.A.; Gonzalez-Aguilar, G.A.; Ayala-Zavala, J.F. Synergistic Mode of Action of Catechin, Vanillic and Protocatechuic Acids to Inhibit the Adhesion of Uropathogenic Escherichia coli on Silicone Surfaces. J. Appl. Microbiol. 2020, 128, 387–400. [Google Scholar] [CrossRef] [PubMed]
- Salinas-Casado, J.; Méndez-Rubio, S.; Esteban-Fuertes, M.; Gómez-Rodríguez, A.; Vírseda-Chamorro, M.; Luján-Galán, M.; Iglesias-García, C.; Rituman, G. Large Study (283 Women) on the Effectiveness of Manosar®: 2 g of d-Mannose + 140 Mg of Proanthocyanidins (PAC), of Prolonged Release. Arch. Esp. Urol. 2020, 73, 491–498. [Google Scholar]
- Salinas-Casado, J.; Méndez-Rubio, S.; Esteban-Fuertes, M.; Gómez-Rodríguez, A.; Vírseda-Chamorro, M.; Luján-Galán, M.; Rituman, G. Efficacy and safety of D-mannose (2 g), 24 h prolonged release, associated with Proanthocyanidin (PAC), versus isolate PAC, in the management of a series of women with recurrent urinary infections. Arch. Esp. Urol. 2018, 71, 169–177. [Google Scholar]
- Lavigne, J.-P.; Ranfaing, J.; Dunyach-Rémy, C.; Sotto, A. Synergistic Effect of Propolis and Antibiotics on Uropathogenic Escherichia coli. Antibiotics 2020, 9, 739. [Google Scholar] [CrossRef]
- Cassano, R.; Curcio, F.; Procopio, D.; Fiorillo, M.; Trombino, S. Multifunctional Microspheres Based on D-Mannose and Resveratrol for Ciprofloxacin Release. Materials 2022, 15, 7293. [Google Scholar] [CrossRef]
- Nasi, G.I.; Georgakopoulou, K.I.; Theodoropoulou, M.K.; Papandreou, N.C.; Chrysina, E.D.; Tsiolaki, P.L.; Iconomidou, V.A. Bacterial Lectin FimH and Its Aggregation Hot-Spots: An Alternative Strategy against Uropathogenic Escherichia coli. Pharmaceutics 2023, 15, 1018. [Google Scholar] [CrossRef]
- Zhou, Z.; Tang, R.; Fang, Y.; Lv, T.; Liu, J.; Wang, X. Facile Synthesis of FimH Antagonist and Its Analogues: Simple Entry to Complex C-Mannoside Inhibitors of E. Coli Adhesion. ACS Med. Chem. Lett. 2024, 15, 1724–1730. [Google Scholar] [CrossRef]
- Faustino, M.; Silva, S.; Costa, E.M.; Pereira, A.M.; Pereira, J.O.; Oliveira, A.S.; Ferreira, C.M.H.; Pereira, C.F.; Durão, J.; Pintado, M.E.; et al. Effect of Mannan Oligosaccharides Extracts in Uropathogenic Escherichia coli Adhesion in Human Bladder Cells. Pathogens 2023, 12, 885. [Google Scholar] [CrossRef]
- Mousavifar, L.; Sarshar, M.; Bridot, C.; Scribano, D.; Ambrosi, C.; Palamara, A.T.; Vergoten, G.; Roubinet, B.; Landemarre, L.; Bouckaert, J.; et al. Insightful Improvement in the Design of Potent Uropathogenic E. coli FimH Antagonists. Pharmaceutics 2023, 15, 527. [Google Scholar] [CrossRef] [PubMed]
- Krammer, E.-M.; De Ruyck, J.; Roos, G.; Bouckaert, J.; Lensink, M.F. Targeting Dynamical Binding Processes in the Design of Non-Antibiotic Anti-Adhesives by Molecular Simulation—The Example of FimH. Molecules 2018, 23, 1641. [Google Scholar] [CrossRef] [PubMed]
- Klemm, P.; Jørgensen, B.J.; van Die, I.; de Ree, H.; Bergmans, H. The Fim Genes Responsible for Synthesis of Type 1 Fimbriae in Escherichia coli, Cloning and Genetic Organization. Mol. Gen. Genet. 1985, 199, 410–414. [Google Scholar] [CrossRef] [PubMed]
- Car, Ž.; Hrenar, T.; Peroković, V.P.; Ribić, R.; Seničar, M.; Tomić, S. Mannosylated N-Aryl Substituted 3-Hydroxypyridine-4-Ones: Synthesis, Hemagglutination Inhibitory Properties, and Molecular Modeling. Chem. Biol. Drug Des. 2014, 84, 393–401. [Google Scholar] [CrossRef]
- Mrázková, J.; Malinovská, L.; Wimmerová, M. Microscopy Examination of Red Blood and Yeast Cell Agglutination Induced by Bacterial Lectins. PLoS ONE 2019, 14, e0220318. [Google Scholar] [CrossRef]
- Bhandari, S.; Khadayat, K.; Poudel, S.; Shrestha, S.; Shrestha, R.; Devkota, P.; Khanal, S.; Marasini, B.P. Phytochemical Analysis of Medicinal Plants of Nepal and Their Antibacterial and Antibiofilm Activities against Uropathogenic Escherichia coli. BMC Complement. Med. Ther. 2021, 21, 116. [Google Scholar] [CrossRef]


| Compound Tested | IT [mM] | Compound Tested | IT [mM] |
|---|---|---|---|
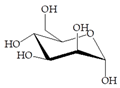 Man | 60 |  Man + diphenylacetic acid | 60 |
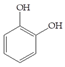 Man + catechol | 60 | 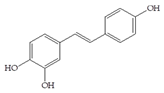 Man + resveratrol | 30 |
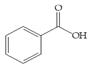 Man + benzoic acid | 30 |  Man + curcumin | 60 |
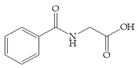 Man + hippuric acid | 30 |  Man + quercetin | 60 |
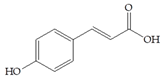 Man + p-coumaric acid | 30 |  Man + (+)-catechin | 30 |
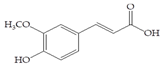 Man + ferulic acid | 30 | 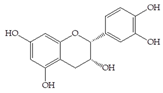 Man + (-)-epicatechin | 60 |
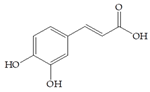 Man + caffeic acid | 30 | 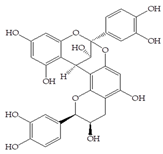 Man + procyanidin A2 | 30 |
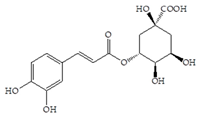 Man + chlorogenic acid | 30 | Man + Vaccinium macrocarpon extract (80 mg/mL) | 30 |
| Compound Tested | IT [mM] |
|---|---|
| benzoic acid | 0.25 |
| hippuric acid | 0.5 |
| p-coumaric acid | 0.5 |
| ferulic acid | 0.5 |
| caffeic acid | 0.25 |
| chlorogenic acid | 0.25 |
| resveratrol | 0.25 |
| (+)-catechin | 0.5 |
| procyanidin A2 | 0.5 |
| Vaccinium macrocarpon extract | 10 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribić, R.; Petrović Peroković, V.; Meštrović, T.; Neuberg, M.; Bradić, N. Cranberry-Derived Phenolic Compounds Contribute to the Inhibition of FimH-Mediated Escherichia coli Hemagglutination. Antibiotics 2025, 14, 418. https://doi.org/10.3390/antibiotics14040418
Ribić R, Petrović Peroković V, Meštrović T, Neuberg M, Bradić N. Cranberry-Derived Phenolic Compounds Contribute to the Inhibition of FimH-Mediated Escherichia coli Hemagglutination. Antibiotics. 2025; 14(4):418. https://doi.org/10.3390/antibiotics14040418
Chicago/Turabian StyleRibić, Rosana, Vesna Petrović Peroković, Tomislav Meštrović, Marijana Neuberg, and Nikola Bradić. 2025. "Cranberry-Derived Phenolic Compounds Contribute to the Inhibition of FimH-Mediated Escherichia coli Hemagglutination" Antibiotics 14, no. 4: 418. https://doi.org/10.3390/antibiotics14040418
APA StyleRibić, R., Petrović Peroković, V., Meštrović, T., Neuberg, M., & Bradić, N. (2025). Cranberry-Derived Phenolic Compounds Contribute to the Inhibition of FimH-Mediated Escherichia coli Hemagglutination. Antibiotics, 14(4), 418. https://doi.org/10.3390/antibiotics14040418








