Diabetic Foot Ulcers: Current Advances in Antimicrobial Therapies and Emerging Treatments
Abstract
:1. Introduction
2. Diabetic Foot Ulcers
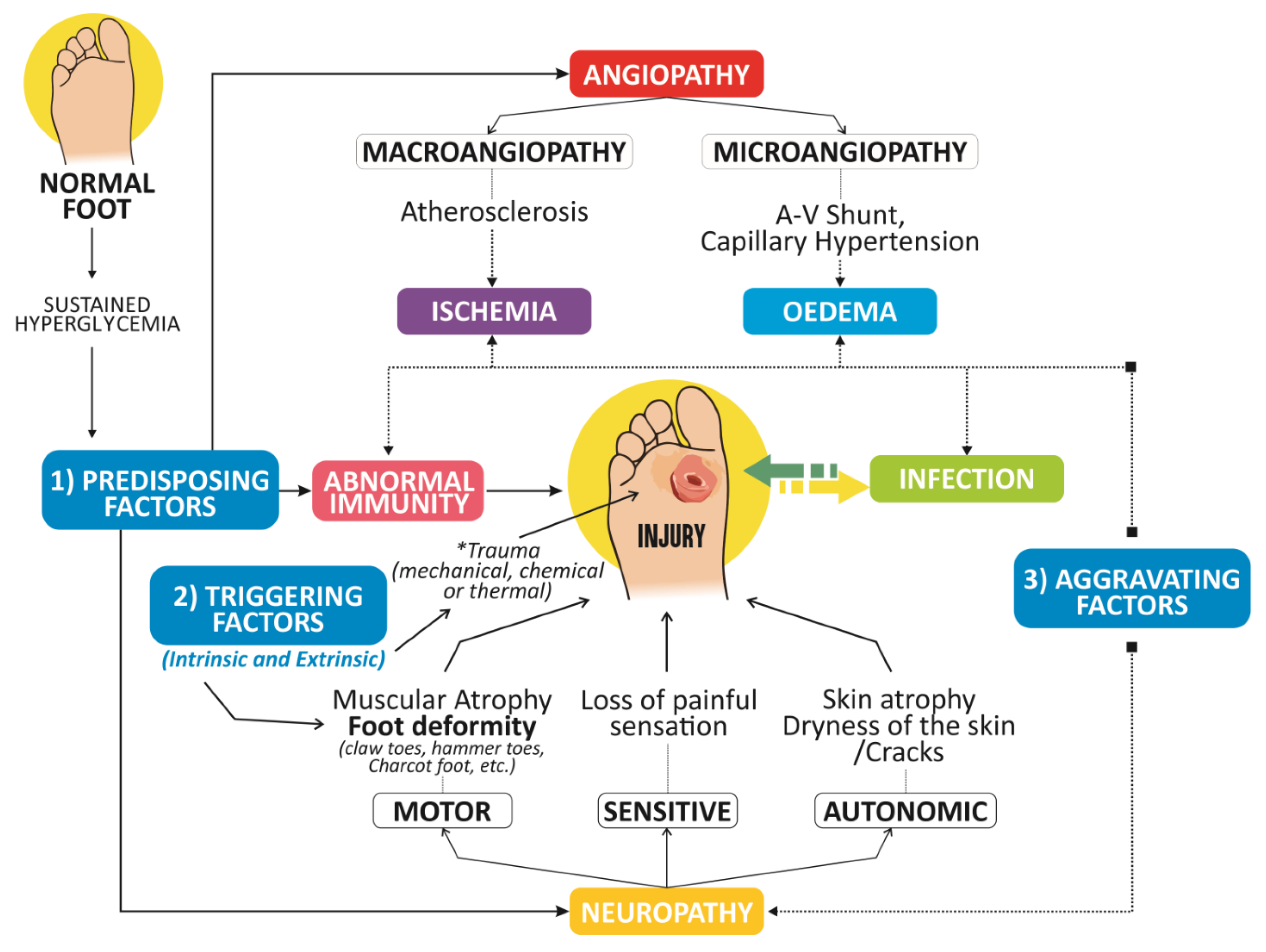
2.1. Physiopathology of DFUs
2.1.1. Diabetic Neuropathy
2.1.2. Immunological Role in the Pathogenesis of DFUs
2.1.3. PAD
2.2. DFU Infection
2.2.1. Microbiota in DFUs
2.2.2. Biofilm
2.2.3. Diagnosis of DFU Infections
Microbiological Approaches
Molecular Approaches
2.2.4. Multidrug-Resistant Bacteria
3. DFU Infection Management Therapeutic Approaches
3.1. Debridement
3.2. Dressings
3.2.1. Hydrogels
3.2.2. Alginate Dressings
3.2.3. Acrylics
3.2.4. Hydrocolloids
3.2.5. Foam Adhesive
3.2.6. Hydrofibers
3.3. Topical Antimicrobials
3.3.1. Povidone Iodine 10% Solution
3.3.2. Chlorhexidine
3.3.3. Acetic Acid 5%
3.3.4. Silver Compounds
3.3.5. Sodium Hypochlorite (Bleach)
3.3.6. Benzalkonium Chloride
3.3.7. Hydrogen Peroxide
3.4. Systemic Antibiotic Therapy
3.5. DFU Emerging Therapies
3.5.1. Drugs
Ciprofloxacin-Loaded Calcium Alginate Wafer
WF10 (Immunokine, Nuvo GmbH)
Pirfenidone (PFD)
Deferoxamine (DFO)
Nitroglycerine (Isosorbide Dinitrate)
3.5.2. Biologics
Growth Factors and Proteins
Growth Factors
Alpha Connexin Carboxy-Terminal (ACT1)
Insulin
Neuropeptides
Antimicrobial Peptides
Platelet-Rich Plasma (PRP)
Cell and Gene Therapy
- Stem Cells
- 2.
- Fibroblast Cultures
- 3.
- Grafting (Bioengineering)
- 4.
- Bovine Fluid Collagen
- 5.
- Acellular Dermal Matrix (ADM)
- 6.
- Human Amniotic Membrane
Honey
Plant Extracts
3.5.3. Ozone Therapy
3.5.4. Devices
3.5.5. Nanomedicine
3.5.6. Others
Energy-Based Therapies
Larval Therapy to Treat Ulcers
4. Collateral Effects of Antimicrobials in Different DFU Therapies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Neville, R.F.; Kayssi, A.; Buescher, T.; Stempel, M.S. The diabetic foot. Curr. Probl. Surg. 2016, 53, 408–437. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Lu, J.; Jing, Y.; Tang, S.; Zhu, D.; Bi, Y. Global epidemiology of diabetic foot ulceration: A systematic review and meta-analysis (dagger). Ann. Med. 2017, 49, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.G.; Boulton, A.J.M.; Bus, S.A. Diabetic Foot Ulcers and Their Recurrence. N. Engl. J. Med. 2017, 376, 2367–2375. [Google Scholar] [CrossRef] [PubMed]
- Jeffcoate, W.J.; Vileikyte, L.; Boyko, E.J.; Armstrong, D.G.; Boulton, A.J.M. Current Challenges and Opportunities in the Prevention and Management of Diabetic Foot Ulcers. Diabetes Care 2018, 41, 645. [Google Scholar] [CrossRef] [PubMed]
- Hinojosa, C.A.; Anaya-Ayala, J.E.; Armstrong, D.G.; Kayssi, A.; Mills, J.L., Sr. The importance of establishing a framework for regional and international collaboration in the management of the diabetic foot. J. Vasc. Surg. 2019, 70, 335–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kruse, I.; Edelman, S. Evaluation and Treatment of Diabetic Foot Ulcers. Clin. Diabetes 2006, 24, 91–93. [Google Scholar] [CrossRef] [Green Version]
- Alavi, A.; Sibbald, R.G.; Mayer, D.; Goodman, L.; Botros, M.; Armstrong, D.G.; Woo, K.; Boeni, T.; Ayello, E.A.; Kirsner, R.S. Diabetic foot ulcers: Part II. Management. J. Am. Acad. Dermatol. 2014, 70, 21.e21–21.e24. [Google Scholar] [CrossRef]
- Everett, E.; Mathioudakis, N. Update on management of diabetic foot ulcers. Ann. N. Y. Acad. Sci. 2018, 1411, 153–165. [Google Scholar] [CrossRef]
- Wienemann, T.; Chantelau, E.A.; Koller, A. Effect of painless diabetic neuropathy on pressure pain hypersensitivity (hyperalgesia) after acute foot trauma. Diabet. Foot Ankle 2014, 5. [Google Scholar] [CrossRef]
- Costa, R.H.R.; Cardoso, N.A.; Procopio, R.J.; Navarro, T.P.; Dardik, A.; de Loiola Cisneros, L. Diabetic foot ulcer carries high amputation and mortality rates, particularly in the presence of advanced age, peripheral artery disease and anemia. Diabetes Metab. Syndr. 2017, 11 (Suppl. 2), S583–S587. [Google Scholar] [CrossRef]
- Cervantes-García, E.; Salazar-Schettino, P.M. Clinical and surgical characteristics of infected diabetic foot ulcers in a tertiary hospital of Mexico. Diabet. Foot Ankle 2017, 8, 1367210. [Google Scholar] [CrossRef] [PubMed]
- Beaney, A.J.; Nunney, I.; Gooday, C.; Dhatariya, K. Factors determining the risk of diabetes foot amputations—A retrospective analysis of a tertiary diabetes foot care service. Diabetes Res. Clin. Pract. 2016, 114, 69–74. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes, A. Consensus Development Conference on Diabetic Foot Wound Care: 7–8 April 1999, Boston, Massachusetts. American Diabetes Association. Diabetes Care 1999, 22, 1354–1360. [Google Scholar]
- Syafril, S. Pathophysiology diabetic foot ulcer. IOP Conf. Ser. Earth Environ. Sci. 2018, 125, 012161. [Google Scholar] [CrossRef]
- Alavi, A.; Sibbald, R.G.; Mayer, D.; Goodman, L.; Botros, M.; Armstrong, D.G.; Woo, K.; Boeni, T.; Ayello, E.A.; Kirsner, R.S. Diabetic foot ulcers: Part, I. Pathophysiology and prevention. J. Am. Acad. Dermatol. 2014, 70, 1.e1–1.e18. [Google Scholar] [CrossRef] [PubMed]
- Aumiller, W.D.; Dollahite, H.A. Pathogenesis and management of diabetic foot ulcers. J. Am. Acad. PAs 2015, 28, 28–34. [Google Scholar] [CrossRef]
- Noor, S.; Zubair, M.; Ahmad, J. Diabetic foot ulcer—A review on pathophysiology, classification and microbial etiology. Diabetes Metab. Syndr. Clin. Res. Rev. 2015, 9, 192–199. [Google Scholar] [CrossRef]
- Chantelau, E.A. Nociception at the diabetic foot, an uncharted territory. World J. Diabetes 2015, 6, 391–402. [Google Scholar] [CrossRef]
- Tresierra-Ayala, M.Á.; García Rojas, A. Association between peripheral arterial disease and diabetic foot ulcers in patients with diabetes mellitus type 2. Med. Univ. 2017, 19, 123–126. [Google Scholar] [CrossRef]
- Schaper, N.C. Diabetic foot ulcer classification system for research purposes: A progress report on criteria for including patients in research studies. Diabetes Metab. Res. Rev. 2004, 20, S90–S95. [Google Scholar] [CrossRef]
- Loesche, M.; Gardner, S.E.; Kalan, L.; Horwinski, J.; Zheng, Q.; Hodkinson, B.P.; Tyldsley, A.S.; Franciscus, C.L.; Hillis, S.L.; Mehta, S.; et al. Temporal Stability in Chronic Wound Microbiota Is Associated With Poor Healing. J. Investig. Dermatol. 2017, 137, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Nelson, E.A.; Backhouse, M.R.; Bhogal, M.S.; Wright-Hughes, A.; Lipsky, B.A.; Nixon, J.; Brown, S.; Gray, J. Concordance in diabetic foot ulcer infection. BMJ Open 2013, 3, e002370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perim, M.C.; Borges, J.D.C.; Celeste, S.R.C.; Orsolin, E.d.F.; Mendes, R.R.; Mendes, G.O.; Ferreira, R.L.; Carreiro, S.C.; Pranchevicius, M.C.D.S. Aerobic bacterial profile and antibiotic resistance in patients with diabetic foot infections. Rev. Soc. Bras. Med. Trop. 2015, 48, 546–554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jneid, J.; Lavigne, J.P.; La Scola, B.; Cassir, N. The diabetic foot microbiota: A review. Hum. Microbiome J. 2017, 5–6, 1–6. [Google Scholar] [CrossRef]
- Martinez De Jesús, F.R.; Ramos-De la Medina, A.; Remes-Troche, J.M.; Armstrong, D.G.; Wu, S.C.; Lázaro Martínez, J.L.; Beneit-Montesinos, J.V. Efficacy and safety of neutral pH superoxidized solution in severe diabetic foot infections. Int. Wound J. 2007, 4, 353–362. [Google Scholar]
- Lipsky, B.A.; Berendt, A.R.; Deery, H.G.; Embil, J.M.; Joseph, W.S.; Karchmer, A.W.; LeFrock, J.L.; Lew, D.P.; Mader, J.T.; Norden, C.; et al. Diagnosis and treatment of diabetic foot infections. Plast Reconstr. Surg. 2006, 117 (Suppl. 7), 212S–238S. [Google Scholar] [CrossRef]
- Jain, S.K.; Barman, R. Bacteriological Profile of Diabetic Foot Ulcer with Special Reference to Drug-resistant Strains in a Tertiary Care Center in North-East India. Indian J. Endocrinol. Metab. 2017, 21, 688–694. [Google Scholar] [CrossRef]
- Banu, A.; Noorul Hassan, M.M.; Rajkumar, J.; Srinivasa, S. Spectrum of bacteria associated with diabetic foot ulcer and biofilm formation: A prospective study. Australas. Med. J. 2015, 8, 280–285. [Google Scholar] [CrossRef]
- Saseedharan, S.; Sahu, M.; Chaddha, R.; Pathrose, E.; Bal, A.; Bhalekar, P.; Sekar, P.; Krishnan, P. Epidemiology of diabetic foot infections in a reference tertiary hospital in India. Braz. J. Microbiol. 2018, 49, 401–406. [Google Scholar] [CrossRef]
- Charles, P.G.P.; Uçkay, I.; Kressmann, B.; Emonet, S.; Lipsky, B.A. The role of anaerobes in diabetic foot infections. Anaerobe 2015, 34, 8–13. [Google Scholar] [CrossRef]
- Haldar, J.; Mukherjee, P.; Mukhopadhyay, S.; Maiti, P.K. Isolation of bacteria from diabetic foot ulcers with special reference to anaerobe isolation by simple two-step combustion technique in candle jar. Indian J. Med. Res. 2017, 145, 97–101. [Google Scholar] [CrossRef] [Green Version]
- Karmaker, M.; Sanyal, S.K.; Sultana, M.; Hossain, M.A. Association of bacteria in diabetic and non-diabetic foot infection—An investigation in patients from Bangladesh. J. Infect. Public Health 2016, 9, 267–277. [Google Scholar] [CrossRef]
- Murali, T.S.; Kavitha, S.; Spoorthi, J.; Bhat, D.V.; Prasad, A.S.; Upton, Z.; Ramachandra, L.; Acharya, R.V.; Satyamoorthy, K. Characteristics of microbial drug resistance and its correlates in chronic diabetic foot ulcer infections. J. Med. Microbiol. 2014, 63, 1377–1385. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Sánchez, M.; Cruz-Pulido, W.L.; Bladinieres-Cámara, E.; Alcalá-Durán, R.; Rivera-Sánchez, G.; Bocanegra-García, V. Bacterial Prevalence and Antibiotic Resistance in Clinical Isolates of Diabetic Foot Ulcers in the Northeast of Tamaulipas, Mexico. Int. J. Low. Extrem. Wounds 2017, 16, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.; Collier, A.; Townsend, E.M.; O’Donnell, L.E.; Bal, A.M.; Butcher, J.; Mackay, W.G.; Ramage, G.; Williams, C. One step closer to understanding the role of bacteria in diabetic foot ulcers: Characterising the microbiome of ulcers. BMC Microbiol. 2016, 16, 54. [Google Scholar] [CrossRef]
- Abbas, M.; Uçkay, I.; Lipsky, B.A. In diabetic foot infections antibiotics are to treat infection, not to heal wounds. Expert Opin. Pharmacother. 2015, 16, 821–832. [Google Scholar] [CrossRef] [PubMed]
- Apelqvist, J.; Larsson, J. What is the most effective way to reduce incidence of amputation in the diabetic foot? Diabetes Metab. Res. Rev. 2000, 16 (Suppl. 1), S75–S83. [Google Scholar] [CrossRef]
- Mark Reglinski, S.S. Chapter 38—Streptococcus pyogenes. In Molecular Medical Microbiology, 2nd ed.; Academic Press: London, UK, 2015; Volume 2, pp. 675–716. ISBN 978-0-12-3971692. [Google Scholar]
- Tayeb, K.A.; Bateman, S.D.; Hampton, S.; Malone, M.; Fletcher, J. Managing infection: A holistic approach. J. Wound Care 2015, 24, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.G.; Lavery, L.A.; Nixon, B.P.; Boulton, A.J. It’s not what you put on, but what you take off: Techniques for debriding and off-loading the diabetic foot wound. Clin. Infect. Dis. 2004, 39 (Suppl. 2), S92–S99. [Google Scholar] [CrossRef]
- Mustatea, P.; Buga, C.; Doran, H.; Mihalache, O.; Bobirca, F.T.; Georgescu, D.E.; Agache, A.; Jauca, C.; Birligea, A.; Chiriac, O.; et al. Soft Tissue Infections in Diabetic Patients. Chirurgia (Bucur) 2018, 113, 651–667. [Google Scholar] [CrossRef]
- Lipsky, B.A.; Armstrong, D.G.; Citron, D.M.; Tice, A.D.; Morgenstern, D.E.; Abramson, M.A. Ertapenem versus piperacillin/tazobactam for diabetic foot infections (SIDESTEP): Prospective, randomised, controlled, double-blinded, multicentre trial. Lancet 2005, 366, 1695–1703. [Google Scholar] [CrossRef]
- Roberts, A.D.; Simon, G.L. Diabetic foot infections: The role of microbiology and antibiotic treatment. Semin. Vasc. Surg. 2012, 25, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Karri, V.V.S.R.; Kuppusamy, G.; Talluri, S.V.; Yamjala, K.; Mannemala, S.S.; Malayandi, R. Current and emerging therapies in the management of diabetic foot ulcers. Curr. Med. Res. Opin. 2016, 32, 519–542. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Getti, G.; Boateng, J. Ciprofloxacin-loaded calcium alginate wafers prepared by freeze-drying technique for potential healing of chronic diabetic foot ulcers. Drug Deliv. Transl. Res. 2017. [Google Scholar] [CrossRef] [PubMed]
- Yingsakmongkol, N. Clinical outcomes of WF10 adjunct to standard treatment of diabetic foot ulcers. J. Wound Care 2013, 22, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Gasca-Lozano, L.E.; Lucano-Landeros, S.; Ruiz-Mercado, H.; Salazar-Montes, A.; Sandoval-Rodríguez, A.; Garcia-Bañuelos, J.; Santos-Garcia, A.; Davila-Rodriguez, J.R.; Navarro-Partida, J.; Bojórquez-Sepúlveda, H.; et al. Pirfenidone Accelerates Wound Healing in Chronic Diabetic Foot Ulcers: A Randomized, Double-Blind Controlled Trial. J. Diabetes Res. 2017, 2017, 12. [Google Scholar] [CrossRef] [PubMed]
- Ram, M.; Singh, V.; Kumawat, S.; Kumar, D.; Lingaraju, M.C.; Uttam Singh, T.; Rahal, A.; Kumar Tandan, S.; Kumar, D. Deferoxamine modulates cytokines and growth factors to accelerate cutaneous wound healing in diabetic rats. Eur. J. Pharmacol. 2015, 764, 9–21. [Google Scholar] [CrossRef]
- Hou, Z.; Nie, C.; Si, Z.; Ma, Y. Deferoxamine enhances neovascularization and accelerates wound healing in diabetic rats via the accumulation of hypoxia-inducible factor-1α. Diabetes Res. Clin. Pract. 2013, 101, 62–71. [Google Scholar] [CrossRef]
- Mikaili, P.; Moloudizargari, M.; Aghajanshakeri, S. Treatment with topical nitroglycerine may promote the healing process of diabetic foot ulcers. Med. Hypotheses 2014, 83, 172–174. [Google Scholar] [CrossRef]
- Maderal, A.D.; Vivas, A.C.; Eaglstein, W.H.; Kirsner, R.S. The FDA and designing clinical trials for chronic cutaneous ulcers. Semin. Cell Dev. Biol. 2012, 23, 993–999. [Google Scholar] [CrossRef]
- Tecilazich, F.; Dinh, T.L.; Veves, A. Emerging Drugs for the Treatment of Diabetic Ulcers. Expert Opin. Emerg. Drugs 2013, 18, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Wieman, T.J.; Smiell, J.M.; Su, Y. Efficacy and safety of a topical gel formulation of recombinant human platelet-derived growth factor-BB (becaplermin) in patients with chronic neuropathic diabetic ulcers. A phase III randomized placebo-controlled double-blind study. Diabetes Care 1998, 21, 822–827. [Google Scholar] [CrossRef] [PubMed]
- Dinh, T.; Tecilazich, F.; Kafanas, A.; Doupis, J.; Gnardellis, C.; Leal, E.; Tellechea, A.; Pradhan, L.; Lyons, T.E.; Giurini, J.M.; et al. Mechanisms involved in the development and healing of diabetic foot ulceration. Diabetes 2012, 61, 2937–2947. [Google Scholar] [CrossRef] [PubMed]
- Uchi, H.; Igarashi, A.; Urabe, K.; Koga, T.; Nakayama, J.; Kawamori, R.; Tamaki, K.; Hirakata, H.; Ohura, T.; Furue, M. Clinical efficacy of basic fibroblast growth factor (bFGF) for diabetic ulcer. Eur. J. Dermatol. 2009, 19, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Montequin, J.I.; Valenzuela-Silva, C.M.; Diaz, O.G.; Savigne, W.; Sancho-Soutelo, N.; Rivero-Fernandez, F.; Sanchez-Penton, P.; Morejon-Vega, L.; Artaza-Sanz, H.; Garcia-Herrera, A.; et al. Intra-lesional injections of recombinant human epidermal growth factor promote granulation and healing in advanced diabetic foot ulcers: Multicenter, randomised, placebo-controlled, double-blind study. Int. Wound J. 2009, 6, 432–443. [Google Scholar] [CrossRef]
- Cruciani, M.; Lipsky, B.A.; Mengoli, C.; de Lalla, F. Are granulocyte colony-stimulating factors beneficial in treating diabetic foot infections?: A meta-analysis. Diabetes Care 2005, 28, 454–460. [Google Scholar] [CrossRef]
- Grek, C.L.; Prasad, G.M.; Viswanathan, V.; Armstrong, D.G.; Gourdie, R.G.; Ghatnekar, G.S. Topical administration of a connexin43-based peptide augments healing of chronic neuropathic diabetic foot ulcers: A multicenter, randomized trial. Wound Repair. Regen. 2015, 23, 203–212. [Google Scholar] [CrossRef]
- Lima, M.H.M.; Caricilli, A.M.; de Abreu, L.L.; Araújo, E.P.; Pelegrinelli, F.F.; Thirone, A.C.P.; Tsukumo, D.M.; Pessoa, A.F.M.; dos Santos, M.F.; de Moraes, M.A.; et al. Topical Insulin Accelerates Wound Healing in Diabetes by Enhancing the AKT and ERK Pathways: A Double-Blind Placebo-Controlled Clinical Trial. PLoS ONE 2012, 7, e36974. [Google Scholar] [CrossRef]
- Toda, M.; Suzuki, T.; Hosono, K.; Kurihara, Y.; Kurihara, H.; Hayashi, I.; Kitasato, H.; Hoka, S.; Majima, M. Roles of calcitonin gene-related peptide in facilitation of wound healing and angiogenesis. Biomed. Pharm. 2008, 62, 352–359. [Google Scholar] [CrossRef]
- Park, J.H.; Suh, D.H.; Kim, H.J.; Lee, Y.I.; Kwak, I.H.; Choi, G.W. Role of procalcitonin in infected diabetic foot ulcer. Diabetes Res. Clin. Pract. 2017, 128, 51–57. [Google Scholar] [CrossRef]
- Michail, M.; Jude, E.; Liaskos, C.; Karamagiolis, S.; Makrilakis, K.; Dimitroulis, D.; Michail, O.; Tentolouris, N. The performance of serum inflammatory markers for the diagnosis and follow-up of patients with osteomyelitis. Int. J. Low. Extrem. Wounds 2013, 12, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Moura, L.I.; Dias, A.M.; Suesca, E.; Casadiegos, S.; Leal, E.C.; Fontanilla, M.R.; Carvalho, L.; de Sousa, H.C.; Carvalho, E. Neurotensin-loaded collagen dressings reduce inflammation and improve wound healing in diabetic mice. Biochim. Biophys. Acta 2014, 1842, 32–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emanuelli, T.; Burgeiro, A.; Carvalho, E. Effects of insulin on the skin: Possible healing benefits for diabetic foot ulcers. Arch. Dermatol. Res. 2016, 308, 677–694. [Google Scholar] [CrossRef] [PubMed]
- da Silva, L.; Carvalho, E.; Cruz, M.T. Role of neuropeptides in skin inflammation and its involvement in diabetic wound healing. Expert Opin. Biol. Ther. 2010, 10, 1427–1439. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Liang, X.; Liu, C.; Cheng, Y.; Zhou, L.; Wang, K.; Zhao, L. Influence of Proline Substitution on the Bioactivity of Mammalian-Derived Antimicrobial Peptide NK-2. Probiotics Antimicrob. Proteins 2017. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.J.; Park, S.J.; Mishig-Ochir, T.; Lee, B.J. Antimicrobial peptides: Therapeutic potentials. Expert Rev. Anti-Infect. 2014, 12, 1477–1486. [Google Scholar] [CrossRef] [PubMed]
- Mangoni, M.L.; McDermott, A.M.; Zasloff, M. Antimicrobial peptides and wound healing: Biological and therapeutic considerations. Exp. Dermatol. 2016, 25, 167–173. [Google Scholar] [CrossRef]
- Germán Alberto Téllez, J.C. Antimicrobial peptides. Asociación Colombiana de Infectología. Rev Infectio 2010, 14, 55–67. [Google Scholar]
- Reddy, K.V.; Yedery, R.D.; Aranha, C. Antimicrobial peptides: Premises and promises. Int. J. Antimicrob. Agents 2004, 24, 536–547. [Google Scholar] [CrossRef]
- Andres, E.; Dimarcq, J.L. Cationic antimicrobial peptides: Update of clinical development. J. Intern. Med. 2004, 255, 519–520. [Google Scholar] [CrossRef]
- Hancock, R.E.; Chapple, D.S. Peptide antibiotics. Antimicrob. Agents Chemother. 1999, 43, 1317–1323. [Google Scholar] [CrossRef] [PubMed]
- Carretero, M.; Escamez, M.J.; Garcia, M.; Duarte, B.; Holguin, A.; Retamosa, L.; Jorcano, J.L.; Rio, M.D.; Larcher, F. In vitro and in vivo wound healing-promoting activities of human cathelicidin LL-37. J. Investig. Dermatol. 2008, 128, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Ramos, R.; Silva, J.P.; Rodrigues, A.C.; Costa, R.; Guardao, L.; Schmitt, F.; Soares, R.; Vilanova, M.; Domingues, L.; Gama, M. Wound healing activity of the human antimicrobial peptide LL37. Peptides 2011, 32, 1469–1476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinstraesser, L.; Hirsch, T.; Schulte, M.; Kueckelhaus, M.; Jacobsen, F.; Mersch, E.A.; Stricker, I.; Afacan, N.; Jenssen, H.; Hancock, R.E.; et al. Innate defense regulator peptide 1018 in wound healing and wound infection. PLoS ONE 2012, 7, e39373. [Google Scholar] [CrossRef]
- Gronberg, A.; Mahlapuu, M.; Stahle, M.; Whately-Smith, C.; Rollman, O. Treatment with LL-37 is safe and effective in enhancing healing of hard-to-heal venous leg ulcers: A randomized, placebo-controlled clinical trial. Wound Repair. Regen. 2014, 22, 613–621. [Google Scholar] [CrossRef]
- Zasloff, M. Magainins, a class of antimicrobial peptides from Xenopus skin: Isolation, characterization of two active forms, and partial cDNA sequence of a precursor. Proc. Natl. Acad. Sci. USA 1987, 84, 5449–5453. [Google Scholar] [CrossRef]
- Lamb, H.M.; Wiseman, L.R. Pexiganan acetate. Drugs 1998, 56, 1047–1052; discussion 1053–1054. [Google Scholar] [CrossRef]
- Nakagami, H.; Sugimoto, K.; Ishikawa, T.; Fujimoto, T.; Yamaoka, T.; Hayashi, M.; Kiyohara, E.; Ando, H.; Terabe, Y.; Takami, Y.; et al. Physician-initiated clinical study of limb ulcers treated with a functional peptide, SR-0379: From discovery to a randomized, double-blind, placebo-controlled trial. NPJ Aging Mech. Dis. 2018, 4, 2. [Google Scholar] [CrossRef]
- Terao, M.; Romao, M.J.; Leimkuhler, S.; Bolis, M.; Fratelli, M.; Coelho, C.; Santos-Silva, T.; Garattini, E. Structure and function of mammalian aldehyde oxidases. Arch. Toxicol. 2016, 90, 753–780. [Google Scholar] [CrossRef]
- Alexiadou, K.; Doupis, J. Management of Diabetic Foot Ulcers. Diabetes Ther. 2012, 3, 4. [Google Scholar] [CrossRef]
- Li, L.; Chen, D.; Wang, C.; Yuan, N.; Wang, Y.; He, L.; Yang, Y.; Chen, L.; Liu, G.; Li, X.; et al. Autologous platelet-rich gel for treatment of diabetic chronic refractory cutaneous ulcers: A prospective, randomized clinical trial. Wound Repair. Regen. 2015, 23, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Kontopodis, N.; Tavlas, E.; Papadopoulos, G.; Pantidis, D.; Kafetzakis, A.; Chalkiadakis, G.; Ioannou, C. Effectiveness of Platelet-Rich Plasma to Enhance Healing of Diabetic Foot Ulcers in Patients With Concomitant Peripheral Arterial Disease and Critical Limb Ischemia. Int. J. Low. Extrem. Wounds 2016, 15, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, I.; Akkaya, S.; Isyar, M.; Batmaz, A.G.; Guler, O.; Oznam, K.; Ugras, A.; Mahiroğullari, M. Is there a treatment protocol in which platelet-rich plasma is effective? J. Orthop. 2016, 13, 316–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez-Zapata, M.J.; Marti-Carvajal, A.J.; Sola, I.; Exposito, J.A.; Bolibar, I.; Rodriguez, L.; Garcia, J.; Zaror, C. Autologous platelet-rich plasma for treating chronic wounds. Cochrane Database Syst. Rev. 2016, CD006899. [Google Scholar] [CrossRef] [PubMed]
- Babaei, V.; Afradi, H.; Gohardani, H.Z.; Nasseri, F.; Azarafza, M.; Teimourian, S. Management of chronic diabetic foot ulcers using platelet-rich plasma. J. Wound Care 2017, 26, 784–787. [Google Scholar] [CrossRef] [PubMed]
- Mehrannia, M.; Vaezi, M.; Yousefshahi, F.; Rouhipour, N. Platelet Rich Plasma for Treatment of Nonhealing Diabetic Foot Ulcers: A Case Report. Can. J. Diabetes 2014, 38, 5–8. [Google Scholar] [CrossRef] [Green Version]
- Mohammadi, M.H.; Molavi, B.; Mohammadi, S.; Nikbakht, M.; Mohammadi, A.M.; Mostafaei, S.; Norooznezhad, A.H.; Ghorbani Abdegah, A.; Ghavamzadeh, A. Evaluation of wound healing in diabetic foot ulcer using platelet-rich plasma gel: A single-arm clinical trial. Transfus. Apher. Sci. 2017, 56, 160–164. [Google Scholar] [CrossRef]
- Ahmed, M.; Reffat, S.A.; Hassan, A.; Eskander, F. Platelet-Rich Plasma for the Treatment of Clean Diabetic Foot Ulcers. Ann. Vasc. Surg. 2017, 38, 206–211. [Google Scholar] [CrossRef]
- Sivan-Loukianova, E.; Awad, O.A.; Stepanovic, V.; Bickenbach, J.; Schatteman, G.C. CD34+ blood cells accelerate vascularization and healing of diabetic mouse skin wounds. J. Vasc. Res. 2003, 40, 368–377. [Google Scholar] [CrossRef]
- Tepper, O.M.; Galiano, R.D.; Capla, J.M.; Kalka, C.; Gagne, P.J.; Jacobowitz, G.R.; Levine, J.P.; Gurtner, G.C. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation 2002, 106, 2781–2786. [Google Scholar] [CrossRef]
- Jackson, W.M.; Nesti, L.J.; Tuan, R.S. Concise review: Clinical translation of wound healing therapies based on mesenchymal stem cells. Stem Cells Transl. Med. 2012, 1, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Sheng, L.; Zhang, T.R.; Li, Q. Stem cell therapy for lower extremity diabetic ulcers: Where do we stand? Biomed. Res. Int. 2013, 2013, 462179. [Google Scholar] [CrossRef]
- Assi, R.; Foster, T.R.; He, H.; Stamati, K.; Bai, H.; Huang, Y.; Hyder, F.; Rothman, D.; Shu, C.; Homer-Vanniasinkam, S.; et al. Delivery of mesenchymal stem cells in biomimetic engineered scaffolds promotes healing of diabetic ulcers. Regen. Med. 2016, 11, 245–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marston, W.A.; Hanft, J.; Norwood, P.; Pollak, R.; Dermagraft Diabetic Foot Ulcer Study Group. The efficacy and safety of Dermagraft in improving the healing of chronic diabetic foot ulcers: Results of a prospective randomized trial. Diabetes Care 2003, 26, 1701–1705. [Google Scholar] [CrossRef] [PubMed]
- Veves, A.; Falanga, V.; Armstrong, D.G.; Sabolinski, M.L.; Apligraf Diabetic Foot Ulcer Study. Graftskin, a human skin equivalent, is effective in the management of noninfected neuropathic diabetic foot ulcers: A prospective randomized multicenter clinical trial. Diabetes Care 2001, 24, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Ingram, R.T.; Patel, J.B.; Pryor, T.J. Flowable Wound Matrix and Its Preparation and Use. U.S. Patent No. 7,993,679, 9 August 2011. [Google Scholar]
- Cazzell, S.; Vayser, D.; Pham, H.; Walters, J.; Reyzelman, A.; Samsell, B.; Dorsch, K.; Moore, M. A randomized clinical trial of a human acellular dermal matrix demonstrated superior healing rates for chronic diabetic foot ulcers over conventional care and an active acellular dermal matrix comparator. Wound Repair. Regen. 2017, 25, 483–497. [Google Scholar] [CrossRef] [Green Version]
- Zelen, C.M.; Serena, T.E.; Denoziere, G.; Fetterolf, D.E. A prospective randomised comparative parallel study of amniotic membrane wound graft in the management of diabetic foot ulcers. Int. Wound J. 2013, 10, 502–507. [Google Scholar] [CrossRef]
- Alam, F.; Islam, M.A.; Gan, S.H.; Khalil, M.I. Honey: A Potential Therapeutic Agent for Managing Diabetic Wounds. Evid.-Based Complement. Altern. Med. 2014, 2014, 169130. [Google Scholar] [CrossRef]
- Jull, A.B.; Cullum, N.; Dumville, J.C.; Westby, M.J.; Deshpande, S.; Walker, N. Honey as a topical treatment for wounds. Cochrane Database Syst. Rev. 2015, CD005083. [Google Scholar] [CrossRef] [Green Version]
- Falanga, V. Wound healing and its impairment in the diabetic foot. Lancet 2005, 366, 1736–1743. [Google Scholar] [CrossRef]
- Zhang, R.P.; Zhang, X.P.; Ruan, Y.F.; Ye, S.Y.; Zhao, H.C.; Cheng, Q.H.; Wu, D.J. Protective effect of Radix Astragali injection on immune organs of rats with obstructive jaundice and its mechanism. World J. Gastroenterol. 2009, 15, 2862–2869. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-Y.; Jiang, M.; Zhang, C.; Wang, Z.; He, D.; Guo, Y.-M.; Tian, J.-P.; Yu, X.-C.; Lu, A.-P. Benefits of Chinese Medicine Among Patients with Diabetic Foot: An Expert Review from Clinical Studies. Curr. Vasc. Pharmacol. 2015, 13, 520–525. [Google Scholar] [CrossRef] [PubMed]
- Lordani, T.V.A.; de Lara, C.E.; Ferreira, F.B.P.; de Souza Terron Monich, M.; Mesquita da Silva, C.; Felicetti Lordani, C.R.; Giacomini Bueno, F.; Vieira Teixeira, J.J.; Lonardoni, M.V.C. Therapeutic Effects of Medicinal Plants on Cutaneous Wound Healing in Humans: A Systematic Review. Mediat. Inflamm. 2018, 2018, 12. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Zhu, B.H. Arnebin-1 promotes the angiogenesis of human umbilical vein endothelial cells and accelerates the wound healing process in diabetic rats. J. Ethnopharmacol. 2014, 154, 653–662. [Google Scholar] [CrossRef]
- Zeng, Z.; Huang, W.D.; Gao, Q.; Su, M.L.; Yang, Y.F.; Liu, Z.C.; Zhu, B.H. Arnebin-1 promotes angiogenesis by inducing eNOS, VEGF and HIF-1alpha expression through the PI3K-dependent pathway. Int. J. Mol. Med. 2015, 36, 685–697. [Google Scholar] [CrossRef]
- Ahmad, M.; Ansari, M.N.; Alam, A.; Khan, T.H. Oral dose of citrus peel extracts promotes wound repair in diabetic rats. Pak. J. Biol. Sci. 2013, 16, 1086–1094. [Google Scholar] [CrossRef]
- Pawar, R.S.; Kumar, S.; Toppo, F.A.; Pk, L.; Suryavanshi, P. Sida cordifolia Linn. accelerates wound healing process in type 2 diabetic rats. J. Acute Med. 2016, 6, 82–89. [Google Scholar] [CrossRef] [Green Version]
- Iabichella, M.L. The use of an extract of Hypericum perforatum and Azadirachta indica in advanced diabetic foot: An unexpected outcome. BMJ Case Rep. 2013, 2013, 007299. [Google Scholar] [CrossRef]
- Iabichella, M.L.; Caruso, C.; Lugli, M. The use of an extract of Hypericum perforatum and Azadirachta indica in a neuropathic patient with advanced diabetic foot. BMJ Case Rep. 2014, 2014, 205706. [Google Scholar] [CrossRef]
- Hussan, F.; Teoh, S.L.; Muhamad, N.; Mazlan, M.; Latiff, A.A. Momordica charantia ointment accelerates diabetic wound healing and enhances transforming growth factor-β expression. J. Wound Care 2014, 23, 400–407. [Google Scholar] [CrossRef]
- Mohajeri, G.; Safaee, M.; Sanei, M.H. Effects of topical Kiwifruit on healing of neuropathic diabetic foot ulcer. J. Res. Med. Sci. 2014, 19, 520–524. [Google Scholar] [PubMed]
- Daburkar, M.; Lohar, V.; Rathore, A.S.; Bhutada, P.; Tangadpaliwar, S. An in vivo and in vitro investigation of the effect of Aloe vera gel ethanolic extract using animal model with diabetic foot ulcer. J. Pharm. Bioallied Sci. 2014, 6, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Najafian, Y.; Mazloum, Z.; Najaf Najafi, M.; Hamedi, S.; Mahjour, M.; Feyzabadi, Z. Efficacy of Aloe vera/Plantago major gel in Diabetic Foot Ulcer: A randomized double-blind clinical trial. Curr. Drug Discov. Technol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Bahar, A.; Saeedi, M.; Kashi, Z.; Akha, O.; Rabiei, K.; Davoodi, M. The Effect of Aleo vera and Honey Gel in Healing Diabetic Foot Ulcers. J. Maz. Univ. Med. Sci. 2015, 25, 113–117. [Google Scholar]
- Nehete, M.N.; Nipanikar, S.; Kanjilal, A.S.; Kanjilal, S.; Tatke, P.A. Comparative efficacy of two polyherbal creams with framycetin sulfate on diabetic wound model in rats. J. Ayurveda Integr. Med. 2016, 7, 83–87. [Google Scholar] [CrossRef] [Green Version]
- Nasiri, M.; Fayazi, S.; Jahani, S.; Yazdanpanah, L.; Haghighizadeh, M.H. The effect of topical olive oil on the healing of foot ulcer in patients with type 2 diabetes: A double-blind randomized clinical trial study in Iran. J. Diabetes Metab. Disord. 2015, 14, 38. [Google Scholar] [CrossRef]
- Sari, Y.; Purnawan, I.; Kurniawan, D.W.; Sutrisna, E. A Comparative Study of the Effects of Nigella sativa Oil Gel and Aloe Vera Gel on Wound Healing in Diabetic Rats. J. Evid.-Based Integr. Med. 2018, 23. [Google Scholar] [CrossRef]
- Singh, A.; Singh, A.K.; Narayan, G.; Singh, T.B.; Shukla, V.K. Effect of Neem oil and Haridra on non-healing wounds. Ayu 2014, 35, 398–403. [Google Scholar] [CrossRef] [Green Version]
- Subbu Lakshmi, S.; Chelladurai, G.; Suresh, B. In vitro studies on medicinal plants used against bacterial diabetic foot ulcer (BDFU) and urinary tract infected (UTI) causing pathogens. J. Parasit. Dis. 2016, 40, 667–673. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, P.; Tian, J.; Li, L.; Li, J.; Tian, J.H.; Yang, K. Ozone therapy for treating foot ulcers in people with diabetes. Cochrane Database Syst. Rev. 2015. [Google Scholar] [CrossRef]
- Fitzpatrick, E.; Holland, O.J.; Vanderlelie, J.J. Ozone therapy for the treatment of chronic wounds: A systematic review. Int. Wound J. 2018. [Google Scholar] [CrossRef] [PubMed]
- Fathi, A.M.; Mawsouf, M.N.; Viebahn-Hänsler, R. Ozone Therapy in Diabetic Foot and Chronic, Nonhealing Wounds. Ozone Sci. Eng. 2012, 34, 438–450. [Google Scholar] [CrossRef]
- Uzun, G.; Mutluoğlu, M.; Karagöz, H.; Memiş, A.; Karabacak, E.; Ay, H. Pitfalls of Intralesional Ozone Injection in Diabetic Foot Ulcers: A Case Study. J. Am. Coll. Clin. Wound Spec. 2012, 4, 81–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosul, M.V.; Patskan, B.M. Ozone therapy effectiveness in patients with ulcerous lesions due to diabetes mellitus. Wiad Lek. 2016, 69, 7–9. [Google Scholar]
- Zhang, J.; Guan, M.; Xie, C.; Luo, X.; Zhang, Q.; Xue, Y. Increased Growth Factors Play a Role in Wound Healing Promoted by Noninvasive Oxygen-Ozone Therapy in Diabetic Patients with Foot Ulcers. Oxid. Med. Cell Longev. 2014, 2014, 273475. [Google Scholar] [CrossRef]
- Nunes, G.A.M.d.A.; Reis, M.d.C.d.; Rosa, M.F.F.; Peixoto, L.R.T.; Rocha, A.F.d.; Rosa, S.d.S.R.F. A system for treatment of diabetic foot ulcers using led irradiation and natural latex. Res. Biomed. Eng. 2016, 32, 3–13. [Google Scholar] [CrossRef] [Green Version]
- Jarl, G.; Tranberg, R. An innovative sealed shoe to off-load and heal diabetic forefoot ulcers—A feasibility study. Diabet. Foot Ankle 2017, 8, 1348178. [Google Scholar] [CrossRef]
- Wang, C.-J.; Cheng, J.-H.; Kuo, Y.-R.; Schaden, W.; Mittermayr, R. Extracorporeal shockwave therapy in diabetic foot ulcers. Int. J. Surg. 2015, 24, 207–209. [Google Scholar] [CrossRef]
- Omar, M.T.A.; Alghadir, A.; Al-Wahhabi, K.K.; Al-Askar, A.B. Efficacy of shock wave therapy on chronic diabetic foot ulcer: A single-blinded randomized controlled clinical trial. Diabetes Res. Clin. Pract. 2014, 106, 548–554. [Google Scholar] [CrossRef]
- Jeppesen, S.M.; Yderstraede, K.B.; Rasmussen, B.S.; Hanna, M.; Lund, L. Extracorporeal shockwave therapy in the treatment of chronic diabetic foot ulcers: A prospective randomised trial. J. Wound Care 2016, 25, 641–649. [Google Scholar] [CrossRef]
- Stoekenbroek, R.M.; Santema, T.B.; Legemate, D.A.; Ubbink, D.T.; van den Brink, A.; Koelemay, M.J.W. Hyperbaric Oxygen for the Treatment of Diabetic Foot Ulcers: A Systematic Review. Eur. J. Vasc. Endovasc. Surg. 2014, 47, 647–655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munjewar, C.; Nabi, I.; Gautam, S.; Ahirwar, N.; Chaudhary, P.; Kumar, R.; Arora, M.P.; Ramteke, V.K. Evaluation of the role of hyperbaric oxygen therapy in the treatment of diabetic foot ulcers: A prospective comparative study. Hell. J. Surg. 2016, 88, 219–224. [Google Scholar] [CrossRef]
- Health Quality Ontario. Hyperbaric Oxygen Therapy for the Treatment of Diabetic Foot Ulcers: A Health Technology Assessment. Ont. Health Technol. Assess. Ser. 2017, 17, 1–142. [Google Scholar]
- Hamdan, S.; Pastar, I.; Drakulich, S.; Dikici, E.; Tomic-Canic, M.; Deo, S.; Daunert, S. Nanotechnology-Driven Therapeutic Interventions in Wound Healing: Potential Uses and Applications. ACS Cent. Sci. 2017, 3, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Almonaci Hernández, C.A.; Juarez-Moreno, K.; Castañeda-Juarez, M.E.; Almanza-Reyes, H.; Pestryakov, A.; Bogdanchikova, N. Silver Nanoparticles for the Rapid Healing of Diabetic Foot Ulcers. Int. J. Med. Nano Res. 2017, 4. [Google Scholar] [CrossRef] [Green Version]
- Mabrouk, M.; Kumar, P.; Choonara, Y.E.; du Toit, L.C.; Pillay, V. Artificial, Triple-Layered, Nanomembranous Wound Patch for Potential Diabetic Foot Ulcer Intervention. Materials 2018, 11, 2128. [Google Scholar] [CrossRef]
- Zarei, F.; Negahdari, B.; Eatemadi, A. Diabetic ulcer regeneration: Stem cells, biomaterials, growth factors. Artif. Cells Nanomed. Biotechnol. 2018, 46, 26–32. [Google Scholar] [CrossRef]
- Kawai, K.; Suzuki, S.; Tabata, Y.; Ikada, Y.; Nishimura, Y. Accelerated tissue regeneration through incorporation of basic fibroblast growth factor-impregnated gelatin microspheres into artificial dermis. Biomaterials 2000, 21, 489–499. [Google Scholar] [CrossRef]
- Merrell, J.G.; McLaughlin, S.W.; Tie, L.; Laurencin, C.T.; Chen, A.F.; Nair, L.S. Curcumin-loaded poly(epsilon-caprolactone) nanofibres: Diabetic wound dressing with anti-oxidant and anti-inflammatory properties. Clin. Exp. Pharmacol. Physiol. 2009, 36, 1149–1156. [Google Scholar] [CrossRef]
- Lemos, H.; Mohamed, E.; Huang, L.; Chandler, P.R.; Ou, R.; Pacholczyk, R.; Mellor, A.L. Stimulator of Interferon Genes Agonists attenuate type I diabetes progression in NOD mice. Immunology 2019. [Google Scholar] [CrossRef]
- Yang, X. Design and optimization of crocetin loaded PLGA nanoparticles against diabetic nephropathy via suppression of inflammatory biomarkers: A formulation approach to preclinical study. Drug Deliv. 2019, 26, 849–859. [Google Scholar] [CrossRef] [PubMed]
- Saggini, R.; Figus, A.; Troccola, A.; Cocco, V.; Saggini, A.; Scuderi, N. Extracorporeal shock wave therapy for management of chronic ulcers in the lower extremities. Ultrasound Med. Biol. 2008, 34, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, M.; Ferraz, J.; Junior, M.; Moura, A.; da Costa, M.; Costa, F.; Neto, V.; Neto, R.; Gama, R. Use of maggot therapy for treating a diabetic foot ulcer colonized by multidrug resistant bacteria in Brazil. Indian J. Med. Res. 2015, 141, 340–342. [Google Scholar] [CrossRef] [PubMed]
- Poppel, A.K.; Vogel, H.; Wiesner, J.; Vilcinskas, A. Antimicrobial peptides expressed in medicinal maggots of the blow fly Lucilia sericata show combinatorial activity against bacteria. Antimicrob. Agents Chemother. 2015, 59, 2508–2514. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Chen, J.A.; Zhang, J.; Wang, W.; Sun, J.; Wang, A. Maggot debridement therapy promotes diabetic foot wound healing by up-regulating endothelial cell activity. J. Diabetes Its Complicat. 2016, 30, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Kasiri, M.M.; Beer, L.; Nemec, L.; Gruber, F.; Pietkiewicz, S.; Haider, T.; Simader, E.M.; Traxler, D.; Schweiger, T.; Janik, S.; et al. Dying blood mononuclear cell secretome exerts antimicrobial activity. Eur. J. Clin. Investig. 2016, 46, 853–863. [Google Scholar] [CrossRef]
- Price, B.L.; Lovering, A.M.; Bowling, F.L.; Dobson, C.B. Development of a Novel Collagen Wound Model To Simulate the Activity and Distribution of Antimicrobials in Soft Tissue during Diabetic Foot Infection. Antimicrob. Agents Chemother. 2016, 60, 6880–6889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mavrogenis, A.F.; Megaloikonomos, P.D.; Antoniadou, T.; Igoumenou, V.G.; Panagopoulos, G.N.; Dimopoulos, L.; Moulakakis, K.G.; Sfyroeras, G.S.; Lazaris, A. Current concepts for the evaluation and management of diabetic foot ulcers. EFORT Open Rev. 2018, 3, 513–525. [Google Scholar] [CrossRef]
- Malone, M.; Johani, K.; Jensen, S.O.; Gosbell, I.B.; Dickson, H.G.; McLennan, S.; Hu, H.; Vickery, K. Effect of cadexomer iodine on the microbial load and diversity of chronic non-healing diabetic foot ulcers complicated by biofilm in vivo. J. Antimicrob. Chemother. 2017, 72, 2093–2101. [Google Scholar] [CrossRef]
- Das, S.; Baker, A.B. Biomaterials and Nanotherapeutics for Enhancing Skin Wound Healing. Front. Bioeng. Biotechnol. 2016, 4, 82. [Google Scholar] [CrossRef]
- Fang, R.C.; Galiano, R.D. A review of becaplermin gel in the treatment of diabetic neuropathic foot ulcers. Biologics 2008, 2, 1–12. [Google Scholar] [PubMed] [Green Version]
- Lopes, L.; Setia, O.; Aurshina, A.; Liu, S.; Hu, H.; Isaji, T.; Liu, H.; Wang, T.; Ono, S.; Guo, X.; et al. Stem cell therapy for diabetic foot ulcers: A review of preclinical and clinical research. Stem Cell Res. 2018, 9, 188. [Google Scholar] [CrossRef] [PubMed]
- Gilligan, A.M.; Waycaster, C.R.; Bizier, R.; Chu, B.C.; Carter, M.J.; Fife, C.E. Comparative Effectiveness of Clostridial Collagenase Ointment to Medicinal Honey for Treatment of Pressure Ulcers. Adv. Wound Care (New Rochelle) 2017, 6, 125–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bingham, M. Timely News and Notes for Primary Care Providers From The American Diabetes Association. Clin. Diabetes USA 2018, 32, 92–96. [Google Scholar] [CrossRef]
- Duscher, D.; Neofytou, E.; Wong, V.W.; Maan, Z.N.; Rennert, R.C.; Inayathullah, M.; Januszyk, M.; Rodrigues, M.; Malkovskiy, A.V.; Whitmore, A.J.; et al. Transdermal deferoxamine prevents pressure-induced diabetic ulcers. Proc. Natl. Acad. Sci. USA 2015, 112, 94–99. [Google Scholar] [CrossRef]
- Hunt, S.D.; Elg, F. Clinical effectiveness of hemoglobin spray (Granulox((R))) as adjunctive therapy in the treatment of chronic diabetic foot ulcers. Diabet. Foot Ankle 2016, 7, 33101. [Google Scholar] [CrossRef]
- Cohn, S.M.; Lipsett, P.A.; Buchman, T.G.; Cheadle, W.G.; Milsom, J.W.; O’Marro, S.; Yellin, A.E.; Jungerwirth, S.; Rochefort, E.V.; Haverstock, D.C.; et al. Comparison of intravenous/oral ciprofloxacin plus metronidazole versus piperacillin/tazobactam in the treatment of complicated intraabdominal infections. Ann. Surg. 2000, 232, 254–262. [Google Scholar] [CrossRef]
- Health Quality Ontario. Management of chronic pressure ulcers: An evidence-based analysis. Ont. Health Technol. Assess. Ser. 2009, 9, 1–203. [Google Scholar]
| Classification | MEGGIT-WAGNER | ANM-SEGAL | TEXAS | S (AD) SAD 1 | SSS 2 | GIBBONS | PEDIS | SEWSS 3 | WIFI 4 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Etiology | Vascular | - | √ | √ | √ | ? | - | √ | √ | √ |
| Neurological | - | √ | - | ? | ? | - | √ | √ | - | |
| Neuroischemic | - | √ | ? | - | ? | - | - | - | - | |
| Size | - | √ | - | √ | - | - | √ | √ | √ | |
| Depth | √ | √ | √ | √ | - | √ | √ | √ | √ | |
| Changes in bone structure | - | √ | √ | - | - | - | √ | √ | - | |
| Infection | Cellulite | - | √ | ? | √ | √ | √ | √ | √ | √ |
| Abscess | - | √ | ? | - | √ | √ | √ | √ | √ | |
| Osteomyelitis | √ | √ | ? | √ | ? | √ | √ | √ | √ | |
| Degrees of severity | - | √ | - | ? | - | - | √ | √ | √ | |
| Topography | ? | ? | ? | ? | ? | ? | - | √ | ? | |
| Edema | - | - | - | - | - | - | - | √ | - | |
| Healing phases | - | - | - | - | - | - | - | √ | - | |
| Metabolic state | - | √ | - | - | - | √ | - | √ | √ | |
| Feature | Gram-Positive Bacteria | Gram-Negative Bacteria | Anaerobes | Reference |
|---|---|---|---|---|
| Main bacteria found in DFUs | 1. Staphylococcus aureus (MSSA and MRSA) 2. Streptococcus β-hemolytic | 1. Pseudomonas aeruginosa 2. Streptococcus β-hemolytic 3. Proteus spp. | 1. Peptostreptococcus spp. 2. Bacteroides spp. 3. Prevotella spp. 4. Clostridium spp. | [23,24,27,28,29] |
| Location of wound | Superficial wounds | Superficial wounds | Deep wounds | [24,30,31] |
| Geographical location | Occidental countries | Eastern and warmer countries | Global | [24,29,32] |
| Diabetic population | Non-predominance | Predominance | Present | [33] |
| Non-diabetic population | Predominance | Non-predominance | Present | [33] |
| Bacteria Isolated from DFI | Less Efficient Antibiotic | More Efficient Antibiotic | Geographic Region | Reference |
|---|---|---|---|---|
| Total isolate | Cephalosporin (ceftazidime, ceftriaxone, cefuroxime), carbapenem (aztreonam) | Not studied | Bangladesh | [32] |
| Gram-positive | Penicillin, dicloxacillin, and vancomycin | Levofloxacin, cefalotin | Mexico | [34] |
| Gram-negative | Cefalotin, penicillin, and vancomycin | Amikacin | Mexico | [34] |
| Anaerobes | Clindamycin, penicillin, and cefoxitin | Imipenem and metronidazole | India | [31] |
| Gram-negative | Not studied | Piperacillin/tazobactam | India | [31] |
| Gram-positive and Gram-negative | Not studied | Imipenem | Brazil | [23] |
| Gram-negative | Not studied | Gentamicin | Brazil | [23] |
| Biological Product | Administration | Reference |
|---|---|---|
| Growth factor derived from platelet-BB | Local | [53,54] |
| Fibroblast growth factor β | Intralesional | [55] |
| Epidermal growth factor | Intralesional | [56] |
| Vascular endothelial growth factor | Intramuscular | [53] |
| Granulocyte colony-stimulating factor | Systemic | [56] |
| Recombinant human epidermal growth factor | Intralesional | [43] |
| Insulin | Local | [59] |
| Neuropeptides | Local, Systemic | [60] |
| C-reactive protein | Systemic | [61,62,63] |
| Procalcitonin | Systemic | [61,62,63] |
| Neurotensin | Systemic | [61,62,63] |
| Characteristic | Conventional Antibiotics | Antimicrobial Peptides |
|---|---|---|
| Spectrum of activity | Bacteria (selectivity) | Bacteria, fungi, viruses, tumors |
| Objective | Class specific (plasminogen-binding peptide “PBP”, topoisomerase, ribosomes) | Relatively non-specific, multiple objectives |
| Resistance | After few passes with minimum inhibitory concentration) | Generally, cannot be selected directly; multiple passes are required for minimum inhibitory concentration; specific proteases |
| Related activities | Few | Include anti-endotoxic mechanisms and increase inn immune response |
| Pharmacokinetics | It varies | Short average life by proteolytic degradation |
| Toxicology | Tends to be safe | No toxicities of topical use are known |
| Production cost | It varies | Expensive, via processes of chemical synthesis |
| Structure | Peptide | Organism | Activity |
|---|---|---|---|
| Linear helical | Cecropin P | Sus scrofa | Antibacterial |
| Seminalplasmin | Bos Taurus | Antibacterial | |
| Non-helical linear | Bac5 | Bos taurus | Antibacterial |
| Indolicidin | Bos Taurus | Antibacterial | |
| Cyclic with one disulfide | Bactenecina | Bos Taurus | Antibacterial |
| Cyclic with two or more disulfides | B-defenders 1, 2, 4 | Bos taurus. | Antibacterial |
| Cryptidine 1, 2, 4, 5 | Mus musculus | Antibacterial | |
| Defenders NP-1, 2, 3A, 3B | Oryctolagus cuniculus | Antibacterial/antifungal | |
| Defenders HNP-1, 2, 3, 5, 6 | Homo sapiens | Antibacterial/antifungal | |
| Defenders MCP-1 | Oryctolagus cuniculus | Antibacterial | |
| Protegrin I, II, and III | Sus scrofa | Antibacterial/antifungal | |
| TAP | Bos Taurus | Antibacterial/antifungal |
| Biological Product | Action | Reference |
|---|---|---|
| Grafting (bioengineering) | Promotes wound healing through the addition of extracellular matrices that induce growth factors and cytokines | [96] |
| Culture of fibroblasts | Creates a three-dimensional dermis that is replaced as a graft; it is used to treat non-ischemic ulcers | [95] |
| Culture of fibroblasts/keratinocytes | Creates a three-dimensional dermis that replaced as a graft; it is used to treat non-ischemic and ischemic ulcers | [96] |
| Bovine fluid collagen | It is a well-refined fluid fibrillar bovine collagen; unlike normal collagen in biological scaffolds (cross-linked collagen), it contains fibrillar collagen, that is, non-cross-linked collagen | [97] |
| Cell dermal matrix | It was used for several years for wound healing, tissue repair, and reconstruction | [98] |
| Human amniotic membrane | It is used as wound coverage | [99] |
| Plant Extract | Presentation | Route of Administration | Action | Reference |
|---|---|---|---|---|
| Arnebin-1 | Unguent | Local | Antidiabetic and healing properties | [107] |
| Momordica charantia | Unguent | Local | Antidiabetic and healing properties | [112] |
| Kiwi | Slices of kiwi | Local | Antimicrobial and pro-angiogenic properties | [113] |
| Aloe vera | Gel | Local | Antimicrobial and pro-angiogenic properties | [114,115,116] |
| Extracts of citrus peel (lemon, grapefruit, and orange) | Liquid formula | Oral | Antimicrobial and pro-angiogenic properties | [108] |
| Sida cordifolia Linn. | Hydrogel | Local | Antimicrobial and pro-angiogenic properties | [109] |
| Polyherbal | Cream | Local | Antimicrobial and pro-angiogenic properties | [117] |
| Olive oil | Topic | Local | Antimicrobial and pro-angiogenic properties | [118] |
| Nigella sativa | Gel | Local | Antimicrobial and pro-angiogenic properties | [119] |
| Neem and Haridra | Liquid formula, gel | Local, oral | Antimicrobial and pro-angiogenic properties | [120] |
| Hypericum and neem oil | Unguent | Local | Antimicrobial and pro-angiogenic properties | [110] |
| Tragia involucrata | In vitro study | - | Antimicrobial properties | [111,121] |
| Type of Therapy | Pharmaceutical Form | Route of Administration | Advantages | Limitations | Reference |
|---|---|---|---|---|---|
| Becaplermin | Gel | Topical | Stimulates different growth factors useful in the treatment of DFUs | Short half-life time | [153] |
| Cell therapy | Injection or gel | Locally | Stimulates different cellular mechanisms for the regeneration of chronic wounds | Short half-life time | [154] |
| Collagenase clostridial | Ointment | Topical | Easy application, minimal blood loss, and proliferation of endothelial tissue | Burning, exudation, and inflammation | [155] |
| Dermapace system | Device | Local shock waves | Stimulates the wound mechanically, for the removal of damaged tissue | Secondary side effects (pain, bruises, etc.) | [156] |
| Deferoxamine | Injectable | Locally | Reduction of ulcers area in less time | Adverse reactions and its lifetime is short | [157] |
| Granulox | Spray | Topical | Accelerating the healing of chronic wounds | Short half-life time | [158] |
| Omnigraft | Device | Topical | Potential for improvement in the DFU | New infections, swelling, and new ulcers, or existing ulcers that would worsen | [156] |
| Piperacillin/tazobactam (Zosyn, Pfizer) | Injectable | Locally | Wide spectrum advantage in infections and low nephrotoxicity | Adverse reactions may include diarrhea | [159] |
| Provant | Device | Locally | It is useful in pressure ulcers | There is little evidence of its efficacy | [160] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramirez-Acuña, J.M.; Cardenas-Cadena, S.A.; Marquez-Salas, P.A.; Garza-Veloz, I.; Perez-Favila, A.; Cid-Baez, M.A.; Flores-Morales, V.; Martinez-Fierro, M.L. Diabetic Foot Ulcers: Current Advances in Antimicrobial Therapies and Emerging Treatments. Antibiotics 2019, 8, 193. https://doi.org/10.3390/antibiotics8040193
Ramirez-Acuña JM, Cardenas-Cadena SA, Marquez-Salas PA, Garza-Veloz I, Perez-Favila A, Cid-Baez MA, Flores-Morales V, Martinez-Fierro ML. Diabetic Foot Ulcers: Current Advances in Antimicrobial Therapies and Emerging Treatments. Antibiotics. 2019; 8(4):193. https://doi.org/10.3390/antibiotics8040193
Chicago/Turabian StyleRamirez-Acuña, Jesus Manuel, Sergio A Cardenas-Cadena, Pedro A Marquez-Salas, Idalia Garza-Veloz, Aurelio Perez-Favila, Miguel A Cid-Baez, Virginia Flores-Morales, and Margarita L Martinez-Fierro. 2019. "Diabetic Foot Ulcers: Current Advances in Antimicrobial Therapies and Emerging Treatments" Antibiotics 8, no. 4: 193. https://doi.org/10.3390/antibiotics8040193
APA StyleRamirez-Acuña, J. M., Cardenas-Cadena, S. A., Marquez-Salas, P. A., Garza-Veloz, I., Perez-Favila, A., Cid-Baez, M. A., Flores-Morales, V., & Martinez-Fierro, M. L. (2019). Diabetic Foot Ulcers: Current Advances in Antimicrobial Therapies and Emerging Treatments. Antibiotics, 8(4), 193. https://doi.org/10.3390/antibiotics8040193







