Design and Characterization of Bioactive Bilayer Films: Release Kinetics of Isopropyl Palmitate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Preparation of Bilayer Films
2.2.1. External Layer: Zein Films
2.2.2. Internal Layer: Pullulan Films Incorporating Licorice Essential Oil
2.3. Characterization of Films
2.3.1. Infrared Spectra
2.3.2. Scanning Electron Microscopy (SEM)
2.3.3. Grammage, Thickness, Mechanical and Optical Properties
2.3.4. Contact Angle Measurements and Surface Free Energy
2.3.5. Barrier Properties
Water Vapor Permeability
Oxygen Permeability
2.3.6. Antioxidant Activity
DPPH (2,2-diphenyl-1-picrylhydrazyl) Free Radical Scavenging Assay
β-Carotene Bleaching Test
2.3.7. Antibacterial Properties
2.4. Study of the Release Kinetics of Isopropyl Palmitate
2.5. Statistical Analysis
3. Results and Discussion
3.1. FTIR Analysis
3.2. Microstructure of Bilayer Films
3.3. Grammage, Thickness, Mechanical and Optical Properties
3.4. Contact Angles and Surface Free Energy
3.5. Barrier Properties
3.6. Antioxidant and Antibacterial Activities
3.7. Release Kinetics of Isopropyl Palmitate
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nithya, V.; Murthy, P.S.K.; Halami, P.M. Development and application of active films for food packaging using antibacterial peptide of Bacillus licheniformis Me1. J. Appl. Microbiol. 2013, 115, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Arcan, İ.; Boyacı, D.; Yemenicioğlu, A. The Use of Zein and Its Edible Films for the Development of Food Packaging Materials. Ref. Modul. Food Sci. 2017. [Google Scholar] [CrossRef]
- Fazeli, M.; Keley, M.; Biazar, E. Preparation and characterization of starch-based composite films reinforced by cellulose nanofibers. Int. J. Biol. Macromol. 2018, 116, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Mastromatteo, M.; Barbuzzi, G.; Conte, A.; Del Nobile, M.A. Controlled release of thymol from zein based film. Innov. Food Sci. Emerg. Technol. 2009, 10, 222–227. [Google Scholar] [CrossRef]
- Sun, H.; Shao, X.; Jiang, R.; Shen, Z.; Ma, Z. Mechanical and barrier properties of corn distarch phosphate-zein bilayer films by thermocompression. Int. J. Biol. Macromol. 2018, 118, 2076–2081. [Google Scholar] [CrossRef]
- Geueke, B.; Groh, K.; Muncke, J. Food packaging in the circular economy: Overview of chemical safety aspects for commonly used materials. J. Clean. Prod. 2018, 193, 491–505. [Google Scholar] [CrossRef]
- Neto, R.J.G.; Genevro, G.M.; Paulo, L.A.; Lopes, P.S.; Moraes, M.A.; Beppu, M.M. Characterization and in vitro evaluation of chitosan/konjac glucomannan bilayer film as a wound dressing. Carbohydr. Polym. 2019, 212, 59–66. [Google Scholar] [CrossRef]
- Luís, Â.; Pereira, L.; Domingues, F.; Ramos, A. Development of a carboxymethyl xylan film containing licorice essential oil with antioxidant properties to inhibit the growth of foodborne pathogens. LWT-Food Sci. Technol. 2019, 111, 218–225. [Google Scholar]
- Luís, Â.; Domingues, F.; Ramos, A. Production of Hydrophobic Zein-Based Films Bioinspired by The Lotus Leaf Surface: Characterization and Bioactive Properties. Microorganisms 2019, 7. [Google Scholar] [CrossRef] [Green Version]
- SCOGS (Select Committee on GRAS Substances); Food and Drug Administration. Available online: https://www.accessdata.fda.gov/scripts/fdcc/?set=SCOGS&sort=Sortsubstance&order=ASC&startrow=1&type=basic&search=licorice (accessed on 20 July 2018).
- EAFUS (Everything Added to Food): A Food Additivie Database. Available online: http://www.cfsan.fda.gov/~dms/eafus.html (accessed on 14 September 2018).
- Zhang, Y.; Cui, L.; Xiaoxia, C.; Zhang, H.; Shi, N.; Li, C.; Chen, Y.; Kong, W. Zein-based films and their usage for controlled delivery: Origin, classes and current landscape. J. Control. Release 2015, 206, 206–219. [Google Scholar] [CrossRef]
- Singh, R.S.; Kaur, N.; Kennedy, J.F. Pullulan production from agro-industrial waste and its applications in food industry: A review. Carbohydr. Polym. 2019, 217, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Chai, Y.; Zhang, G. Synergistic effect of oleic acid and glycerol on zein film plasticization. J. Agric. Food Chem. 2012, 60, 10075–10081. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Xu, T.; Gao, C.; Liu, X.; Zhang, N.; Feng, X.; Liu, X.; Shen, X.; Tang, X. Evaluations of physicochemical and biological properties of pullulan-based films incorporated with cinnamon essential oil and Tween 80. Int. J. Biol. Macromol. 2019, 122, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Morsy, M.K.; Khalaf, H.H.; Sharoba, A.M.; El-Tanahi, H.H.; Cutter, C.N. Incorporation of Essential Oils and Nanoparticles in Pullulan Films to Control Foodborne Pathogens on Meat and Poultry Products. J. Food Sci. 2014, 79, M676–M684. [Google Scholar] [CrossRef]
- Silva, Â.; Duarte, A.; Sousa, S.; Ramos, A.; Domingues, F.C. Characterization and antimicrobial activity of cellulose derivatives films incorporated with a resveratrol inclusion complex. LWT–Food Sci. Technol. 2016, 73, 481–489. [Google Scholar] [CrossRef]
- Queirós, L.C.C.; Sousa, S.C.L.; Duarte, A.F.S.; Domingues, F.C.; Ramos, A.M.M. Development of carboxymethyl xylan films with functional properties. J. Food Sci. Technol. 2017, 54, 9–17. [Google Scholar] [CrossRef] [Green Version]
- Luís, Â.; Silva, F.; Sousa, S.; Duarte, A.P.; Domingues, F. Antistaphylococcal and biofilm inhibitory activities of gallic, caffeic, and chlorogenic acids. Biofouling 2014, 30, 69–79. [Google Scholar] [CrossRef]
- Good, R.; van Oss, C. The modern theory of contact angles and the hydrogen bond components of surface energies. In Modern Approaches to Wettability Applications; Schrader, M., Loeb, G., Eds.; Plenum Press: New York, NY, USA, 1991; pp. 1–27. [Google Scholar]
- Ramos, A.; Sousa, S.; Evtuguin, D.V.; Gamelas, J.A.F. Functionalized xylans in the production of xylan-coated paper laminates. React. Funct. Polym. 2017, 117, 89–96. [Google Scholar] [CrossRef]
- Nostro, A.; Scaffaro, R.; D’Arrigo, M.; Botta, L.; Filocamo, A.; Marino, A.; Bisignano, G. Study on carvacrol and cinnamaldehyde polymeric films: Mechanical properties, release kinetics and antibacterial and antibiofilm activities. Appl. Microbiol. Biotechnol. 2012, 96, 1029–1038. [Google Scholar] [CrossRef]
- Zhang, W.; Shu, C.; Chen, Q.; Cao, J.; Jiang, W. The multi-layer film system improved the release and retention properties of cinnamon essential oil and its application as coating in inhibition to penicillium expansion of apple fruit. Food Chem. 2019, 299, 125109. [Google Scholar] [CrossRef]
- Santos, E.S.; Luís, Â.; Gonçalves, J.; Rosado, T.; Pereira, L.; Gallardo, E.; Duarte, A.P. Julbernardia paniculata and Pterocarpus angolensis: From Ethnobotanical Surveys to Phytochemical Characterization and Bioactivities Evaluation. Molecules 2020, 25, 1828. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Liu, Z.; Li, J.; Nie, S.; Pan, W. Effects of isopropyl palmitate on the skin permeation of drugs. Biol. Pharm. Bull. 2006, 29, 2324–2326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tay, B.Y.P.; Yung, S.C.; Teoh, T.Y. Determination and confirmation of isopropyl p-toluenesulfonate in cosmetics by HPLC-diode array detector method and GC–MS. Int. J. Cosmet. Sci. 2016, 38, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Tay, B.; Ping, Y.; Aziz, H.A. An HPLC method for low-level detection and quantification of isopropyl p-toluenesulfonate in palm- based isopropyl esters. Trends Pharm. Biomed. Anal. Res. 2018, 1, 1–5. [Google Scholar]
- Fu, Y.; Kao, W.J. Drug Release Kinetics and Transport Mechanisms of Non-degradable and Degradable Polymeric Delivery Systems. Expert Opin. Drug Deliv. 2010, 7, 429–444. [Google Scholar] [CrossRef]
- Messager, S.; Hammer, K.A.; Carson, C.F.; Riley, T.V. Assessment of the antibacterial activity of tea tree oil using the European EN 1276 and EN 12054 standard suspension tests. J. Hosp. Infect. 2005, 59, 113–125. [Google Scholar] [CrossRef]
- Evangelho, J.A.; Dannenberg, G.S.; Biduski, B.; Halal, S.L.M.; Kringel, D.H.; Gularte, M.A.; Fiorentini, A.M.; Zavareze, E.R. Antibacterial activity, optical, mechanical, and barrier properties of corn starch films containing orange essential oil. Carbohydr. Polym. 2019, 222, 114981. [Google Scholar] [CrossRef]
- Yeddes, W.; Djebali, K.; Aidi, W.W.; Horchani-Naifer, K.; Hammami, M.; Younes, I.; Saidani, M.T. Gelatin-chitosan-pectin films incorporated with rosemary essential oil: Optimized formulation using mixture design and response surface methodology. Int. J. Biol. Macromol. 2020, 154, 92–103. [Google Scholar] [CrossRef]
- Nunes, M.R.; Castilho, M.S.M.; Veeck, A.P.L.; Rosa, C.G.; Noronha, C.M.; Maciel, M.V.O.B.; Barreto, P.M. Antioxidant and antimicrobial methylcellulose films containing Lippia alba extract and silver nanoparticles. Carbohydr. Polym. 2018, 192, 37–43. [Google Scholar] [CrossRef]
- Dong, F.; Padua, G.W.; Wang, Y. Controlled formation of hydrophobic surfaces by self-assembly of an amphiphilic natural protein from aqueous solutions. Soft Matter 2013, 9, 5933–5941. [Google Scholar] [CrossRef]
- Liu, B.; Wang, W.; Jiang, G.; Mei, X.; Wang, Z.; Wang, K.; Cui, J. Study on hierarchical structured PDMS for surface super-hydrophobicity using imprinting with ultrafast laser structured models. Appl. Surf. Sci. 2016, 364, 528–538. [Google Scholar] [CrossRef]
- Pastor, C.; Sánchez-González, L.; Chiralt, A.; Cháfer, M.; González-Martínez, C. Physical and antioxidant properties of chitosan and methylcellulose based films containing resveratrol. Food Hydrocoll. 2013, 30, 272–280. [Google Scholar] [CrossRef] [Green Version]
- Escamilla-García, M.; Caldéron-Dominguéz, G.; Chanona-Pérez, J.J.; Mendoza-Madrigal, A.G.; Di Pierro, P.; García-Alméndarez, B.E.; Amaro-Reyes, A.; Regalado-González, C. Physical, structural, barrier, and antifungal characterization of chitosan-zein edible films with added essential oils. Int. J. Mol. Sci. 2017, 18, 2370. [Google Scholar] [CrossRef] [Green Version]
- Shi, W.; Dumont, M.J. Review Bio-based films from zein, keratin, pea, and rapeseed protein feedstocks. J. Mater. Sci. 2014, 49, 1915–1930. [Google Scholar] [CrossRef]
- Bridier, A.; Sanchez-Vizuete, P.; Guilbaud, M.; Piard, J.C.; Naïtali, M.; Briandet, R. Biofilm-associated persistence of food-borne pathogens. Food Microbiol. 2015, 45, 167–178. [Google Scholar] [CrossRef]
- Collignon, P.C.; Conly, J.M.; Andremont, A.; McEwen, S.A.; Aidara-Kane, A. World Health Organization Ranking of Antimicrobials According to Their Importance in Human Medicine: A Critical Step for Developing Risk Management Strategies to Control Antimicrobial Resistance from Food Animal Production. Clin. Infect. Dis. 2016, 63, 1087–1093. [Google Scholar] [CrossRef] [Green Version]
- Mild, R.M.; Joens, L.A.; Friedman, M.; Olsen, C.W.; Mchugh, T.H.; Law, B.; Ravishankar, S. Antimicrobial edible apple films inactivate antibiotic resistant and susceptible Campylobacter jejuni strains on chicken breast. J. Food Sci. 2011, 76, M163–M168. [Google Scholar] [CrossRef]
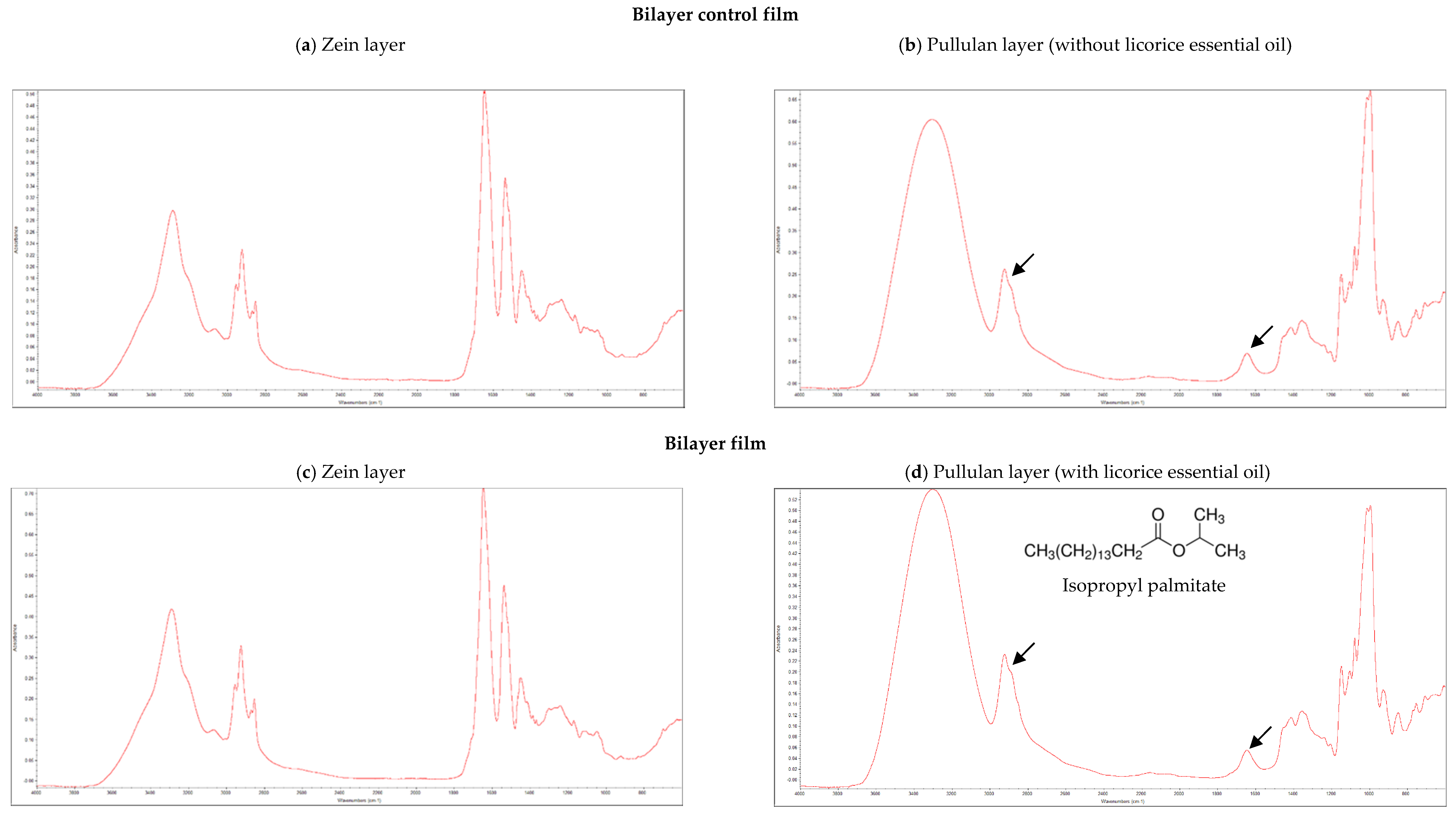
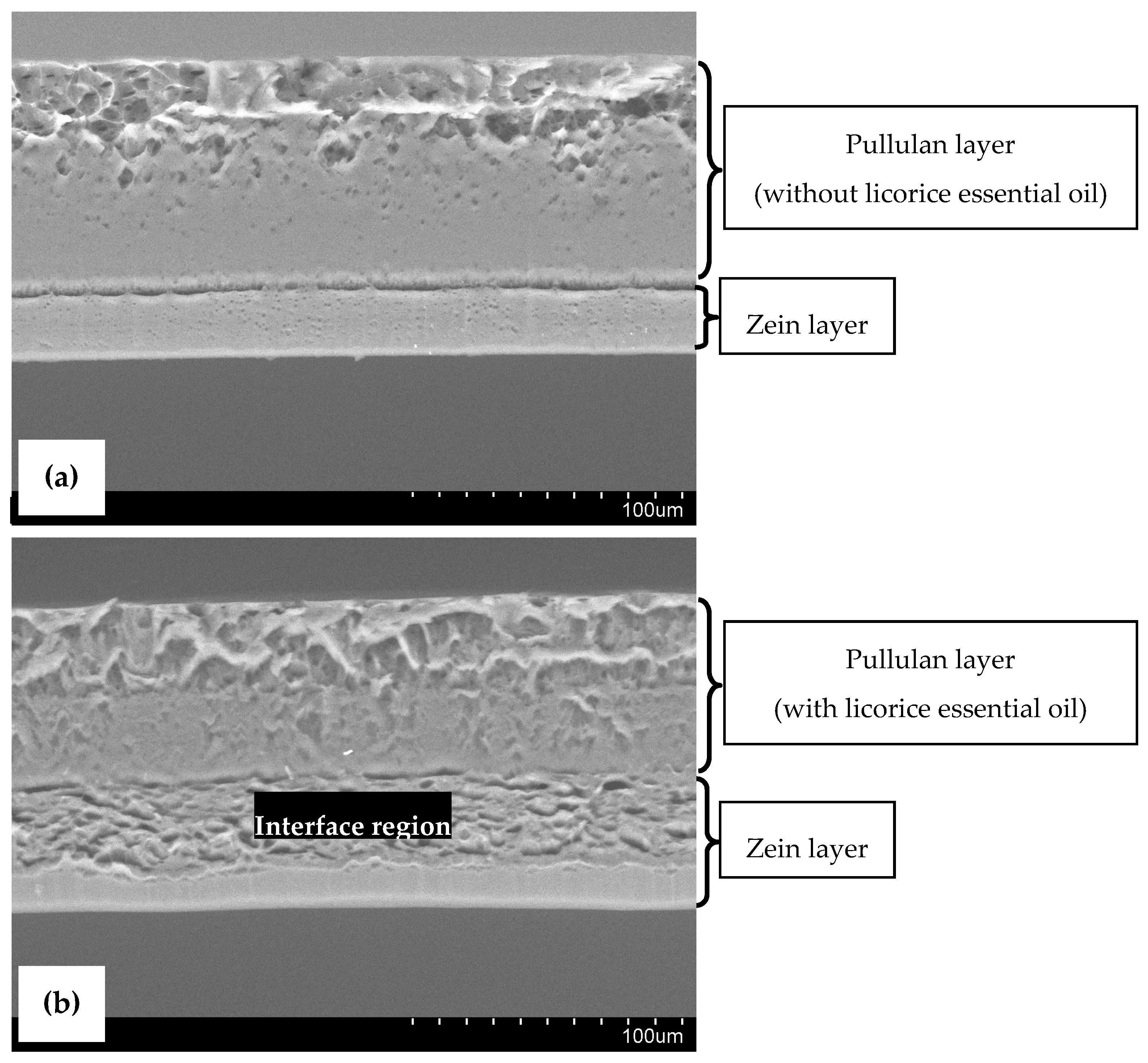
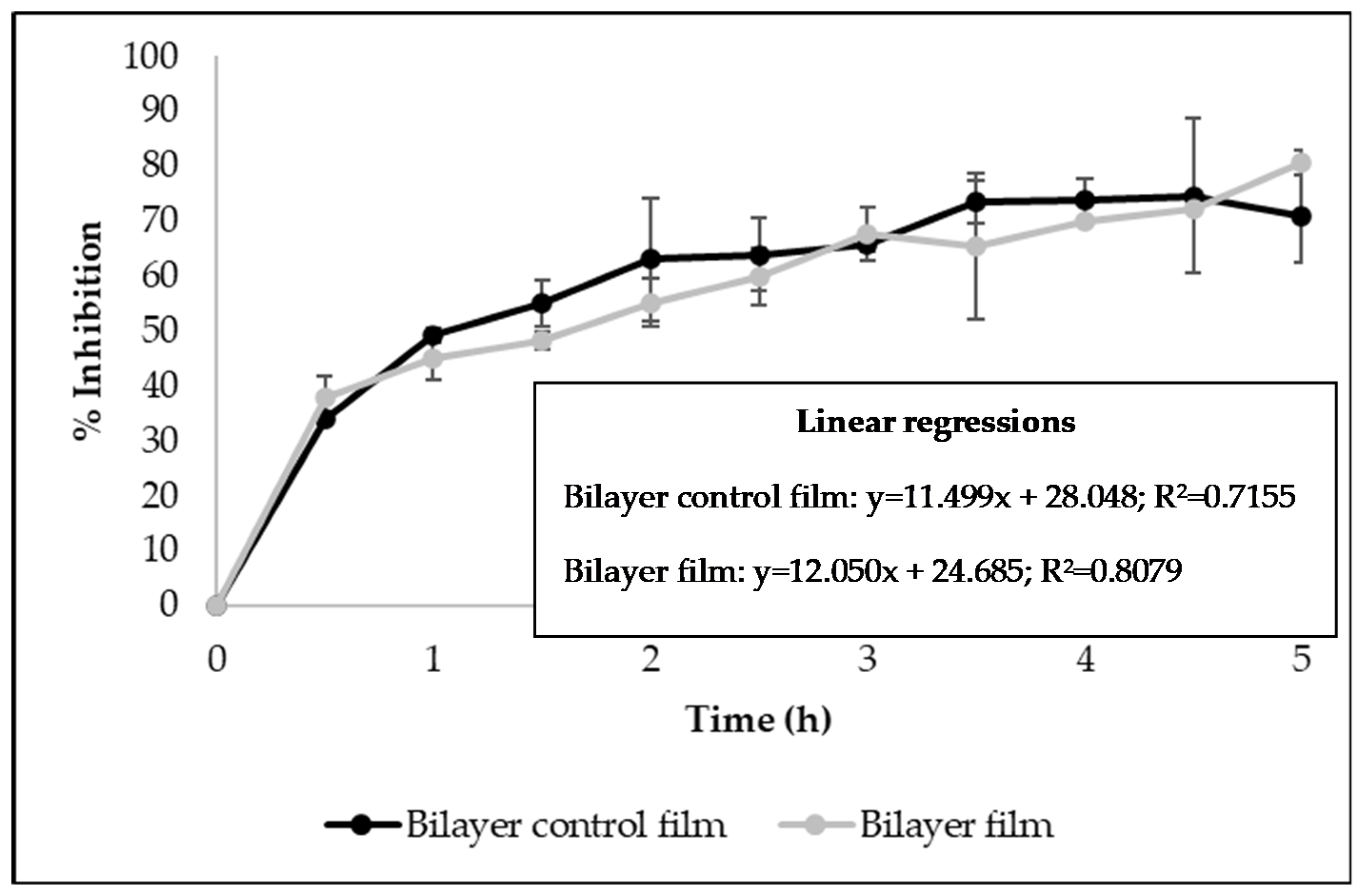
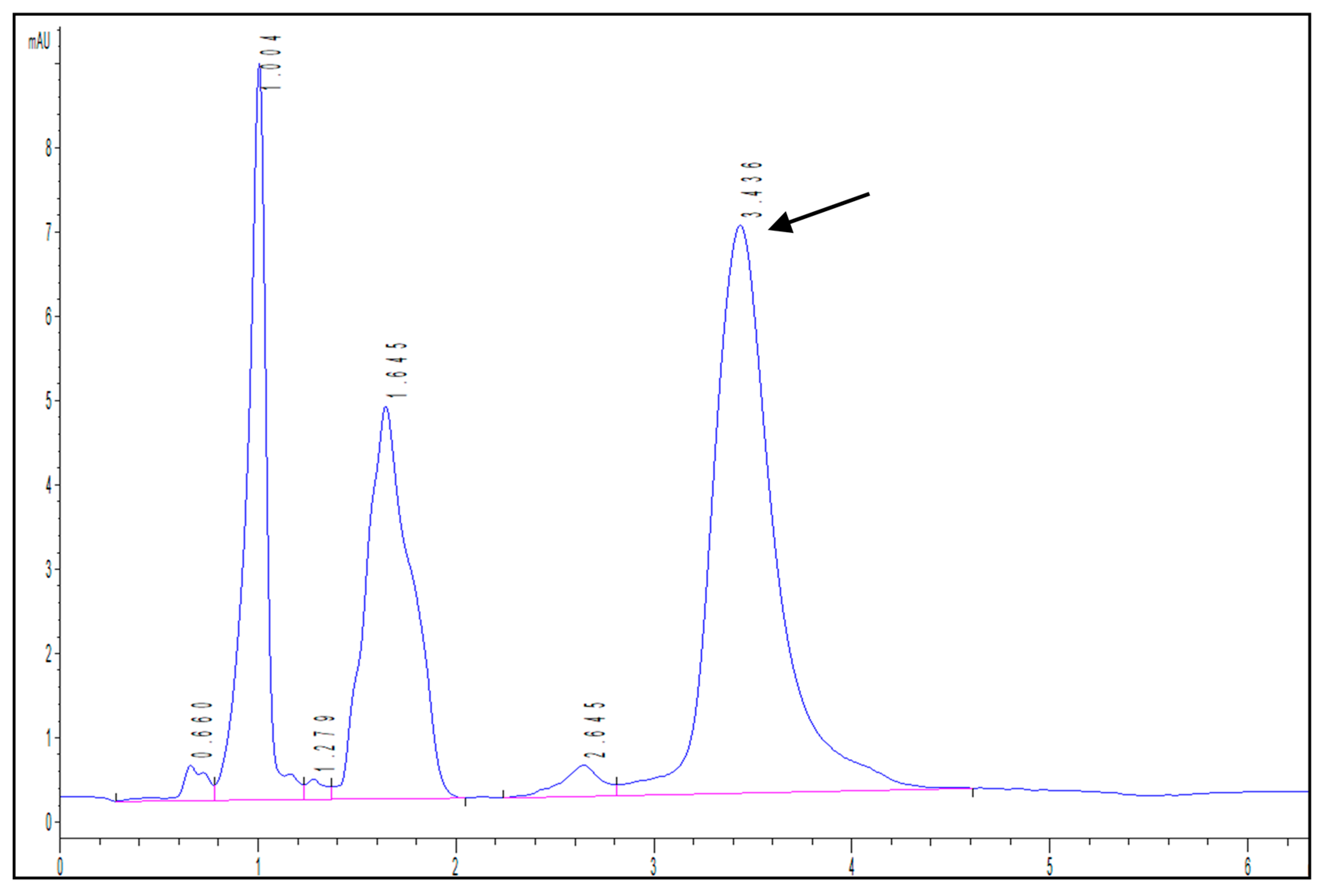

| Properties | Bilayer Control Films | Bilayer Films (with Licorice Essential Oil) | p-Value | |
|---|---|---|---|---|
| Grammage (g/m2) | 134.38 ± 2.13 | 149.16 ± 1.74 | 0.001 * | |
| Thickness (µm) | 154.16 ± 6.63 | 168.37 ± 5.42 | 0.047 * | |
| Mechanical properties | Elongation at break (%) | 3.00 ± 0.01 | 2.39 ± 0.36 | 0.099 |
| Tensile index (N·m/g) | 25.33 ± 0.93 | 20.33 ± 1.67 | 0.018 * | |
| Elastic modulus (MPa) | 1196.92 ± 149.41 | 1119.66 ± 62.99 | 0.476 | |
| Optical properties | L* (lightness) | 31.87 ± 0.37 | 38.45 ± 0.44 | <0.001 * |
| a* (redness) | −0.61 ± 0.03 | −0.17 ± 0.03 | <0.001 * | |
| b* (yellowness) | 17.68 ± 0.88 | 22.95 ± 1.04 | 0.003 * | |
| Transparency (%) | 92.10 ± 0.21 | 88.05 ± 0.17 | <0.001 * | |
| Properties | Bilayer Control Films | Bilayer Films | p-Values | ||
|---|---|---|---|---|---|
| Zein Layer a | Pullulan Layer (without Licorice Essential Oil) b | Zein Layer c | Pullulan Layer (with Licorice Essential Oil) d | ||
| Water contact angle (°) | 68.79 ± 1.25 | 44.22 ± 1.11 | 71.73 ± 2.29 | 43.71 ± 1.41 | 0.143 ac 0.650 bd |
| Ethylene glycol contact angle (°) | 58.64 ± 1.86 | 61.97 ± 2.78 | 61.23 ± 0.61 | 61.76 ± 2.49 | 0.127 ac 0.927 bd |
| Diiodomethane contact angle (°) | 36.20 ± 1.70 | 37.20 ± 1.29 | 36.35 ± 1.94 | 38.22 ± 2.07 | 0.925 ac 0.516 bd |
| Total surface free energy, ɤT (mN/m) | 39.65 ± 1.98 | 52.89 ± 2.64 | 38.05 ± 1.90 | 53.14 ± 2.66 | 0.370 ac 0.914 bd |
| Dispersive component, ɤD (mN/m) | 28.41 ± 1.42 | 16.74 ± 0.84 | 28.17 ± 1.41 | 16.60 ± 0.83 | 0.846 ac 0.847 bd |
| Polar component, ɤP (mN/m) | 11.24 ± 0.56 | 36.15 ± 1.81 | 9.88 ± 0.66 | 36.54 ± 1.83 | 0.054 ac 0.806 bd |
| Permeability | Bilayer Control Films | Bilayer Films (with Licorice Essential Oil) | p-Value | |
|---|---|---|---|---|
| Water vapor | WVTR (g/m2·day) | 16.24 ± 0.68 | 15.53 ± 0.34 | 0.206 |
| WVP (g/Pa·day·m) (×10−6) | 1.89 ± 0.08 | 1.98 ± 0.04 | 0.182 | |
| Oxygen | OTR (cm3/m2·day) | 1845.15 ± 164.25 | 1161.33 ± 6.69 | 0.019 * |
| OP (cm3·µm/m2·day·kPa) | 3041.00 ± 246.89 | 2143.00 ± 168.29 | 0.009 * | |
| Properties | Bilayer Control Films | Bilayer Films (with Licorice Essential Oil) | p-Value | |
|---|---|---|---|---|
| β-carotene bleaching test | % Inhibition | 39.86 ± 0.83 | 91.89 ± 1.07 | <0.001 * |
| Inhibition zones (mm) | E. faecalis ATCC 29212 | 6.00 ± 0.00 (−) | 7.02 ± 0.56 (+) | 0.087 |
| L. monocytogenes LMG 16779 | 6.00 ± 0.00 (−) | 6.84 ± 0.48 (+) | 0.094 | |
| Conditions | Release Constant, k (s−1) (×10−5) | Error (×10−14) | |
|---|---|---|---|
| 10% ethanol | room temperature | 9.43 | 1.28 |
| 4 °C | 6.29 | 1.00 | |
| 50% ethanol | room temperature | 45.40 | 1.00 |
| 4 °C | 9.00 | 1.28 | |
| Conditions | Diffusion Coefficient, D (m2/s) (×10−9) | Error (×10−5) | |
|---|---|---|---|
| 10% ethanol | room temperature | 0.363 | 8.472 |
| 4 °C | 0.250 | 5.706 | |
| 50% ethanol | room temperature | 1.004 | 8.089 |
| 4 °C | 0.142 | 1.642 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luís, Â.; Gallardo, E.; Ramos, A.; Domingues, F. Design and Characterization of Bioactive Bilayer Films: Release Kinetics of Isopropyl Palmitate. Antibiotics 2020, 9, 443. https://doi.org/10.3390/antibiotics9080443
Luís Â, Gallardo E, Ramos A, Domingues F. Design and Characterization of Bioactive Bilayer Films: Release Kinetics of Isopropyl Palmitate. Antibiotics. 2020; 9(8):443. https://doi.org/10.3390/antibiotics9080443
Chicago/Turabian StyleLuís, Ângelo, Eugenia Gallardo, Ana Ramos, and Fernanda Domingues. 2020. "Design and Characterization of Bioactive Bilayer Films: Release Kinetics of Isopropyl Palmitate" Antibiotics 9, no. 8: 443. https://doi.org/10.3390/antibiotics9080443
APA StyleLuís, Â., Gallardo, E., Ramos, A., & Domingues, F. (2020). Design and Characterization of Bioactive Bilayer Films: Release Kinetics of Isopropyl Palmitate. Antibiotics, 9(8), 443. https://doi.org/10.3390/antibiotics9080443








