The Bactericidal Activity of Protein Extracts from Loranthus europaeus Berries: A Natural Resource of Bioactive Compounds
Abstract
:1. Introduction
2. Results
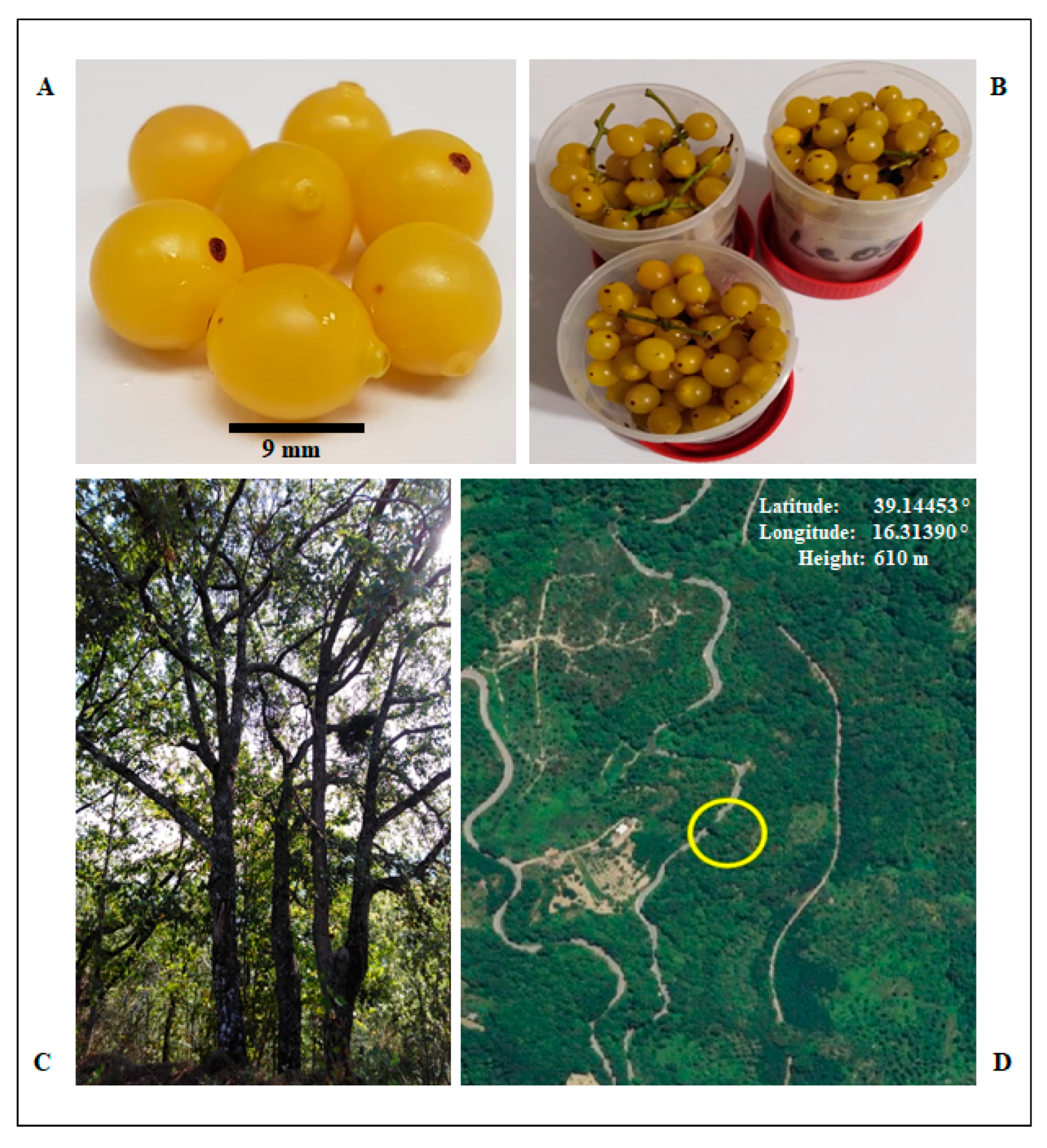
2.1. Samples Collection
2.2. Preparation of Protein Extracts from Loranthus europaeus Berries and Plant Extracts Yield
2.3. Antifungal Activity
2.4. Antibacterial Activity
2.5. Spectroscopic Analysis
2.6. Partial Purification of the Active Compounds
3. Materials and Methods
3.1. Collection of Plant Material
3.2. Preparation of Crude Extracts
3.3. Antifungal Activity Assays
3.4. Bacterial Culture and Inoculum Preparation
3.5. Antibacterial Activity Assay of Plant Fruit Extracts
3.6. Antibacterial Activity Assay of Partially Purified Samples
3.7. Partial Purification of the Active Components
3.8. SDS-PAGE Analysis
3.9. Spectroscopic Analyses
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pliny. Loeb Classical Library 330. In Natural History; Rackham, H., Ed.; Harvard University Press: Cambridge, MA, USA, 1938; Volume I: Books 1–2. [Google Scholar]
- Schurè, E. Rama (le cycle aryen). In Les Grands Initiés. Esquisse de l’histoire secrète des religions; Librairie académique Perrin et Cie: Paris, France, 1889; pp. 3–38. [Google Scholar]
- Frazer, J.G. The Golden Bough: A Study in Magic and Religion. Boringhieri, Ed; Macmillan and Co., Ltd.: London, UK, 1890; pp. 407–409. [Google Scholar]
- Alexander, N. The Germanic word for ‘sword’ and delocatival derivation in Proto-IndoEuropean. JIES 2009, 37, 462–488. [Google Scholar]
- Watson, D.M. Mistletoe—A keystone resource in forests and woodlands worldwide. Annu. Rev. Ecol. Syst. 2001, 32, 219–249. [Google Scholar] [CrossRef] [Green Version]
- Stead, I.M; Bourke, J.B.; Brothwell, D. Lindow Man: The body in the Bog; British Museum Publications: London, UK, 1986. [Google Scholar]
- Ramm, H. Mistletoe through Cultural and Medical History: The All-Healing Plant Proves to Be a Cancer-Specific Remedy. In Mistletoe: From Mythology to Evidence-Based Medicine; Zänker, K.S., Kaveri, S.V., Eds.; Transl Res Biomed: Basel, Switzerland, 2015; Volume 4, pp. 1–10. [Google Scholar]
- Liu, B.; Le, C.T.; Barrett, R.L.; Nickrent, D.L.; Chen, Z.; Lu, L.; Vidal-Russell, R. Historical biogeography of Loranthaceae (Santalales): Diversification agrees with emergence of tropical forests and radiation of songbirds. Mol. Phylogenet. Evol. 2018, 124, 199–212. [Google Scholar] [CrossRef] [Green Version]
- Hegi, G. Angiospermae, dicotyledones 1. In Illustrierte Flora von Mitteleuropa, Band III, Teil 2; Parey, P., Ed.; Verlag Paul Parey: Berlin, Germany, 1981. [Google Scholar]
- Nickrent, D.L.; Malécot, V.; Vidal-Russell, R.; Der, J.P. A revised classification of Santalales. TAXON 2010, 59, 538–558. [Google Scholar] [CrossRef]
- Shahi Shavvon, R.; Saeidi Mehrvarz, S.; Golmohammadi, N. Evidence from micromorphology and gross morphology of the genus Loranthus (Loranthaceae) in Iran. Turk. J. Bot. 2012, 36, 655–666. [Google Scholar]
- Hibberd, J.M.; Jeschke, W.D. Solute flux into parasitic plants. J. Exp. Bot. 2001, 52, 2043–2049. [Google Scholar] [CrossRef] [Green Version]
- Gebauer, R.; Volarik, D.; Urban, J. Quercus pubescens and its hemiparasite Loranthus europaeus: Nutrient dynamics of leaves and twigs. Acta Physiol. Plant 2012, 34, 1801–1809. [Google Scholar] [CrossRef]
- Sharquie, K.E.; Noaimi, A.A.; Saleh, B.M.; Sharara, Z.A.; Al-Salam, W.S. Topical 40% Loranthus europaeus Ointment as an Alternative Medicine in the Treatment of Acute Cutaneous Leishmaniasis versus Topical 25% Podophyllin Solution. J.C.D.S.A. 2017, 7, 148–163. [Google Scholar] [CrossRef] [Green Version]
- Vitasović Kosić, I.; Juračak, J.; Łuczaj, Ł. Using Ellenberg-Pignatti values to estimate habitat preferences of wild food and medicinal plants: An example from northeastern Istria (Croatia). J. Ethnobiol. Ethnomed. 2017, 13, 31. [Google Scholar] [CrossRef] [Green Version]
- Leporatti, M.L.; Corradi, L. Ethnopharmacobotanical remarks on the Province of Chieti town (Abruzzo, Central Italy). J. Ethnopharmacol. 2001, 74, 17–40. [Google Scholar] [CrossRef]
- Leporatti, M.L.; Pavesi, A. Usi nuovi, rari o interessanti di piante officinali di alcune zone della Calabria. Webbia 1989, 43, 269–289. [Google Scholar] [CrossRef]
- Harvala, E. Flavonoids of Loranthus europaeus. J. Nat. Prod. 1984, 47, 1054–1055. [Google Scholar] [CrossRef]
- Katsarou, A.; Rhizopoulou, S.; Kefalas, P. Antioxidant Potential of the Aerial Tissues of the Mistletoe Loranthus europaeus Jacq. Rec. Nat. Prod. 2012, 6, 394–397. [Google Scholar]
- Cholakova, M.; Christov, V.; Dimitrova, D.; Evstatieva, L.; Alexandrova, R.; Nikolova, E. Flavonoid and terpenoid isolated from Loranthus europaeus with stimulatory effect on lymphocyte proliferation. Experimental Pathology and Parasitology 2002, 5/9, 45–48. [Google Scholar]
- Larsson, S. Mistletoes and Thionins as Selection Models in Natural Products Drug Discovery. Digital Comprehensive Summaries of Uppsala Dissertations from the Faculty of Pharmacy 2007, 49, 65. [Google Scholar]
- Parisi, K.; Shafee, T.M.A.; Quimbar, P.; van der Weerden, N.L.; Bleackley, M.R.; Anderson, M.A. The evolution, function and mechanisms of action for plant defensins. Semin. Cell. Dev. Biol. 2019, 88, 107–118. [Google Scholar] [CrossRef]
- Sathoff, A.E.; Velivelli, S.; Shah, D.M.; Samac, D.A. Plant Defensin Peptides have Antifungal and Antibacterial Activity Against Human and Plant Pathogens. Phytopathology 2019, 109, 402–408. [Google Scholar] [CrossRef]
- Tiwari, P.; Kumar, B.; Kaur, M.; Kaur, G.; Kaur, H. Phytochemical screening and Extraction: A Review. I.P.S. 2011, 1, 98–106. [Google Scholar]
- Cowan, M.M. Plant Products as Antimicrobial Agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef] [Green Version]
- Tattersall, D.B.; van Heeswijck, R.; Høj, P.B. Identification and characterization of a fruit-specific, thaumatin-like protein that accumulates at very high levels in conjunction with the onset of sugar accumulation and berry softening in grapes. Plant Physiol. 1997, 114, 759–769. [Google Scholar] [CrossRef] [Green Version]
- Jammer, A.; Gasperl, A.; Luschin-Ebengreuth, N.; Heyneke, E.; Chu, H.; Cantero-Navarro, E.; Großkinsky, D.K.; Albacete, A.A.; Stabentheiner, E.; Franzaring, J.; et al. Simple and robust determination of the activity signature of key carbohydrate metabolism enzymes for physiological phenotyping in model and crop plants. J. Exp. Bot. 2015, 66, 5531–5542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palaniyandi, U.; Muthuswamy, A. An efficient protein extraction method for proteomic analysis of black pepper (‘Piper nigrum’ L.) and generation of protein map using nano LC-LTQ Orbitrap mass spectrometry. Plant Omics 2015, 8, 500–507. [Google Scholar]
- Wang, W.; Tai, F.; Chen, S. Optimizing protein extraction from plant tissues for enhanced proteomics analysis. J. Sep. Sci. 2008, 31, 2032–2039. [Google Scholar] [CrossRef] [PubMed]
- Friedenauer, S.; Berlet, H.H. Sensitivity and variability of the Bradford protein assay in the presence of detergents. Anal. Biochem. 1989, 178, 263–268. [Google Scholar] [CrossRef]
- Arachea, B.T.; Sun, Z.; Potente, N.; Malik, R.; Isailovic, D.; Viola, R.E. Detergent selection for enhanced extraction of membrane proteins. Protein Expr. Purif. 2012, 86, 12–20. [Google Scholar] [CrossRef]
- Smith, J.V. Antimicrobial activity of different sodium and potassium salts of carboxylic acid against some common foodborne pathogens and spoilage-associated bacteria. International Journal of GEOMATE 2016, 11, 2671–2678. [Google Scholar]
- Cabezas-Pizarro, J.; Redondo-Solano, M.; Umaña-Gamboa, C.; Arias-Echandi, M.L. Antimicrobial activity of different sodium and potassium salts of carboxylic acid against some common foodborne pathogens and spoilage-associated bacteria. Rev. Argent. Microbiol. 2018, 50, 56–61. [Google Scholar] [CrossRef]
- Agrillo, B.; Mirino, S.; Tatè, R.; Gratino, L.; Gogliettino, M.; Cocca, E.; Tabli, N.; Nabti, E.; Palmieri, G. An alternative biocontrol agent of soil-borne phytopathogens: A new antifungal compound produced by a plant growth promoting bacterium isolated from North Algeria. Microbiol. Res. 2019, 221, 60–69. [Google Scholar] [CrossRef]
- Tabli, N.; Raia, A.; Bensidhoum, L.; Palmieri, G.; Gogliettino, M.; Cocca, E.; Consiglio, C.; Cillo, F.; Bubici, G.; Nabti, E. Plant growth promoting and inducible antifungal activities of irrigation well water-bacteria. Biol. Control 2018, 117, 78–86. [Google Scholar] [CrossRef]
- Hee-Ock, B.; Sung-Jin, H.; Chun-Sik, B.; Su-Hyun, P.; Buk-Gu, H.; Gorinstein, S. Extraction and characterization of some natural plant pigments. Ind. Crops Prod. 2012, 40, 129–135. [Google Scholar]
- Kumar, P.; Ramakritinan, C.M.; Kumaraguru, A.K. Solvent Extraction and Spectrophotometric Determination of Pigments of Some Algal Species from the Shore of Puthumadam, Southeast Coast of India. I.J.O.O. 2010, 4, 29–34. [Google Scholar]
- Scheetz, M.H.; Qi, C.; Warren, J.R.; Postelnick, M.J.; Zembower, T.; Obias, A.; Noskin, G.A. In vitro activities of various antimicrobials alone and in combination with tigecycline against carbapenem-intermediate or -resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 2007, 51, 1621–1626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Owuama, C. Determination of minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) using a novel dilution tube method. Afr. J. Microbiol. Res. 2017, 11, 977–980. [Google Scholar]
- Palmieri, G.; Tatè, R.; Gogliettino, M.; Balestrieri, M.; Rea, I.; Terracciano, M.; Proroga, Y.T.; Capuano, F.; Anastasio, A.; De Stefano, L. Small Synthetic Peptides Bioconjugated to Hybrid Gold Nanoparticles Destroy Potentially Deadly Bacteria at Submicromolar Concentrations. Bioconjug. Chem. 2018, 29, 3877–3885. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Bondi, M.; Lauková, A.; de Niederhausern, S.; Messi, P.; Papadopoulou, C. Natural Preservatives to Improve Food Quality and Safety. J. Food Qual. 2017, 2017, 3. [Google Scholar] [CrossRef] [Green Version]
- Delesa, D.A. Traditional Medicinal Plants for Industrial Application as Natural Food Preservatives. Int. J. Adv. Res. Biol. Sci. 2018, 5, 85–94. [Google Scholar]







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ambrosio, R.L.; Gratino, L.; Mirino, S.; Cocca, E.; Pollio, A.; Anastasio, A.; Palmieri, G.; Balestrieri, M.; Genovese, A.; Gogliettino, M. The Bactericidal Activity of Protein Extracts from Loranthus europaeus Berries: A Natural Resource of Bioactive Compounds. Antibiotics 2020, 9, 47. https://doi.org/10.3390/antibiotics9020047
Ambrosio RL, Gratino L, Mirino S, Cocca E, Pollio A, Anastasio A, Palmieri G, Balestrieri M, Genovese A, Gogliettino M. The Bactericidal Activity of Protein Extracts from Loranthus europaeus Berries: A Natural Resource of Bioactive Compounds. Antibiotics. 2020; 9(2):47. https://doi.org/10.3390/antibiotics9020047
Chicago/Turabian StyleAmbrosio, Rosa Luisa, Lorena Gratino, Sara Mirino, Ennio Cocca, Antonino Pollio, Aniello Anastasio, Gianna Palmieri, Marco Balestrieri, Angelo Genovese, and Marta Gogliettino. 2020. "The Bactericidal Activity of Protein Extracts from Loranthus europaeus Berries: A Natural Resource of Bioactive Compounds" Antibiotics 9, no. 2: 47. https://doi.org/10.3390/antibiotics9020047
APA StyleAmbrosio, R. L., Gratino, L., Mirino, S., Cocca, E., Pollio, A., Anastasio, A., Palmieri, G., Balestrieri, M., Genovese, A., & Gogliettino, M. (2020). The Bactericidal Activity of Protein Extracts from Loranthus europaeus Berries: A Natural Resource of Bioactive Compounds. Antibiotics, 9(2), 47. https://doi.org/10.3390/antibiotics9020047






