Navigating Vancomycin and Acute Kidney Injury: AUC- vs. Trough-Guided Monitoring in Initial and Steady-State Therapy
Abstract
1. Introduction
2. Results
2.1. Demographic and Clinical Data
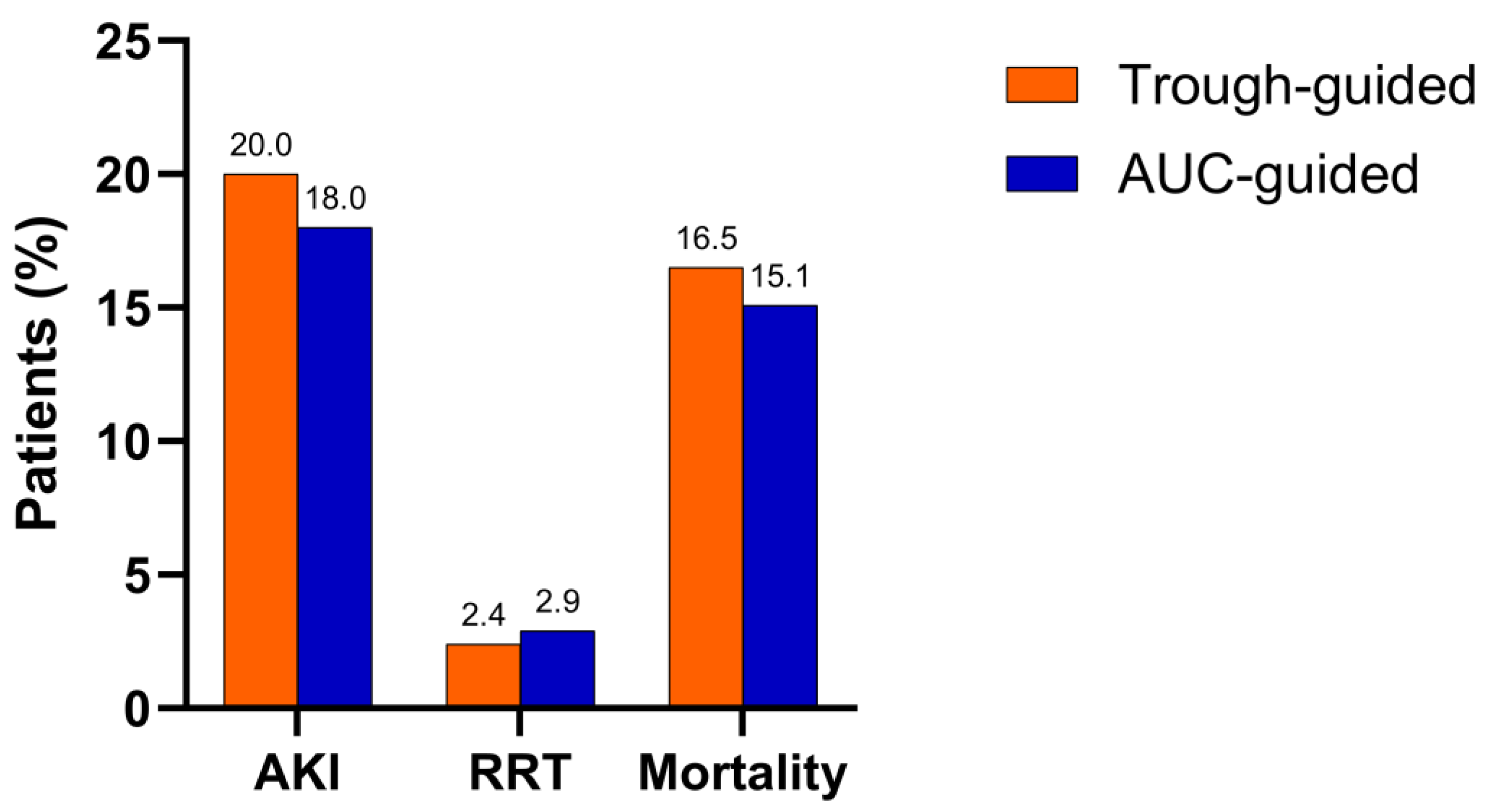
2.2. Primary and Secondary Outcomes
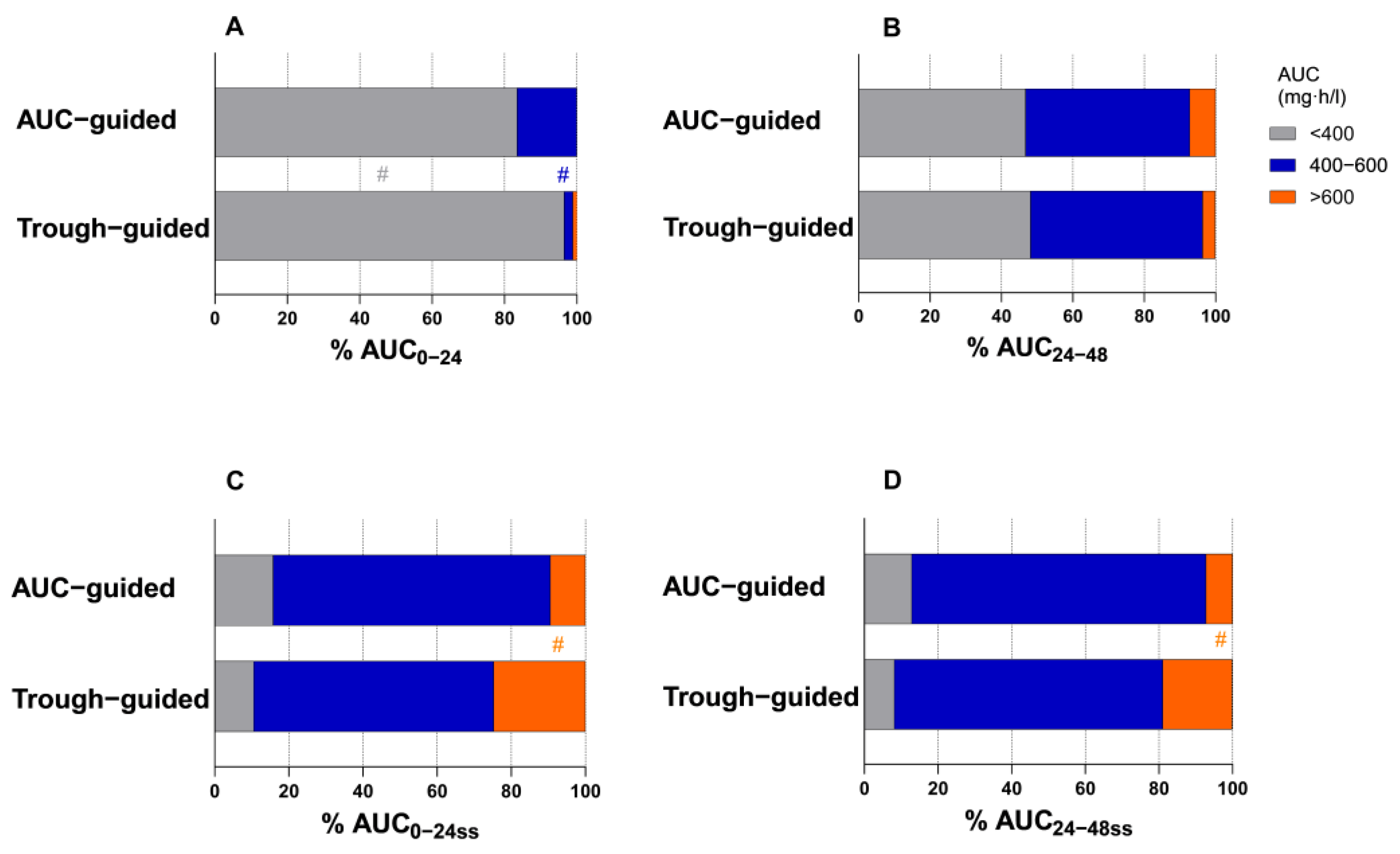
2.3. Pharmacokinetic Parameters
2.4. Concomitant Nephrotoxic Therapy
2.5. Risk Factors for Vancomycin-Associated AKI
3. Discussion
4. Materials and Methods
4.1. Study Design and Population
4.2. Data Collection
4.3. Outcomes
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Biondi, S.; Chugunova, E.; Panunzio, M. From Natural Products to Drugs. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2016; Volume 50, pp. 249–297. [Google Scholar] [CrossRef]
- Levine, D.P. Vancomycin: A History. Clin. Infect. Dis. 2006, 42 (Suppl. 1), S5–S12. [Google Scholar] [CrossRef]
- Rybak, M.; Lomaestro, B.; Rotschafer, J.C.; Moellering, R.; Craig, W.; Billeter, M.; Dalovisio, J.R.; Levine, D.P. Therapeutic Monitoring of Vancomycin in Adult Patients: A Consensus Review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am. J. Health Syst. Pharm. 2009, 66, 82–98. [Google Scholar] [CrossRef] [PubMed]
- Rybak, M.J. The Pharmacokinetic and Pharmacodynamic Properties of Vancomycin. Clin. Infect. Dis. 2006, 42 (Suppl. 1), S35–S39. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.L.; Lalla, C.D.; Masselink, A.J. AUC Versus Peak–Trough Dosing of Vancomycin: Applying New Pharmacokinetic Paradigms to an Old Drug. Ther. Drug Monit. 2013, 35, 443–449. [Google Scholar] [CrossRef]
- Pai, M.P.; Neely, M.; Rodvold, K.A.; Lodise, T.P. Innovative Approaches to Optimizing the Delivery of Vancomycin in Individual Patients. Adv. Drug Deliv. Rev. 2014, 77, 50–57. [Google Scholar] [CrossRef]
- Neely, M.N.; Kato, L.; Youn, G.; Kraler, L.; Bayard, D.; Van Guilder, M.; Schumitzky, A.; Yamada, W.; Jones, B.; Minejima, E. Prospective Trial on the Use of Trough Concentration versus Area under the Curve to Determine Therapeutic Vancomycin Dosing. Antimicrob. Agents Chemother. 2018, 62, e02042-17. [Google Scholar] [CrossRef]
- Finch, N.A.; Zasowski, E.J.; Murray, K.P.; Mynatt, R.P.; Zhao, J.J.; Yost, R.; Pogue, J.M.; Rybak, M.J. A Quasi-Experiment to Study the Impact of Vancomycin Area under the Concentration-Time Curve-Guided Dosing on Vancomycin-Associated Nephrotoxicity. Antimicrob. Agents Chemother. 2017, 61, e01293-17. [Google Scholar] [CrossRef] [PubMed]
- Neely, M.N.; Youn, G.; Jones, B.; Jelliffe, R.W.; Drusano, G.L.; Rodvold, K.A.; Lodise, T.P. Are Vancomycin Trough Concentrations Adequate for Optimal Dosing? Antimicrob. Agents Chemother. 2014, 58, 309–316. [Google Scholar] [CrossRef]
- Lim, A.S.; Foo, S.H.W.; Benjamin Seng, J.J.; Magdeline Ng, T.T.; Chng, H.T.; Han, Z. Area-Under-Curve–Guided Versus Trough-Guided Monitoring of Vancomycin and Its Impact on Nephrotoxicity: A Systematic Review and Meta-Analysis. Ther. Drug Monit. 2023, 45, 519–532. [Google Scholar] [CrossRef]
- Abdelmessih, E.; Patel, N.; Vekaria, J.; Crovetto, B.; SanFilippo, S.; Adams, C.; Brunetti, L. Vancomycin Area under the Curve versus Trough Only Guided Dosing and the Risk of Acute Kidney Injury: Systematic Review and Meta-analysis. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2022, 42, 741–753. [Google Scholar] [CrossRef]
- D’Amico, H.; Wallace, K.L.; Burgess, D.; Burgess, D.S.; Cotner, S.; Mynatt, R.; Li, N.; Stromberg, A.; VanHoose, J. Acute Kidney Injury Associated with Area under the Curve versus Trough Monitoring of Vancomycin in Obese Patients. Antimicrob. Agents Chemother. 2022, 66, e00886-21. [Google Scholar] [CrossRef] [PubMed]
- Aljefri, D.M.; Avedissian, S.N.; Rhodes, N.J.; Postelnick, M.J.; Nguyen, K.; Scheetz, M.H. Vancomycin Area Under the Curve and Acute Kidney Injury: A Meta-Analysis. Clin. Infect. Dis. 2019, 69, 1881–1887. [Google Scholar] [CrossRef] [PubMed]
- Rybak, M.J.; Le, J.; Lodise, T.P.; Levine, D.P.; Bradley, J.S.; Liu, C.; Mueller, B.A.; Pai, M.P.; Wong-Beringer, A.; Rotschafer, J.C.; et al. Therapeutic Monitoring of Vancomycin for Serious Methicillin-Resistant Staphylococcus aureus Infections: A Revised Consensus Guideline and Review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am. J. Health Syst. Pharm. 2020, 77, 835–864. [Google Scholar] [CrossRef]
- Bosso, J.A.; Nappi, J.; Rudisill, C.; Wellein, M.; Bookstaver, P.B.; Swindler, J.; Mauldin, P.D. Relationship between Vancomycin Trough Concentrations and Nephrotoxicity: A Prospective Multicenter Trial. Antimicrob. Agents Chemother. 2011, 55, 5475–5479. [Google Scholar] [CrossRef]
- Hanrahan, T.P.; Kotapati, C.; Roberts, M.J.; Rowland, J.; Lipman, J.; Roberts, J.A.; Udy, A. Factors Associated with Vancomycin Nephrotoxicity in the Critically Ill. Anaesth. Intensiv. Care 2015, 43, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, L.; Baker, C.; Leggett, J.; Sehdev, P.; Brown, A.; Bayley, K.B. Increasing Vancomycin Serum Trough Concentrations and Incidence of Nephrotoxicity. Am. J. Med. 2010, 123, 1143–1149. [Google Scholar] [CrossRef]
- Lodise, T.P.; Patel, N.; Lomaestro, B.M.; Rodvold, K.A.; Drusano, G.L. Relationship between Initial Vancomycin Concentration-Time Profile and Nephrotoxicity among Hospitalized Patients. Clin. Infect. Dis. 2009, 49, 507–514. [Google Scholar] [CrossRef]
- Cano, E.L.; Haque, N.Z.; Welch, V.L.; Cely, C.M.; Peyrani, P.; Scerpella, E.G.; Ford, K.D.; Zervos, M.J.; Ramirez, J.A.; Kett, D.H. Incidence of Nephrotoxicity and Association With Vancomycin Use in Intensive Care Unit Patients With Pneumonia: Retrospective Analysis of the IMPACT-HAP Database. Clin. Ther. 2012, 34, 149–157. [Google Scholar] [CrossRef]
- Van Hal, S.J.; Paterson, D.L.; Lodise, T.P. Systematic Review and Meta-Analysis of Vancomycin-Induced Nephrotoxicity Associated with Dosing Schedules That Maintain Troughs between 15 and 20 Milligrams per Liter. Antimicrob. Agents Chemother. 2013, 57, 734–744. [Google Scholar] [CrossRef]
- Tsutsuura, M.; Moriyama, H.; Kojima, N.; Mizukami, Y.; Tashiro, S.; Osa, S.; Enoki, Y.; Taguchi, K.; Oda, K.; Fujii, S.; et al. The Monitoring of Vancomycin: A Systematic Review and Meta-Analyses of Area under the Concentration-Time Curve-Guided Dosing and Trough-Guided Dosing. BMC Infect. Dis. 2021, 21, 153. [Google Scholar] [CrossRef]
- Holmes, N.E.; Turnidge, J.D.; Munckhof, W.J.; Robinson, J.O.; Korman, T.M.; O’Sullivan, M.V.N.; Anderson, T.L.; Roberts, S.A.; Warren, S.J.C.; Gao, W.; et al. Vancomycin AUC/MIC Ratio and 30-Day Mortality in Patients with Staphylococcus aureus Bacteremia. Antimicrob. Agents Chemother. 2013, 57, 1654–1663. [Google Scholar] [CrossRef] [PubMed]
- Jumah, M.T.B.; Vasoo, S.; Menon, S.R.; De, P.P.; Neely, M.; Teng, C.B. Pharmacokinetic/Pharmacodynamic Determinants of Vancomycin Efficacy in Enterococcal Bacteremia. Antimicrob. Agents Chemother. 2018, 62, e01602-17. [Google Scholar] [CrossRef] [PubMed]
- Vance-Bryan, K.; Rotschafer, J.C.; Gilliland, S.S.; Rodvold, K.A.; Fitzgerald, C.M.; Guay, D.R.P. A Comparative Assessment of Vancomycin-Associated Nephrotoxicity in the Young versus the Elderly Hospitalized Patient. J. Antimicrob. Chemother. 1994, 33, 811–821. [Google Scholar] [CrossRef]
- Hall, R.G.; Hazlewood, K.A.; Brouse, S.D.; Giuliano, C.A.; Haase, K.K.; Frei, C.R.; Forcade, N.A.; Bell, T.; Bedimo, R.J.; Alvarez, C.A. Empiric Guideline-Recommended Weight-Based Vancomycin Dosing and Nephrotoxicity Rates in Patients with Methicillin-Resistant Staphylococcus aureus Bacteremia: A Retrospective Cohort Study. BMC Pharmacol. Toxicol. 2013, 14, 12. [Google Scholar] [CrossRef]
- Xi, L.; Li, S.; Chen, M.; Huang, X.; Li, N.; Chen, N.; Wu, H.; Bian, Q.; Bian, X.; Li, X.; et al. Age-Related Differences in Vancomycin-Associated Nephrotoxicity and Efficacy in Methicillin-Resistant Staphylococcus aureus Infection: A Comparative Study between Elderly and Adult Patients. Antibiotics 2024, 13, 324. [Google Scholar] [CrossRef]
- Zasowski, E.J.; Murray, K.P.; Trinh, T.D.; Finch, N.A.; Pogue, J.M.; Mynatt, R.P.; Rybak, M.J. Identification of Vancomycin Exposure-Toxicity Thresholds in Hospitalized Patients Receiving Intravenous Vancomycin. Antimicrob. Agents Chemother. 2018, 62, e01684-17. [Google Scholar] [CrossRef]
- Chavada, R.; Ghosh, N.; Sandaradura, I.; Maley, M.; Van Hal, S.J. Establishment of an AUC0–24 Threshold for Nephrotoxicity Is a Step towards Individualized Vancomycin Dosing for Methicillin-Resistant Staphylococcus aureus Bacteremia. Antimicrob. Agents Chemother. 2017, 61, e02535-16. [Google Scholar] [CrossRef] [PubMed]
- Goetz, M.B.; Sayers, J. Nephrotoxicity of Vancomycin and Aminoglycoside Therapy Separately and in Combination. J. Antimicrob. Chemother. 1993, 32, 325–334. [Google Scholar] [CrossRef]
- Hidayat, L.K.; Hsu, D.I.; Quist, R.; Shriner, K.A.; Wong-Beringer, A. High-Dose Vancomycin Therapy for Methicillin-Resistant Staphylococcus aureus Infections: Efficacy and Toxicity. Arch. Intern. Med. 2006, 166, 2138. [Google Scholar] [CrossRef]
- Kim, J.Y.; Yee, J.; Yoon, H.Y.; Han, J.M.; Gwak, H.S. Risk Factors for Vancomycin-associated Acute Kidney Injury: A Systematic Review and Meta-analysis. Br. J. Clin. Pharmacol. 2022, 88, 3977–3989. [Google Scholar] [CrossRef]
- Hashimoto, N.; Kimura, T.; Hamada, Y.; Niwa, T.; Hanai, Y.; Chuma, M.; Fujii, S.; Matsumoto, K.; Shigemi, A.; Kawamura, H.; et al. Candidates for Area under the Concentration–Time Curve (AUC)-Guided Dosing and Risk Reduction Based on Analyses of Risk Factors Associated with Nephrotoxicity in Vancomycin-Treated Patients. J. Glob. Antimicrob. Resist. 2021, 27, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Lodise, T.P.; Lomaestro, B.; Graves, J.; Drusano, G.L. Larger Vancomycin Doses (at Least Four Grams per Day) Are Associated with an Increased Incidence of Nephrotoxicity. Antimicrob. Agents Chemother. 2008, 52, 1330–1336. [Google Scholar] [CrossRef]
- Diebold, M.; Zimmermann, T.; Dickenmann, M.; Schaub, S.; Bassetti, S.; Tschudin-Sutter, S.; Bingisser, R.; Heim, C.; Siegemund, M.; Osswald, S.; et al. Comparison of Acute Kidney Injury in Patients with COVID-19 and Other Respiratory Infections: A Prospective Cohort Study. J. Clin. Med. 2021, 10, 2288. [Google Scholar] [CrossRef] [PubMed]
- Ronco, C.; Reis, T.; Husain-Syed, F. Management of Acute Kidney Injury in Patients with COVID-19. Lancet Respir. Med. 2020, 8, 738–742. [Google Scholar] [CrossRef]
- Fu, E.L.; Janse, R.J.; De Jong, Y.; Van Der Endt, V.H.W.; Milders, J.; Van Der Willik, E.M.; De Rooij, E.N.M.; Dekkers, O.M.; Rotmans, J.I.; Van Diepen, M. Acute Kidney Injury and Kidney Replacement Therapy in COVID-19: A Systematic Review and Meta-Analysis. Clin. Kidney J. 2020, 13, 550–563. [Google Scholar] [CrossRef] [PubMed]
- Cockcroft, D.W.; Gault, H. Prediction of Creatinine Clearance from Serum Creatinine. Nephron 1976, 16, 31–41. [Google Scholar] [CrossRef]
- Van Walraven, C.; Austin, P.C.; Jennings, A.; Quan, H.; Forster, A.J. A Modification of the Elixhauser Comorbidity Measures into a Point System for Hospital Death Using Administrative Data. Med. Care 2009, 47, 626–633. [Google Scholar] [CrossRef]
| Characteristic | Trough-Guided (n = 85) | AUC-Guided (n = 139) | p-Value |
|---|---|---|---|
| Demographics | |||
| Sex | |||
| Male (%) | 47 (55.3%) | 97 (69.8%) | 0.032 |
| Female (%) | 38 (44.7%) | 42 (30.2%) | N/A |
| Age (years), median (IQR) | 63 (47–79) | 64 (45–83) | 0.856 |
| BMI (kg/m2), median (IQR) | 26.5 (20.9–32.1) | 26.3 (20.0–32.6) | 0.746 |
| Clinical data | |||
| Baseline SCr (µmol/L), mean (±SD) | 62.8 (±17.5) | 63.6 (±17.2) | 0.718 |
| Baseline ClCr (mL/min), median (IQR) | 94.7 (45.3–80.3) | 102.7 (50.4–155.0) | 0.507 |
| Duration of therapy (days), median (IQR) | 13.0 (2.0–24.0) | 14.0 (8.0–20.0) | 0.656 |
| Concomitant SARS-CoV-2 infection (%) | 0 (0.0%) | 5 (3.6%) | 0.159 |
| ICU residence (%) | 18 (21.2%) | 23 (15.8%) | 0.369 |
| Comorbidities | |||
| Elixhauser Comorbidity Index, median (IQR) | 4.0 (−4.5–12.5) | 3.0 (−6.0–12.0) | 0.835 |
| Hypertension (%) | 38 (44.7%) | 79 (56.8%) | 0.098 |
| Obesity (%) | 20 (23.5%) | 36 (25.9%) | 0.752 |
| Diabetes (%) | 14 (16.5%) | 22 (15.8%) | 1.000 |
| Cardiac arrhythmias (%) | 7 (8.2%) | 27 (19.4%) | 0.034 |
| Solid tumor without metastasis (%) | 16 (18.8%) | 16 (11.5%) | 0.168 |
| Congestive heart failure (%) | 13 (15.3%) | 16 (11.5%) | 0.539 |
| Valvular disease (%) | 10 (11.8%) | 18 (12.9%) | 0.838 |
| Chronic pulmonary disease (%) | 6 (7.1%) | 21 (15.1%) | 0.091 |
| Anemia (%) | 6 (7.1%) | 18 (12.9%) | 0.189 |
| Liver disease (%) | 9 (10.6%) | 14 (10.1%) | 1.000 |
| Type of vancomycin treatment | |||
| Targeted (%) | 58 (68.2%) | 90 (64.7%) | 0.663 |
| Empiric (%) | 27 (31.8%) | 49 (35.3%) | N/A |
| Infection site | |||
| Bloodstream infection (%) | 36 (42.4%) | 49 (35.3%) | 0.322 |
| Pneumonia (%) | 4 (4.7%) | 19 (13.7%) | 0.040 |
| Bone and joint infection (%) | 6 (7.1%) | 11 (7.9%) | 1.000 |
| Abdominal infection (%) | 12 (14.1%) | 12 (8.6%) | 0.265 |
| CNS infection (%) | 11 (12.9%) | 19 (13.7%) | 1.000 |
| Isolated bacterial species | |||
| Staphylococci (%) | 14 (16.5%) | 27 (19.4%) | 0.663 |
| Streptococci (%) | 5 (5.9%) | 9 (6.5%) | 1.000 |
| Enterococci (%) | 30 (35.3%) | 38 (27.3%) | 0.232 |
| Anaerobes (%) | 12 (14.1%) | 19 (13.7%) | 1.000 |
| Methicillin-resistant staphylococci (%) | 37 (43.5%) | 49 (35.3%) | 0.258 |
| Pharmacokinetic Parameter | Trough-Guided (n= 85) | AUC-Guided (n = 139) | p-Value |
|---|---|---|---|
| AUC0–24 (mg·h/L), median (IQR) | 260.6 (169.5–351.7) | 295.4 (179.1–411.7) | 0.004 |
| AUC24–48 (mg·h/L), mean (±SD) | 411.5 (±114.9) | 419.9 (±118.0) | 0.602 |
| AUC0–48 (mg·h/L), median (IQR) | 667.6 (432.5–902.7) | 707.9 (425.4–990.4) | 0.116 |
| AUC0–24ss (mg·h/L), median (IQR) | 509.7 (368.7–650.7) | 473.4 (357.0–589.8) | 0.001 |
| AUC24–48ss (mg·h/L), median (IQR) | 504.0 (364.6–643.4) | 466.6 (368.0–565.2) | 0.001 |
| AUC0–48ss (mg·h/L), median (IQR) | 1017.0 (791.4–1242.6) | 943.7 (752.2–1135.2) | <0.001 |
| Cmin24 (mg/L), median (IQR) | 10.9 (4.6–17.2) | 9.3 (5.3–13.3) | 0.170 |
| Cmin48 (mg/L), median (IQR) | 12.7 (4.8–20.6) | 13.7 (7.3–20.1) | 0.833 |
| Cmin24ss (mg/L), median (IQR) | 16.0 (9.1–22.9) | 14.7 (8.9–20.5) | 0.581 |
| Cmin48ss (mg/L), median (IQR) | 17.0 (10.6–23.4) | 15.1 (10.3–19.9) | 0.049 |
| Concomitant Nephrotoxic Therapy | Trough-Guided (n = 64) | AUC-Guided (n = 118) | p-Value |
|---|---|---|---|
| Aminoglycosides (%) | 3 (4.7%) | 6 (5.1%) | 1.000 |
| Loop diuretics (%) | 22 (34.4%) | 47 (39.8%) | 0.524 |
| Thiazide diuretics (%) | 10 (15.6%) | 16 (13.6%) | 0.825 |
| Vasopressors (%) | 8 (12.5%) | 12 (10.2%) | 0.628 |
| RAAS inhibitors (%) | 24 (37.5%) | 40 (33.9%) | 0.630 |
| NSAID (%) | 6 (9.4%) | 17 (14.4%) | 0.362 |
| Duration of nephrotoxic therapy (days), median (IQR) | 10 (6.3–14.8) | 10 (7.0–14.0) | 0.842 |
| Characteristic | Univariate Model OR (95% CI) | p-Value | Multivariate Model OR (95% CI) | p-Value |
|---|---|---|---|---|
| Age | 1.04 (1.01–1.07) | 0.003 | 1.04 (1.00–1.07) | 0.042 |
| Sex | 1.00 (0.50–2.01) | 1.000 | / | / |
| Baseline SCr | 0.99 (0.97–1.01) | 0.516 | / | / |
| ICU residence | 2.57 (1.19–5.57) | 0.016 | 2.36 (0.91–6.17) | 0.079 |
| Type of vancomycin treatment | 2.54 (1.11–5.79) | 0.027 | 1.26 (0.47–3.39) | 0.652 |
| AUC0–24ss | 1.01 (1.01–1.01) | <0.001 | 1.01 (1.00–1.01) | <0.001 |
| AUC24–48ss | 1.01 (1.01–1.02) | <0.001 | / a | / a |
| AUC0–48ss | 1.01 (1.00–1.01) | <0.001 | / b | / b |
| Duration of nephrotoxic therapy | 1.07 (1.03–1.12) | <0.001 | 1.06 (1.01–1.12) | 0.019 |
| Aminoglycosides | 0.00 (0.00–/) | 0.999 | / | / |
| Loop diuretics | 3.85 (1.79–8.26) | <0.001 | 2.46 (1.02–5.95) | 0.045 |
| RAAS inhibitors | 0.77 (0.35–1.69) | 0.519 | / | / |
| NSAID | 1.15 (0.40–3.33) | 0.801 | / | / |
| Elixhauser Comorbidity Index | 1.02 (0.97–1.06) | 0.508 | / | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marovič, A.; Vovk, T.; Petre, M. Navigating Vancomycin and Acute Kidney Injury: AUC- vs. Trough-Guided Monitoring in Initial and Steady-State Therapy. Antibiotics 2025, 14, 438. https://doi.org/10.3390/antibiotics14050438
Marovič A, Vovk T, Petre M. Navigating Vancomycin and Acute Kidney Injury: AUC- vs. Trough-Guided Monitoring in Initial and Steady-State Therapy. Antibiotics. 2025; 14(5):438. https://doi.org/10.3390/antibiotics14050438
Chicago/Turabian StyleMarovič, Astrid, Tomaž Vovk, and Maja Petre. 2025. "Navigating Vancomycin and Acute Kidney Injury: AUC- vs. Trough-Guided Monitoring in Initial and Steady-State Therapy" Antibiotics 14, no. 5: 438. https://doi.org/10.3390/antibiotics14050438
APA StyleMarovič, A., Vovk, T., & Petre, M. (2025). Navigating Vancomycin and Acute Kidney Injury: AUC- vs. Trough-Guided Monitoring in Initial and Steady-State Therapy. Antibiotics, 14(5), 438. https://doi.org/10.3390/antibiotics14050438






