GC-MS Profile and Enhancement of Antibiotic Activity by the Essential Oil of Ocotea odorífera and Safrole: Inhibition of Staphylococcus aureus Efflux Pumps
Abstract
1. Introduction
2. Results
2.1. Chemical Composition of the Essential Oil of Ocotea Odorífera
2.2. Antibacterial Activities of the EOOO and Safrole
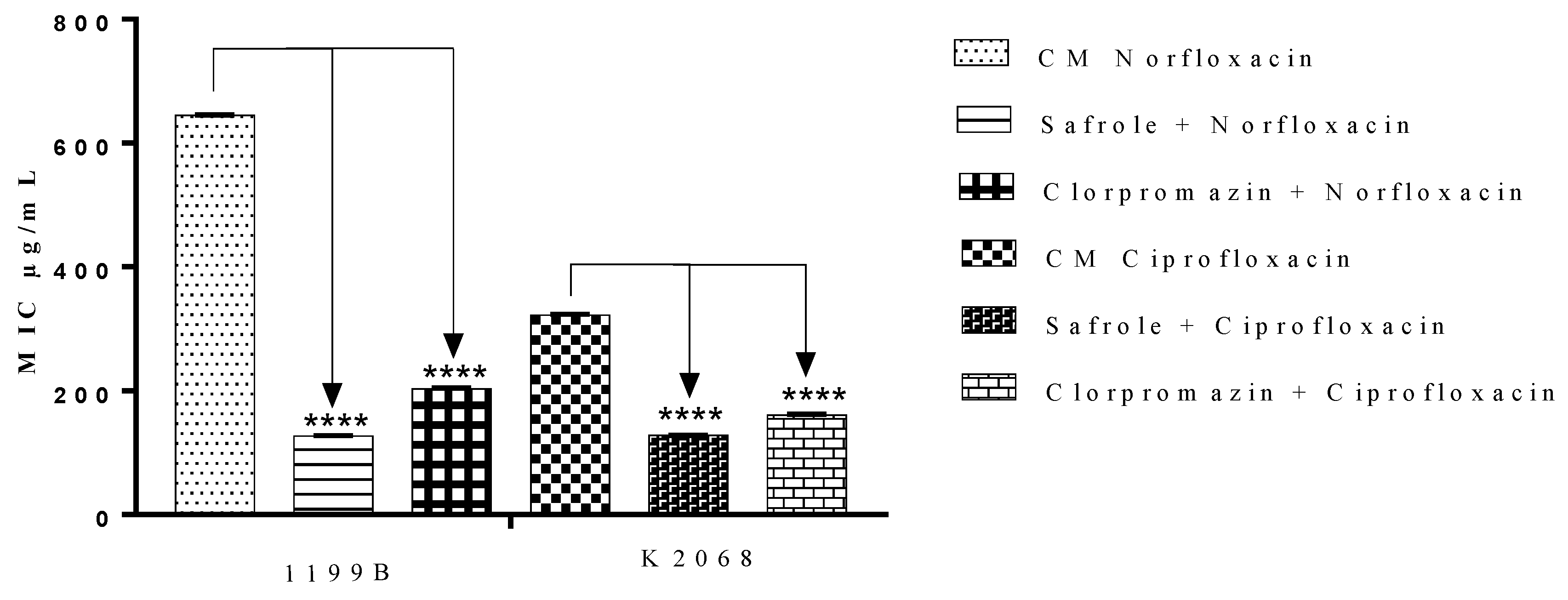
2.3. Antibiotic-Potentiating Effects of the EOOO and Safrole
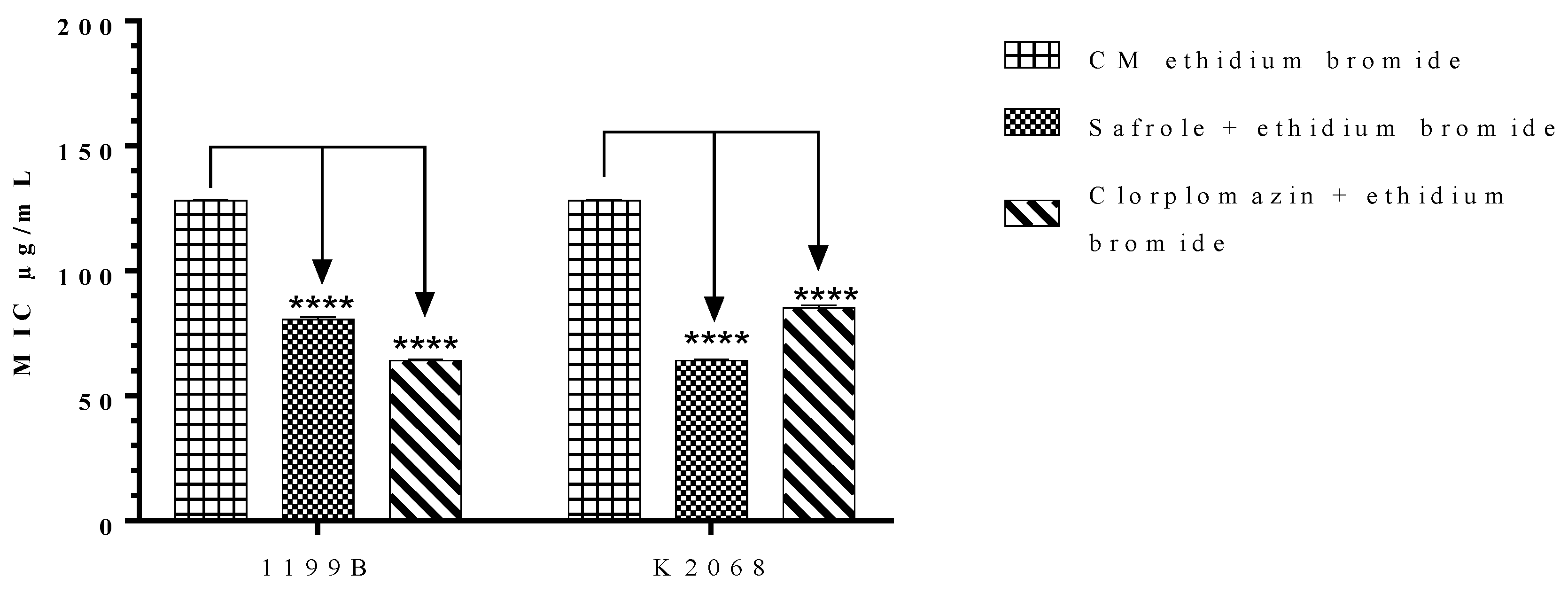
2.4. Effects of Safrole on the S. aureus NorA and MepA Efflux Proteins
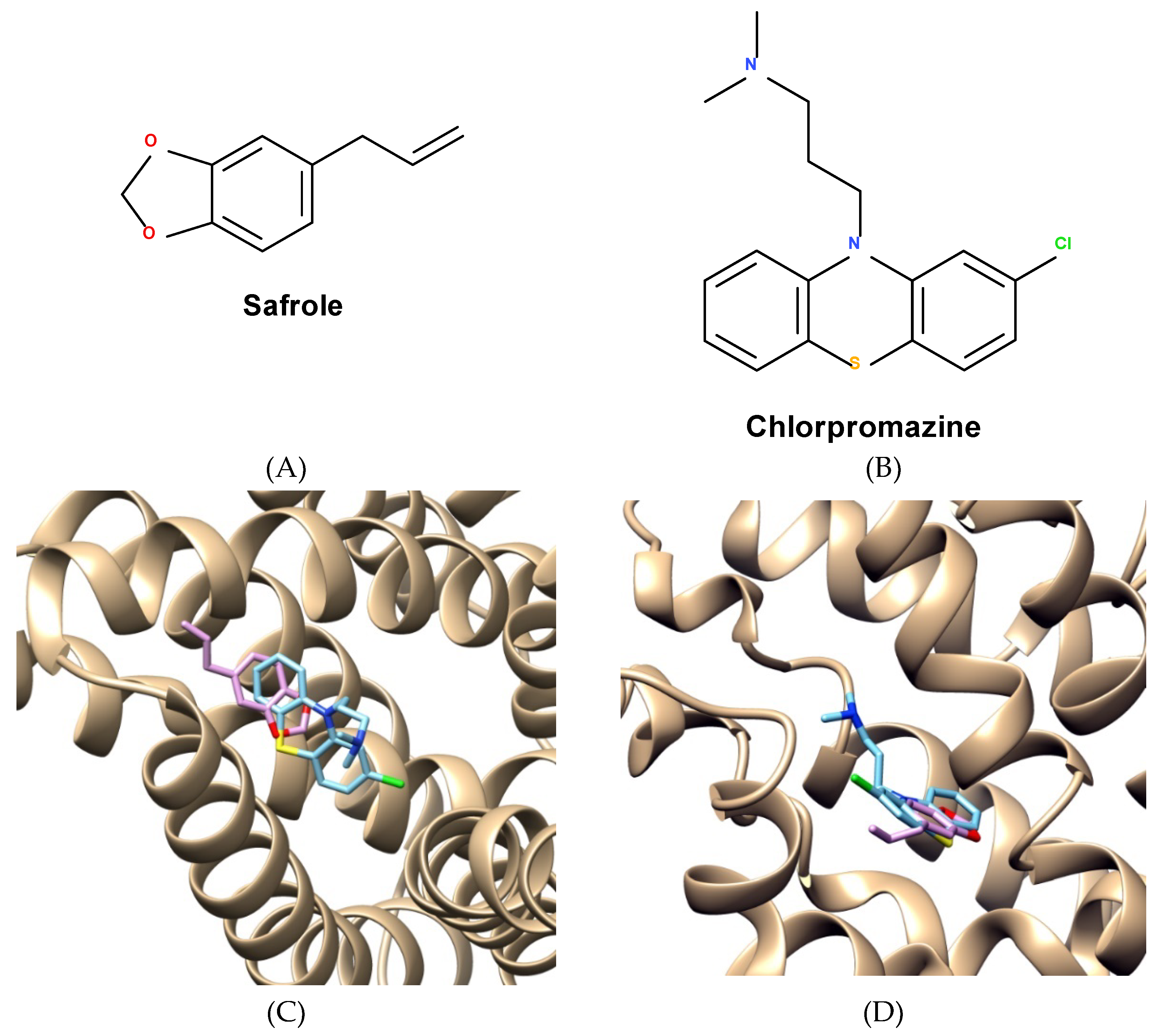
2.5. Molecular Docking and Analysis of Knteractions between Safrole and Efflux Proteins
3. Discussion
4. Materials and Methods
4.1. Collection and Identification of the Botanic Material
4.2. Essential Oil Extraction
4.3. Calculation of Essential Oil Yield
4.4. Phytochemical Analysis
4.5. Bacterial Cultures
4.6. Drugs
4.7. Determination of Minimum Inhibitory Concentration (MIC)
4.8. Analysis of Antibiotic Resistance Modulation
4.9. Efflux Pump Inhibition Analysis Using an Ethidium Bromide Assay
4.10. Efflux Pump Inhibition Analysis Using an Antibiotic Resistance Modulation Assay
4.11. Molecular Modelling and Docking Studies
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods: A review. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Khan, U.; Kumar, P.; Bhatt, R.P.; Manzoor, N. Antifungal activity of Coriaria nepalensis essential oil by disrupting ergosterol biosynthesis and membrane integrity against Candida. Yeast 2011, 28, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Pérez, S.G.; Ramos-López, M.A.; Sánchez-Miranda, E.; Fresán-Orozco, M.C.; Pérez-Ramos, J. Antiprotozoa activity of some essential oils. J. Med. Plants Res. 2012, 6, 2901–2908. [Google Scholar] [CrossRef]
- Sadekuzzaman, M.; Yang, S.; Mizan, M.F.R.; Ha, S.D. Current and recent advanced strategies for combating biofilms. Comp. Rev. Food Sci. Food Saf. 2015, 14, 491–509. [Google Scholar] [CrossRef]
- Franz, C.M. Essential oil research: Past, present and future. Flavour. Fragr. J. 2010, 25, 112–113. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Dubey, V.S.; Bhalla, R.; Luthra, R. An overview of the non-mevalonate pathway for terpenoid biosynthesis in plants. J. Biosci. 2003, 28, 637–646. [Google Scholar] [CrossRef]
- Peixoto-Neves, D.; Silva-Alves, K.S.; Gomes, M.D.; Lima, F.C.; Lahlou, S.; Magalhães, P.J.; Ceccatto, V.M.; Coelho-de-Souza, A.N.; Leal-Cardoso, J.H. Vasorelaxant effects of the monoterpenic phenol isomers, carvacrol and thymol, on rat isolated aorta. Fundam. Clin. Pharmacol. 2010, 24, 341–350. [Google Scholar] [CrossRef]
- Santos, T.G.; Laemmle, J.; Rebelo, R.A.; Dalmarco, E.M.; Cruz, A.B.; Schmitd, A.P.; Cruz, R.C.B.; Zeni, A.L.B. Chemical composition and antimicrobial activity of Aloysia gratíssima (Verbenaceae) leaf essential oil. J. Essent. Oil Res. 2015, 2, 125–130. [Google Scholar] [CrossRef]
- Priscilla, R.F.; Ana Carolina, J.A.; Cristina, R.S.B.; Débora, F.M.; Silva, A.C.A.; Rocha, J.E.; Oliveira-Tintino, C.D.M.; Ribeiro-Filho, J.; Silva, L.E.; Camila Confortin, C.; et al. GC-MS-FID and potentiation of the antibiotic activity of the essential oil of Baccharis reticulata (ruiz & pav.) pers. And α-pinene. Ind. Crops Prod. 2020, 145, 112106. [Google Scholar] [CrossRef]
- Oliveira, B.V.; Yamada, L.T.; Fagg, C.W.; Brandão, M.G.L. Native foods from Brazilian biodiversity as a source of bioactive compounds. Food Res. Int. 2012, 48, 170–179. [Google Scholar] [CrossRef]
- Bolsaris, A.S. Plants used traditionally to treat malaria in Brazil: The archives of Flora Medicinal. J. Ethnobiol. Ethnomed. 2007, 3, 1–8. [Google Scholar] [CrossRef]
- Tribess, B.; Pintarelli, G.M.; Bini, L.A.; Camargo, A.; Funez, L.A.; de Gasper, A.L.; Zeni, A.L. Ethnobotanical study of plants used for therapeutic purposes in the Atlantic Forest region, Southern Brazil. J. Ethnopharmacol. 2015, 164, 136–146. [Google Scholar] [CrossRef]
- Castro, R.D.; Lima, E.O. Antifungal activity of Brazilian sassafras (Ocotea odorifera Vell.) and Rosemary (Rosmarinus officinalis L.) essential oils against genus Candida. Braz. J. Med. Plant. 2011, 13, 203–208. [Google Scholar] [CrossRef][Green Version]
- Junior, A.R.P.; de Carvalho, R.I.N.; Netto, S.P.; Weber, S.H.; de Souza, E.; Furiatti, R.S. Bioactivity of essential oils of Brazilian implese and eucalyptus against lesser mealworm. Ciência Rural 2010, 40, 637–643. [Google Scholar] [CrossRef]
- Riva, D.; Simionatto, E.L.; Junior, A.W.; Salerno, A.R.; Schallenberger, T.H. Estudo da adaptação da espécie Piper hispidinervum C. DC. (pimenta longa) à região do Vale do Itajaí–SC, através da composição química do óleo essencial obtido por hidrodestilação por micro-ondas e convencional. Act. Amazonica 2011, 41, 297. [Google Scholar] [CrossRef]
- Costa, P.R.R. Safrol e eugenol: Estudo da reatividade química e uso em síntese de produtos naturais biologicamente ativos e seus derivados. Quím. Nova 2000, 23, 357. [Google Scholar] [CrossRef]
- Khayyat, A.S.; Al-Zahrani, S.H. Thermal, photosynthesis and antibacterial studies of bioactive safrole derivative as precursor for natural flavor and fragrance. Arab. J. Chem. 2014, 7, 800–804. [Google Scholar] [CrossRef]
- Barbosa, Q.P.; da Câmara, C.A.G.; Ramos, C.S.; Nascimento, D.C.O.; Lima-Filho, J.V.; Guimarães, E.F. Chemical composition, circadian rhythm and antibacterial activity of essential oils of Piper divaricatum: A new source of safrole. Quím. Nova 2012, 35, 1806–1808. [Google Scholar] [CrossRef]
- Alterthum, F. Microbiologia, 6th ed.; Atheneu: São Paulo, Brazil, 2017. [Google Scholar]
- Loureiro, R.J.; Roque, F.; Rodrigues, A.T.; Herdeiro, M.T.; Ramalheira, E. O uso de antibióticos e as resistências bacterianas: Breves notas sobre a sua evolução. Ver. Port. Ver. Pub. 2016, 34, 77–84. [Google Scholar] [CrossRef]
- Kohl, T.; Pontarolo, G.H.; Pedrassani, D. Resistência Antimicrobiana De Bactérias Isoladas De Amostras De Animais Atendidos Em Hospital Veterinário. Saúde. Meio. Ambiente 2016, 5, 115–127. [Google Scholar] [CrossRef]
- Tortora, G.J.; CASE, C.L.; Funke, B.R. Microbiologia, 10th ed.; Artmed Editora: Porto Alegre, Brazil, 2012. [Google Scholar]
- Blair, J.M.A.; Baylay, A.J.; Ogbolu, D.O.; Piddock, L.J. Molecular mechanisms of antibiotic resistance. Nat. Ver. Microbiol. 2015, 13, 42. [Google Scholar] [CrossRef] [PubMed]
- Tintino, S.R.; Oliveira-Tintino, C.D.M.; Campina, F.F.; Limaverde, P.W.; Pereira, P.S.; Siqueira-Junior, J.P.; Coutinho, H.D.M.; Quintans-Júnior, L.J.; da Silva, T.G.; Leal-Balbino, T.C.; et al. Vitamin K enhances the effect of antibiotics inhibiting the efflux pumps of Staphylococcus aureus strains. Med. Chem. Rese. 2018, 27, 261–267. [Google Scholar] [CrossRef]
- Bahmani, M.; Schmidt, O. Plant essential oils for environment-friendly protection of wood objects against fungi. Maderas Cienc. Tecnol. 2018, 20, 325–332. [Google Scholar] [CrossRef]
- Souza, L.M.; Fonseca, F.S.A.; Silva, J.C.R.L.; Silva, A.M.; Silva, J.R.; Martins, E.R. Essential oil composition in natural population of Lippia origanoides (Verbenaceae) during dry and rainy seasons. Rev. Biol. Trop. 2019, 67, 278–285. [Google Scholar] [CrossRef]
- Hoefel, H.H.K.; Lautert, L. Administração endovenosa de antibióticos e resistência bacteriana: Responsabilidade da enfermagem. Rev. Eletrônica. Enferm. 2006, 8, 441–449. [Google Scholar] [CrossRef][Green Version]
- Alós, J.I. Resistencia bacteriana a los antibióticos: Ver crisis global. Enferm. Infecc. Microbiol. Clin. 2015, 33, 692–699. [Google Scholar] [CrossRef]
- Mota, R.A.; da Silva, K.P.C.; de Freitas, M.F.L.; Porto, W.J.N.; da Silva, L.B.G. Utilização indiscriminada de anmicrobianos e sua contribuição a 14imple14se14stência bacteriana. Braz. J. Vet. Res. Anim. Sci. 2005, 42, 465–470. [Google Scholar] [CrossRef][Green Version]
- Mossi, J.A.; Zanella, C.A.; Kubiak, G.B.; Lerin, L.A.; Cansian, R.L.; Frandoloso, F.S.; Prá, V.D.; Mazutti, M.A.; Costa, J.A.V.; Treichel, H. Essential oil of Ocotea odorifera: An alternative against Sitophilus zeamais. Renew. Agric. Food Syst. 2014, 29, 1–6. [Google Scholar] [CrossRef]
- Lorenzi, H.; Matos, F.J.A. Plantas medicinais no Brasil: Nativas e exóticas. Inst. Plant. 2002. Available online: http://www.sidalc.net/cgibin/wxis.exe/?IsisScript=LIBROS.xis&method=post&formato=2&cantidad=1&expresion=mfn=008440 (accessed on 10 May 2020).
- Silva, D.C.; Blank, A.F.; Nizio, D.A.C.; Sampaio, T.S.; Nogueira, P.C.L.; Arrigoni-blank, M.F. Chemical diversity of essential oils from native populations of Eplingiella implese. Crop Breed. Appl. Biot. 2018, 18, 205–214. [Google Scholar] [CrossRef]
- Nazzaro, F.; Frantianni, F.; de Martino, L.; Coppola, R.; de Feo, V. Effect of Essential Oils on Pathogenic Bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef] [PubMed]
- Betim, F.C.M.; Souza, A.M.; Szabo, E.M.; Zanin, S.M.W.; Miguel, O.G.; Miguel, M.D.; Dias, J.F.G. Ocotea nutans (Nees) Mez (LAURACEAE): Chemical composition, antioxidante capacity and biological properties of essential oil. Braz. J. Pharm. Sci. 2019, 55, 1–10. [Google Scholar] [CrossRef]
- Damascemo, C.S.B.; de Oliveira, L.F.; Szabo, E.M.; Souza, A.M.; Dias, J.F.G.; Miguel, M.D.; Miguel, O.G. Chemical composition, antioxidant and biological activity of Ocotea odorífera Vattimo-Gil (LAURACEAE) essential oil. Braz. J. Pharm. Sci. 2017, 53, 1–8. [Google Scholar] [CrossRef]
- Cansian, R.L.; Mossi, A.J.; Paroul, N.; Toniazzo, G.; Zboralski, F.; Prichoa, F.C.; Kubiak, G.B.; Lerin, L.A. Atividade antioxidante e antimicrobiana de extratos de canela-sassafrás (Ocotea odorífera (VELL) Rowher). Perspect. Erechim. 2010, 34, 123–133. [Google Scholar]
- Pereira, P.S.; Lima, M.D.C.A.; Neto, P.P.M.; Oliveira-Tintino, C.D.M.; Tintino, S.R.; Menezes, I.R.A.; de Oliveira, J.F.; Marchand, P.; Coutinho, H.D.M.; Rodrigues, M.D.D.; et al. Thiazolidinedione and thiazole derivatives potentiate norfloxacin activity against NorA efflux pump over expression in Staphylococcus aureus 1199B strains. Bioorg. Med. Chem. 2019, 27, 3797–3804. [Google Scholar] [CrossRef] [PubMed]
- Tintino, S.R.; Oliveira-Tintino, C.D.; Campina, F.F.; Silva, R.L.; Costa, M.do.S.; Menezes, I.R.; Calixto-Júnior, J.T.; Siqueira-Junior, J.P.; Coutinho, H.D.M.; Leal-Balbino, T.C.; et al. Evaluation of the tannic acid inhibitory effect against the NorA efflux pump of Staphylococcus aureus. Microb. Pathog. 2016, 97, 9–13. [Google Scholar] [CrossRef]
- Oliveira-Tintino, C.D.M.; Tintino, S.R.; Limaverde, P.W.; Figueredo, F.G.; Campina, F.F.; da Cunha, F.A.B.; da Costa, R.H.S.; Pereira, P.S.; Lima, L.F.; de Matos, Y.M.L.S.; et al. Inhibition of the essential oil from Chenopodium ambrosioides L. and α-terpinene on the NorA efflux-pump of Staphylococcus aureus. Food Chem. 2018, 226, 72–77. [Google Scholar] [CrossRef]
- Tintino, S.R.; Oliveira-Tintino, C.D.M.; Campina, F.F.; Costa, M.D.S.; Menezes, I.R.A.; Matos, Y.M.L.S.; Calixto-Júnior, J.T.; Pereira, P.S.; Siqueira-Junior, J.P.; Leal-Balbino, T.C.; et al. Tannic acid affects the phenotype of Staphylococcus aureus resistant to tetracycline and erythromycin by inhibition of efflux pumps. Bioorg. Chem. 2017, 74, 197–200. [Google Scholar] [CrossRef]
- Costa, S.S.; Falcão, C.; Viveiros, H.; Machado, D.; Martins, H.; Melo, C.J.; Amaral, G. Exploring the contribution of efflux on the resistance to fluoroquinolones in clinical isolates of Staphylococcus aureus. BMC Microbiol. 2011, 11, 241. [Google Scholar] [CrossRef]
- Schindler, B.D.; Jacinto, P.; Kaatz, G.W. Inhibition of drug efflux pumps in Staphylococcus aureus: Current status of potentiating existing antibiotics. Future. Microbiol. 2013, 8, 491–507. [Google Scholar] [CrossRef]
- Kaatz, G.W. Phenothiazines and thioxanthenes inhibit multidrug efflux pump activity in Staphylococcus aureus. Antimicrob. Agents Chemother. 2003, 47, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Sabatini, S.; Kaatz, G.W.; Rossolini, G.M.; Brandini, D.; Fravolini, A. From phenothiazine to 3-phenyl-1, 4-benzothiazine derivatives as inhibitors of the Staphylococcus aureus NorA multidrug efflux pump. J. Med. Chem. 2008, 51, 4321–4330. [Google Scholar] [CrossRef] [PubMed]
- Alemão, N.; Wei, P.; Kaatz, G.W.; Kerns, R.J. Synthesis and evaluation of fluoroquinolone derivatives as substrate-based inhibitors of bacterial efflux pumps. Eur. J. Med. Chem. 2008, 43, 2453–2463. [Google Scholar] [CrossRef]
- Wasicky, R. Uma modificação do aparelho de clevenger para extração de óleos essenciais. Rev. Fac. Farmácia Bioquímica 1963, 1, 77–81. [Google Scholar]
- Girard, E.A.; Koehler, H.S.; Netto, S.P. Volume, biomassa e rendimento de óleos essenciais do craveiro (pimenta pseudocaryophyllus (gomes) landrum. Ver. Acad. Curitiba 2007, 5, 147–165. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/mass Spectroscopy; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- National Committee for Clinical Laboratory Standards (NCCLS). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, 6th ed.; NCCLS: Pennsylvania, PA, USA, 2003; Available online: https://clsi.org/media/1632/m07a10_sample.pdf (accessed on 10 January 2020).
- Mann, C.M.; Markham, J.L. A new method for determining the minimum inhibitory concentration of essential oils. J. Appl. Microb. 1998, 84, 538–544. [Google Scholar] [CrossRef]
- Palomino, J.C.; Martin, A.; Camacho, H.; Guerra, H.; Swings, J.; Portaels, F. Resazurin microtiter assay plate: Imple and unexpensive method for detection of drug resistance in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2002, 46, 2720–2722. [Google Scholar] [CrossRef]
- Coutinho, H.D.M.; Costa, J.G.; Lima, E.O.; Falcão-Silva, V.S.; Siqueira-Júnior, J.P. Enhancement of the antibiotic activity against a multiresistant Escherichia coli by Mentha arvensis L. and chlorpromazine. Chemotherapy 2008, 54, 328–330. [Google Scholar] [CrossRef]
- Tintino, S.R.; Oliveira-Tintino, C.D.; Campina, F.F.; Costa, M.S.; Cruz, R.P.; Pereira, R.L.; Andrade, J.C.; Sousa, E.O.; Siqueira-Junior, J.P.; Coutinho, H.D.M.; et al. Cholesterol and ergosterol affect the activity of Staphylococcus aureus antibiotic efflux pumps. Microb. Pathog. 2017, 104, 133–136. [Google Scholar] [CrossRef]
- Laskowski, R.A.; MacArthur, M.W.; Moss, D.S.; Thornton, J.M. PROCHECK–a program to check the stereochemical quality of protein structures. J. App. Cryst. 1993, 26, 283–291. [Google Scholar] [CrossRef]
- Wass, M.N.; Kelley, L.A.; Sternberg, M.J. 3DLigandSite: Predicting ligand-binding sites using similar structures. Nucleic. Acids Res. 2010, 38, W469–W473. [Google Scholar] [CrossRef] [PubMed]
- 3D Structure Generator CORINA Classic. 3D Structure Generator CORINA Classic. Nürnberg: Molecular Networks GmbH. 2019. Available online: http://www.mn-am.com (accessed on 10 May 2020).
- Trott, A.J. Olson, AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [PubMed]
- Onawole, A.T.; Kolapo, T.U.; Sulaiman, K.O.; Adegoke, R.O. Structure based virtual screening of the Ebola virus trimeric glycoprotein using consensus scoring. Comput. Biol. chem. 2018, 72, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Nissink, J.W.M. Simple size-independent measure of ligand efficiency. J. Chem. Inform. Model. 2009, 49, 1617–1622. [Google Scholar] [CrossRef] [PubMed]



| RI | Compound | % |
|---|---|---|
| 936 | Alpha-pinene | 0.3 |
| 951 | Camphene | 0.2 |
| 978 | Beta-pinene | 0.1 |
| 1005 | Alpha-felandrene | 1.9 |
| 1026 | Ortho-cymene | 3.0 |
| 1033 | 1,8-Cineole | 0.9 |
| 1145 | Camphor | 0.4 |
| 1189 | Alfa-terpineol | 0.3 |
| 1292 | Safrole | 77.9 |
| 1356 | Eugenol | 0.6 |
| 1414 | (E)-caryophyllene | 0.4 |
| 1476 | Gama-muurolene | 0.3 |
| 1487 | Delta-selinene | 0.5 |
| 1491 | Bicyclogemacrene | 1.1 |
| 1572 | Spathulenol | 4.0 |
| 1648 | 11-selinen-4-alpha-ol | 1.2 |
| Total composition identified | 93.1 | |
| Bacterial Strain | EOOO MIC(μg/mL) | SafroleMIC (μg/mL) |
|---|---|---|
| S. aureus 10 | 512 | 512 |
| E. coli 06 | ≥1024 | ≥1024 |
| P. aeruginosa 24 | ≥1024 | ≥1024 |
| Compound | MIC (µg/mL) | IE (Kcal/mol) | Ki (µM) | SILE |
|---|---|---|---|---|
| Ethidium Bromide | 128 | −7.8 | 1.95 | 0.74 |
| Chlorpromazine | 64 | −6.4 | 20.64 | 7.86 |
| Safrole | 80.63 | −5.9 | 47.95 | 18.72 |
| Compound | MIC (µg/mL) | IE (Kcal/mol) | Ki (µM) | SILE |
|---|---|---|---|---|
| Ethidium Bromide | 128 | −8.6 | 0.51 | 0.19 |
| Chlorpromazine | 85.33 | −6.9 | 8.88 | 3.47 |
| Safrole | 64 | −6.1 | 34.23 | 15.19 |
| Bacterial Strain | Origin | Resistance Profile |
|---|---|---|
| S. aureus 10 | Rectum swab | Amc, Amox, Amp, Asb, Azi, Cefa Cef, Cf, Cip, Cla, Clin, Ery, Lev, Mox, Oxa, Pen |
| E. coli 06 | Urine | Asb, Cefa, Cef, Cfo, Cpm, Ctx |
| P. aeruginosa 24 | Nasal discharge | Ami, Cip, Ctz, Imi, Lev, Mer, Ptz |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almeida, R.S.; Freitas, P.R.; Araújo, A.C.J.; Alencar Menezes, I.R.; Santos, E.L.; Tintino, S.R.; Moura, T.F.; Filho, J.R.; Ferreira, V.A.; Silva, A.C.A.; et al. GC-MS Profile and Enhancement of Antibiotic Activity by the Essential Oil of Ocotea odorífera and Safrole: Inhibition of Staphylococcus aureus Efflux Pumps. Antibiotics 2020, 9, 247. https://doi.org/10.3390/antibiotics9050247
Almeida RS, Freitas PR, Araújo ACJ, Alencar Menezes IR, Santos EL, Tintino SR, Moura TF, Filho JR, Ferreira VA, Silva ACA, et al. GC-MS Profile and Enhancement of Antibiotic Activity by the Essential Oil of Ocotea odorífera and Safrole: Inhibition of Staphylococcus aureus Efflux Pumps. Antibiotics. 2020; 9(5):247. https://doi.org/10.3390/antibiotics9050247
Chicago/Turabian StyleAlmeida, Ray S., Priscilla R. Freitas, Ana Carolina J. Araújo, Irwin R. Alencar Menezes, Eduardo L. Santos, Saulo R. Tintino, Talysson F. Moura, Jaime R. Filho, Vitória A. Ferreira, Ana Cristina A. Silva, and et al. 2020. "GC-MS Profile and Enhancement of Antibiotic Activity by the Essential Oil of Ocotea odorífera and Safrole: Inhibition of Staphylococcus aureus Efflux Pumps" Antibiotics 9, no. 5: 247. https://doi.org/10.3390/antibiotics9050247
APA StyleAlmeida, R. S., Freitas, P. R., Araújo, A. C. J., Alencar Menezes, I. R., Santos, E. L., Tintino, S. R., Moura, T. F., Filho, J. R., Ferreira, V. A., Silva, A. C. A., Silva, L. E., do Amaral, W., Deschamps, C., Iriti, M., & Melo Coutinho, H. D. (2020). GC-MS Profile and Enhancement of Antibiotic Activity by the Essential Oil of Ocotea odorífera and Safrole: Inhibition of Staphylococcus aureus Efflux Pumps. Antibiotics, 9(5), 247. https://doi.org/10.3390/antibiotics9050247









