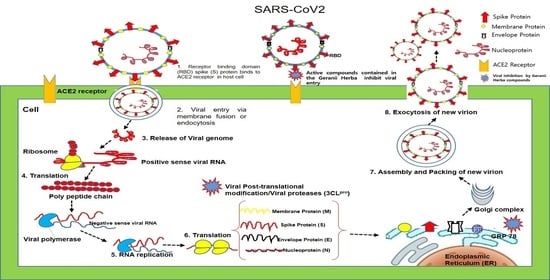
Geranii Herba as a Potential Inhibitor of SARS-CoV-2 Main 3CLpro, Spike RBD, and Regulation of Unfolded Protein Response: An In Silico Approach
Abstract
:1. Introduction
2. Results and Discussion
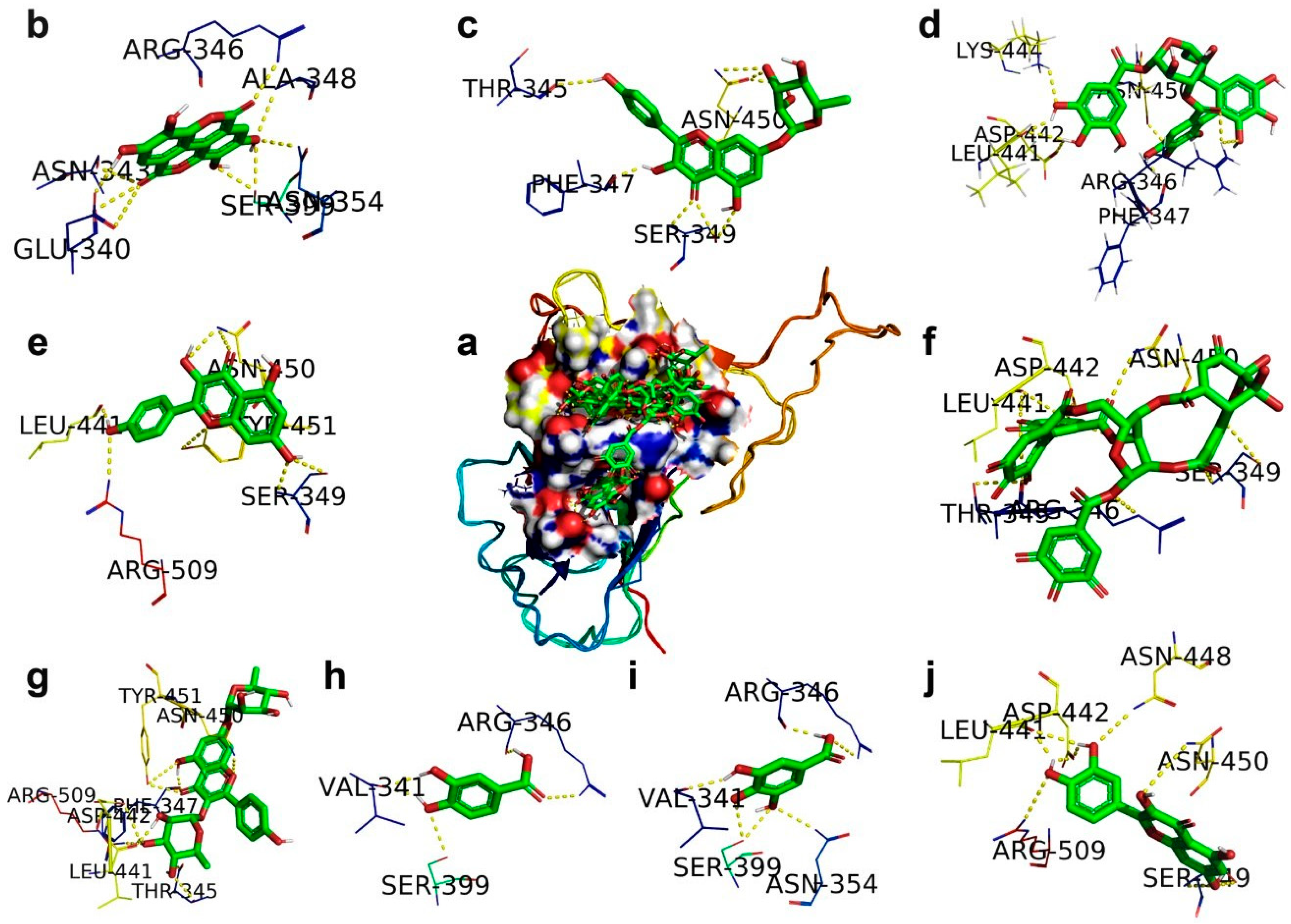
2.1. SARS-CoV-2 Spike RBD
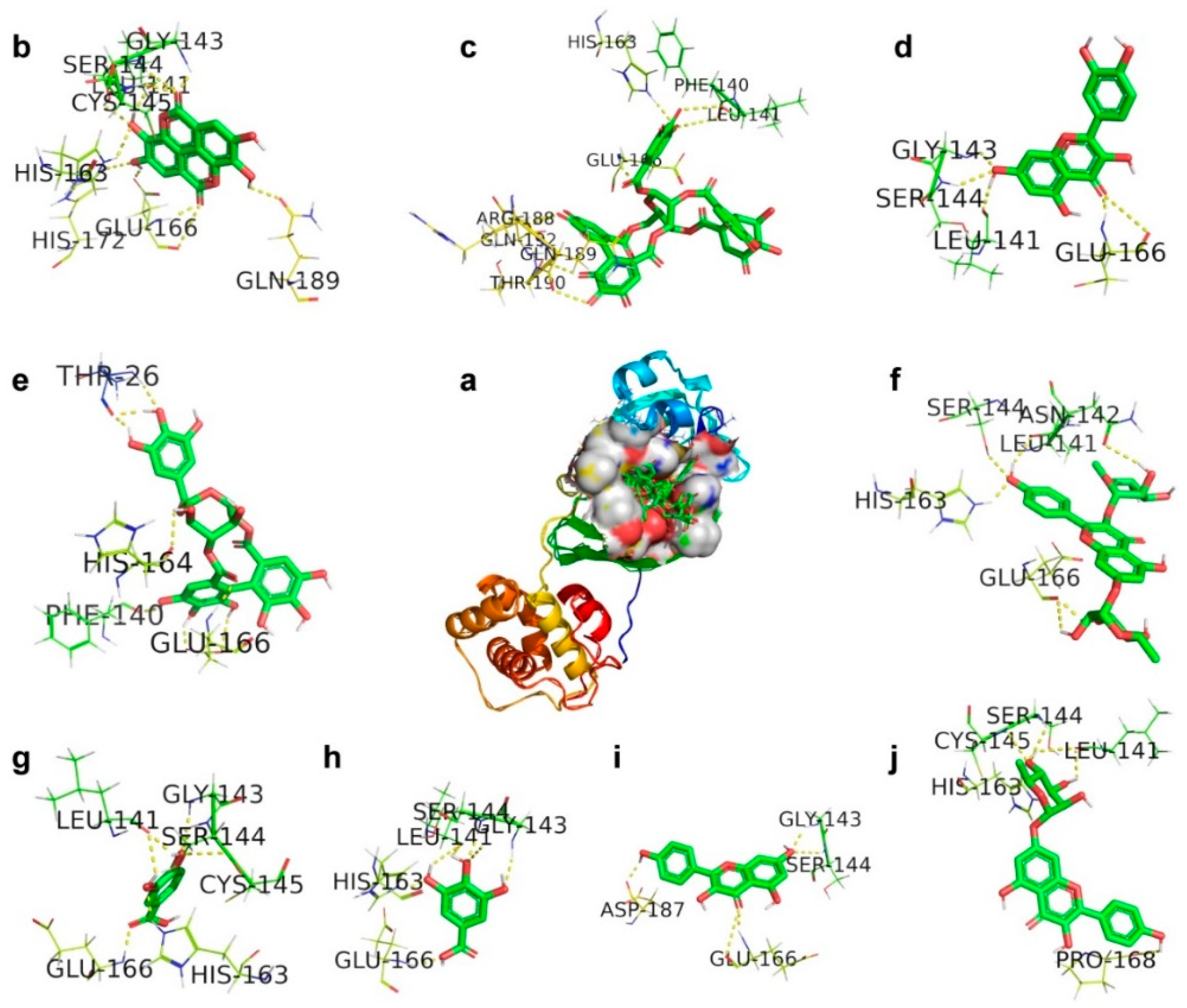
2.2. CLpro, Main Protease
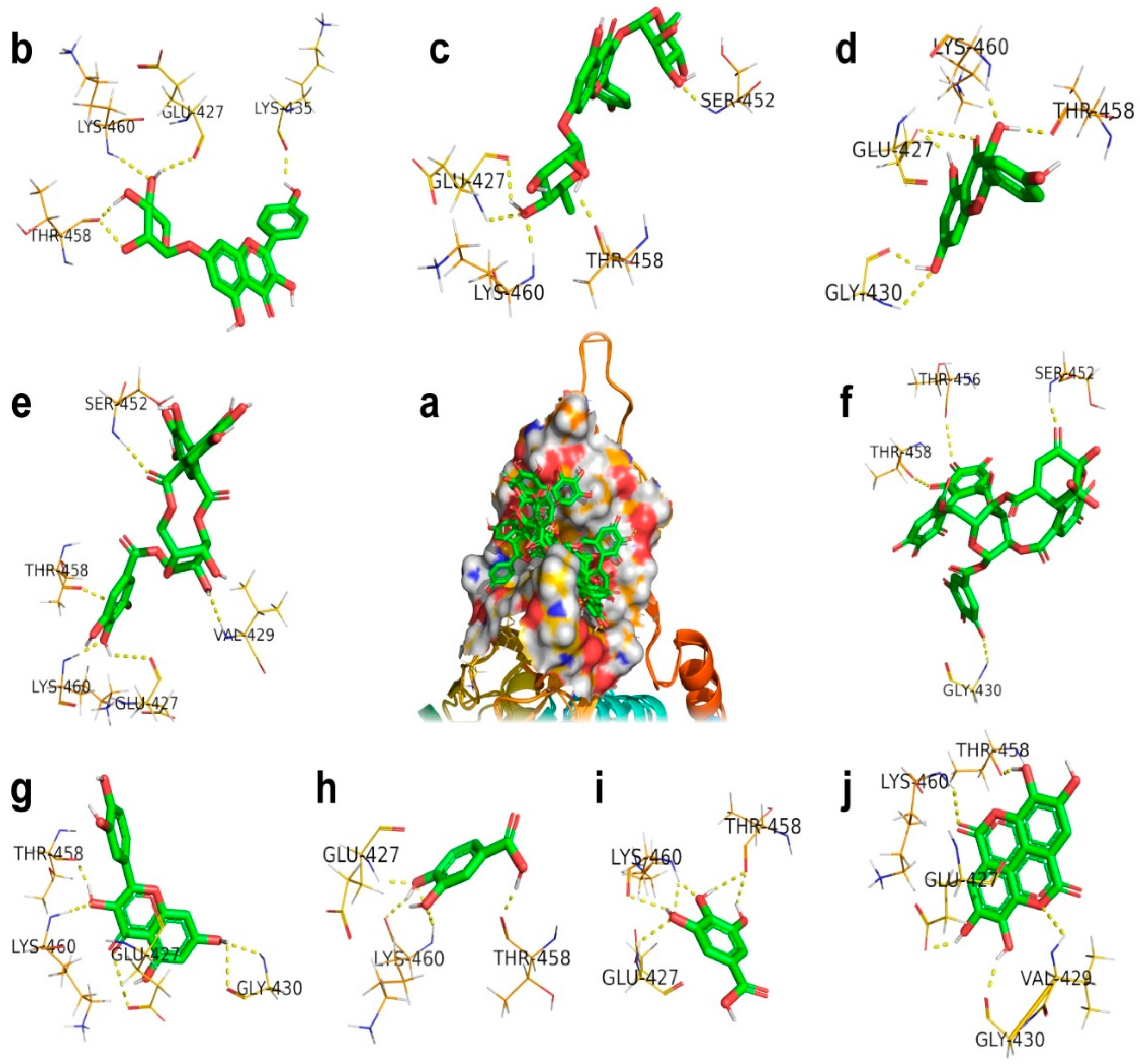
2.3. Glucose-Regulated Protein 78 (GRP78)
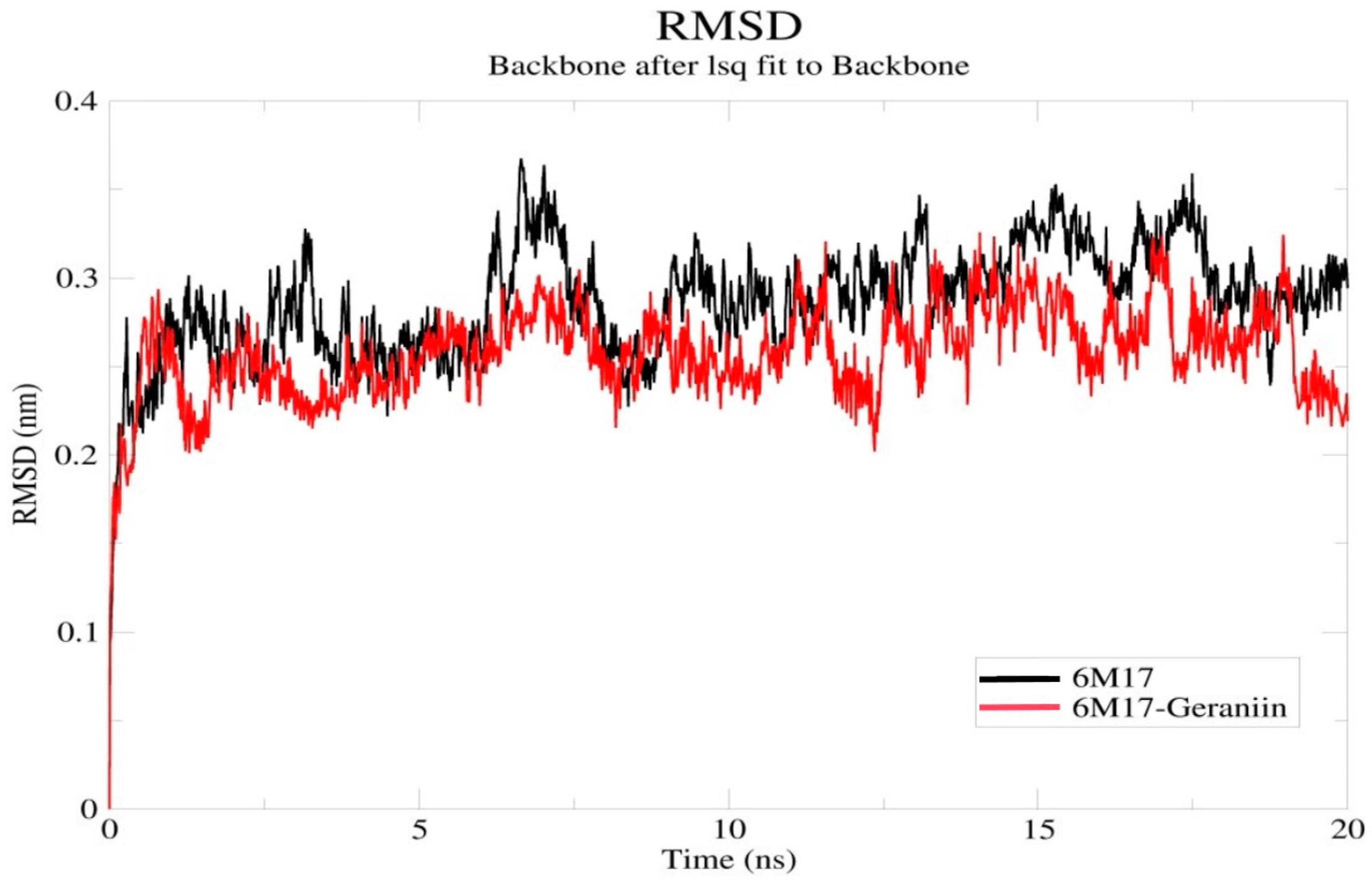
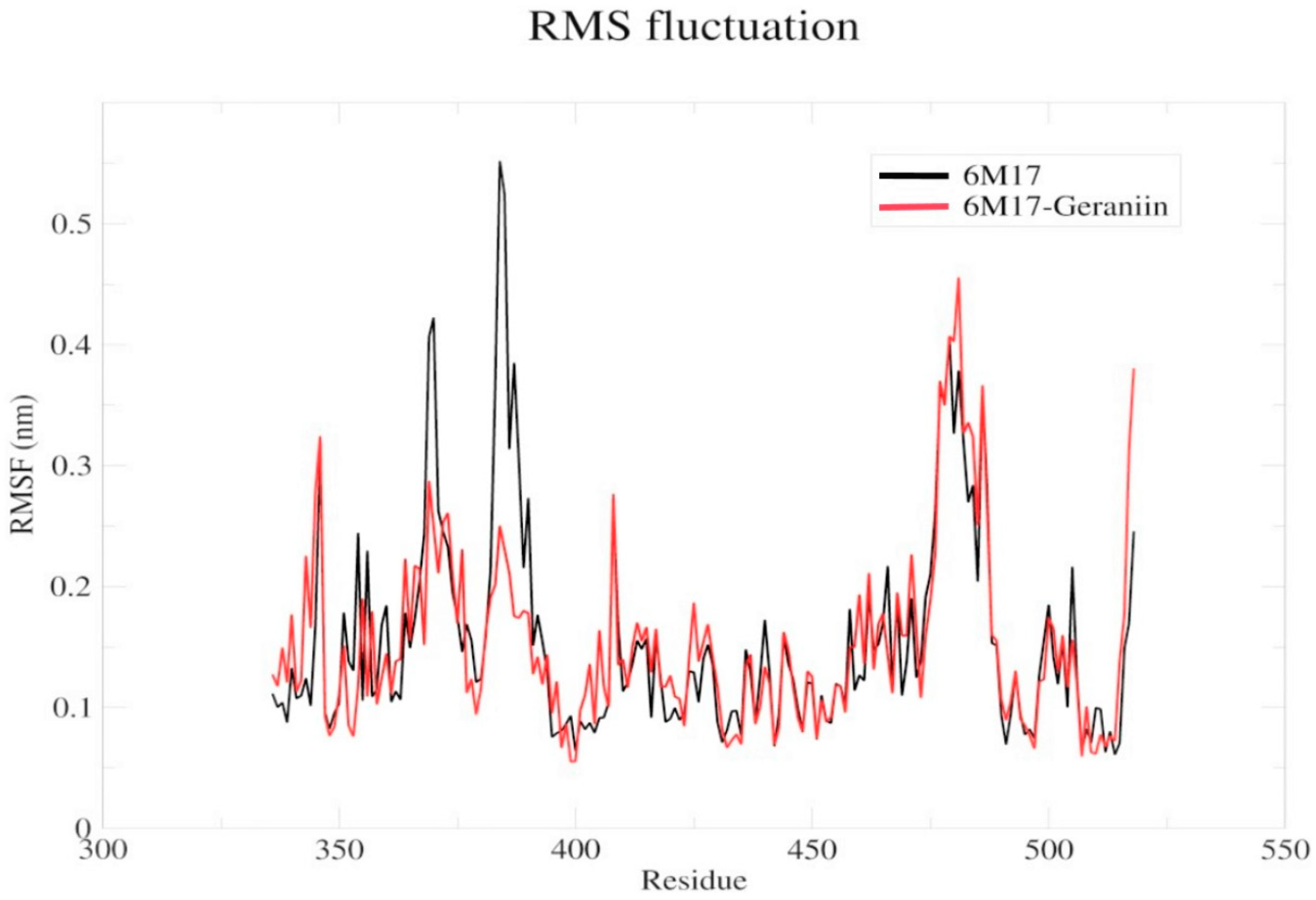
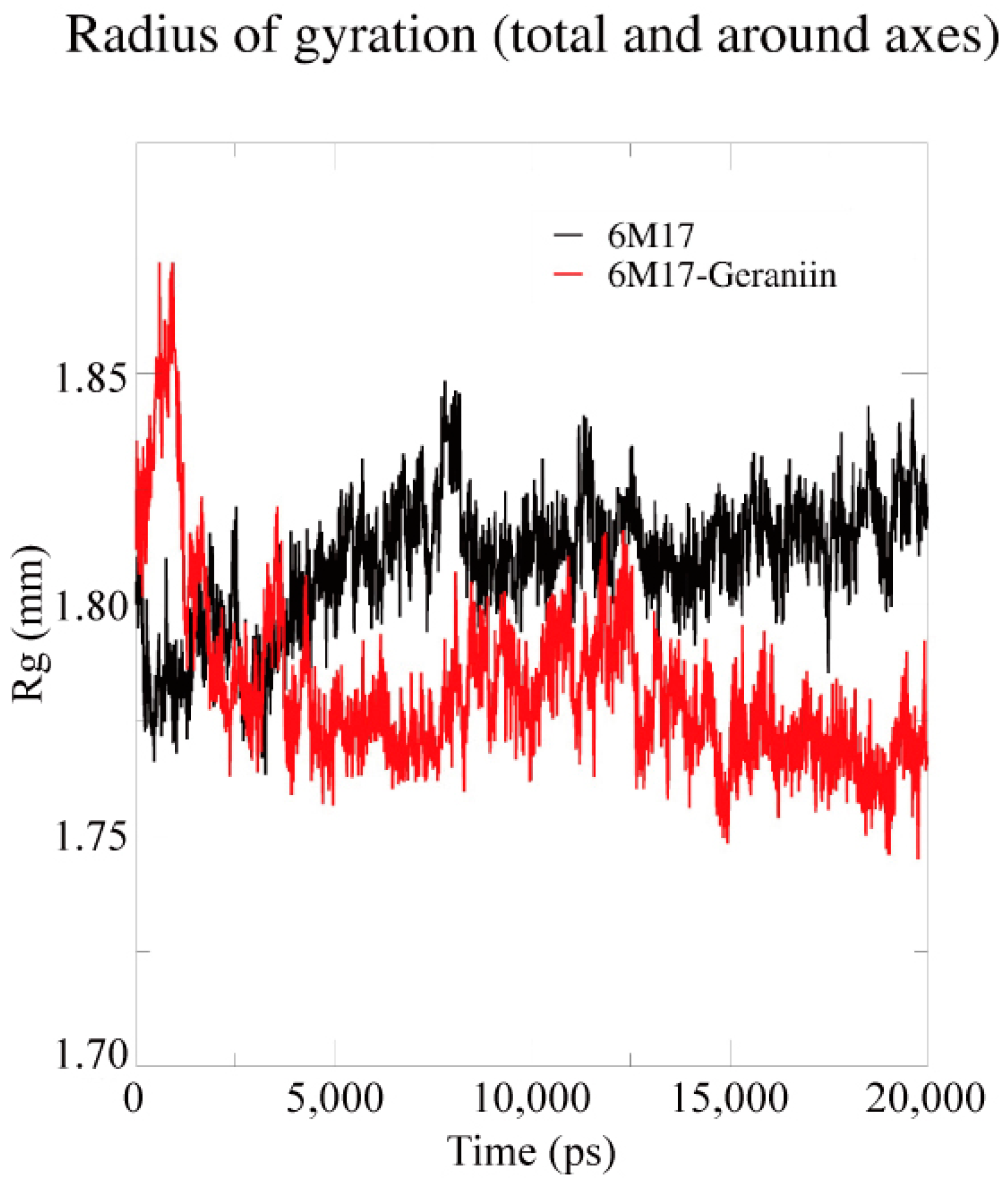
2.4. Molecular Dynamics Simulation
3. Materials and Methods
3.1. Ligand and Protein Preparation
3.2. Molecular Docking
3.3. Molecular Dynamics Simulation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hui, D.S.; Azhar, E.I.; Madani, T.A.; Ntoumi, F.; Kock, R.; Dar, O.; Ippolito, G.; McHugh, T.D.; Memish, Z.A.; Drosten, C.; et al. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health—The latest 2019 novel coronavirus outbreak in Wuhan, China. Int. J. Infect. Dis. 2020, 91, 264–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, R.; Zhao, X.; Li, J.; Niu, P.; Yang, B.; Wu, H.; Wang, W.; Song, H.; Huang, B.; Zhu, N.; et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet 2020, 395, 565–574. [Google Scholar] [CrossRef] [Green Version]
- Ren, L.L.; Wang, Y.M.; Wu, Z.Q.; Xiang, Z.C.; Guo, L.; Xu, T.; Jiang, Y.Z.; Xiong, Y.; Li, Y.J.; Li, X.W.; et al. Identification of a novel coronavirus causing severe pneumonia in human: A descriptive study. Chin. Med. J. (Engl.) 2020, 133, 1015–1024. [Google Scholar] [CrossRef] [PubMed]
- Elfiky, A.A. Anti-HCV, nucleotide inhibitors, repurposing against COVID-19. Life Sci. 2020, 248, 117477. [Google Scholar] [CrossRef] [PubMed]
- Elfiky, A.A.; Mahdy, S.M.; Elshemey, W.M. Quantitative structure-activity relationship and molecular docking revealed a potency of anti-hepatitis C virus drugs against human corona viruses. J. Med. Virol. 2017, 89, 1040–1047. [Google Scholar] [CrossRef] [Green Version]
- Magrone, T.; Magrone, M.; Jirillo, E. Focus on Receptors for Coronaviruses with Special Reference to Angiotensin-converting Enzyme 2 as a Potential Drug Target—A Perspective. Endocr. Metab. Immune Disord. Drug Targets 2020. [Google Scholar] [CrossRef]
- Chamsi-Pasha, M.A.; Shao, Z.; Tang, W.H. Angiotensin-converting enzyme 2 as a therapeutic target for heart failure. Curr. Heart Fail. Rep. 2014, 11, 58–63. [Google Scholar] [CrossRef]
- Xia, S.; Zhu, Y.; Liu, M.; Lan, Q.; Xu, W.; Wu, Y.; Ying, T.; Liu, S.; Shi, Z.; Jiang, S.; et al. Fusion mechanism of 2019-nCoV and fusion inhibitors targeting HR1 domain in spike protein. Cell Mol. Immunol. 2020, 17, 765–767. [Google Scholar] [CrossRef]
- Moreira, R.A.; Chwastyk, M.; Baker, J.L.; Guzman, H.V.; Poma, A.B. Quantitativ edetermination of mechanical stability in the novel coronavirus spike protein. Nanoscale 2020, 12, 16409–16413. [Google Scholar] [CrossRef]
- Armijos-Jaramillo, V.; Yeager, J.; Muslin, C.; Perez-Castillo, Y. SARS-CoV-2, an evolutionary perspective of interaction with human ACE2 reveals undiscovered amino acids necessary for complex stability. Evol. Appl. 2020, 13, 2168–2178. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Xiao, G.; Chen, Y.; He, Y.; Niu, J.; Escalante, C.R.; Xiong, H.; Farmar, J.; Debnath, A.K.; Tien, P.; et al. Interaction between heptad repeat 1 and 2 regions in spike protein of SARS-associated coronavirus: Implications for virus fusogenic mechanism and identification of fusion inhibitors. Lancet 2004, 363, 938–947. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.W.; Yiu, C.B.; Wong, K.Y. Prediction of the SARS-CoV-2 (2019-nCoV) 3C-like protease (3CL (pro)) structure: Virtual screening reveals velpatasvir, ledipasvir, and other drug repurposing candidates. F1000Res 2020, 9, 129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; He, S.; Deng, W.; Zhang, Y.; Li, G.; Sun, J.; Zhao, W.; Guo, Y.; Yin, Z.; Li, D.; et al. Comprehensive Insights into the Catalytic Mechanism of Middle East Respiratory Syndrome 3C-Like Protease and Severe Acute Respiratory Syndrome 3C-Like Protease. ACS Catal. 2020, 10, 5871–5890. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Hu, S.; Gao, J. Discovering drugs to treat coronavirus disease 2019 (COVID-19). Drug Discov. Ther. 2020, 14, 58–60. [Google Scholar] [CrossRef] [Green Version]
- Wu, R.; Wang, L.; Kuo, H.D.; Shannar, A.; Peter, R.; Chou, P.J.; Li, S.; Hudlikar, R.; Liu, X.; Liu, Z.; et al. An Update on Current Therapeutic Drugs Treating COVID-19. Curr. Pharmacol. Rep. 2020, 1–15. [Google Scholar] [CrossRef]
- Khaerunnisa, S.K.; Awaluddin, R.; Suhartati, S.; Soetjipto, S. Potential Inhibitor of COVID-19 Main Protease (Mpro) From Several Medicinal Plant Compounds by Molecular Docking Study. Preprints 2020, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Aanouz, I.; Belhassan, A.; El-Khatabi, K.; Lakhlifi, T.; El-Ldrissi, M.; Bouachrine, M. Moroccan Medicinal plants as inhibitors against SARS-CoV-2 main protease: Computational investigations. J. Biomol. Struct. Dyn. 2020, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Gurung, A.B.; Ali, M.A.; Lee, J.; Farah, M.A.; Al-Anazi, K.M. Unravelling lead antiviral phytochemicals for the inhibition of SARS-CoV-2 M(pro) enzyme through in silico approach. Life Sci. 2020, 255, 117831. [Google Scholar] [CrossRef]
- Ul Qamar, M.T.; Alqahtani, S.M.; Alamri, M.A.; Chen, L.L. Structural basis of SARS-CoV-2 3CL(pro) and anti-COVID-19 drug discovery from medicinal plants. J. Pharm. Anal. 2020. [Google Scholar] [CrossRef]
- Scalbert, A.; Manach, C.; Morand, C.; Remesy, C.; Jimenez, L. Dietary polyphenols and the prevention of diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. [Google Scholar] [CrossRef]
- Su, X.; D’Souza, D.H. Naturally occurring flavonoids against human norovirus surrogates. Food Environ. Virol. 2013, 5, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, S.; Faris, A.N.; Comstock, A.T.; Wang, Q.; Nanua, S.; Hershenson, M.B.; Sajjan, U.S. Quercetin inhibits rhinovirus replication in vitro and in vivo. Antivir. Res. 2012, 94, 258–271. [Google Scholar] [CrossRef] [PubMed]
- Sithisarn, P.; Michaelis, M.; Schubert-Zsilavecz, M.; Cinatl, J., Jr. Differential antiviral and anti-inflammatory mechanisms of the flavonoids biochanin A and baicalein in H5N1 influenza A virus-infected cells. Antivir. Res. 2013, 97, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.G.; Kim, Y.S.; Kim, J.H.; Chung, H.S. Antiviral activity of ethanol extract of Geranii Herba and its components against influenza viruses via neuraminidase inhibition. Sci. Rep. 2019, 9, 12132. [Google Scholar] [CrossRef] [PubMed]
- Fanunza, E.; Iampietro, M.; Distinto, S.; Corona, A.; Quartu, M.; Maccioni, E.; Horvat, B.; Tramontano, E. Quercetin Blocks Ebola Virus Infection by Counteracting the VP24 Interferon-Inhibitory Function. Antimicrob. Agents Chemother. 2020, 64. [Google Scholar] [CrossRef] [PubMed]
- Falsaperla, M.; Morgia, G.; Tartarone, A.; Ardito, R.; Romano, G. Support ellagic acid therapy in patients with hormone refractory prostate cancer (HRPC) on standard chemotherapy using vinorelbine and estramustine phosphate. Eur. Urol. 2005, 47, 449–454, discussion 454–455. [Google Scholar] [CrossRef]
- Senathilake, K.S.; Tennekoon, K. Virtual Screening of Inhibitors Against Spike Glycoprotein of 2019 Novel Corona Virus: A Drug Repurposing Approach. Preprints 2020, 2020030042. [Google Scholar] [CrossRef]
- Anand, K.; Palm, G.J.; Mesters, J.R.; Siddell, S.G.; Ziebuhr, J.; Hilgenfeld, R. Structure of coronavirus main proteinase reveals combination of a chymotrypsin fold with an extra alpha-helical domain. EMBO J. 2002, 21, 3213–3224. [Google Scholar] [CrossRef]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V.; Hayashi, Y.; Jung, S.H. An Overview of Severe Acute Respiratory Syndrome-Coronavirus (SARS-CoV) 3CL Protease Inhibitors: Peptidomimetics and Small Molecule Chemotherapy. J. Med. Chem. 2016, 59, 6595–6628. [Google Scholar] [CrossRef]
- Zhang, X.W.; Yap, Y.L. Old drugs as lead compounds for a new disease? Binding analysis of SARS coronavirus main proteinase with HIV, psychotic and parasite drugs. Bioorg. Med. Chem. 2004, 12, 2517–2521. [Google Scholar] [CrossRef]
- Wang, F.; Chen, C.; Tan, W.; Yang, K.; Yang, H. Structure of Main Protease from Human Coronavirus NL63: Insights for Wide Spectrum Anti-Coronavirus Drug Design. Sci. Rep. 2016, 6, 22677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.Q.; Meng, S.; Li, Z.R.; Peng, Z.G.; Han, Y.X.; Guo, S.S.; Cui, X.L.; Li, Y.H.; Jiang, J.D. The antiviral effect of 7-hydroxyisoflavone against Enterovirus 71 in vitro. J. Asian Nat. Prod. Res. 2013, 15, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, G.; Misra, U.K.; Gawdi, G.; Cianciolo, G.; Pizzo, S.V. Inducible expression of the alpha2-macroglobulin signaling receptor in response to antigenic stimulation: A study of second messenger generation. J. Cell Biochem. 2001, 82, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Chen, X.; Hendershot, L.; Prywes, R. ER stress regulation of ATF6 localization by dissociation of BiP/GRP78 binding and unmasking of Golgi localization signals. Dev. Cell 2002, 3, 99–111. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, I.M.; Abdelmalek, D.H.; Elfiky, A.A. GRP78: A cell’s response to stress. Life Sci. 2019, 226, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Lillo, A.M.; Steiniger, S.C.; Liu, Y.; Ballatore, C.; Anichini, A.; Mortarini, R.; Kaufmann, G.F.; Zhou, B.; Felding-Habermann, B.; et al. Targeting heat shock proteins on cancer cells: Selection, characterization, and cell-penetrating properties of a peptidic GRP78 ligand. Biochemistry 2006, 45, 9434–9444. [Google Scholar] [CrossRef]
- Yang, J.; Nune, M.; Zong, Y.; Zhou, L.; Liu, Q. Close and Allosteric Opening of the Polypeptide-Binding Site in a Human Hsp70 Chaperone BiP. Structure 2015, 23, 2191–2203. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, L.T.; Lee, Y.H.; Sharma, A.R.; Park, J.B.; Jagga, S.; Sharma, G.; Lee, S.S.; Nam, J.S. Quercetin induces apoptosis and cell cycle arrest in triple-negative breast cancer cells through modulation of Foxo3a activity. Korean J. Physiol. Pharmacol. 2017, 21, 205–213. [Google Scholar] [CrossRef] [Green Version]
- Clemente-Soto, A.F.; Salas-Vidal, E.; Milan-Pacheco, C.; Sanchez-Carranza, J.N.; Peralta-Zaragoza, O.; Gonzalez-Maya, L. Quercetin induces G2 phase arrest and apoptosis with the activation of p53 in an E6 expressionindependent manner in HPVpositive human cervical cancerderived cells. Mol. Med. Rep. 2019, 19, 2097–2106. [Google Scholar] [CrossRef] [Green Version]
- Del Carmen Juarez-Vazquez, M.; Josabad Alonso-Castro, A.; Garcia-Carranca, A. Kaempferitrin induces immunostimulatory effects in vitro. J. Ethnopharmacol. 2013, 148, 337–340. [Google Scholar] [CrossRef]
- Sudeep, H.V.; Gouthamchandra, K.; Shyamprasad, K. Molecular docking analysis of Withaferin A from Withania somnifera with the Glucose regulated protein 78 (GRP78) receptor and the SARS-CoV-2 main protease. Bioinformation 2020, 16, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Utomo, R.Y.; Ikawati, M.; Meiyanto, E. Revealing the Potency of Citrus and Galangal Constituents to Halt SARS-CoV-2 Infection. Preprints 2020, 2020030214. [Google Scholar] [CrossRef] [Green Version]
- Sung, Y.Y.; Yoon, T.; Yang, W.K.; Kim, S.J.; Kim, H.K. Anti-obesity effects of Geranium thunbergii extract via improvement of lipid metabolism in high-fat diet-induced obese mice. Mol. Med. Rep. 2011, 4, 1107–1113. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, G.; Kumar, V. In-silico design of a potential inhibitor of SARS-CoV-2 S protein. PLoS ONE 2020, 15, e0240004. [Google Scholar] [CrossRef] [PubMed]
- Toelzer, C.; Gupta, K.; Yadav, S.K.N.; Borucu, U.; Davidson, A.D.; Kavanagh Williamson, M.; Shoemark, D.K.; Garzoni, F.; Staufer, O.; Milligan, R.; et al. Free fatty acid binding pocket in the locked structure of SARS-CoV-2 spike protein. Science 2020, 370, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, X.; Luo, G.; Zhang, X.; Lu, F.; Qiao, L.; He, W.; Li, G.; Zhang, Y. Discovery of Potential Inhibitors of Squalene Synthase from Traditional Chinese Medicine Based on Virtual Screening and In Vitro Evaluation of Lipid-Lowering Effect. Molecules 2018, 23, 1040. [Google Scholar] [CrossRef] [Green Version]
- Schuttelkopf, A.W.; van Aalten, D.M. PRODRG: A tool for high-throughput crystallography of protein-ligand complexes. Acta Crystallogr. D Biol. Crystallogr. 2004, 60, 1355–1363. [Google Scholar] [CrossRef] [Green Version]
- Tian, W.; Chen, C.; Lei, X.; Zhao, J.; Liang, J. CASTp 3.0: Computed atlas of surface topography of proteins. Nucleic Acids Res. 2018, 46, W363–W367. [Google Scholar] [CrossRef] [Green Version]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [Green Version]
- Halperin, I.; Ma, B.; Wolfson, H.; Nussinov, R. Principles of docking: An overview of search algorithms and a guide to scoring functions. Proteins 2002, 47, 409–443. [Google Scholar] [CrossRef]
- Stierand, K.; Rarey, M. Drawing the PDB: Protein-Ligand Complexes in Two Dimensions. ACS Med. Chem. Lett. 2010, 1, 540–545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanommeslaeghe, K.; Hatcher, E.; Acharya, C.; Kundu, S.; Zhong, S.; Shim, J.; Darian, E.; Guvench, O.; Lopes, P.; Vorobyov, I.; et al. CHARMM general force field: A force field for drug-like molecules compatible with the CHARMM all-atom additive biological force fields. J. Comput. Chem. 2010, 31, 671–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brooks, B.R.; Brooks, C.L., 3rd; Mackerell, A.D., Jr.; Nilsson, L.; Petrella, R.J.; Roux, B.; Won, Y.; Archontis, G.; Bartels, C.; Boresch, S.; et al. CHARMM: The biomolecular simulation program. J. Comput. Chem. 2009, 30, 1545–1614. [Google Scholar] [CrossRef] [PubMed]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High-performance molecular simulations through multi-level parallelism from laptops tosupercomputers. SoftwareX 2015, 1–2, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Darden, T.D.; Pedersen, L. Particle mesh Ewald: An N.log(N) method for Ewald sums in large systems. J. Chem. Phys. 1993, 98, 10089–10092. [Google Scholar] [CrossRef] [Green Version]
- Berendsen, H.J.C.; Postma, J.P.M.; van Gunsteren, W.F.; DiNola, A.; Haak, J.R. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 1984, 81, 3684–3690. [Google Scholar] [CrossRef] [Green Version]
- Stalin, A.; Lin, D.; Josephine Princy, J.; Feng, Y.; Xiang, H.; Ignacimuthu, S.; Chen, Y. Computational analysis of single nucleotide polymorphisms (SNPs) in PPAR gamma associated with obesity, diabetes and cancer. J. Biomol. Struct. Dyn. 2020, 1–15. [Google Scholar] [CrossRef]
Sample Availability: Not applicable. |
| S.No. | Drugs | Mode of Action |
|---|---|---|
| 1 | Remdesivir | Conceals the RNA polymerase and evades viral endonuclease proofreading, thereby reducing the viral RNA production inside the host cell |
| 2 | Hydroxychloroquine and Chloroquine | Alter the pH of the lysosome, thereby deteriorating the viral proteins; also inhibit the entry of the virus into cells by interfering with the phage–host fusion through glycosylation of the ACE2 receptor and spike proteins |
| 3 | Lopinavir–Ritonavir | Restricts the protease activity |
| 4 | Umifenovir (Arbidol) | Blocks the virus–host cell membrane fusion through hydrogen bonding with phospholipids |
| 5 | Favipiravir (Avigan) | Restricts the RNA-dependent RNA polymerase |
| 6 | Oseltamivir (Tamiflu) | Inhibits the neuraminidase enzyme on the viral surface |
| 7 | Immune Interferon-alpha (IFN-α) | Inhibits the protein synthesis from viral RNA |
| 8 | Ribavirin | Interferes with the RNA metabolism |
| Ligand | Protein PDB ID | Binding Amino Acid Residues | Binding Energy (kcal/mol) | Inhibition Constant μM | VDW_HB Desolv_Energy (kcal/mol) | Ligand Efficiency |
|---|---|---|---|---|---|---|
| Corilagin | Spike RBD (6M17) | ARG346/1HH1, PHE347/O, LEU441/O, ASP442/OD1, LYS444/HZ1, ASN450/C | −3.62 | 2.23 (mM) | −7.43 | 0.08 |
| Ellagic acid | Spike RBD (6M17) | GLU340/O, ASN343/O, ARG346/NH2, ALA348/N, ASN354/ND2, SER399/OG | −5.22 | 149.02 | −6.21 | 0.24 |
| Gallic acid | Spike RBD (6M17) | VAL341/O, ARG346/NH2/O, ASN354/ND2, SER399/OG | −4.21 | 817.47 | −5.01 | 0.25 |
| Geraniin | Spike RBD (6M17) | THR345/OG1/O, ARG346/NE, SER349/N.OG, LEU441/O, ASP442/OD1, ASN450/ND2 | −7.58 | 2.79 | −8.86 | 0.31 |
| Kaempferitrin | Spike RBD (6M17) | THR345/OG1, PHE347/O, LEU441/O, ASP442/OD1, ASN450/ND2, TYR451/OH, ARG509/NH2, | −5.98 | 79.26 | −9.24 | 0.28 |
| Kaempferol 7-O-rhamnoside | Spike RBD (6M17) | THR345/O, PHE347/O, SER349/OG/N, 450/OD1/ND2 | −5.69 | 66.94 | −8.27 | 0.18 |
| Kaempferol | Spike RBD (6M17) | SER349/OG/N, LEU441/O, ASN450/ND2, TYR451/OH, ARG509/NH2 | −5.69 | 67.86 | −7.07 | 0.26 |
| Protocatechuic acid | Spike RBD (6M17) | VAL341/O, ARG346/NH2/O, SER399/OG | −4.18 | 857.2 | −4.53 | 0.24 |
| Quercetin | Spike RBD (6M17) | SER349/OD1/N, LEU441/O, ASP442/OD1, ASN448/ND2, ASN450/ND2, ARG509/NH2 | −5.71 | 65.57 | −7.16 | 0.26 |
| Ligand | Protein PDB ID | Binding Amino Acid Residues | Binding Energy (kcal/mol) | Inhibition Constant μM | VDW_HB Desolv_Energy (kcal/mol) | Ligand Efficiency |
|---|---|---|---|---|---|---|
| Corilagin | 3CLpro (6LU7) | THR26/HN/O, PHE140/O, HIS164/O, GLU166/HN/OE2, | −5.82 | 54.33 | −9.44 | 0.13 |
| Ellagic acid | 3CLpro (6LU7) | LEU141/O, GLY143/HN, SER144/HN/HG, CYS145/HN, HIS163/HE2, GLU166/OE2/HN/O, HIS172/HE2, GLN189/OE1, | −6.37 | 21.54 | −7.18 | 0.29 |
| Gallic acid | 3CLpro (6LU7) | LEU141/OGLY143/HN, SER144/HN, HIS163/HE2, GLU166/HN | −4.46 | 535.39 | −4.86 | 0.32 |
| Geraniin | 3CLpro (6LU7) | PHE140/O, LEU141/O, HIS163/HE2, GLU166/HN, ARG188/O, GLN189/O, THR190/O, GLN192/1HE2 | −9.78 | 67.61 | −10.76 | 0.38 |
| Kaempferitrin | 3CLpro (6LU7) | LEU141/O, ASN142/OD1, SER144/OG, HIS163/HE2, GLU166/O | −7.83 | 286.47 | −8.48 | 0.28 |
| Kaempferol7-O-rhamnoside | 3CLpro (6LU7) | LEU141/O, SER144/HN, CYS145/HN, HIS163/HE2, PRO168/O | −5.87 | 49.91 | −8.42 | 0.19 |
| Kaempferol | 3CLpro (6LU7) | GLY143/HN, SER144/N, GLU166/O/HN, ASP187/O | −7.76 | 2.04 | −9.14 | 0.36 |
| Protocatechuic acid | 3CLpro (6LU7) | LEU141/O, GLY143/HN, SER144/HN, CYS145/HN, HIS163/HE2, GLU166/HN | −4.32 | 677.36 | −4.59 | 0.34 |
| Quercetin | 3CLpro (6LU7) | LEU141/O, GLY143/HN, SER144/HN, GLU166/HN | −6.49 | 3.24 | −9.2 | 0.30 |
| Ligand | Protein PDB ID | Binding Amino Acid Residues | Binding Energy (kcal/mol) | Inhibition Constant μM | VDW_HB Desolv_Energy (kcal/mol) | Ligand Efficiency |
|---|---|---|---|---|---|---|
| Corilagin | GRP78(5E84) | GLU427/O, VAL429/HN, SER452/HN, THR458/O, LYS460/HN | −4.39 | 606.67 | −8.32 | 0.10 |
| Ellagic acid | GRP78(5E84) | GLU427/OE2, VAL429/HN, GLY430/O, THR458/O, LYS460/HN | −6.47 | 18.05 | −7.24 | 0.29 |
| Gallic acid | GRP78(5E84) | GLU427/HN, THR458/O, LYS460/HN/O | −3.19 | 4.45 (mM) | −3.42 | 0.27 |
| Geraniin | GRP78(5E84) | GLY430/HN, SER452/HN, THR456/O, THR458/O | −9.55 | 100.03 (nM) | −10.38 | 0.34 |
| Kaempferitrin | GRP78(5E84) | GLU427/O, SER452/HN, THR458/HN/O, LYS460/HN | −4.36 | 639.8 | −8.0 | 0.11 |
| Kaempferol 7-O-rhamnoside | GRP78(5E84) | GLU427/O, LYS435/O, THR458/O, LYS460/HN | −5.45 | 100.92 | −7.91 | 0.18 |
| Kaempferol | GRP78(5E84) | GLU427/OE1, GLY430/HN, THR458/O, LYS460/HN | −6.27 | 25.23 | −7.4 | 0.30 |
| Protocatechuic acid | GRP78(5E84) | GLU427/HN, THR458/O, LYS460/O | −3.78 | 1.68 (mM) | −3.33 | 0.24 |
| Quercetin | GRP78(5E84) | GLU427/OE1, GLY430/HN/O, THR458/O, LYS460/HN | −5.52 | 90.35 | −6.98 | 0.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arokiyaraj, S.; Stalin, A.; Kannan, B.S.; Shin, H. Geranii Herba as a Potential Inhibitor of SARS-CoV-2 Main 3CLpro, Spike RBD, and Regulation of Unfolded Protein Response: An In Silico Approach. Antibiotics 2020, 9, 863. https://doi.org/10.3390/antibiotics9120863
Arokiyaraj S, Stalin A, Kannan BS, Shin H. Geranii Herba as a Potential Inhibitor of SARS-CoV-2 Main 3CLpro, Spike RBD, and Regulation of Unfolded Protein Response: An In Silico Approach. Antibiotics. 2020; 9(12):863. https://doi.org/10.3390/antibiotics9120863
Chicago/Turabian StyleArokiyaraj, Selvaraj, Antony Stalin, Balakrishnan Senthamarai Kannan, and Hakdong Shin. 2020. "Geranii Herba as a Potential Inhibitor of SARS-CoV-2 Main 3CLpro, Spike RBD, and Regulation of Unfolded Protein Response: An In Silico Approach" Antibiotics 9, no. 12: 863. https://doi.org/10.3390/antibiotics9120863
APA StyleArokiyaraj, S., Stalin, A., Kannan, B. S., & Shin, H. (2020). Geranii Herba as a Potential Inhibitor of SARS-CoV-2 Main 3CLpro, Spike RBD, and Regulation of Unfolded Protein Response: An In Silico Approach. Antibiotics, 9(12), 863. https://doi.org/10.3390/antibiotics9120863