Abstract
This work reports on the synthesis of eight new 2′-hydroxy-chalcones with potential anti-phytopathogenic applications in agroindustry, AMONG others, via Claisen–Schmidt condensation and ultrasound assisted reaction. Assays showed three chalcones with allyl moieties strongly inhibited growth of phytopathogenic oomycete Phytophthora infestans; moreover, compound 8a had a half maximal effective concentration (EC50) value (32.5 µg/mL) similar to that of metalaxyl (28.6 µg/mL). A software-aided quantitative structure–activity relationship (QSAR) analysis of the whole series suggests that the structural features of these new chalcones—namely, the fluoride, hydroxyl, and amine groups over the carbon 3′ of the chalcone skeleton—increase anti-oomycete activity.
1. Introduction
In recent years, worldwide per capita food consumption has increased while growth rates in global agricultural production and crop yields have declined [1]; this latter is largely due to phytosanitary problems like pests, fungi, bacteria, and, mainly in the post-harvest phase, diseases produced by oomycetes [2,3,4]. Of the diseases caused by oomycetes, root rot and fruit blight [5] are estimated to render up to 30% of the harvested vegetables and fruits inedible during postharvest handling [6]. As the main causal agent of stem, root, and fruit rot in commercial cultivars, genus Phytophthora [7] is present scattered in almost all producing regions worldwide, causing heavy economic losses and disastrous consequences for natural ecosystems [8].
Specifically, oomycete Phytophthora infestans is renowned as the most significant pathogen of the genus, and is responsible for the grave potato and tomato disease known as late blight or potato blight [9]. While there are several fungicides presently used as pre-and post-harvest treatments for control of P. infestans [10], their use leaves residues on food and in cultivar soils, increases pathogen resistance, and damages human health and the environment [11]. The prevalence of P. infestans and the downsides to chemical controls have stimulated modern agriculture research into alternative microorganism control methods. In addressing these shortcomings, natural product derivatives appear to be a viable approach to reducing late blight disease [12].
Chalcones are well documented as biologically active natural products [13], with potential use in diseases caused by Phytophthora spp. In this context, several studies have shown that natural and synthetic chalcones with –OH and –O-alkyl chains linked to different positions of the A and B rings are known to have bactericidal, antifungal, anthelmintic, antiviral, and anti-oomycetes activities [14,15,16,17,18]. These reports illustrate that antimicrobial activity depends on the number, type, and length of side chain, and position of these substituents in the aromatic ring. Due to the importance of hydroxyl groups and alkyl side chains, this work reports on a facile sonochemical synthesis of a series of 2′-hydroxy-chalcone derivatives, in which oxyalkyl chains were attached to the A aromatic ring in different positions and variable lengths. The anti-oomycete activities of these chalcone derivatives were tested against P. infestans to evaluate the effect of the 2′ position of the hydroxyl group as well as the position and length of the alkyl chain.
2. Results and Discussion
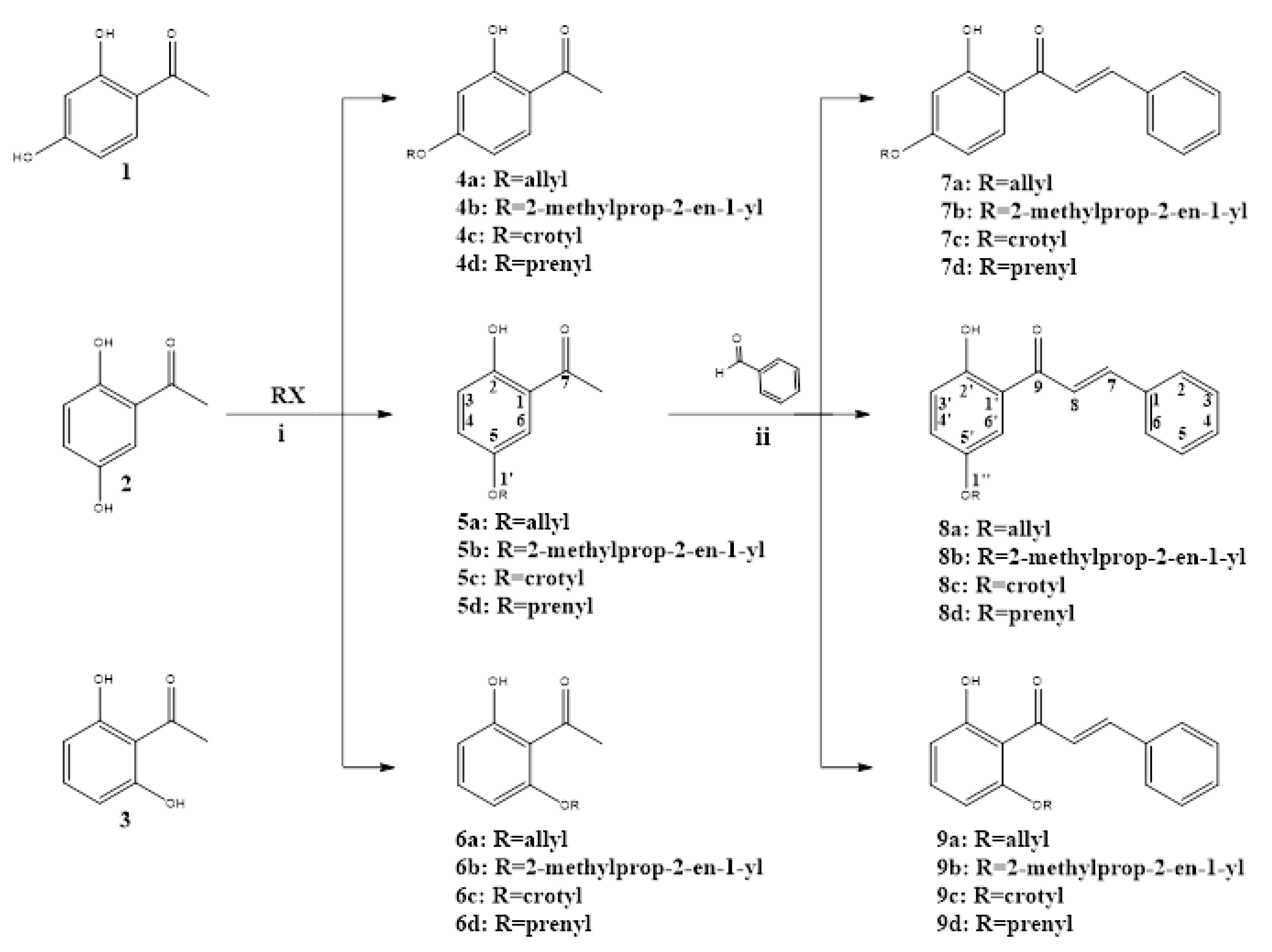
This study synthesized twelve derivatives of 2′-hydroxy-chalcone (7a–d, 8a–d, and 9a–d), characterized by spectroscopy. The base structure was manipulated to include different oxyalkyl chains—allyl, 2-methyl-3propenyl, crotyl, and prenyl—in the 4′, 5′, and 6′ positions, as shown in Scheme 1.
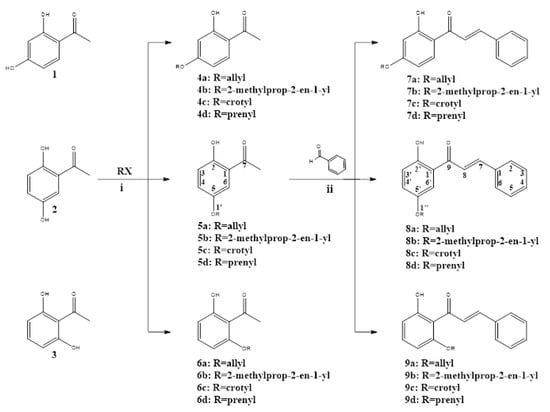
Scheme 1.
Route for the synthesis of oxyalkylated chalcones. Reagents and conditions: (i) K2CO3, acetone, ultrasound at 45–50 °C for 20–30 min. (ii) NaOH, ethanol, ultrasound at 30–35 °C for 3 h.
As shown Scheme 1, alkoxy acetophenone (4a–d, 5a–d, and 6a–d) synthesis had good yield for the alkylation of the corresponding acetophenone, with the desired alkyl halide in the presence of K2CO3 under ultrasound irradiation. Alkoxy acetophenones obtained were characterized by nuclear magnetic resonance (NMR) spectra analysis. This stage produced five molecules never before described, which, due mainly to the use of ultrasound irradiation, were synthesized nearly stoichiometric. This was notably true for those derived from 2′,6′-dihydroxyacetophenone, whose double hydrogen bridge, under normal synthesis conditions, is obtained inefficiently [19]. From here, 2′-hydroxy-chalcone derivatives were prepared by Claisen–Schmidt condensation of the different alkoxy acetophenones with benzaldehyde under ultrasound irradiation [20], resulting in a known series (7a–d) and two new series of molecules (8a–d and 9a–d). The mild reaction conditions, shorter reaction time, and high yields make this reaction more efficient than the classic reflux agitation method used for chalcone synthesis [21].
Furthermore, synthesized chalcones derivatives (7a–d, 8a–d, and 9a–d), at purity values over 92%, were evaluated for anti-oomycete activity against P. infestans, demonstrating significant mycelial growth inhibition (Table 1).

Table 1.
Inhibition of mycelial growth by 2′-hydroxy-chalcone derivatives against P. infestans.
Furthermore, all compounds tested showed strong anti-oomycete activity and were superior to difenoconazole. Among them, compound 8a was the most potent derivative of the series, with activity similar to that of metalaxyl.
A membrane damage experiment—based on the direct action of the compounds on the formation of sterol in the cells—was performed to establish the possible death pathway of P. infestans strains. Compound effects were compared to 2% sodium dodecyl sulfate (SDS), an anionic surfactant that produces 100% cell lysis. Percentage of membrane lysis of P. infestans strains are summarized in Table 2.

Table 2.
Percentage of membrane lysis of the synthetic compounds 7a–d, 8a–d, and 9a–d.
This type of test is based on the direct action of the compounds on the formation of sterol in the cells of the oomycete membranes’ paper anterior.
The membrane damage test showed antifungal effectiveness of compound 8a against P. infestans similar to a chaotropic agent, like SDS. These results are consistent with previous reports that short oxyalkylated chains significantly enhance and improve antimicrobial activities [22].
Additionally, some structure–activity relationship studies suggest that the antimicrobial effect of chalcones is mainly attributable to the presence of hydroxyl phenolic groups (and their high affinity for membrane proteins), especially in the 2′ position of the A ring [23,24]. Furthermore, alkyl group substitution of the A ring, especially O-alkyl, is thought to increase lipophilicity, and consequently, enhance antimicrobial activity [25,26,27].
To this end, quantitative structure–activity relationship (QSAR) analysis (i.e., a series of multivariate linear regressions between biological activity (pEC50, dependent variable) and various descriptors (physicochemical, steric, or other, independent variables)) was performed following [28,29,30], in order to better describe activity and aid in the design and prediction of new compounds [31].
The multivariate equations obtained in the gaseous and condensed phase (Equations (1) and (2), respectively) showed that the inhibitory activity of 2′-hydroxy-chalcone derivatives on P. infestans depends on the molecular area as well as on the atomic charge of carbon C3′. Indeed, the atomic charge of chalcone derivatives has previously been discussed for antifungal activity against B. cinerea and M. fructicola [28,32]. Moreover, the atomic charge of 2-allylphenol derivatives has been related to inhibition activity against other Phytopthora strains [32]. Furthermore, while the molecular surface (MS) has been used to explain lipophilicity [7], this descriptor has not been reported as an important feature of P. infestans inhibition activity.
pEC50 = 5.49 − 0.005MS + 0.464C3′
N = 12; r = 0.925; r2 = 0.856; SD = 0.032; F = 26.8; q2 = 0.832
N = 12; r = 0.925; r2 = 0.856; SD = 0.032; F = 26.8; q2 = 0.832
pEC50 = 5.51 − 0.006MS + 0.472C3′
N = 12; r = 0.934; r2 = 0.871; SD = 0.029; F = 30.5; q2 = 0.852
N = 12; r = 0.934; r2 = 0.871; SD = 0.029; F = 30.5; q2 = 0.852
The QSAR model obtained in the condensed phase was more robust than that of the gaseous phase (Equation (2) greater values of r, r2, F, and q2 than Equation (1)). Equation (2) suggests that the growth inhibition activity of 2′-hydroxy-chalcone derivatives against P. infestans decreases when molecular surface increases (negative slope MS). However, the most important descriptor is the atomic charge on C3′ (~79-folds more than MS). Thus, the atomic charge on C3′ very likely provides more active compounds against P. infestans.
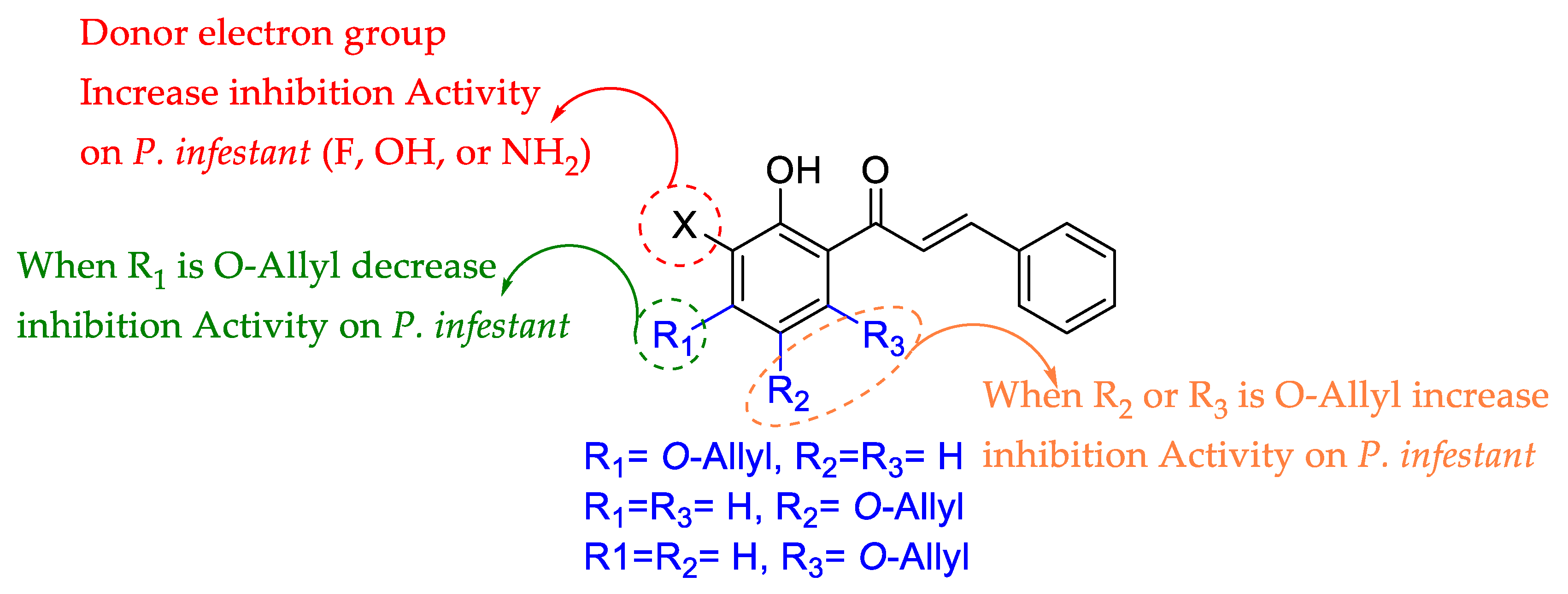
The most active compounds of the series are 7a, 8a, and 9a; while these compounds have a similar molecular surface, the atomic charge on C3′ is different due to the substitution pattern (see Table S2). Thus, new compounds with substitution on C3′ were calculated using the compounds 7a, 8a, and 9a as core (see Tables S3–S5). These new compounds differed in calculated pIC50, depending on the stereo-electronic properties. For example, using compounds 7a, 8a, and 9a as the core caused the electronegative substituent –F, –OH, and NH2 bonded to C3′ to increase in positive atomic charge, allowing for increased inhibition growth activity against P. infestans (see Tables S3–S5). Interestingly, the derivatives calculated from 7a showed less activity than derivatives from cores 8a and 9a. This phenomenon is likely related to substituent proximity in the 7a derivatives, which causes stereo-electronic repulsions (see Tables S3–S5). Thus, the most active compound against P. infestans (8a, see Table 1), the most promising substituents for the inhibition activity of 2-hydroxychalcones against P. infestans bonded to C3′ were –F and –OH, which allow for ~1.7-folds.
In sum, QSAR analysis yielded important structural information as well as allowed for calculations of other compounds (see Tables S3–S5) summarized in Figure 1.
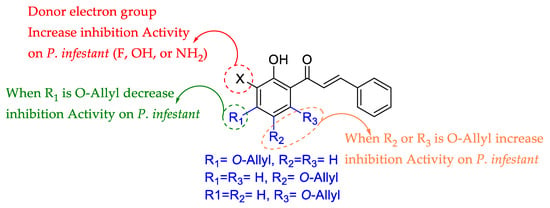
Figure 1.
Summary of QSAR analysis.
3. Materials and Methods
3.1. Chemicals and Reagents
All chemicals were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA), GIBCO BRL Life Technologies (Grand Island, NY, USA) and Santa Cruz Biotechnology (Santa Cruz, CA, USA). Spectra were recorded in CDCl3 solutions and were referenced to the residual peaks of CHCl3, δ = 7.26 ppm, and δ = 77.0 ppm for 1H and 13C, respectively, on a Bruker Avance 400 Digital NMR spectrometer (Bruker, Rheinstetten, Germany), operating at 400.1 MHz for 1 H and 100.6 MHz for 13C. Chemical shifts were reported in δ ppm and coupling constants (J) were given in Hz. HRMS were measured on Termo Finnigan MAT95XL mass spectrometers. Silica gel (Merck 200–300 mesh) was used for column chromatography.
3.2. General Procedure: Synthesis
3.2.1. Alkoxy Acetophenones
A mixture of the respective dihydroxyacetophenone, alkyl bromide, and anhydrous potassium carbonate at a ratio of 1:1.2:1 in 2 mL of acetone and a ratio of 1:2:4 in 2 mL of acetonitrile for monoalkylated and dialkylated chalcones, respectively. The reaction was irradiated in a water bath of an ultrasonic cleaner (Elmasonic S 10 H, Elma Schmidbauer GmbH, Sigen, Germany) with frequency of 37 KHz and a nominal power of 240 W at 45–50 °C for 20–30 min. After the reaction time the extraction, separation and identification protocols was performed according to the reported procedures [33]. This procedure made it possible to obtain known compounds 4a–d, 5a, 6a, and 6d [34,35,36]. NMR data for 4a–d, 5a, 6a, and 6d were consistent with those previously reported. In addition, new compounds such as 5b–d and 6b–d were obtained. The structure of these compounds was established by NMR data.
1-{2-Hydroxy-5-[(2-methylprop-2-en-1-yl)oxy]phenyl}ethanone (5b). Dark yellow oil. Yield: 90%. 1H NMR (400 MHz, CDCl3): δ 11.85 (s, 1H, 2-OH); 7.22 (d, J = 3.0 Hz, 1H, H-6); 7.13 (dd, J = 9.0 and 3.0 Hz, 1H, H-4); 6.91 (d, J = 9.0 Hz, 1H, H-3); 5.10 (s, 1H, H-3α’); 5.01 (s, 1H, H-3β’); 4.42 (s, 2H, H-1′); 2.61 (s, 3H, H-8); 1.84 (s, 3H, H-4′). 13C NMR (100 MHz, CDCl3): δ 203.9 (C-7); 156.8 (C-2); 150.8 (C-5); 140.9 (C-2′); 125.0 (C-4); 119.2 (C-3); 115.0 (C-6); 113.1 (C-3′); 72.9 (C-1′); 26.7 (C-8); 19.4 (C-4′).
1-[5-(Crotyloxy)-2-hydroxyphenyl]ethanone (5c). Brown oil. Yield: 93%. 1H NMR (400 MHz, CDCl3): δ 11.84 (s, 1H, 2-OH); 7.21 (d, J = 3.0 Hz, 1H, H-6); 7.12 (dd, J = 9.0 and 3.0 Hz, 1H, H-4); 6.91 (d, J = 8.9 Hz, 1H, H-3); 5.87 (m, 1H, H-2′); 5.72 (m, 1H, H-3′); 4.43 (d, J = 6.0 Hz, 2H, H-1′); 2.61 (s, 3H, H-8); 1.57 (s, 3H, H-4′). 13C NMR (100 MHz, CDCl3): δ 205.4 (C-7); 153.7 (C-2); 148.6 (C-5); 129.9 (C-2′); 125.0 (C-3′); 122.4 (C-4); 120.0 (C-1); 119.0 (C-3); 114.0 (C-6); 68.1 (C-1′); 26.7 (C-8); 17.3 (C-4′).
1-[2-Hydroxy-5-(prenyloxy)phenyl]ethanone (5d). Dark yellow oil. Yield: 93%. 1H NMR (400 MHz, CDCl3): δ 11.82 (s, 1H, 2-OH); 7.18 (d, J = 2.9 Hz, 1H, H-6); 7.10 (dd, J = 9.0 and 2.9 Hz, 1H, H-4); 6.88 (d, J = 9.0 Hz, 1H, H-3); 5.45 (m, 1H, H-2′); 4.46 (d, J = 6.8 Hz, 2H, H-1′); 2.58 (s, 3H, H-8); 1.79 (s, 3H, H-4′); 1.73 (s, 3H, H-5′). 13C NMR (100 MHz, CDCl3): δ 205.5 (C-7); 153.7 (C-2); 148.1 (C-5); 138.4 (C-3′); 122.4 (C-6); 121.4 (C-4); 119.8 (C-2′); 118.9 (C-1); 114.4 (C-3); 65.5 (C-1′); 26.7 (C-8); 25.8 (C-5′); 18.3 (C-4′).
1-{2-Hydroxy-6-[(2-methylprop-2-en-1-yl)oxy]phenyl}ethanone (6b). Yellow oil. Yield: 86%. 1H NMR (400 MHz, CDCl3): δ 13.24 (s, 1H, 2-OH); 7.31 (t, J = 8.3 Hz, 1H, H-4); 6.57 (d, J = 8.2 Hz, 1H, H-3); 6.38 (d, J = 8.3 Hz, 1H, H-5); 5.1 (s, 1H, H-3α’); 5.05 (s, 1H, H-3β’); 4.52 (s, 2H, H-1′); 2.71 (s, 3H, H-8); 1.87 (s, 3H, H-4′). 13C NMR (100 MHz, CDCl3): δ 202.5 (C-7); 162.0 (C-2); 155.4 (C-6); 139.1 (C-2′); 136.0(C-4); 113.8 (C-1); 111.6 (C-3′); 111.1 (C-5); 101.5 (C-3); 72.4 (C-1′); 33.2 (C-8); 18.8 (C-4′).
1-[6-(Crotyloxy)-2-hydroxyphenyl]ethanone (6c). Brown oil. Yield: 86%. 1H NMR (400 MHz, CDCl3): δ 13.34 (s, 1H, 2-OH); 7.12 (d, J = 8.3 Hz, 1H, H-4); 6.57 (d, J = 8.2 Hz, 1H, H-3); 6.34 (d, J = 8.3 Hz, 1H, H-5); 5.62 (m, 2H, H-2′ and H-3′); 4.38 (d, J = 5.6 Hz, 2H, H-1′); 2.72 (s, 3H, H-8); 1.72 (s, 3H, H-4′). 13C NMR (100 MHz, CDCl3): δ 202.5 (C-7); 162.0 (C-2); 159.8 (C-6); 136.0 (C-4); 127.8 (C-2′); 125.0 (C-3′); 112,0 (C-1); 111.1 (C-5); 106.8 (C-3); 64.0 (C-1′); 33.2 (C-8); 13.3 (C-4′).
3.2.2. Oxyalkyl Chalcones
To a solution of benzaldehyde (1.5 mmol) and the corresponding oxyalkyl acetophenone (1.5 mmol) in ethanol (3 mL) taken in a flask (25 mL), a catalytic quantity of sodium hydroxide (0.75 mmol) was added and the reaction mixture was irradiated in the water bath of an ultrasonic cleaner with a frequency of 37 KHz and a nominal power of 240 W at 30–35 °C for 3 h, after which 10% HCl solution was added until pH ~ 7 to end the reaction, and the mixture was extracted with EtOAc (3 × 30 mL). The organic layer was dried with Na2CO3, filtered, and separated with column chromatography on silica gel.
This procedure made it possible to obtain four known chalcones, 7a, 7b, 7c, and 7d, with high percentages of yield 9%, 85.3%, 86.0%, and 81.1%, respectively. NMR data for 7a–7d were consistent with those previously reported [37]. The structure of the new compounds obtained was established by NMR and mass data, as detailed below:
(2E)-1-[5-(Allyloxy)-2-hydroxyphenyl]-3-phenylprop-2-en-1-one (8a). Solid orange. Yield: 91%. m.p.: 59 °C. 1H NMR (400 MHz, CDCl3): δ 12.37 (s, 1H, 2′-OH); 7.92 (d, J = 15.5 Hz, 1H, H-7); 7.67 (m, 2H, H-2 and H-6); 7.59 (d, J = 15.5 Hz, 1H, H-8); 7.44 (m, 3H, H-3, H-4 and H-5); 7.41 (s, 1H, H-6′); 7.17 (d, J = 9.0 Hz, H-4′); 6.98 (d, J = 9.0 Hz, 1H, H-3′); 6.08 (m, 1H, H-2″); 5.45 (d, J = 17.2 Hz, 1H, H-3α″); 5,32 (d, J = 10.6 Hz, 1H, H-3β″); 4.56 (d, J = 5.3 Hz, 2H, H-1″). 13C NMR (100 MHz, CDCl3): δ 193.4 (C-9); 158.1 (C-2′); 150.7 (C-5′); 145.6 (C-7); 134.6 (C-1); 133.2 (C-2″); 131.0 (C-4); 129.1 (C-3 and C-5); 128.7 (C-2 and C-6); 124.7 (C-4′); 120. 1 (C-8); 119.7 (C-1′); 119.3 (C-3′); 118.0 (C-3″); 114.5 (C-6′); 70.0 (C-1″). HREIMS: M+H ion m/z 280.3179 (C18H16O3: 280.3178).
(2E)-1-{2-Hydroxy-5-[(2-methylprop-2-en-1-yl)oxy]phenyl}-3-phenylprop-2-en-1-one (8b). Dark yellow oil. Yield: 90%. 1H NMR (400 MHz, CDCl3): δ 12.36 (s, 1H, 2′-OH); 7.92 (d, J = 15.5 Hz, 1H, H-7); 7.67 (m, 2H, H-2 and H-6); 7.59 (d, J = 15.5 Hz, 1H, H-8); 7.45 (m, 3H, H-3, H-4, and H-5); 7.42 (s, 1H, H-6′); 7.16 (d, J = 9.0 Hz, 1H, H-4′); 6.97 (d, J = 9.0 Hz, 1H, H-3′); 5.14 (s, 1H, H-3β″); 5.04 (s, 1H, H-3α″); 4.46 (s, 2H, H-1″); 1.86 (s, 3H, H-4″). 13C NMR (100 MHz, CDCl3): δ 193.4 (C-9); 158.0 (C-2′); 150.8 (C-5′); 145.5 (C-7); 141.0 (C-2″); 134.6 (C-1); 130.9 (C-4); 129.1 (C-3 and C-5); 128.6 (C-2 and C-6); 124.7 (C-4′); 120.2 (C-8); 119.7 (C-1′); 119.2 (C-3′); 114.3 (C-6′); 113.1 (C-3″); 73.0 (C-1″); 19.4 (C-4″). HRMS: M+H ion m/z 294.3447 (C19H18 O3: 294.3444).
(2E)-1-[5-(Crotyloxy)-2-hydroxyphenyl]-3-phenylprop-2-en-1-one (8c). Solid red. Yield: 89%. m.p.: 104 °C. 1H RMN (400 MHz, CDCl3): δ 12.35 (s, 1H, 2′-OH); 7.92 (d, J = 15.5 Hz, 1H, H-7); 7.66 (m, 2H, H-2 and H-6); 7.60 (d, J = 15.5 Hz, 1H, H-8); 7.45 (m, 3H, H-3, H-4, and H-5); 7.39 (s, 1H, H-6′); 7.16 (d, J = 9.0 Hz, 1H, H-4′); 6.97 (d, J = 9.0 Hz, 1H, H-3′); 5.91 (m, 1H, H-2″); 5.84 (m, 1H, H-3″); 4.46 (d, J = 6.0 Hz, 2H, H-1″); 1.56 (s, 3H, H-4″). 13C NMR (100 MHz, CDCl3): δ 192.0 (C-9); 156.3 (C-2′); 153.3 (C-5′); 145.3 (C-7); 138.9 (C-1); 130.9 (C-4); 129.0 (C-2″); 128.8 (C-3 and C-5); 128.7 (C-2 and C-6); 126.0 (C-3″); 125.7 (C-4′); 120.7 (C-8); 119.4 (C-3′); 108.6 (C-6′); 69.3 (C-1″); 17.9 (C-4″). HRMS: M+H ion m/z 294.3448 (C19H18O3: 294.3445).
(2E)-1-[2-Hydroxy-5-(prenyloxy)phenyl]-3-phenylprop-2-en-1-one (8d). Solid pale brown. Yield: 85%. m.p.: 63.5 °C. 1H NMR (400 MHz, CDCl3): δ 12.35 (s, 1H, 2′-OH); 7.92 (d, J = 15.5 Hz, 1H, H-7); 7.66 (m, 2H, H-2, and H-6); 7.60 (d, J = 15.5 Hz, 1H, H-8); 7.45 (m, 3H, H-3, H-4, and H-5); 7.39 (s, 1H, H-6′); 7.16 (m, 1H, H-4′); 6.97 (d, J = 9.0 Hz, 1H, H-3′); 5.51 (m, 1H, H-2″); 4.53 (d, J = 6.7 Hz, 2H, H-1″); 1.82 (s, 3H, H-4″); 1.77 (s, 3H, H-5″). 13C NMR (100 MHz, CDCl3): δ 193,4 (C-9); 157,9 (C-2′); 150,9 (C-5′); 145,5 (C-7); 138,4 (C-3″); 134.6 (C-1); 130.9 (C-4); 130.6 (C-4); 129.1 (C-3 and C-5); 128.6 (C-2 and C-6); 126.6 (C-4′); 120.2 (C-8); 119.7 (C-2″); 119.5 (C-3′); 114.4 (C-6′); 65.8 (C-1″); 25.8 (C-5″); 18.3 (C-4″). HRMS: M+H ion m/z 308.3715 (C20H20O3: 308.3710).
(2E)-1-[2-(Allyloxy)-6-hydroxyphenyl]-3-phenylprop-2-en-1-one (9a). Solid dark yellow. Yield: 90%. m.p.: 78.5 °C. 1H NMR (400 MHz, CDCl3): δ 13.39 (s, 1H, 2′-OH); 7.95 (d, J = 15.6 Hz, 1H, H-7); 7.81 (d, J = 15.6 Hz, 1H, H-8); 7.61(m, 2H, H-2, and H-6); 7.39 (m, 3H, H-3, H-4 and H-5); 7.23 (m, 1H, H-4′); 6.62 (d, J = 8.5 Hz, 1H, H-5′); 6.38 (d, J = 8.5 Hz, 1H, H-3′); 5.99 (m, 1H, H-2″); 5.47 (d, J =17.2 Hz, 1H, H-3α″); 5.32 (d, J = 10.6 Hz, 1H, H-3β″); 4.63 (d, J = 5.4 Hz, 2H, H-1″). 13C RMN (100 MHz, CDCl3): δ 194.8 (C-9); 162.3 (C-2′); 158.4 (C-6′); 142.6 (C-7); 136.6 (C-1); 135.8 (C-4′); 132.5 (C-2″); 130.2 (C-4); 128.8 (C-3 and C-5); 128.5 (C-2 and C-6); 127.9 (C-8); 118.6 (C-3″); 115.6 (C-3′); 110.8 (C-1′); 102.1 (C-5′); 69.7 (C-1″). HRMS: M+H ion m/z 280.3173 (C18H16O3: 280.3178).
(2E)-1-{2-Hydroxy-6-[(2-methylprop-2-en-1-yl)oxy]phenyl}-3-phenylprop-2-en-1-one (9b). Solid dark green. Yield: 83%. m.p.: 68–69 °C. 1H NMR (400 MHz. CDCl3): 13.02 (s, 1H, 2′-OH); 7.92 (d, J = 15.6 Hz, 1H, H-7); 7.81 (d, J = 15.6 Hz, 1H, H-8); 7. 59 (m, 2H, H-2 and H-6); 7.38 (m, 3H, H-3, H-4 and H-5); 7.33 (m, 1H, H-4′); 6.62 (d, J = 8.5 Hz, 1H, H-5′); 6.42 (d, J = 8.5 Hz, 1H, H-3′); 5.17 (s, 1H, H-3β″); 5.04 (s, 1H, H-3α″); 4.54 (s, 2H, H-1″); 1.83 (s, 3H, H-4″). 13C NMR (100 MHz, CDCl3): δ 194.5 (C-9); 164.7 (C-2′); 160.1 (C-6′); 142.8 (C-7); 140.1 (C-2″); 135.9 (C-4′); 135.2 (C-1); 130.3 (C-4); 128.8 (C-3 and C-5); 128.6 (C-2 and C-6); 127.8 (C-8); 114.1 (C-3″); 111.0 (C-3′); 102.5 (C-5′); 73.0 (C-1″); 19.7 (C-4″). HRMS: M+H ion m/z 294.3441 (C19H18 O3: 294.3444).
(2E)-1-[6-(Crotyloxy)-2-hydroxyphenyl]-3-phenylprop-2-en-1-one (9c). Red oil. Yield: 86%. 1H NMR (400 MHz, CDCl3): δ 13.25 (s, 1H, 2′-OH); 8.00 (d, J = 15.6 Hz, 1H, H-7); 7.79 (d, J = 15.6 Hz, 1H, H-8); 7.61 (m, 2H, H-2 and H-6); 7.39 (m, 3H, H-3, H-4 and H-5); 7.33 (m, 1H, H-4′); 6.61 (d, J = 8.5 Hz, 1H, H-5′); 6.41 (d, J = 8.5 Hz, 1H, H-3′); 5.94 (m, 1H, H-2″); 5.82 (m, 1H, H-3″); 4.56 (d, J = 6.0 Hz, 2H, H-1″); 1.77 (s, 3H, H-4″). 13C RMN (100 MHz, CDCl3): δ 194.6 (C-9); 165.0 (C-2′); 160.2 (C-6′); 142.6 (C-7); 135.9 (C-4′); 135.4 (C-1); 131.7 (C-4); 130.2 (C-2″); 128.9 (C-3 and C-5); 128.5 (C-2 and C-6); 128.1 (C-8); 125.3 (C-3″); 112.0 (C-3′); 111.0 (C-1′); 102.5 (C-5′); 69.6 (C-1″); 17.9 (C-4″). HRMS: M+H ion m/z 294.3447 (C19H18O3: 294.3445).
(2E)-1-[2-Hydroxy-6-(prenyloxy)phenyl]-3-phenylprop-2-en-1-one (9d). Solid dark yellow. Yield: 80%. m.p.: 54.3 °C. 1H NMR (400 MHz, CDCl3): δ 13.42 (s, 1H, 2′-OH); 8.06 (d, J = 15.6 Hz, 1H, H-7); 7.78 (d, J = 15.6 Hz, 1H, H-8); 7.60 (m, 2H, H-2 and H-6); 7.38 (m, 3H, H-3, H-4 and H-5); 7.34 (m, 1H, H-4′); 6.61 (d, J = 8.5 Hz, 1H, H-5′); 6.44 (d, J = 8.5 Hz, 1H, H-3′); 5.62 (m, 1H, H-2″); 4.60 (d, J = 6.7 Hz, 2H, H-1″); 1.84 (s, 3H, H-4″); 1.77 (s, 3H, H-5″). 13C NMR (100 MHz, CDCl3): δ 194.7 (C-9); 165.3 (C-2′); 160.6 (C-6′); 142.6 (C-7); 139.7 (C-4′); 136.0 (C-3″); 135.5 (C-1); 130.1 (C-4); 128.8 (C-3 and C-5); 128.4 (C-2 and C-6); 128.0 (C-8); 118.8 (C-2″); 111.9 (C-3′); 110.8 (C-1′); 102.4 (C-5′); 65.6 (C-1″); 25.8 (C-5″); 18.2 (C-4″). HRMS: M+H ion m/z 308.3712 (C20H20O3: 308.3710).
3.3. Anti-Oomycete Activity
All oxyalkyl chalcones 7a–d, 8a–d, and 9a–d were subjected to antifungal assays against Phytophthora infestans, obtained from the collection maintained in our Laboratory of Biochemistry and Environmental Microbiology in Chillan, Chile.
Mycelial discs (4 mm in diameter) of test fungi grown on rye B agar [38] were cut from the margins of the colony and placed on the same medium containing different concentrations of the test oxyalkyl chalcones (12.5–400 µg/mL). After incubation at 20 °C for seven days, radial mycelial growth was measured and was compared with the controls [39]. Control plates were treated with difenoconazole and metalaxil. Activity was expressed as EC50 (the concentration inhibiting growth by 50%), MIC (the minimum concentration inhibition of mycelial growth), and MOC was defined as the lowest concentration of the chemicals that prevented visible growth or germination of mycelium. The experiments were repeated three times with three replicates.
3.4. Membrane Damage
P. infestans strains were cultured by shaking at 20 °C and then washed twice and diluted to approximately (3 × 104 zoospores/mL with cold 3-(N-morpholino)propanesulfonic acid (MOPS) buffer, pH 6.0. Cells were aliquoted to tubes, and 7a–d, 8a–d, and 9a–d was added at a final concentration of 100 µg/mL. SDS (2%) was used as the reference compound, which produced 100% cellular oomycete leakage. P. infestans was incubated at 20 °C, and samples were taken at time intervals (6, 12, 24, and 48 h) and spun at 3500 rpm for 7 min in microcentrifugetubes. The supernatants were collected for absorbance analysis at 260 nm in a Beckman DU-600 spectrophotometer [37]. Results are the means of values from at least two independent assays.
3.5. Computational Details
All compounds (7a–d, 8a–d, and 9a–d) were optimized using DFT-B3LYP-6-31G (d,p) level of theory calculations, and the optimized structures were verified by frequency calculations (obtaining no imaginary frequencies) in the gas phase and using the integral equation formalism (IEFPCM) (water) model as the solvent phase according to a previous report [27]. The descriptors obtained from quantum mechanical calculations such as the dipolar moment (DM), atomic charge from the electrostatic potential (C1, C2, C3, C4, C5, C6, C1′, C2′, C3′, C4′, C5′, C6′, Cα, Cβ, CO), highest occupied molecular orbital (HOMO), and lowest unoccupied molecular orbital (LUMO) were obtained directly from the output file, while the chemical potential (µ), hardness (η), softness (S), and electrophilic global index (ω) values were calculated using the following equations.
In addition, steric and topological descriptors such as molecular weight (MW), molecular surface (MS), molecular volume (MV), hydrogen bonding acceptor (HA), hydrogen bonding donor (HD), rotatable bonds (RT), and topological diameter (TD), lipophilicity index (CLogP), molar refractivity (MR) were obtained using molecular mechanics (MM) optimization carried out with the ChemDraw software.
3.6. Quantitative Structure–Activity Relationship (QSAR)
The structure–activity relationship study was carried out using multiple linear regressions as described in our previous report with small changes [30,40]. We developed several regression models using pEC50 (−log10(EC50)) in M units as the dependent variable and all descriptors above-mentioned in the gas phase and in the solvent phase as independent variables (DM, C1, C2, C3, C4, C5, C6, C1′, C2′, C3′, C4′, C5′, C6′, Cα, Cβ, CO, HOMO, LUMO, ΔLH, µ, η, S, ω, MW, MS, MV, HA, HD, RT, TD, CLogP, MR). In addition, to avoid random correlations between pEC50 and any descriptor, cross-validation was carried out using the Golbraikh method as described in Equation (7) [41]:
where yobs is the experimental pEC50; ycalc is the pEC50 calculated by the QSAR model; and yave is the average pEC50 of all of the compounds used in the QSAR model. An acceptable value of q2 was equal to or higher than 0.5.
3.7. Statistical
In vitro assays were performed in triplicate and the results expressed as mean values ± SD. The results were analyzed using the standard method [39].
4. Conclusions
The results of this research show that the presence of a short alkyl chain improves biological activity. Data so far suggest that oxyalkylated chalcones may represent a new frontier and a challenge for the development of new anti-oomycete compounds against Phytophthora spp. in the near future. Indeed, our results show that research into 2′-hydroxy-chalcone derivate compounds can generate new applications with important impacts for productive sectors, specifically Solanaceae crops (tomatoes, potatoes, and chili).
Supplementary Materials
The following are available online at https://www.mdpi.com/2079-6382/9/9/576/s1, SpectraS1: 1 H and 13 C NMR of compounds 5b–5d and 6b–6c. SpectraS2: 1H, 13C NMR, and MS of compounds 8a–8d and 9a–9d, Table S1: Result of quantitative structure–activity relationship in gas phase, Table S2: Result of quantitative structure activity relationship in condensed phase, Table S3: Proposal compounds based in QSAR analysis based in 7a core, Table S4: Proposal compounds based in QSAR analysis based in 8a core, Table S5: Proposal compounds based in QSAR analysis based in 9a core.
Author Contributions
A.M. supervised the whole study. G.L. and B.S. performed synthesis of all compounds. N.C. performed the spectroscopic data. I.M. conceived and designed the biologic experiments; M.M. conceived and designed the computational methodologies. P.G. and X.B. performed the biologic experiments. A.M., E.W. and I.M. collaborated in the discussion and interpretation of the results. A.M., E.W. and I.M. wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The authors wish to thank FONDECYT (grant no. 1190424).
Acknowledgments
The authors wish to acknowledge the staff of the laboratory at LPNSO, UPLA.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fuglie, K.O. Is agricultural productivity slowing? Glob. Food Sec. 2018, 17, 73–83. [Google Scholar] [CrossRef]
- Okonya, J.S.; Ocimati, W.; Nduwayezu, A.; Kantungeko, D.; Niko, N.; Blomme, G.; Legg, J.P.; Kroschel, J. Farmer Reported Pest and Disease Impacts on Root, Tuber, and Banana Crops and Livelihoods in Rwanda and Burundi. Sustainability 2019, 11, 1592. [Google Scholar] [CrossRef]
- Picard, C.; Alonso, T.; Benko-Beloglavec, A.; Karadjova, O.; Matthews-Berry, S.; Paunovic, S.A.; Pietsch, M.; Reed, P.; van der Gaag, D.J.; Ward, M. Recommended regulated non-quarantine pests (RNQP s), associated thresholds and risk management measures in the European and Mediterranean region. Bull. OEPP 2018, 48, 552–568. [Google Scholar] [CrossRef]
- Pandey, S. International and Regional Institutions and Instruments for Agricultural Policy, Research, and Development. In Encyclopedia of Agriculture and Food Systems, 1st ed.; Van Alfen, N.K., Ed.; Academic Press: London, UK, 2014; pp. 44–48. [Google Scholar]
- Judelson, H.S. Sexual Reproduction in Oomycetes: Biology, Diversity, and Contributions to Fitness. In Oomycete Genetics and Genomics: Diversity, Interactions, and Research Tools, 1st ed.; Lamour, K., Kamoun, S., Eds.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2009; pp. 121–138. [Google Scholar]
- Singh, D.; Sharma, R.R. Postharvest Disinfection of Fruits and Vegetables. In Postharvest Diseases of Fruits and Vegetables and Their Management, 1st ed.; Siddiqui, M.W., Ed.; Academic Press: New York, NY, USA, 2018; pp. 1–52. [Google Scholar]
- Meszka, B.; Michalecka, M. Identification of Phytophthora spp. isolated from plants and soil samples on strawberry plantations in Poland. J. Plant. Dis Prot. 2016, 123, 29–36. [Google Scholar] [CrossRef]
- Scott, P.; Bader, M.; Burgess, T.; Hardy, G.; Williams, N. Global biogeography and invasion risk of the plant pathogen genus Phytophthora. Environ. Sci. Policy 2019, 101, 175–182. [Google Scholar] [CrossRef]
- Fry, W.E.; Grunwald, N.J.; Cooke, D.; McLeod, A.; Forbes, G.A.; Cao, K. Population Genetics and Population Diversity of Phytophthora infestans. In Oomycete Genetics and Genomics: Diversity, Interactions, and Research Tools, 1st ed.; Lamour, K., Kamoun, S., Eds.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2009; pp. 139–164. [Google Scholar]
- Leadbeater, A.J. Plant Health Management: Fungicides and Antibiotics. In Encyclopedia of Agriculture and Food Systems, 2nd ed.; Van Alfen, N.K., Ed.; Academic Press: New York, NY, USA, 2018; pp. 408–424. [Google Scholar]
- Bena, Y.; Fua, C.; Hua, M.; Liua, L.; Wonga, M.H.; Zheng, C. Human health risk assessment of antibiotic resistance associated with antibiotic residues in the environment: A review. Environ. Res. 2019, 169, 483–493. [Google Scholar] [CrossRef]
- Joshi, R.K. Role of Natural Products against Microorganisms. Am. J. Clin. Microbiol. Antimicrob. 2018, 1, 1005. [Google Scholar]
- Chen, X.; Mukwaya, E.; Wong, M.S.; Zhang, Y. A systematic review on biological activities of prenylated flavonoids. Pharm. Biol. 2014, 52, 655–660. [Google Scholar] [CrossRef]
- Cheenpracha, S.; Karalai, C.; Ponglimanont, C.; Subhadhirasakul, S.; Tewtrakul, S. Anti-HIV-1 protease activity of compounds from Boesenbergia pandurata. Bioorg. Med. Chem. 2006, 14, 1710–1714. [Google Scholar] [CrossRef]
- Hans, R.; Guantai, E.M.; Lategan, C.; Smith, P.J.; Wan, B.; Franzblau, S.G.; Gut, J.; Rosenthal, P.J.; Chibale, K. Synthesis, antimalarial and antitubercular activity of acetylenic chalcones. Bioorg. Med. Chem. Lett. 2010, 20, 942–944. [Google Scholar] [CrossRef]
- Tadigoppula, N.; Korthikunta, V.; Gupta, S.; Kancharla, P.; Khaliq, T.; Soni, A.; Srivastava, R.K.; Srivastava, K.; Puri, S.K.; Raju, K.S.R. Synthesis and insight into the structure-activity relationships of chalcones as antimalarial agents. J. Med. Chem. 2012, 56, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Muškinja, J.; Burmudžija, A.; Ratkovic, Z.; Rankovic, B.; Kosanic, M.; Bogdanovic, G.A.; Novakovic, S.B. Ferrocenyl chalcones with O-alkylated vanillins: Synthesis, spectral characterization, microbiological evaluation, and single-crystal X-ray analysis. Med. Chem. Res. 2016, 25, 1744–1753. [Google Scholar] [CrossRef]
- Escobar, B.; Montenegro, I.; Villena, J.; Werner, E.; Godoy, P.; Olguín, Y.; Madrid, A. Hemi-Synthesis and Anti-Oomycete Activity of Analogues of Isocordoin. Molecules 2017, 22, 968. [Google Scholar] [CrossRef] [PubMed]
- Miles, C.O.; Main, L.; Nicholson, B.K. Synthesis of 2′,6′-Dihydroxychalcones by Using Tetrahydropyran-2-yl and Trialkylsilyl Protective Groups: The Crystal Structure Determination of 2′,6′-Dihydroxy-2,4,6-trimethoxychalcone. Aust. J. Chem. 1989, 42, 1103–1113. [Google Scholar] [CrossRef]
- Li, J.T.; Yang, W.Z.; Wang, S.X.; Li, S.H.; Li, T.S. Improved synthesis of chalcones under ultrasound irradiation. Ultrason. Sonochem. 2002, 9, 237–239. [Google Scholar] [CrossRef]
- Marquina, S.; Maldonado-Santiagoa, M.; Sánchez-Carranza, J.N.; Antúnez-Mojica, M.; González-Maya, L.; Razo-Hernández, R.S.; Alvarez, L. Design, synthesis and QSAR study of 2′-hydroxy-4′-alkoxy chalcone derivatives that exert cytotoxic activity by the mitochondrial apoptotic pathway. Bioorg. Med. Chem. 2019, 27, 43–54. [Google Scholar] [CrossRef]
- Ngaini, Z.; Fadzillah, S.M.H.; Hussain, H. Synthesis and antimicrobial studies of hydroxylated chalcone derivatives with variable chain length. Nat. Prod. Res. 2012, 26, 892–902. [Google Scholar] [CrossRef]
- Prusky, D.; Keen, N.T. Involvement of preformed antifungal compounds in the resistance of subtropical fruits to fungal decay. Plant. Dis. 1993, 77, 114–119. [Google Scholar] [CrossRef]
- Medina-Alarcón, K.P.; Singulani, J.; Dutra, L.; Pitangui, N.S.; Pereira-da-Silva, M.A.; dos Santos, M.B.; Ayusso, G.M.; Regasini, L.O.; Soares, C.P.; Chorilli, M.; et al. Antifungal activity of 2-hydroxychalcone loaded in nanoemulsion against Paracoccidioides spp. Future Microbiol. 2020, 15, 1. [Google Scholar] [CrossRef]
- Barron, D.; Ibrahim, R. Isoprenylated flavonoids—A survey. Phytochemistry 1996, 43, 921–982. [Google Scholar] [CrossRef]
- Marcos, I.S.; Escola, M.A.; Moro, R.F.; Basabe, P.; Díez, D.; Sanz, F.; Mollinedo, F.; de la Iglesia-Vicente, J.; Sierra, B.G.; Urones, J.G. Synthesis of novel antitumoural analogues of dysidiolide from ent-halimic acid. Bioorg. Med. Chem. 2007, 15, 5719–5737. [Google Scholar] [CrossRef] [PubMed]
- Basabe, P.; de Román, M.; Díez, D.; Marcos, I.S.; Blanco, A.; Bodero, O.; Mollinedo, F.; Sierra, B.G.; Urones, J.G. Prenylflavonoids and prenyl/alkyl-phloroacetophenones: Synthesis and antitumour biological evaluation. Eur. J. Med. Chem. 2010, 45, 4258–4269. [Google Scholar] [CrossRef]
- Mellado, M.; Espinoza, L.; Madrid, A.; Mella, J.; Chávez-Weisser, E.; Diaz, K.; Cuellar, M. Design, synthesis, antifungal activity, and structure–activity relationship studies of chalcones and hybrid dihydrochromane–chalcones. Mol. Divers. 2020. [Google Scholar] [CrossRef] [PubMed]
- Mellado, M.; Madrid, A.; Reyna, M.; Weinstein-Oppenheimer, C.; Mella, J.; Salas, C.O.; Sánchez, E.; Cuellar, M. Synthesis of chalcones with antiproliferative activity on the SH-SY5Y neuroblastoma cell line: Quantitative Structure–Activity Relationship Models. Med. Chem. Res. 2018, 27, 2414–2425. [Google Scholar] [CrossRef]
- Montenegro, I.; Muñoz, O.; Villena, J.; Werner, E.; Mellado, M.; Ramírez, I.; Caro, N.; Flores, S.; Madrid, A. Structure-Activity Relationship of Dialkoxychalcones to Combat Fish Pathogen Saprolegnia australis. Molecules 2018, 23, 1377. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, I.; Mellado, M.; Russo, A.; Said, B.; Besoain, X.; Godoy, P.; Werner, E.; Caro, N.; Madrid, A. Carveoylphenols and Their Antifungal Potential against Pathogenic Yeasts. Antibiotics 2019, 8, 185. [Google Scholar] [CrossRef]
- Olea, A.F.; Espinoza, L.; Sedan, C.; Thomas, M.; Martínez, R.; Mellado, M.; Carrasco, H.; Díaz, K. Synthesis and In Vitro Growth Inhibition of 2-Allylphenol Derivatives Against Phythopthora cinnamomi Rands. Molecules 2019, 24, 4196. [Google Scholar] [CrossRef]
- Da Silva, G.D.; Da Silva, M.G.; Souza, E.; Barison, A.; Simões, S.C.; Varotti, F.P.; Barbosa, L.A.; Viana, G.; Villar, J. Design and Synthesis of New Chacones Substituted with Azide/Triazole Groups and Analysis of Their Cytotoxicity Towards HeLa Cells. Molecules 2012, 17, 10331–10343. [Google Scholar] [CrossRef]
- Martin, R. Handbook of Hydroxyacetophenones, 1st ed.; Springer: Dordrecht, The Netherlands, 1997; pp. 200–296. [Google Scholar]
- Venkateswararao, E.; Sharma, V.K.; Yun, J.; Kim, Y.; Jung, S. Anti-proliferative effect of chalcone derivatives through inactivation of NF-jB in human cancer cells. Bioorg. Med. Chem 2014, 22, 3386–3392. [Google Scholar] [CrossRef]
- Nguyen, H.D.; Okada, T.; Kitamura, S.; Yamaoka, S.; Horaguchi, Y.; Kasanami, Y.; Sekiguchi, F.; Tsubota, M.; Yoshida, S.; Nishikawa, H.; et al. Design and synthesis of novel anti-hyperalgesic agents based on 6-prenylnaringenin as the T-type calcium channel blockers. Bioorg. Med. Chem 2018, 26, 4410–4427. [Google Scholar] [CrossRef]
- Flores, S.; Montenegro, I.; Villena, J.; Cuellar, M.; Werner, E.; Godoy, P.; Madrid, A. Synthesis and Evaluation of novel oxyalkylated derivatives of 2′,4′-dihydroxychalcone as anti-oomycete agents against bronopol resistant strains of Saprolegnia sp. Int. J. Mol. Sci. 2016, 17, 1366. [Google Scholar] [CrossRef]
- Mitani, S.; Araki, S.; Yamaguchi, T.; Takii, Y.; Ohshima, T.; Matsuo, N. Antifungal Activity of the Novel Fungicide Cyazofamid against Phytophthora infestans and Other Plant Pathogenic Fungi in Vitro. Pestic. Biochem. Physiol. 2001, 70, 92–99. [Google Scholar] [CrossRef]
- Madrid-Villegas, A.; Díaz, K.; González, C.; Catalán, K.; Espinoza, L. Antiphytopathogenic Activity of Psoralea glandulosa (Fabaceae) Against Botrytis cinerea and Phytophthora cinnamomi. Nat. Prod. Res. 2015, 29, 586–588. [Google Scholar] [CrossRef] [PubMed]
- Mellado, M.; Madrid, A.; Martinez, U.; Mella, J.; Salas, C.O.; Cuellar, M. Hansch’s analysis application to chalcone synthesis by Claisen-Schmidt reaction based in DFT methodology. Chem. Pap. 2018, 72, 703–709. [Google Scholar] [CrossRef]
- Golbraikh, A.; Tropsha, A. Beware of q2! J. Mol. Graph. Model. 2002, 20, 269–276. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).