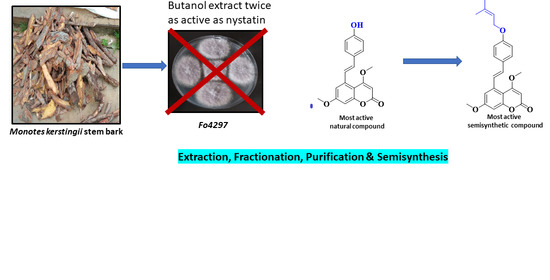
Synthesis of Novel Stilbene–Coumarin Derivatives and Antifungal Screening of Monotes kerstingii-Specialized Metabolites Against Fusarium oxysporum
Abstract
1. Introduction
2. Results and Discussion
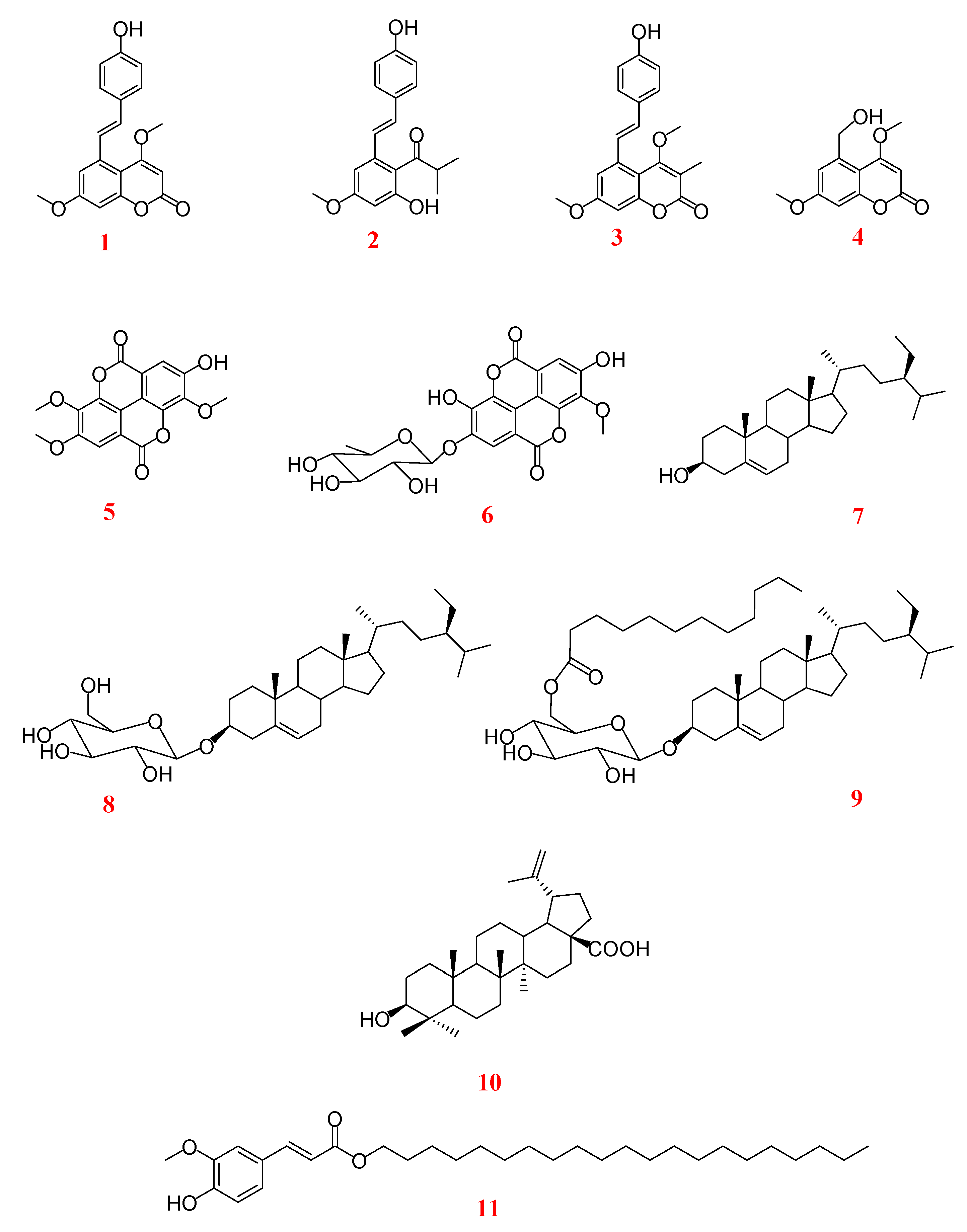
2.1. Antifungal Activity of Crude Extract, Fractions, and Specialized metabolites from Monotes kerstingii against Fo32931 and Fo4287
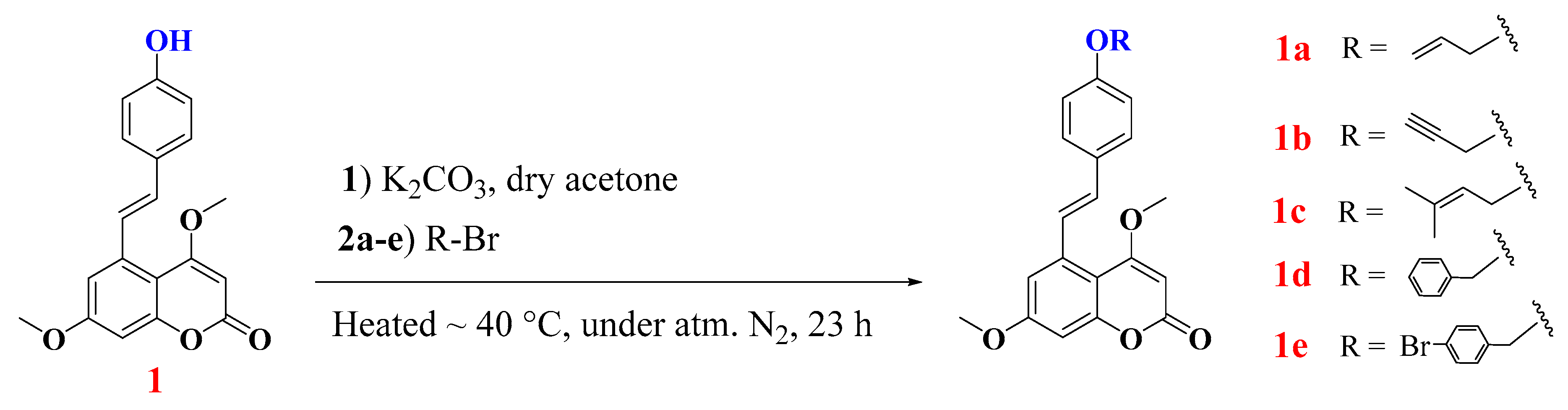
2.2. Alkylation of Compound 1: Semisynthesis of Allylated, Propargylated, Prenylated, and Benzylated Derivatives 1a–e
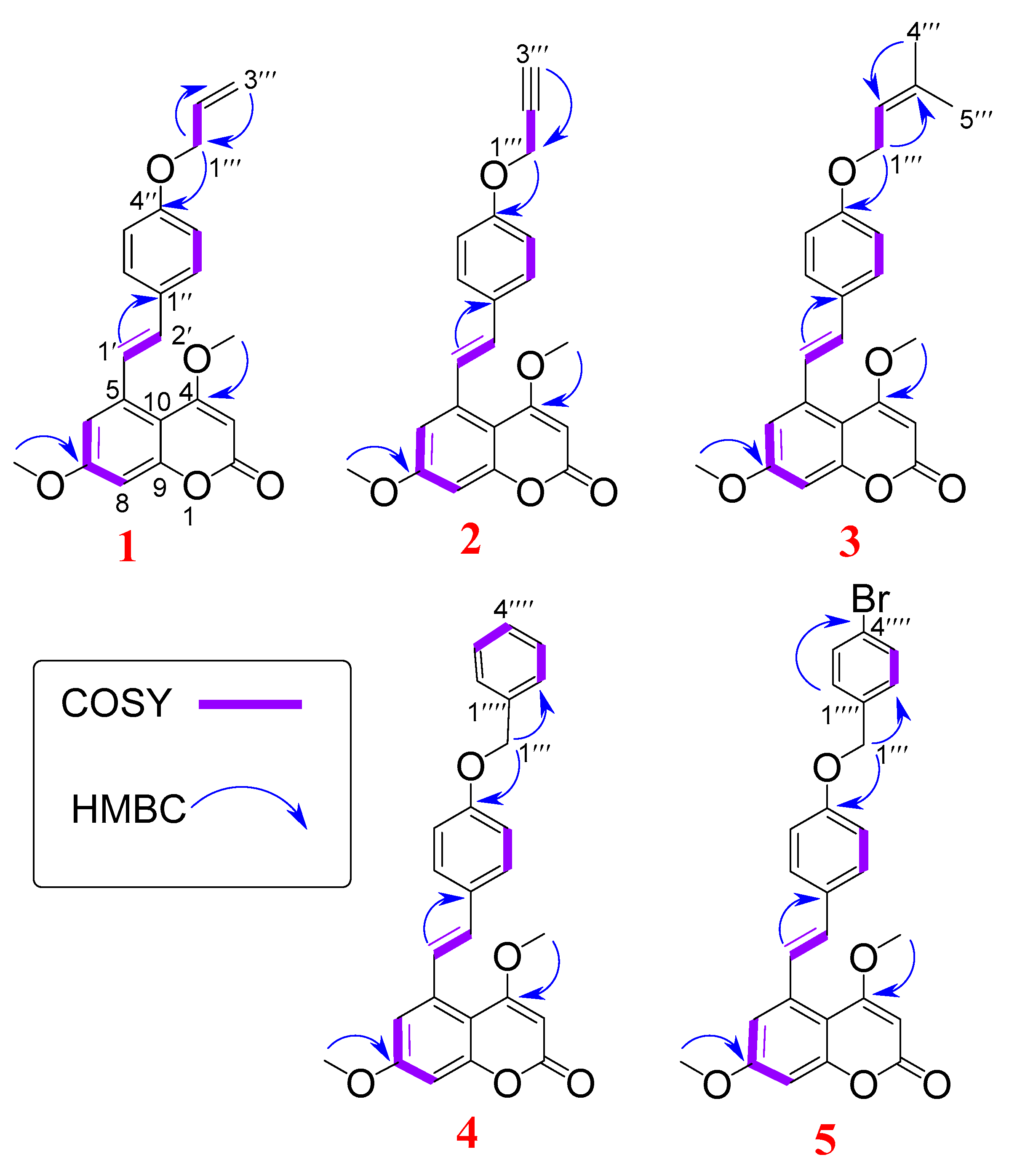
2.2.1. Characterization of Compounds 1a–e
2.2.2. Antifungal Activity of Semi-Synthetic Derivatives against Fo32931 and Fo4287
3. Materials and Methods
3.1. General Methods
3.2. Plant Material
3.3. Extraction of M. kerstingii Stem Barks and Isolation of Specialized Metabolites
3.4. Alkylation of Compound 1
3.5. Antifungal Bioassay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shuping, D.S.S.; Eloff, J.N. The use of plants to protect plants and food against fungal pathogens: A Review. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 120–127. [Google Scholar] [CrossRef]
- Ramaiah, A.K.; Garampalli, R.k.H. In vitro antifungal activity of plant extracts against Fusarium oxysporum f. sp. lycopersici. Asian J. Plant Sci. Res. 2015, 5, 22–27. [Google Scholar]
- Wickern, G.M. Fusarium allergic fungal sinusitis. J. Allergy Clin. Immunol. 1993, 92, 624–625. [Google Scholar] [CrossRef]
- Kredics, L.; Narendran, V.; Shobana, C.S.; Vágvölgyi, C.; Manikandan, P.; Indo-Hungarian Fungal Keratitis Working Group. Filamentous fungal infections of the cornea: A global overview of epidemiology and drug sensitivity. Mycoses 2015, 58, 243–260. [Google Scholar] [CrossRef]
- Al-Hatmi, A.M.; Meis, J.F.; de Hoog, G.S. Fusarium: Molecular Diversity and Intrinsic Drug Resistance. PLoS Pathog 2016, 12, e1005464. [Google Scholar] [CrossRef]
- Boulenouar, N.; Marouf, A.; Cheriti, A.; Belboukhari, N. Medicinal Plants Extracts as Source of Antifungal Agents against Fusarium oxysporum f. sp. Albedinis. J. Agric. Sci. Technol. 2012, 14, 659–669. [Google Scholar]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Butler, M.S. The role of natural product chemistry in drug discovery. J. Nat. Prod. 2004, 67, 2141–2153. [Google Scholar] [CrossRef]
- Cowan, M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef]
- Guo, Z. The modification of natural products for medical use. Acta Pharm. Sin. B 2017, 7, 119–136. [Google Scholar] [CrossRef]
- Taffou, J.B.; Fokou, H.; Zeuko’o, E.M.; Tchokouaha, L.R.T.; Mfopa, A.N.; Kamdem, M.S.; Ngouana, V.; Kenfack, I.F.; Boyom, F.F. Anti-yeast potential of some Annonaceae species from Camerronian biodiveristy. Int. J. Biol.Chem Sci. 2017, 11, 15–31. [Google Scholar] [CrossRef]
- Mambe, F.T.; Na-Iya, J.; Fotso, G.W.; Ashu, F.; Ngameni, B.; Ngadjui, B.T.; Beng, V.P.; Kuete, V. Antibacterial and Antibiotic Modifying Potential of Crude Extracts, Fractions, and Compounds from. Evid. Based Complement. Altern. Med. 2019, 2019, 7507549. [Google Scholar] [CrossRef]
- Kuete, V.; Wabo, H.K.; Eyong, K.O.; Feussi, M.T.; Wiench, B.; Krusche, B.; Tane, P.; Folefoc, G.N.; Efferth, T. Anticancer activities of six selected natural compounds of some Cameroonian medicinal plants. PLoS ONE 2011, 6, e21762. [Google Scholar] [CrossRef]
- Tabopda, T.K.; Fotso, G.W.; Ngoupayo, J.; Mitaine-Offer, A.C.; Ngadjui, B.T.; Lacaille-Dubois, M.A. Antimicrobial dihydroisocoumarins from Crassocephalum biafrae. Planta Med. 2009, 75, 1258–1261. [Google Scholar] [CrossRef]
- Fotso, G.W.; Mogue Kamdem, L.; Dube, M.; Fobofou, S.A.; Ndjie Ebene, A.; Arnold, N.; Tchaleu Ngadjui, B. Antimicrobial secondary metabolites from the stem barks and leaves of Monotes kerstingii Gilg (Dipterocarpaceae). Fitoterapia 2019, 137, 104239. [Google Scholar] [CrossRef]
- Morales, G.; Paredes, A.; Sierra, P.; Loyola, L.A. Antimicrobial activity of three baccharis species used in the traditional medicine of Northern Chile. Molecules 2008, 13, 790–794. [Google Scholar] [CrossRef]
- Khan, N.; Martínez-Hidalgo, P.; Ice, T.A.; Maymon, M.; Humm, E.A.; Nejat, N.; Sanders, E.R.; Kaplan, D.; Hirsch, A.M. Antifungal Activity of. Front. Microbiol. 2018, 9, 2363. [Google Scholar] [CrossRef]
- Nefzi, A.; Jabnoun-Khiareddine, H.; Abdallah, R.A.B.; Ammar, N.; Medimagh-Saidana, S.; Haouala, R.; Daami-Remadi, M. Suppressing Fusarium Crown and Root Rot infections and enhancing the growth of tomato plants by Lycium arabicum Schweinf. Ex Boiss. extracts. S. Afr. J. Bot. 2017, 113, 288–299. [Google Scholar] [CrossRef]
- Montagner, C.; de Souza, S.M.; Groposoa, C.; Delle Monache, F.; Smânia, E.F.; Smânia, A. Antifungal activity of coumarins. Z. Naturforsch. C J. Biosci. 2008, 63, 21–28. [Google Scholar] [CrossRef]
- Wu, H.-S.; Raza, W.; Liu, D.-Y.; Wu, C.-L.; Mao, Z.-S.; Xu, Y.-C.; Shen, Q.-R. Allelopathic impact of applied coumarin on Fusarium oxysporum f.sp. niveum. World J. Microbiol. Biotechnol. 2008, 24, 1297–1304. [Google Scholar] [CrossRef]
- Rongai, D.; Pulcini, P.; Pesce, B.; Milano, F. Antifungal activity of pomegranate peel extract against fusarium wilt of tomato. Eur. J. Plant Pathol. 2017, 147, 229–238. [Google Scholar] [CrossRef]
- Mbambo, B.; Odhav, B.; Mohanlall, V. Antifungal activity of stigmasterol, sitosterol and ergosterol from Bulbine natalensis Baker (Asphodelaceae). J. Med. Plants Res. 2012, 6, 5135–5141. [Google Scholar] [CrossRef]
- Guo, J.; Hu, H.; Zhao, Q.; Wang, T.; Zou, Y.; Yu, S.; Wu, Q.; Guo, Z. Synthesis and antifungal activities of glycosylated derivatives of the cyclic peptide fungicide caspofungin. ChemMedChem 2012, 7, 1496–1503. [Google Scholar] [CrossRef]
- Moore, D. Effects of hexose analogues on fungi: Mechanisms of inhibition and resistance. New Phytol. 1981, 87, 487–515. [Google Scholar] [CrossRef]
- Ahmad, B.; Khan, I.; Bashir, S.; Azam, S.; Ali, N. The antifungal, cytotoxic, antitermite and insecticidal activities of Zizyphus jujube. Pak. J. Pharm. Sci. 2011, 24, 489–493. [Google Scholar]
- Sunita, P.; Jha, S.; Pattanayak, S.P.; Mishra, S. Antimicrobial activity of a halophytic plant Cressa cretica L. J.Sci. Res. 2012, 4, 203–212. [Google Scholar] [CrossRef]
- Teodoro, G.R.; Ellepola, K.; Seneviratne, C.J.; Koga-Ito, C.Y. Potential Use of Phenolic Acids as Anti-Candida Agents: A Review. Front. Microbiol. 2015, 6, 1420. [Google Scholar] [CrossRef]
- Schöneberg, T.; Kibler, K.; Sulyok, M.; Musa, T.; Bucheli, T.D.; Mascher, F.; Bertossa, M.; Voegele, R.T.; Vogelgsang, S. Can plant phenolic compounds reduce Fusarium growth and mycotoxin production in cereals? Food Addit. Contam. Part A Chem. Anal. Control Expo Risk Assess. 2018, 35, 2455–2470. [Google Scholar] [CrossRef]
- Heneczkowski, M.; Kopacz, M.; Nowak, D.; Kuźniar, A. Infrared spectrum analysis of some flavonoids. Acta Pol. Pharm. 2001, 58, 415–420. [Google Scholar]
- Chavez, D.; Chai, H.-B.; Chagwedera, T.E.; Gao, Q.; Farnsworth, N.R.; Cordell, G.A.; Pezzuto, J.M.; Kinghorn, A.D. Novel stilbenes isolated from the root bark of Ekebergia benguelensis. Tetrahedron Lett. 2001, 42, 3685–3688. [Google Scholar] [CrossRef]
- Ngameni, B.; Fotso, W.G.; Ngachussi, E.; Poumale, H.; Ngadjui, B.; Shiono, Y.; Murayama, T. Hemisynthesis and spectroscopic characterization of two novel O-allylated benzophenones from Garcinia punctata Oliv. (Clusiaceae). Asian J. Chem. 2014, 26, 6943–6949. [Google Scholar] [CrossRef]
- Hearn, M. Carbon-13 chemical shifts of some propargyl alchohol derivatives. Tetrahedron 1975, 32, 115–120. [Google Scholar] [CrossRef]
- Uliniuc, A.; Popa, M.; Drockenmuller, E.; Boisson, F.; Leonard, D.; Hamaide, T. Toward tunable amphiphilic copolymers via CuAAC click chemistry of oligocaprolactones onto starch backbone. Carbohydr. Polym. 2013, 96, 259–269. [Google Scholar] [CrossRef]
- Ngnintedo, D.; Fotso, G.W.; Kuete, V.; Nana, F.; Sandjo, L.P.; Karaosmanoğlu, O.; Sivas, H.; Keumedjio, F.; Kirsch, G.; Ngadjui, B.T.; et al. Two new pterocarpans and a new pyrone derivative with cytotoxic activities from. Chem. Cent. J. 2016, 10, 58. [Google Scholar] [CrossRef]
- Xiao, Y.; Lee, I.S. Microbial transformation of quercetin and its prenylated derivatives. Nat. Prod. Res. 2018, 32, 902–908. [Google Scholar] [CrossRef]
- Al-Douh, M.H.; Hamid, S.A.; Osman, H. 1D and 2D NMR Studies of benzyl O-vanillin. Indones. J. Chem. 2008, 8, 411–417. [Google Scholar] [CrossRef]
- Badertscher, M.; Buhlmann, P.; Pretsch, E. Structure Determination of Organic Compounds; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Yang, X.; Yang, J.; Jiang, Y.; Yang, H.; Yun, Z.; Rong, W.; Yang, B. Regiospecific synthesis of prenylated flavonoids by a prenyltransferase cloned from Fusarium oxysporum. Sci. Rep. 2016, 6, 24819. [Google Scholar] [CrossRef]
- Alhassan, A.M.; Abdullahi, M.I.; Uba, A.; Umar, A. Prenylation of aromatic secondary metabolites: A new frontier for development of novel drugs. Trop. J. Pharm. Res. 2014, 13, 307–314. [Google Scholar] [CrossRef]
- Botta, B.; Vitali, A.; Menendez, P.; Misiti, D.; Monache, G.D. Prenylated flavonoids: Pharmacology and Biotechnology. Curr. Med. Chem. 2005, 12, 713–739. [Google Scholar] [CrossRef]
- Konuk, H.B.; Ergüden, B. Phenolic -OH group is crucial for the antifungal activity of terpenoids via disruption of cell membrane integrity. Folia Microbiol. (Praha) 2020. [Google Scholar] [CrossRef]
- Campbell, J. High-throughput assessment of bacterial growth inhibition by optical density measurements. Curr. Protoc. Chem. Biol. 2011, 3. [Google Scholar] [CrossRef]
- Rodriguez-Tudela, J.; Barchiesi, F.; Bille, J.; Chryssanthou, E.; Cuenca-Estrella, M.; Denning, D.; Donnelly, J.; Dupont, B.; Fegeler, W.; Moore, C.; et al. Method for the determination of minimum inhibitory concentration (MIC) by broth dilution of fermentative yeasts. Clin. Microbiol. Infect. 2002, 9, 1–8. [Google Scholar] [CrossRef]
| Type | Code | Fo32931 MIC (µg/mL) | Fo4287 MIC (µg/mL) |
|---|---|---|---|
| Extract | MKS | 841 | 679 |
| Fractions | MKSHEX | >1000 | 331 |
| MKSEA | >1000 | 171 | |
| MKSBUT | 448 | 23 | |
| Hemisynthetic | 1a | 477 | >1000 |
| 1b | 281 | 658 | |
| 1c | 103 | >1000 | |
| 1d | 708 | >1000 | |
| 1e | 341 | 621 | |
| Natural | 1 | 116 | 96 |
| 2 | 337 | 286 | |
| 3 | 327 | 330 | |
| 4 | 553 | 273 | |
| 5 | 98 | 180 | |
| 6 | 116 | 361 | |
| 7 | 408 | 915 | |
| 8 | 174 | 457 | |
| 9 | 473 | >1000 | |
| 10 | >1000 | >1000 | |
| 11 | 523 | >1000 | |
| Pop | Nystatin | 50 | 50 |
| N° | Compound 1a δH (m, J in Hz) | Compound 1b δH (m, J in Hz) | Compound 1c δH (m, J in Hz) | Compound 1d δH (m, J in Hz) | Compound 1e δH (m, J in Hz) |
|---|---|---|---|---|---|
| 3 | 5.49 (1H, s) | 5.52 (1H, s) | 5.49 (1H, s) | 5.51 (1H, s) | 5.62 (1H, s) |
| 4 | - | - | - | - | - |
| 5 | - | - | - | - | - |
| 6 | 6.87 (1H, d, 2.6) | 6.89 (1H, d, 2.4) | 6.87 (1H, d, 2.6) | 6.89 (1H, d, 2.6) | 6.99 (1H, d, 2.5) |
| 7 | - | - | - | - | - |
| 8 | 6.66 (1H, d, 2.5) | 6.68 (1H, d, 2.6) | 6.65 (1H, d, 2.6) | 6.67 (1H, d, 2.6) | 6.79 (1H, d, 2.5) |
| 9 | - | - | - | - | - |
| 10 | - | - | - | - | - |
| 1′ | 7.68 (1H, d, 16.0) | 7.71 (1H, d, 16.0) | 7.67 (1H, d, 15.9) | 7.69 (1H, d, 16.0) | 7.80 (1H, d, 15.9) |
| 2′ | 6.70 (1H, d, 16.0) | 6.73 (1H, d, 16.0) | 6.71 (1H, d, 15.9) | 6.73 (1H, d, 16.0) | 6.81 (1H, d, 15.9) |
| 1″ | - | - | - | - | - |
| 2″, 6″ | 7.35 (2H, d, 8.7) | 7.39 (2H, d, 8.6) | 7.35 (2H, d, 8.7) | 7.35 (2H, d, 8.8) | 7.47 (2H, d, 8.7) |
| 4″ | - | - | - | - | - |
| 3″, 5″ | 6.86 (2H, d, 8.7) | 6.93 (2H, d, 8.8) | 6.85 (2H, d, 8.7) | 6.92 (2H, d, 8.8) | 6.99 (2H, d, 8.7) |
| 1‴ | 4.50 (2H, d, 2.6) | 4.66 (2H, d, 2.4) | 4.46 (2H, d, 6.7) | 5.04 (2H, s) | 5.09 (2H, s) |
| 2‴ | 5.99 (1H, m) | 5.43 (1H, t, 2.7) | |||
| 3‴ | 5.36 (1H, dd, 17.3; 1.5) 5.24 (1H, d, 11.8) | 2.48 (1H, d, 2.4) | |||
| 4‴ | 1.73 (3H, s) | ||||
| 5‴ | 1.68 (3H, s) | ||||
| 2⁗, 6⁗ | 7.25–7.40 m | 7.35 (2H, d, 8.5) | |||
| 3⁗, 5⁗ | 7.55 (2H, d, 8.5) | ||||
| 4⁗ | - | ||||
| 4-OMe | 3.88 (3H, s) | 3.92 (3H, s) | 3.87 (3H, s) | 3.90 (3H, s) | 3.99 (3H, s) |
| 7-OMe | 3.80 (3H, s) | 3.89 (3H, s) | 3.80 (3H, s) | 3.82 (3H, s) | 3.92 (3H, s) |
| N° | Compound 1a δC (m) | Compound 1b δc (m) | Compound 1c δc (m) | Compound 1d δc (m) | Compound 1e δc (m) |
|---|---|---|---|---|---|
| 2 | 162.1 (s) | 162.1 (s) | 162.1 (s) | 162.0 (s) | 162.1 (s) |
| 3 | 88.2 (d) | 88.2 (d) | 88.2 (d) | 88.0 (d) | 88.2 (d) |
| 4 | 169.2 (s) | 169.2 (s) | 169.3 (s) | 169.2 (s) | 169.2 (s) |
| 5 | 131.0 (s) | 131.0 (s) | 130.0 (s) | 130.2 (s) | 130.5 (s) |
| 6 | 111.7 (d) | 111.9 (d) | 111.7 (d) | 111.6 (d) | 111.8 (d) |
| 7 | 162.9 (s) | 162.9 (s) | 162.9 (s) | 162.8 (s) | 162.9 (s) |
| 8 | 100.0 (d) | 100.1 (d) | 100.0 (d) | 100.0 (d) | 100.1 (d) |
| 9 | 156.4 (s) | 156.4 (s) | 156.4 (s) | 156.4 (s) | 156.5 (s) |
| 10 | 106.7 (s) | 106.7 (s) | 106.7 (s) | 106.6 (s) | 106.7 (s) |
| 1′ | 126.7 (d) | 127.2 (d) | 126.5 (d) | 126.7 (d) | 127.0 (d) |
| 2′ | 131.4 (d) | 131.2 (d) | 131.4 (d) | 131.3 (d) | 131.3 (d) |
| 1″ | 138.8 (s) | 138.8 (s) | 138.9 (s) | 138.9 (s) | 138.9 (s) |
| 2″, 6″ | 127.9 (d) | 115.2 (d) | 127.9 (d) | 127.9 (d) | 128.0 (d) |
| 4″ | 157.5 (s) | 157.5 (s) | 158.9 (s) | 158.7 (s) | 158.5 |
| 3″, 5″ | 115.1 (d) | 127.9 (d) | 115.0 (d) | 115.1 (d) | 115.2 (d) |
| 1‴ | 68.9 (t) | 55.9 (t) | 64.9 (t) | 70.0 (t) | 69.3 |
| 2‴ | 133.1 (d) | 78.4 (s) | 119.5 (d) | ||
| 3‴ | 117.4 (t) | 75.7 (d) | 138.4 (s) | ||
| 4‴ | 25.9 (q) | ||||
| 5‴ | 18.2 (q) | ||||
| 1⁗ | 136.7 (s) | 135.9 | |||
| 2⁗ | 128.6 (d) | 129.0 | |||
| 3⁗ | 127.9 (d) | 131.8 | |||
| 4⁗ | 128.0 (d) | 129.0 | |||
| 5⁗ | 127.9 (d) | 131.8 | |||
| 6⁗ | 128.6 (d) | 122.0 | |||
| 4-OMe | 56.3 (q) | 56.4 (q) | 56.3 (q) | 56.4 (q) | 56.4 |
| 7-OMe | 55.7 (q) | 55.7 (q) | 55.7 (q) | 55.7 (q) | 55.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fotso, G.W.; Ngameni, B.; Storr, T.E.; Ngadjui, B.T.; Mafu, S.; Stephenson, G.R. Synthesis of Novel Stilbene–Coumarin Derivatives and Antifungal Screening of Monotes kerstingii-Specialized Metabolites Against Fusarium oxysporum. Antibiotics 2020, 9, 537. https://doi.org/10.3390/antibiotics9090537
Fotso GW, Ngameni B, Storr TE, Ngadjui BT, Mafu S, Stephenson GR. Synthesis of Novel Stilbene–Coumarin Derivatives and Antifungal Screening of Monotes kerstingii-Specialized Metabolites Against Fusarium oxysporum. Antibiotics. 2020; 9(9):537. https://doi.org/10.3390/antibiotics9090537
Chicago/Turabian StyleFotso, Ghislain Wabo, Bathelemy Ngameni, Thomas E. Storr, Bonaventure Tchaleu Ngadjui, Sibongile Mafu, and G. Richard Stephenson. 2020. "Synthesis of Novel Stilbene–Coumarin Derivatives and Antifungal Screening of Monotes kerstingii-Specialized Metabolites Against Fusarium oxysporum" Antibiotics 9, no. 9: 537. https://doi.org/10.3390/antibiotics9090537
APA StyleFotso, G. W., Ngameni, B., Storr, T. E., Ngadjui, B. T., Mafu, S., & Stephenson, G. R. (2020). Synthesis of Novel Stilbene–Coumarin Derivatives and Antifungal Screening of Monotes kerstingii-Specialized Metabolites Against Fusarium oxysporum. Antibiotics, 9(9), 537. https://doi.org/10.3390/antibiotics9090537