A Review on the Use of Antimicrobial Peptides to Combat Porcine Viruses
Abstract
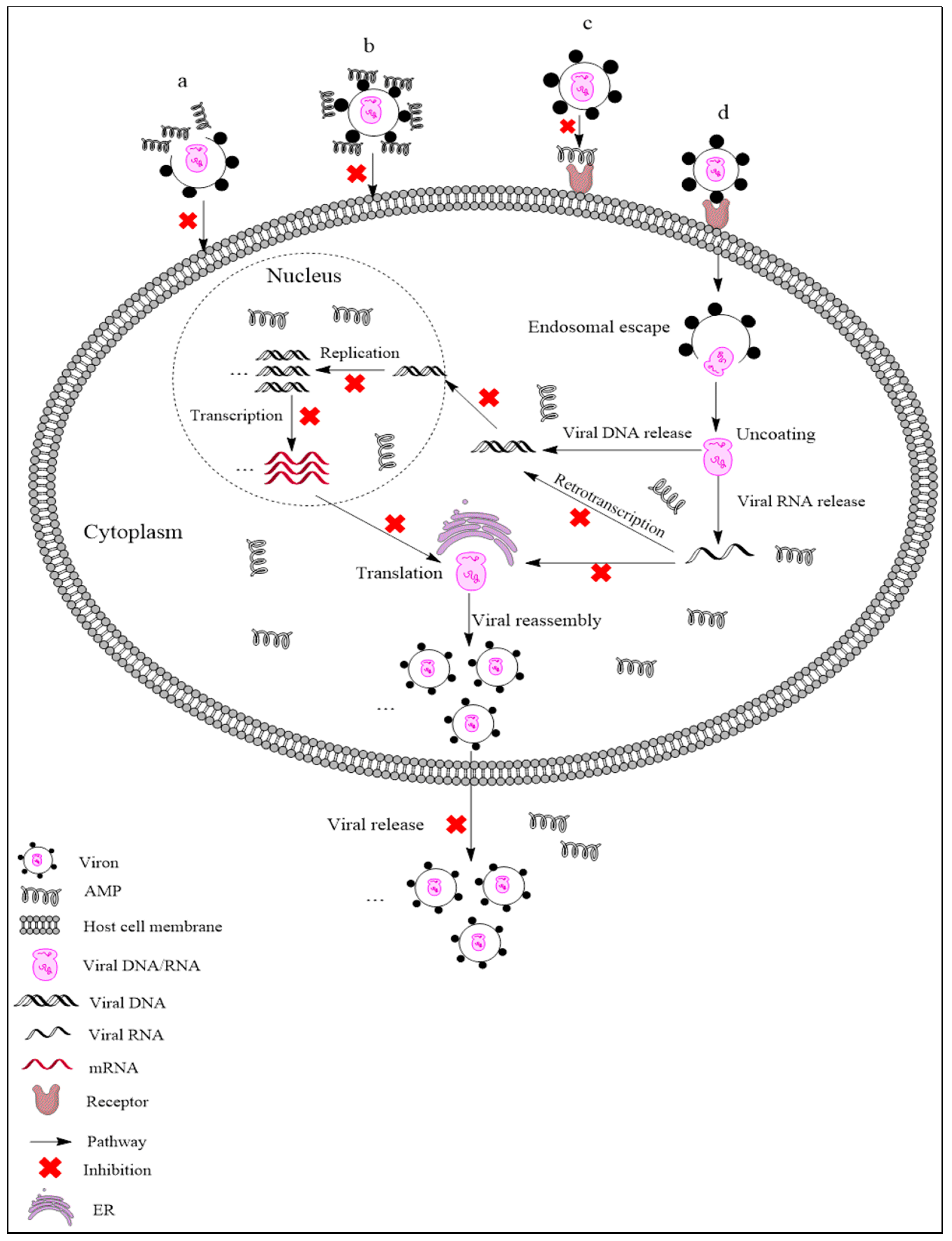
:1. Introduction
2. Antimicrobial Peptides Used against Viruses in Swine
2.1. AMPs Active against PRV and PEDV
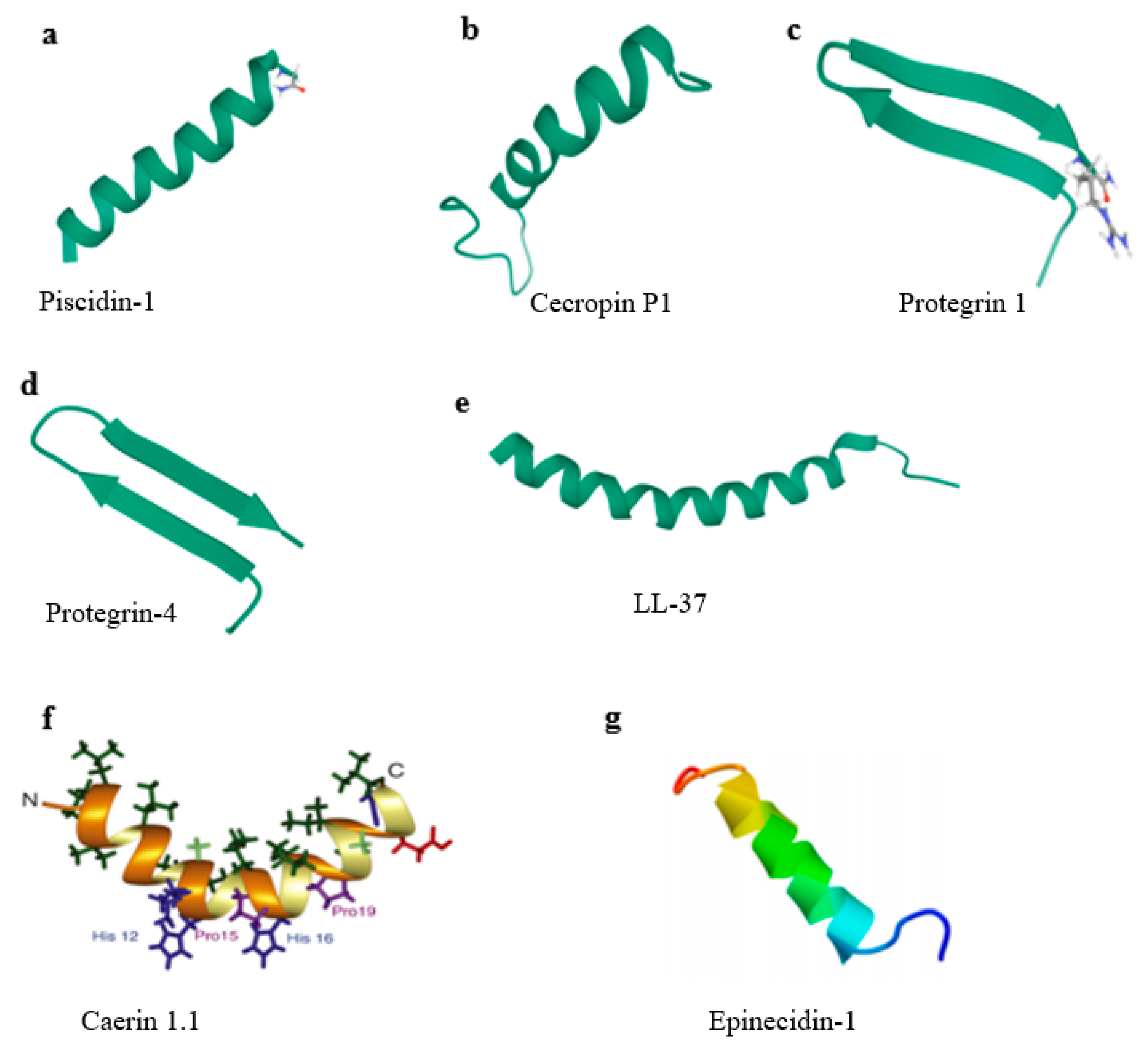
2.1.1. Piscidin-1
2.1.2. Caerin 1.1
2.1.3. Porcine β-Defensin-2 (pBD-2)
2.2. AMPs Active against PRRSV
2.2.1. Cecropins
2.2.2. Host Defense Peptides (HDPs)
2.3. Epinecidin-1 (Epi-1) Fights against FMDV
2.4. Synthesized Peptides Fight against ASFV
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ye, C.; Chen, J.; Wang, T.; Xu, J.; Zheng, H.; Wu, J.; Li, G.; Yu, Z.; Tong, W.; Cheng, X.; et al. Generation and characterization of UL41 null pseudorabies virus variant in vitro and in vivo. Virol. J. 2018, 15, 119. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Zhu, Y.; Wu, M.; Ku, X.; Ye, S.; Li, Z.; Guo, X.; He, Q. Comparative genomic analysis of classical and variant virulent parental/attenuated strains of porcine epidemic diarrhea virus. Viruses 2015, 7, 5525–5538. [Google Scholar] [CrossRef] [PubMed]
- Snijder, E.J.; Meulenberg, J.J. The molecular biology of arteriviruses. J. Gen. Virol. 1998, 79, 961–979. [Google Scholar] [CrossRef] [PubMed]
- Renukaradhya, G.J.; Meng, X.J.; Calvert, J.G.; Roof, M.; Lager, K.M. Inactivated and subunit vaccines against porcine reproductive and respiratory syndrome: Current status and future direction. Vaccine 2015, 33, 3065–3072. [Google Scholar] [CrossRef] [PubMed]
- Paton, D.J.; Taylor, G. Developing vaccines against foot-and-mouth disease and some other exotic viral diseases of livestock. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2011, 366, 2774–2781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ansari, I.H.; Kwon, B.; Osorio, F.A.; Pattnaik, A.K. Influence of N-linked glycosylation of porcine reproductive and respiratory syndrome virus GP5 on virus infectivity, antigenicity, and ability to induce neutralizing antibodies. J. Virol. 2006, 80, 3994–4004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Yang, J.; Zhu, Z.; Zheng, H. Porcine epidemic diarrhea virus and the host innate immune response. Pathogens 2020, 9, 367. [Google Scholar] [CrossRef] [PubMed]
- An, T.Q.; Peng, J.M.; Tian, Z.J.; Zhao, H.Y.; Li, N.; Liu, Y.M.; Chen, J.Z.; Leng, C.L.; Sun, Y.; Chang, D.; et al. Pseudorabies virus variant in Bartha-K61-vaccinated pigs, China, 2012. Emerg. Infect. Dis. 2013, 19, 1749–1755. [Google Scholar] [CrossRef]
- Li, W.; Li, H.; Liu, Y.; Pan, Y.; Deng, F.; Song, Y.; Tang, X.; He, Q. New variants of porcine epidemic diarrhea virus, China, 2011. Emerg. Infect. Dis. 2012, 18, 1350–1353. [Google Scholar] [CrossRef]
- Sánchez-Cordón, P.J.; Montoya, M.; Reis, A.L.; Dixon, L.K. African swine fever: A re-emerging viral disease threatening the global pig industry. Vet. J. 2018, 233, 41–48. [Google Scholar] [CrossRef]
- Mahmoud, A. New vaccines: Challenges of discovery. Microb. Biotechnol. 2016, 9, 549–552. [Google Scholar] [CrossRef] [PubMed]
- Bahar, A.A.; Ren, D. Antimicrobial peptides. Pharmaceuticals (Basel) 2013, 6, 1543–1575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubos, R.J. Studies on a bactericidal agent extracted from a soil bacillus: I. Preparation of the agent. Its activity in vitro. J. Exp. Med. 1939, 70, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubos, R.J. Studies on a bactericidal agent extracted from a soil bacillus: II. Protective effect of the bactericidal agent against experimental pneumococcus infections in mice. J. Exp. Med. 1939, 70, 11–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Powers, J.P.; Hancock, R.E. The relationship between peptide structure and antibacterial activity. Peptides 2003, 24, 1681–1691. [Google Scholar] [CrossRef]
- Zasloff, M. Antimicrobial peptides: Do they have a future as therapeutics? In Antimicrobial Peptides; Springer International Publishing: Cham, Switzerland, 2016; pp. 147–154. [Google Scholar]
- Ganz, T. Defensins: Antimicrobial peptides of innate immunity. Nat. Rev. Immunol. 2003, 3, 710–720. [Google Scholar] [CrossRef]
- VanCompernolle, S.E.; Taylor, R.J.; Oswald-Richter, K.; Jiang, J.; Youree, B.E.; Bowie, J.H.; Tyler, M.J.; Conlon, J.M.; Wade, D.; Aiken, C.; et al. Antimicrobial peptides from amphibian skin potently inhibit human immunodeficiency virus infection and transfer of virus from dendritic cells to T cells. J. Virol. 2005, 79, 11598–11606. [Google Scholar] [CrossRef] [Green Version]
- Matanic, V.C.A.; Castilla, V. Antiviral activity of antimicrobial cationic peptides against Junin virus and herpes simplex virus. Int. J. Antimicrob. Agents 2004, 23, 382–389. [Google Scholar] [CrossRef]
- Gao, Y.; Cui, T.; Lam, Y. Synthesis and disulfide bond connectivity-activity studies of a kalata B1-inspired cyclopeptide against dengue NS2B-NS3 protease. Bioorg. Med. Chem. 2010, 18, 1331–1336. [Google Scholar] [CrossRef]
- Bastian, A.; Schäfer, H. Human alpha-defensin 1 (HNP-1) inhibits adenoviral infection in vitro. Regul. Pept. 2001, 101, 157–161. [Google Scholar] [CrossRef]
- Horne, W.S.; Wiethoff, C.M.; Cui, C.; Wilcoxen, K.M.; Amorin, M.; Ghadiri, M.R.; Nemerow, G.R. Antiviral cyclic D,L-alpha-peptides: Targeting a general biochemical pathway in virus infections. Bioorg. Med. Chem. 2005, 13, 5145–5153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, W.E., Jr.; McDougall, B.; Tran, D.; Selsted, M.E. Anti-hiv-1 activity of indolicidin, an antimicrobial peptide from neutrophils. J. Leukoc. Biol. 1998, 63, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Yasin, B.; Wang, W.; Pang, M.; Cheshenko, N.; Hong, T.; Waring, A.J.; Herold, B.C.; Wagar, E.A.; Lehrer, R.I. Theta defensins protect cells from infection by herpes simplex virus by inhibiting viral adhesion and entry. J. Virol. 2004, 78, 5147–5156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersen, J.H.; Jenssen, H.; Sandvik, K.; Gutteberg, T.J. Anti-hsv activity of lactoferrin and lactoferricin is dependent on the presence of heparan sulphate at the cell surface. J. Med. Virol. 2004, 74, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Zapata, W.; Aguilar-Jiménez, W.; Feng, Z.; Weinberg, A.; Russo, A.; Potenza, N.; Estrada, H.; Rugeles, M.T. Identification of innate immune antiretroviral factors during in vivo and in vitro exposure to HIV-1. Microbes Infect. 2016, 18, 211–219. [Google Scholar] [CrossRef]
- Liu, X.; Guo, C.; Huang, Y.; Zhang, X.; Chen, Y. Inhibition of porcine reproductive and respiratory syndrome virus by Cecropin D in vitro. Infect. Genet. Evol. 2015, 34, 7–16. [Google Scholar] [CrossRef]
- Jiao, J.; Mao, R.; Teng, D.; Wang, X.; Hao, Y.; Yang, N.; Wang, X.; Feng, X.; Wang, J. In vitro and in vivo antibacterial effect of NZ2114 against Streptococcus suis type 2 infection in mice peritonitis models. AMB Express 2017, 7, 44. [Google Scholar] [CrossRef] [Green Version]
- Zhao, F.; Yang, N.; Wang, X.; Mao, R.; Hao, Y.; Li, Z.; Wang, X.; Teng, D.; Fan, H.; Wang, J. In vitro/vivo mechanism of action of MP1102 with low/nonresistance against Streptococcus suis type 2 strain CVCC 3928. Front. Cell. Infect. Microbiol. 2019, 9, 48. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Yang, N.; Mao, R.; Teng, D.; Hao, Y.; Wang, X.; Wang, J. A new high-yielding antimicrobial peptide NZX and its antibacterial activity against Staphylococcus hyicus in vitro/vivo. Appl. Microbiol. Biotechnol. 2020, 104, 1555–1568. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, F.; Huang, Z.; Liu, H.; Xie, C.; Zhang, J.; Thacker, P.A.; Qiao, S. Effects of the antimicrobial peptide cecropin AD on performance and intestinal health in weaned piglets challenged with Escherichia coli. Peptides 2012, 35, 225–230. [Google Scholar] [CrossRef]
- Pomeranz, L.E.; Reynolds, A.E.; Hengartner, C.J. Molecular biology of pseudorabies virus: Impact on neurovirology and veterinary medicine. Microbiol. Mol. Biol. Rev. 2005, 69, 462–500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pensaert, M.B.; de Bouck, P. A new coronavirus-like particle associated with diarrhea in swine. Arch. Virol. 1978, 58, 243–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silphaduang, U.; Noga, E.J. Peptide antibiotics in mast cells of fish. Nature 2001, 414, 268–269. [Google Scholar] [CrossRef] [PubMed]
- Noga, E.J.; Silphaduang, U. Piscidins: A novel family of peptide antibiotics from fish. Drug News Perspect. 2003, 16, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Shin, A.; Jeong, K.W.; Jin, B.; Jnawali, H.N.; Shin, S.; Shin, S.Y.; Kim, Y. Role of phenylalanine and valine10 residues in the antimicrobial activity and cytotoxicity of piscidin-1. PLoS ONE 2014, 9, e114453. [Google Scholar] [CrossRef] [Green Version]
- Chinchar, V.G.; Bryan, L.; Silphadaung, U.; Noga, E.; Wade, D.; Rollins-Smith, L. Inactivation of viruses infecting ectothermic animals by amphibian and piscine antimicrobial peptides. Virology 2004, 323, 268–275. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Watson, K.M.; Peterkofsky, A.; Buckheit, R.W., Jr. Identification of novel human immunodeficiency virus type 1-inhibitory peptides based on the antimicrobial peptide database. Antimicrob. Agents Chemother. 2010, 54, 1343–1346. [Google Scholar] [CrossRef] [Green Version]
- Lei, Z.; Liu, Q.; Zhu, Q.; Yang, B.; Khaliq, H.; Sun, A.; Qi, Y.; Moku, G.K.; Su, Y.; Wang, J.; et al. Comparative pharmacokinetics and preliminary pharmacodynamics evaluation of Piscidin 1 against PRV and PEDV in rats. Front. Chem. 2018, 6, 244. [Google Scholar] [CrossRef] [Green Version]
- Hu, H.; Guo, N.; Chen, S.; Guo, X.; Liu, X.; Ye, S.; Chai, Q.; Wang, Y.; Liu, B.; He, Q. Antiviral activity of Piscidin 1 against pseudorabies virus both in vitro and in vivo. Virol. J. 2019, 16, 95. [Google Scholar] [CrossRef] [Green Version]
- Wong, H.; Bowie, J.H.; Carver, J.A. The solution structure and activity of caerin 1.1, an antimicrobial peptide from the Australian green tree frog, Litoria splendida. Eur. J. Biochem. 1997, 247, 545–557. [Google Scholar] [CrossRef] [Green Version]
- Veldhuizen, E.J.; Rijnders, M.; Claassen, E.A.; van Dijk, A.; Haagsman, H.P. Porcine beta-defensin 2 displays broad antimicrobial activity against pathogenic intestinal bacteria. Mol. Immunol. 2008, 45, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Sang, Y.; Patil, A.A.; Zhang, G.; Ross, C.R.; Blecha, F. Bioinformatic and expression analysis of novel porcine beta-defensins. Mamm. Genome 2006, 17, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Hultmark, D.; Engström, A.; Bennich, H.; Kapur, R.; Boman, H.G. Insect immunity: Isolation and structure of cecropin D and four minor antibacterial components from Cecropia pupae. Eur. J. Biochem. 1982, 127, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Boman, A.; Sun, C.X.; Andersson, M.; Jörnvall, H.; Mutt, V.; Boman, H.G. Antibacterial peptides from pig intestine: Isolation of a mammalian cecropin. Proc. Natl. Acad. Sci. USA 1989, 86, 9159–9162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, C.; Huang, Y.; Cong, P.; Liu, X.; Chen, Y.; He, Z. Cecropin P1 inhibits porcine reproductive and respiratory syndrome virus by blocking attachment. BMC Microbiol. 2014, 14, 273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kokryakov, V.N.; Harwig, S.S.; Panyutich, E.A.; Shevchenko, A.A.; Aleshina, G.M.; Shamova, O.V.; Korneva, H.A.; Lehrer, R.I. Protegrins: Leukocyte antimicrobial peptides that combine features of corticostatic defensins and tachyplesins. FEBS Lett. 1993, 327, 231–236. [Google Scholar] [CrossRef] [Green Version]
- Sang, Y.; Ruchala, P.; Lehrer, R.I.; Ross, C.R.; Rowland, R.R.; Blecha, F. Antimicrobial host defense peptides in an arteriviral infection: Differential peptide expression and virus inactivation. Viral. Immunol. 2009, 22, 235–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, C.; Liu, L.; Lehrer, R.I. Identification of a new member of the protegrin family by cDNA cloning. FEBS Lett. 1994, 346, 285–288. [Google Scholar]
- Guo, C.; Cong, P.; He, Z.; Mo, D.; Zhang, W.; Chen, Y.; Liu, X. Inhibitory activity and molecular mechanism of protegrin-1 against porcine reproductive and respiratory syndrome virus in vitro. Antivir. Ther. 2015, 20, 573–582. [Google Scholar] [CrossRef] [Green Version]
- Wang, G. Structures of human host defense cathelicidin LL-37 and its smallest antimicrobial peptide KR-12 in lipid micelles. J. Biol. Chem. 2008, 283, 32637–32643. [Google Scholar] [CrossRef] [Green Version]
- Levast, B.; Hogan, D.; van Kessel, J.; Strom, S.; Walker, S.; Zhu, J.; Meurens, F.; Gerdts, V. Synthetic cationic peptide IDR-1002 and human cathelicidin LL37 modulate the cell innate response but differentially impact PRRSV replication in vitro. Front. Vet. Sci. 2019, 6, 233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, C.Y.; Chen, J.Y.; Cheng, Y.S.; Chen, C.Y.; Ni, I.H.; Sheen, J.F.; Pan, Y.L.; Kuo, C.M. Gene expression and localization of the epinecidin-1 antimicrobial peptide in the grouper (Epinephelus coioides), and its role in protecting fish against pathogenic infection. DNA Cell Biol. 2007, 26, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.N.; Pan, C.Y.; Chen, J.Y. Grouper (Epinephelus coioides) antimicrobial peptide epinecidin-1 exhibits antiviral activity against foot-and-mouth disease virus in vitro. Peptides 2018, 106, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Hernáez, B.; Tarragó, T.; Giralt, E.; Escribano, J.M.; Alonso, C. Small peptide inhibitors disrupt a high-affinity interaction between cytoplasmic dynein and a viral cargo protein. J. Virol. 2010, 84, 10792–10801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haney, E.F.; Hunter, H.N.; Matsuzaki, K.; Vogel, H.J. Solution NMR studies of amphibian antimicrobial peptides: Linking structure to function? Biochim. Biophys. Acta 2009, 1788, 1639–1655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neshani, A.; Zare, H.; Eidgahi, M.R.A.; Khaledi, A.; Ghazvini, K. Epinecidin-1, a highly potent marine antimicrobial peptide with anticancer and immunomodulatory activities. BMC Pharmacol. Toxicol. 2019, 20, 33. [Google Scholar] [CrossRef] [Green Version]
- Pukala, T.L.; Brinkworth, C.S.; Carver, J.A.; Bowie, J.H. Investigating the importance of the flexible hinge in caerin 1.1: Solution structures and activity of two synthetically modified caerin peptides. Biochemistry 2004, 43, 937–944. [Google Scholar] [CrossRef]
- Mechler, A.; Praporski, S.; Atmuri, K.; Boland, M.; Separovic, F.; Martin, L.L. Specific and selective peptide-membrane interactions revealed using quartz crystal microbalance. Biophys. J. 2007, 93, 3907–3916. [Google Scholar] [CrossRef] [Green Version]
- Guo, N.; Zhang, B.; Hu, H.; Ye, S.; Chen, F.; Li, Z.; Chen, P.; Wang, C.; He, Q. Caerin1.1 suppresses the growth of porcine epidemic diarrhea virus in vitro via direct binding to the virus. Viruses 2018, 10, 507. [Google Scholar] [CrossRef] [Green Version]
- Holly, M.K.; Diaz, K.; Smith, J.G. Defensins in viral infection and pathogenesis. Annu. Rev. Virol. 2017, 4, 369–391. [Google Scholar] [CrossRef]
- Selsted, M.E.; Ouellette, A.J. Mammalian defensins in the antimicrobial immune response. Nat. Immunol. 2005, 6, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Fellermann, K.; Stange, E.F. Defensins innate-immunity at the epithelial frontier. Eur. J. Gastroenterol. Hepatol. 2001, 13, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhong, F.; Zhang, Y.; Zhang, J.; Huo, S.; Lin, H.; Wang, L.; Cui, D.; Li, X. Construction of Bacillus subtilis strain engineered for expression of porcine β-defensin-2/cecropin P1 fusion antimicrobial peptides and its growth-promoting effect and antimicrobial activity. Asian Australas. J. Anim. Sci. 2017, 30, 576–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, F.; Zhang, H.; Xia, X.; Xiong, H.; Song, D.; Zong, X.; Wang, Y. Porcine β-defensin 2 attenuates inflammation and mucosal lesions in dextran sodium sulfate-induced colitis. J. Immunol. 2015, 194, 1882–1893. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Qi, Y.; Wang, A.; Huang, C.; Liu, X.; Yang, X.; Li, L.; Zhou, R. Porcine β-defensin 2 inhibits proliferation of pseudorabies virus in vitro and in transgenic mice. Virol. J. 2020, 17, 18. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Liu, S.; Li, C.; Yang, L.; Zu, Y. In vitro evaluation of the antiviral activity of the synthetic epigallocatechin gallate analog-epigallocatechin gallate (EGCG) palmitate against porcine reproductive and respiratory syndrome virus. Viruses 2014, 6, 938–950. [Google Scholar] [CrossRef] [PubMed]
- Neumann, E.J.; Kliebenstein, J.B.; Johnson, C.D.; Mabry, J.W.; Bush, E.J.; Seitzinger, A.H.; Green, A.L.; Zimmerman, J.J. Assessment of the economic impact of porcine reproductive and respiratory syndrome on swine production in the United States. J. Am. Vet. Med. Assoc. 2005, 227, 385–392. [Google Scholar] [CrossRef] [Green Version]
- Wensvoort, G.; Terpstra, C.; Pol, J.M.; ter Laak, E.A.; Bloemraad, M.; de Kluyver, E.P.; Kragten, C.; van Buiten, L.; den Besten, A.; Wagenaar, F. Mystery swine disease in The Netherlands: The isolation of Lelystad virus. Vet. Q. 1991, 13, 121–130. [Google Scholar] [CrossRef]
- Dokland, T. The structural biology of PRRSV. Virus Res. 2010, 154, 86–97. [Google Scholar] [CrossRef]
- Kuzemtseva, L.; de la Torre, E.; Martín, G.; Soldevila, F.; Ait-Ali, T.; Mateu, E.; Darwich, L. Regulation of toll-like receptors 3, 7 and 9 in porcine alveolar macrophages by different genotype 1 strains of porcine reproductive and respiratory syndrome virus. Vet. Immunol. Immunopathol. 2014, 158, 189–198. [Google Scholar] [CrossRef]
- Deaton, M.K.; Spear, A.; Faaberg, K.S.; Pegan, S.D. The vOTU domain of highly-pathogenic porcine reproductive and respiratory syndrome virus displays a differential substrate preference. Virology 2014, 454–455, 247–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thanawongnuwech, R.; Suradhat, S. Taming PRRSV: Revisiting the control strategies and vaccine design. Virus Res. 2010, 154, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xiao, S.; Gao, J.; Liu, M.; Zhang, X.; Li, M.; Zhao, G.; Mo, D.; Liu, X.; Chen, Y. Inhibition of replication of porcine reproductive and respiratory syndrome virus by hemin is highly dependent on heme oxygenase-1, but independent of iron in MARC-145 cells. Antivir. Res. 2014, 105, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Huang, Y.; Zheng, H.; Tang, L.; He, J.; Xiang, L.; Liu, D.; Jiang, H. Secretion and activity of antimicrobial peptide cecropin D expressed in Pichia pastoris. Exp. Ther. Med. 2012, 4, 1063–1068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steiner, H.; Hultmark, D.; Engström, A.; Bennich, H.; Boman, H.G. Sequence and specificity of two antibacterial proteins involved in insect immunity. Nature 1981, 292, 246–248. [Google Scholar] [CrossRef] [PubMed]
- Hultmark, D.; Steiner, H.; Rasmuson, T.; Boman, H.G. Insect immunity. Purification and properties of three inducible bactericidal proteins from hemolymph of immunized pupae of Hyalophora cecropia. Eur. J. Biochem. 1980, 106, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Andersson, M.; Boman, A.; Boman, H.G. Ascaris nematodes from pig and human make three antibacterial peptides: Isolation of cecropin P1 and two ASABF peptides. Cell. Mol. Life Sci. 2003, 60, 599–606. [Google Scholar] [CrossRef]
- Sipos, D.; Andersson, M.; Ehrenberg, A. The structure of the mammalian antibacterial peptide cecropin P1 in solution, determined by proton-NMR. Eur. J. Biochem. 1992, 209, 163–169. [Google Scholar] [CrossRef]
- Lee, I.H.; Cho, Y.; Lehrer, R.I. Effects of pH and salinity on the antimicrobial properties of clavanins. Infect. Immun. 1997, 65, 2898–2903. [Google Scholar] [CrossRef] [Green Version]
- Kandasamy, S.K.; Larson, R.G. Binding modes of protegrin-1, a beta-strand antimicrobial peptide, in lipid bilayers. Mol. Simul. 2007, 33, 799–807. [Google Scholar] [CrossRef]
- Vandamme, D.; Landuyt, B.; Luyten, W.; Schoofs, L. A comprehensive summary of LL-37, the factotum human cathelicidin peptide. Cell. Immunol. 2012, 280, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Sousa, F.H.; Casanova, V.; Findlay, F.; Stevens, C.; Svoboda, P.; Pohl, J.; Proudfoot, L.; Barlow, P.G. Cathelicidins display conserved direct antiviral activity towards rhinovirus. Peptides 2017, 95, 76–83. [Google Scholar] [CrossRef] [PubMed]
- James, A.D.; Rushton, J. The economics of foot and mouth disease. Rev. Sci. Tech. 2002, 21, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Wernery, U.; Kinne, J. Foot and mouth disease and similar virus infections in camelids: A review. Rev. Sci. Tech. 2012, 31, 907–918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sáiz, M.; Núñez, J.I.; Jimenez-Clavero, M.A.; Baranowski, E.; Sobrino, F. Foot-and-mouth disease virus: Biology and prospects for disease control. Microbes Infect. 2002, 4, 1183–1192. [Google Scholar] [CrossRef]
- Rweyemamu, M.; Roeder, P.; MacKay, D.; Sumption, K.; Brownlie, J.; Leforban, Y. Planning for the progressive control of foot-and-mouth disease worldwide. Transbound. Emerg. Dis. 2008, 55, 73–87. [Google Scholar] [CrossRef]
- Knight-Jones, T.J.; Rushton, J. The economic impacts of foot and mouth disease—What are they, how big are they and where do they occur? Prev. Vet. Med. 2013, 112, 161–173. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y. Adjuvants for foot-and-mouth disease virus vaccines: Recent progress. Expert Rev. Vaccines 2014, 13, 1377–1385. [Google Scholar] [CrossRef]
- Yin, Z.X.; He, W.; Chen, W.J.; Yan, J.H.; Yang, J.N.; Chan, S.M.; He, J.G. Cloning, expression and antimicrobial activity of an antimicrobial peptide, epinecidin-1, from the orange-spotted grouper, Epinephelus coioides. Aquaculture 2006, 253, 204–211. [Google Scholar] [CrossRef]
- Masso-Silva, J.A.; Diamond, G. Antimicrobial peptides from fish. Pharmaceuticals (Basel) 2014, 7, 265–310. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.C.; Pan, C.Y.; Chen, J.Y. The antimicrobial peptide, epinecidin-1, mediates secretion of cytokines in the immune response to bacterial infection in mice. Peptides 2012, 36, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.Y.; Chen, J.Y.; Lin, T.L.; Lin, C.H. In vitro activities of three synthetic peptides derived from epinecidin-1 and an anti-lipopolysaccharide factor against Propionibacterium acnes, Candida albicans, and Trichomonas vaginalis. Peptides 2009, 30, 1058–1068. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.D.; Kung, C.W.; Chi, S.C.; Chen, J.Y. Inactivation of nervous necrosis virus infecting grouper (Epinephelus coioides) by epinecidin-1 and hepcidin 1–5 antimicrobial peptides, and downregulation of M × 2 and M × 3 gene expressions. Fish Shellfish Immunol. 2010, 28, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Galindo, I.; Alonso, C. African Swine Fever Virus: A Review. Viruses 2017, 9, 103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazur-Panasiuk, N.; Woźniakowski, G.; Niemczuk, K. The first complete genomic sequences of African swine fever virus isolated in Poland. Sci. Rep. 2019, 9, 4556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, E.C.; Hutchings, G.H.; Mukarati, N.; Wilkinson, P.J. African swine fever virus infection of the bushpig (Potamochoerus porcus) and its significance in the epidemiology of the disease. Vet. Microbiol. 1998, 62, 1–15. [Google Scholar] [CrossRef]
- Alonso, C.; Miskin, J.; Hernáez, B.; Fernandez-Zapatero, P.; Soto, L.; Cantó, C.; Rodríguez-Crespo, I.; Dixon, L.; Escribano, J.M. African swine fever virus protein p54 interacts with the microtubular motor complex through direct binding to light-chain dynein. J. Virol. 2001, 75, 9819–9827. [Google Scholar] [CrossRef] [Green Version]
- Hernaez, B.; Alonso, C. Dynamin- and clathrin-dependent endocytosis in African swine fever virus entry. J. Virol. 2010, 84, 2100–2109. [Google Scholar] [CrossRef] [Green Version]
- Döhner, K.; Gel, C.H.; Sodeik, B. Viral stop-and-go along microtubules: Taking a ride with dynein and kinesins. Trends Microbiol. 2005, 13, 320–327. [Google Scholar] [CrossRef]
- Raux, H.; Flamand, A.; Blondel, D. Interaction of the rabies virus P protein with the LC8 dynein light chain. J. Virol. 2000, 74, 10212–10216. [Google Scholar] [CrossRef] [Green Version]
- Jacob, Y.; Badrane, H.; Ceccaldi, P.E.; Tordo, N. Cytoplasmic dynein LC8 interacts with lyssavirus phosphoprotein. J. Virol. 2000, 74, 10217–10222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.G.; Jiang, L.; Liu, L.L.; Sun, L.; Zhao, W.G.; Chen, Y.Q.; Qi, T.M.; Han, Z.X.; Shao, Y.H.; Liu, S.W.; et al. Induction of Avian β-defensin 2 is possibly mediated by the p38 MAPK signal pathway in chicken embryo fibroblasts after newcastle disease virus infection. Front. Microbiol. 2018, 9, 751. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Lin, L.; Zhang, K.; Han, Z.X.; Shao, Y.H.; Liu, X.L.; Liu, S.W. Three novel Anas platyrhynchos avian β-defensins, upregulated by duck hepatitis virus, with antibacterial and antiviral activities. Mol. Immunol. 2011, 49, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, D.S.; Kovacs, N.J.; Snider, M.; Babiuk, L.A.; Hurk, S. Inclusion of the bovine neutrophil beta-defensin 3 with glycoprotein D of bovine herpesvirus 1 in a DNA vaccine modulates immune responses of mice and cattle. Clin. Vaccine Immunol. 2014, 21, 463–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monteiro, J.M.C.; Oliveira, M.D.; Dias, R.S.; Marcal, L.N.; Feio, R.N.; Ferreira, S.O.; Oliveira, L.L.; Silva, C.C.; Paula, S.O. The antimicrobial peptide HS-1 inhibits dengue virus infection. Virology 2018, 514, 79–87. [Google Scholar] [CrossRef]
- Boas, L.C.P.V.; Campos, M.L.; Berlanda, R.L.A.; Neves, N.D.C.; Franco, O.L. Antiviral peptides as promising therapeutic drugs. Cell. Mol. Life Sci. CMLS 2019, 76, 3525–3542. [Google Scholar] [CrossRef] [PubMed]
- Rai, A.; Pinto, S.; Velho, T.; Ferreira, A.; Moita, C.; Trivedi, U.; Evangelista, M.; Comune, M.; Rumbaugh, K.P.; Simoes, P.N.; et al. One-step synthesis of high-density peptide-conjugated gold nanoparticles with antimicrobial efficacy in a systemic infection model. Biomaterials 2016, 85, 99–110. [Google Scholar] [CrossRef]
- Water, J.J.; Smart, S.; Franzyk, H.; Foged, C.; Nielsen, H.M. Nanoparticle-mediated delivery of the antimicrobial peptide plectasin against Staphylococcus aureus in infected epithelial cells. Eur. J. Pharm. Biopharm. 2015, 92, 65–73. [Google Scholar] [CrossRef]
- Chen, H.X.; Mao, R.Y.; Teng, D.; Wang, X.M.; Hao, Y.; Feng, X.J.; Wang, J.H. Design and pharmacodynamics of recombinant NZ2114 histidine mutants with improved activity against methicillin-resistant Staphylococcus aureus. AMB Express 2017, 7, 46. [Google Scholar] [CrossRef]
- Silva, T.; Silva, T.; Magalhães, B.; Maia, S.; Gomes, P.; Gomes, M.S. Killing of Mycobacterium avium by lactoferricin peptides: Improved activity of arginine-and D-amino-acid-containing molecules. Antimicrob. Agents Chemother. 2014, 58, 3461–3467. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, L.T.; Chau, J.K.; Perry, N.A.; Boer, L.; Zaat, S.A.J.; Vogel, H.J. Serum stabilities of short tryptophan-and arginine-rich antimicrobial peptide analogs. PLoS ONE 2010, 5, e12684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, L.Y.; Zhang, V.M.; Huang, Y.H.; Waters, N.C.; Bansal, P.S.; Craik, D.J.; Daly, N.L. Cyclization of the antimicrobial peptide gomesin with native chemical ligation: Influences on stability and bioactivity. ChemBioChem 2013, 14, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Mao, R.Y.; Teng, D.; Wang, X.M.; Xi, D.; Zhang, Y.; Hu, X.Y.; Yang, Y.L.; Wang, J.H. Design, expression, and characterization of a novel targeted plectasin against methicillin-resistant Staphylococcus aureus. Appl. Microbiol. Biotechnol. 2013, 97, 3991–4002. [Google Scholar] [CrossRef] [PubMed]
- Kuriakose, J.; Hernandez, G.V.; Nepal, M.; Brezden, A.; Pozzi, V.; Seleem, M.N.; Chmielewski, J. Targeting intracellular pathogenic bacteria with unnatural proline-rich peptides: Coupling antibacterial activity with macrophage penetration. Angew. Chem. 2013, 125, 9846–9849. [Google Scholar] [CrossRef]
- Wang, X.; Teng, D.; Wang, X.M.; Hao, Y.; Chen, H.X.; Mao, R.Y.; Wang, J.H. Internalization, distribution, and activity of peptide H2 against the intracellular multidrug-resistant bovine mastitis-causing bacterium Staphylococcus aureus. Sci. Rep. 2019, 9, 7968. [Google Scholar] [CrossRef]
- Li, Z.; Teng, D.; Mao, R.Y.; Wang, X.; Hao, Y.; Wang, X.M.; Wang, J.H. Improved antibacterial activity of the marine peptide N6 against intracellular Salmonella typhimurium by conjugating with the cell-penetrating peptide Tat11 via a cleavable linker. J. Med. Chem. 2018, 61, 7991–8000. [Google Scholar] [CrossRef]
- Gomarasca, M.; Martins, T.F.C.; Greune, L.; Hardwidge, P.R.; Rüter, C. Bacterium-derived cell-penetrating peptides deliver gentamicin to kill intracellular pathogens. Antimicrob. Agents Chemother. 2014, 61, e02545-16. [Google Scholar] [CrossRef] [Green Version]
- Richard, J.P.; Melikov, K.; Brooks, H.; Prevot, P.; Lebleu, B.; Chernomordik, L.V. Cellular uptake of unconjugated TAT peptide involves clathrin-dependent endocytosis and heparan sulfate receptors. J. Biol. Chem. 2005, 280, 15300–15306. [Google Scholar] [CrossRef] [Green Version]
- Yang, N.; Teng, D.; Mao, R.Y.; Hao, Y.; Wang, X.; Wang, Z.L.; Wang, X.M.; Wang, J.H. A recombinant fungal defensin-like peptide-P2 combats multidrug-resistant Staphylococcus aureus and biofilms. Appl. Microbiol. Biotechnol. 2019, 103, 5193–5213. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.M.; Teng, D.; Mao, R.Y.; Hao, Y.; Yang, N.; Li, Z.; Wang, J.H. Increased intracellular activity of MP1102 and NZ2114 against Staphylococcus aureus in vitro and in vivo. Sci. Rep. 2018, 8, 4204. [Google Scholar] [CrossRef]
- Li, Z.; Wang, X.; Teng, D.; Mao, R.Y.; Hao, Y.; Yang, N.; Chen, H.X.; Wang, X.M.; Wang, J.H. Improved antibacterial activity of a marine peptide-N2 against intracellular Salmonella typhimurium by conjugating with cell-penetrating peptides-bLFcin6/Tat11. Eur. J. Med. Chem. 2017, 145, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.L.; Liu, X.H.; Teng, D.; Mao, R.Y.; Hao, Y.; Yang, N.; Wang, X.; Li, Z.Z.; Wang, X.M.; Wang, J.H. Development of chimeric peptides to facilitate the neutralisation of lipopolysaccharides during bactericidal targeting of multidrug-resistant Escherichia coli. Commun. Biol. 2020, 3, 41. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.J. The Research and Application of New Alternatives to Antibiotics (ATA) for Feed Usage; Chinese Academy of Agricultural Sciences (CAAS) Annual Report (2017); China Agricultural Science and Technology Press: Beijing, China, 2018; p. 29. ISBN 978-7-5116-3602-7. [Google Scholar]
- Zhang, Y.; Teng, D.; Mao, R.Y.; Wang, X.M.; Xi, D.; Hu, X.Y.; Wang, J.H. High expression of a plectasin-derived peptide NZ2114 in Pichia pastoris and its pharmacodynamics, postantibiotic and synergy against Staphylococcus aureus. Appl. Microbiol. Biotechnol. 2014, 98, 681–694. [Google Scholar] [CrossRef] [PubMed]
- Czaplewski, L.; Bax, R.; Clokie, M.; Dawson, M.; Fairhead, H.; Fischetti, V.A.; Foster, S.; Gilmore, B.F.; Hancock, R.E.W.; Harper, D.; et al. Alternatives to antibiotics—A pipeline portfolio review. Lancet Infect. Dis. 2016, 16, 239–251. [Google Scholar] [CrossRef] [Green Version]

| Peptide | Sequence | No. of Amino Acids | Virus | Secondary Structure | Reference |
|---|---|---|---|---|---|
| Piscidin-1 | FFHHIFRGIVHVGKTIHRLVTG | 22 | PRV, PEDV, PRRSV, TGEV, RV | α-Helix | [35,39,40] |
| Caerin 1.1 | GLLSVLGSVAKHVLPHVVPVIAEHL | 25 | PRV, PEDV, PRRSV, TGEV, RV | α-Helix | [40,41] |
| pBD-2 | DHYICAKKGGTCNFSPCPLFNRIEGTCYSGKAKCCIR | 37 | PRV, PRRSV | Combine helix and beta structure | [42] |
| pBD-3 | RYYCKIRRGRCAVLGCLPKEEQIGSCSVSGRKCCRKRK | 38 | PRRSV | Combine helix and beta structure | [43] |
| Cecropin D | WNPFKELEKVGQRVRDAVISAGPAVATVAQATALAK | 36 | PRRSV | α-Helix | [27,44] |
| Cecropin P1 | SWLSKTAKKLENSAKKRISEGIAIAIQGGPR | 31 | PRRSV | α-Helix | [45,46] |
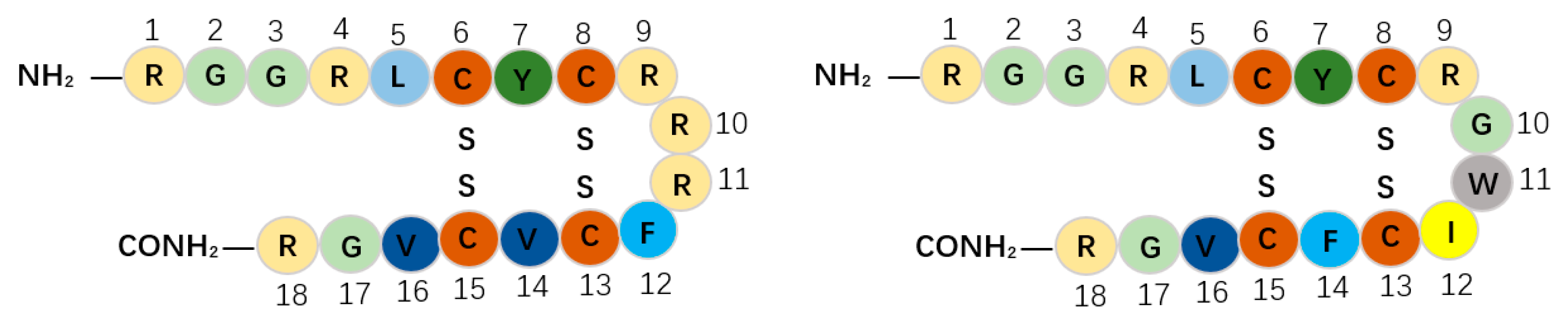
| Protegrin-1 | RGGRLCYCRRRFCVCVGR | 18 | PRRSV | β-Strand | [47,48] |
| Protegrin-4 | RGGRLCYCRGWICFCVGR | 18 | PRRSV | β-Strand | [49,50] |
| LL-37 | LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES | 37 | PRRSV | α-Helix | [51,52] |
| Epinecidin-1 | GFIFHIIKGLFHAGKMIHGLV | 21 | FMDV | α-Helix | [53,54] |
| DNBLK1 | RRRRRRRRHPAEPGSTVTTQNTASQTMS | 28 | ASFV | -- | [55] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pen, G.; Yang, N.; Teng, D.; Mao, R.; Hao, Y.; Wang, J. A Review on the Use of Antimicrobial Peptides to Combat Porcine Viruses. Antibiotics 2020, 9, 801. https://doi.org/10.3390/antibiotics9110801
Pen G, Yang N, Teng D, Mao R, Hao Y, Wang J. A Review on the Use of Antimicrobial Peptides to Combat Porcine Viruses. Antibiotics. 2020; 9(11):801. https://doi.org/10.3390/antibiotics9110801
Chicago/Turabian StylePen, Guihong, Na Yang, Da Teng, Ruoyu Mao, Ya Hao, and Jianhua Wang. 2020. "A Review on the Use of Antimicrobial Peptides to Combat Porcine Viruses" Antibiotics 9, no. 11: 801. https://doi.org/10.3390/antibiotics9110801
APA StylePen, G., Yang, N., Teng, D., Mao, R., Hao, Y., & Wang, J. (2020). A Review on the Use of Antimicrobial Peptides to Combat Porcine Viruses. Antibiotics, 9(11), 801. https://doi.org/10.3390/antibiotics9110801







