A Localized Phage-Based Antimicrobial System: Effect of Alginate on Phage Desorption from β-TCP Ceramic Bone Substitutes
Abstract
:1. Introduction
2. Results
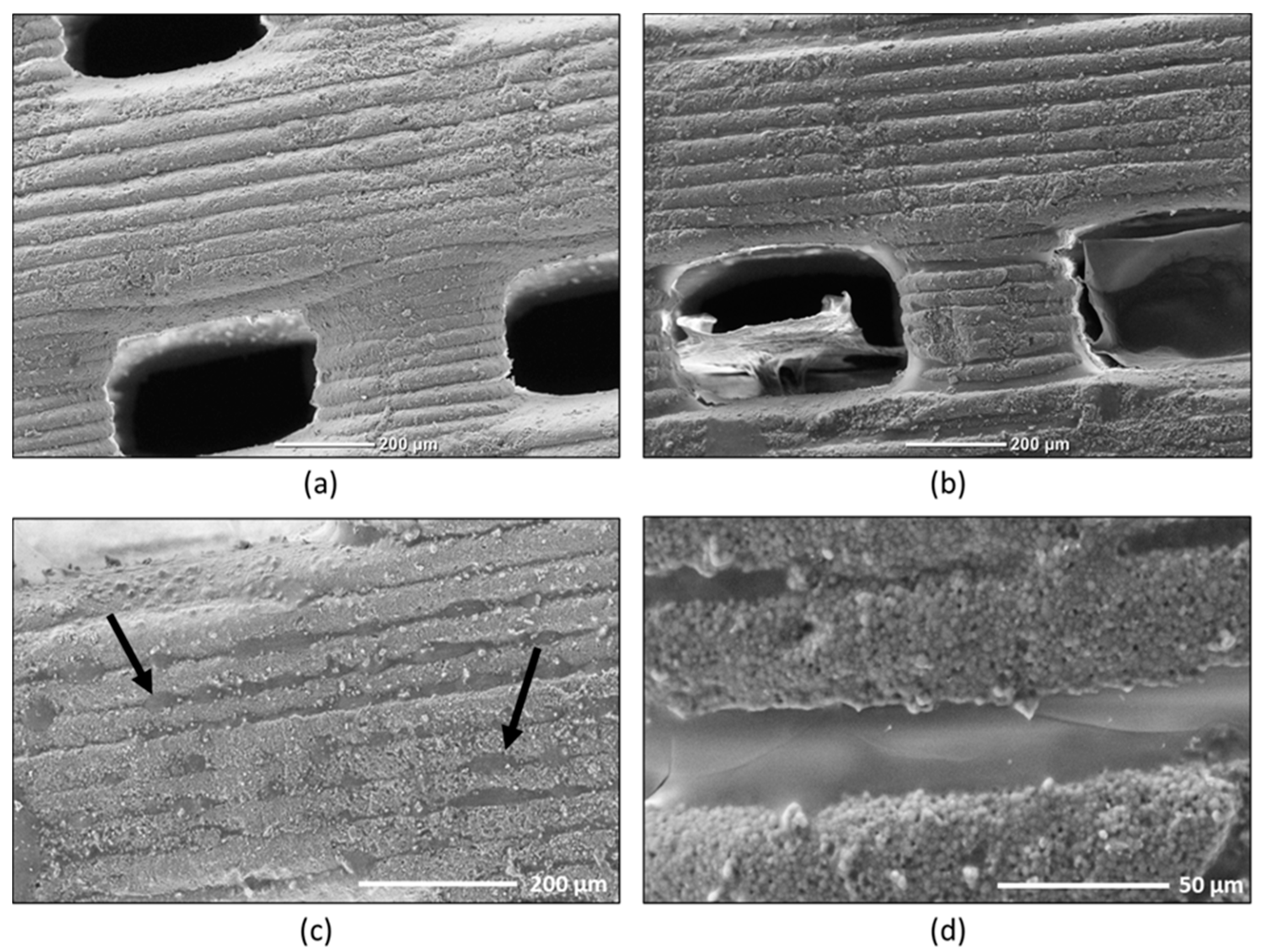
2.1. SEM Observations of the Hydrogel on Alginate Covered Pellets
2.2. Phage Loading of the Inoculated Ceramic Pellets
2.3. Influence of the Percentage of Alginate on the Phage Retention on the Pellets
2.4. Impact of Alginate on the Phage Retention within Two Weeks
3. Discussion
4. Materials and Methods
4.1. Bacteria Growth Conditions and Phage-Killing Titer Preparation
4.1.1. Bacteria Strain, Media, and Growth Conditions
4.1.2. λvir-Phage Stock and Titration
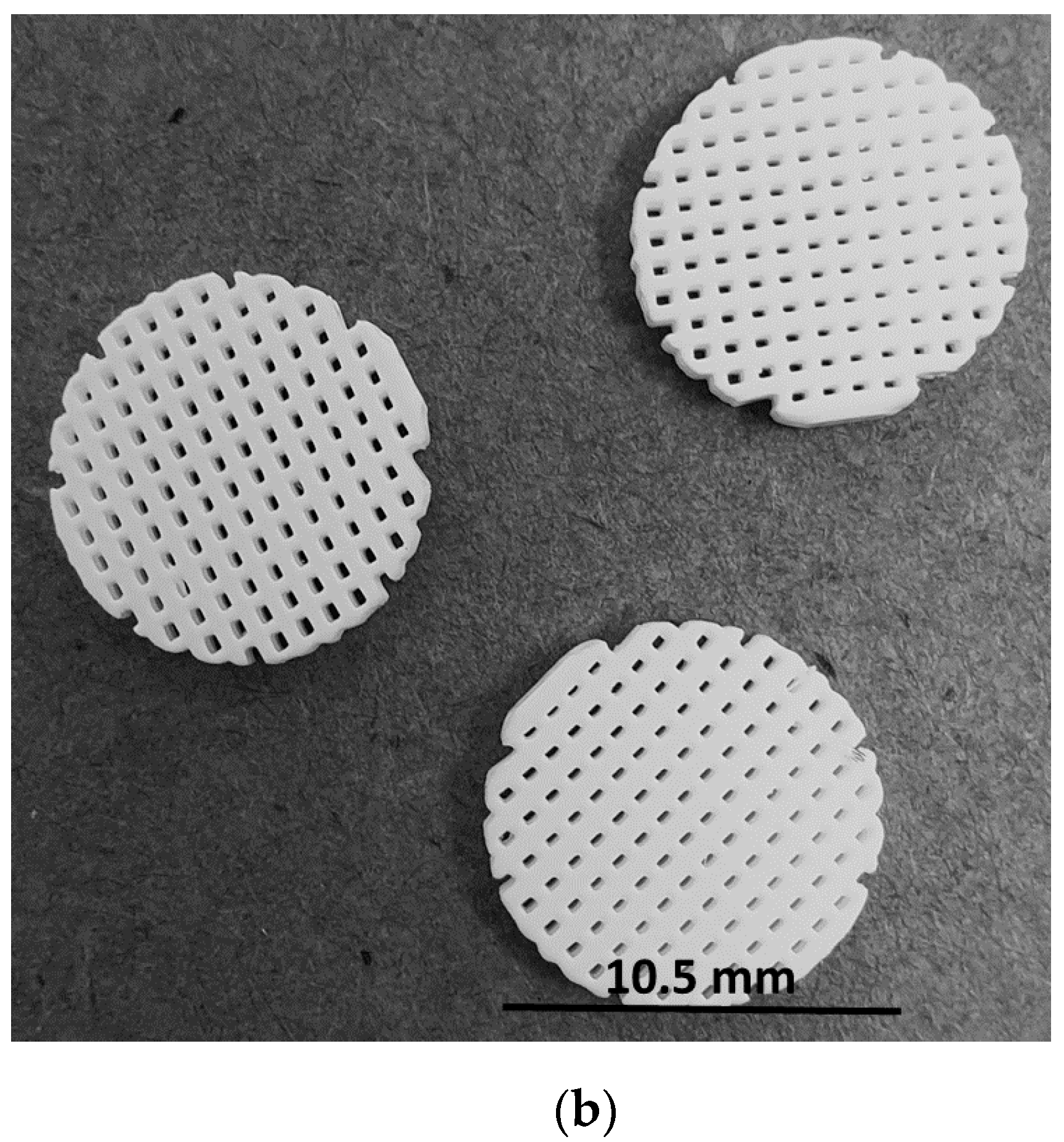
4.2. β-TCP Pellet Preparation
4.2.1. Synthesis and Stereolithographic Shaping of the β-TCP Pellets
4.2.2. Pellet Characterization
4.2.3. Scanning Electron Microscopy
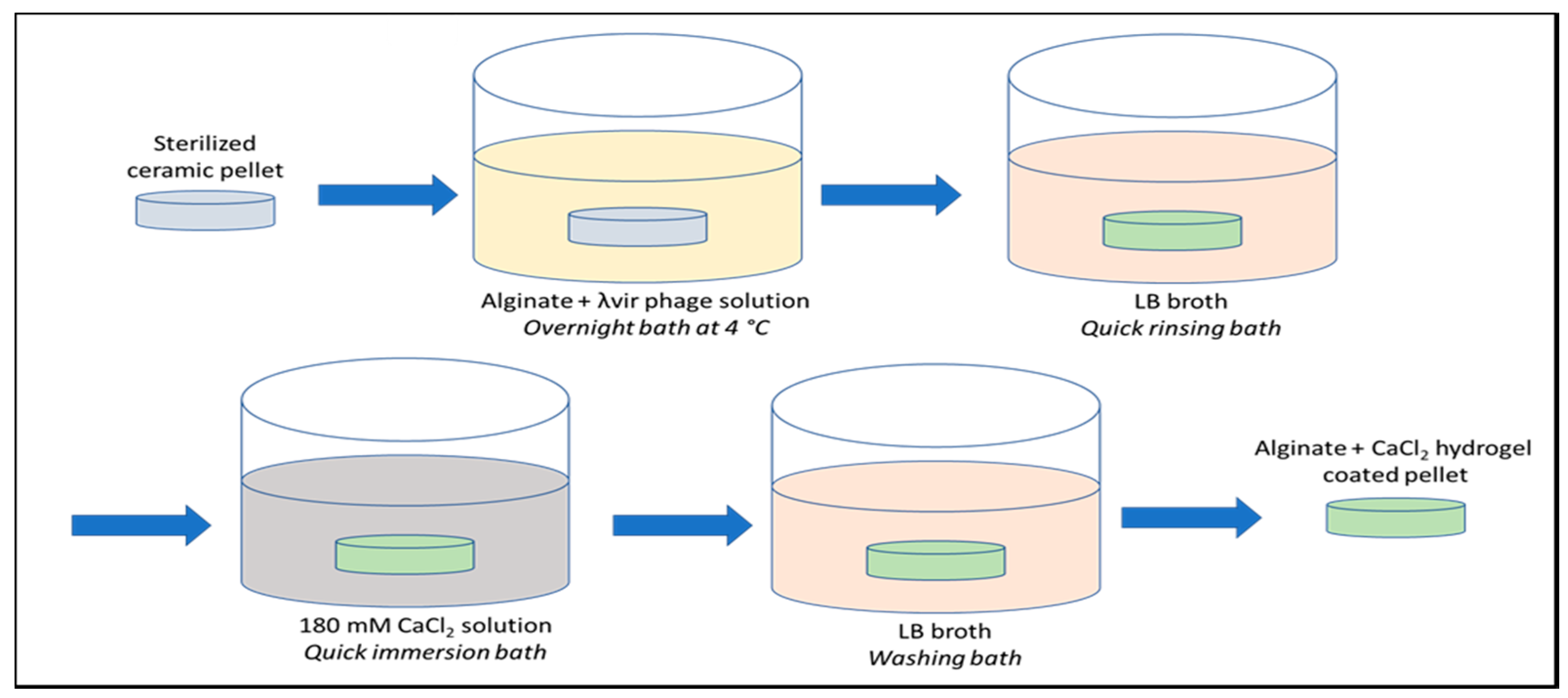
4.2.4. Loading of λvir Phages and Alginate Coating of the β-TCP Pellets
4.3. Washing Tests
4.4. Optical Density Measurements of Bacteria Kinetics
4.5. Determination of the Phage Titer Load of the Pellets
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Twort, F.W. An investigation on the nature of ultra-microscopic viruses. Lancet 1915, 186, 1241–1243. [Google Scholar] [CrossRef] [Green Version]
- D’Herelle, F.; D’Herelle, F.; Smith, G.H. The Bacteriophage and Its Behavior; The Williams & Wilkins Company: Baltimore, MD, USA, 1926; pp. 1–654. [Google Scholar]
- D’Herelle, F. Publications service on an invisible microbe antagonistic toward dysenteric bacilli: Brief note by Mr. F. D’Herelle, presented by Mr. Roux. Res. Microbiol. 2007, 158, 553–554. [Google Scholar] [CrossRef] [PubMed]
- Ho, K. Bacteriophage Therapy for Bacterial Infections: Rekindling a Memory from the Pre-Antibiotics Era. Perspect. Biol. Med. 2001, 44, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Summers, W.C. The strange history of phage therapy. Bacteriophage 2012, 2, 130–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sulakvelidze, A.; Alavidze, Z.; Morris, J.G. Bacteriophage Therapy. Antimicrob. Agents Chemother. 2001, 45, 649–659. [Google Scholar] [CrossRef] [Green Version]
- Górski, A.; Międzybrodzki, R.; Węgrzyn, G.; Jończyk-Matysiak, E.; Borysowski, J.; Weber-Dąbrowska, B. Phage therapy: Current status and perspectives. Med. Res. Rev. 2020, 40, 459–463. [Google Scholar] [CrossRef] [PubMed]
- Pires, D.P.; Oliveira, H.; Melo, L.D.R.; Sillankorva, S.; Azeredo, J. Bacteriophage-encoded depolymerases: Their diversity and biotechnological applications. Appl. Microbiol. Biotechnol. 2016, 100, 2141–2151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dublanchet, A.; Fruciano, E. Brève histoire de la phagothérapie. Médecine Mal. Infect. 2008, 38, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Ansaldi, M.; Boulanger, P.; Brives, C.; Debarbieux, L.; Dufour, N.; Froissart, R.; Gandon, S.; Le Hénaff, C.; Petit, M.-A.; Rocha, E.; et al. Antibacterial applications of bacteriophages. Virologie 2018, 24, 23–36. [Google Scholar] [CrossRef]
- Abedon, S.T.; Kuhl, S.J.; Blasdel, B.G.; Kutter, E.M. Phage treatment of human infections. Bacteriophage 2011, 1, 66–85. [Google Scholar] [CrossRef] [Green Version]
- Loc-Carrillo, C.; Abedon, S.T. Pros and cons of phage therapy. Bacteriophage 2011, 1, 111–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCallin, S.; Sacher, J.C.; Zheng, J.; Chan, B.K. Current State of Compassionate Phage Therapy. Viruses 2019, 11, 343. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, S.; Baker, K.; Padman, B.S.; Patwa, R.; Dunstan, R.A.; Weston, T.A.; Schlosser, K.; Bailey, B.; Lithgow, T.; Lazarou, M.; et al. Bacteriophage Transcytosis Provides a Mechanism To Cross Epithelial Cell Layers. mBio 2017, 8, e01874-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lehti, T.A.; Pajunen, M.I.; Skog, M.S.; Finne, J. Internalization of a polysialic acid-binding Escherichia coli bacteriophage into eukaryotic neuroblastoma cells. Nat. Commun. 2017, 8, 1915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Górski, A.; Międzybrodzki, R.; Jończyk-Matysiak, E.; Borysowski, J.; Letkiewicz, S.; Weber-Dąbrowska, B. The fall and rise of phage therapy in modern medicine. Expert Opin. Biol. Ther. 2019, 19, 1115–1117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pires, D.P.; Costa, A.R.; Pinto, G.; Meneses, L.; Azeredo, J. Current challenges and future opportunities of phage therapy. FEMS Microbiol. Rev. 2020. [Google Scholar] [CrossRef] [PubMed]
- Darouiche, R.O. Treatment of Infections Associated with Surgical Implants. N. Engl. J. Med. 2004, 350, 1422–1429. [Google Scholar] [CrossRef]
- Ma, Y.; Pacan, J.C.; Wang, Q.; Xu, Y.; Huang, X.; Korenevsky, A.; Sabour, P.M. Microencapsulation of Bacteriophage Felix O1 into Chitosan-Alginate Microspheres for Oral Delivery. Appl. Environ. Microbiol. 2008, 74, 4799–4805. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Pacan, J.C.; Wang, Q.; Sabour, P.M.; Huang, X.; Xu, Y. Enhanced alginate microspheres as means of oral delivery of bacteriophage for reducing Staphylococcus aureus intestinal carriage. Food Hydrocoll. 2012, 26, 434–440. [Google Scholar] [CrossRef]
- Meurice, E.; Rguiti, E.; Brutel, A.; Hornez, J.; Leriche, A.; Descamps, M.; Bouchart, F. New antibacterial microporous CaP materials loaded with phages for prophylactic treatment in bone surgery. J. Mater. Sci. Mater. Med. 2012, 23, 2445–2452. [Google Scholar] [CrossRef]
- Remminghorst, U.; Rehm, B.H.A. Bacterial alginates: From biosynthesis to applications. Biotechnol. Lett. 2006, 28, 1701–1712. [Google Scholar] [CrossRef] [PubMed]
- Chapman, V.J.; Chapman, D.J. Algin and Alginates. In Seaweeds and Their Uses; Springer: Dordrecht, The Netherlands, 1980; pp. 194–225. ISBN 978-94-009-5808-1. [Google Scholar]
- Goh, C.H.; Heng, P.W.S.; Chan, L.W. Alginates as a useful natural polymer for microencapsulation and therapeutic applications. Carbohydr. Polym. 2012, 88, 1–12. [Google Scholar] [CrossRef]
- Gasperini, L.; Mano, J.F.; Reis, R.L. Natural polymers for the microencapsulation of cells. J. R. Soc. Interface 2014, 11, 20140817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.; Jo, A.; Ahn, J. Application of chitosan-alginate microspheres for the sustained release of bacteriophage in simulated gastrointestinal conditions. Int. J. Food Sci. Technol. 2015, 50, 913–918. [Google Scholar] [CrossRef]
- Barros, J.A.R.; de Melo, L.D.R.; da Silva, R.A.R.; Ferraz, M.P.; Azeredo, J.C.V.d.R.; Pinheiro, V.M.d.C.; Colaço, B.J.A.; Fernandes, M.H.R.; Gomes, P.d.S.; Monteiro, F.J. Encapsulated bacteriophages in alginate-nanohydroxyapatite hydrogel as a novel delivery system to prevent orthopedic implant-associated infections. Nanomed. Nanotechnol. Biol. Med. 2020, 24, 102145. [Google Scholar] [CrossRef] [Green Version]
- Bouchart, F.; Vidal, O.; Lacroix, J.-M.; Spriet, C.; Chamary, S.; Brutel, A.; Hornez, J.-C. 3D printed bioceramic for phage therapy against bone nosocomial infections. Mater. Sci. Eng. C 2020, 111, 110840. [Google Scholar] [CrossRef]
- Strasser, V.; Matijaković, N.; Mihelj Josipović, T.; Kontrec, J.; Lyons, D.M.; Kralj, D.; Dutour Sikirić, M. Factors affecting calcium phosphate mineralization within bulk alginate hydrogels. J. Polym. Res. 2019, 26, 262. [Google Scholar] [CrossRef]
- Gordillo Altamirano, F.L.; Barr, J.J. Phage Therapy in the Postantibiotic Era. Clin. Microbiol. Rev. 2019, 32, e00066-18. [Google Scholar] [CrossRef] [Green Version]
- Alves, D.; Cerqueira, M.A.; Pastrana, L.M.; Sillankorva, S. Entrapment of a phage cocktail and cinnamaldehyde on sodium alginate emulsion-based films to fight food contamination by Escherichia coli and Salmonella Enteritidis. Food Res. Int. 2020, 128, 108791. [Google Scholar] [CrossRef] [Green Version]
- Lusk, J.E.; Williams, R.J.; Kennedy, E.P. Magnesium and the growth of Escherichia coli. J. Biol. Chem. 1968, 243, 2618–2624. [Google Scholar]
- Biedenbach, D.J.; Jones, R.N. Disk diffusion test interpretive criteria and quality control recommendations for testing linezolid (U-100766) and eperezolid (U-100592) with commercially prepared reagents. J. Clin. Microbiol. 1997, 35, 3198–3202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- AFNOR. (French Standardization Association) Détermination Quantitative du Rapport Ca/P de Phosphates de Calcium, Désignation NFS94-066; AFNOR: Paris, France, 1998. [Google Scholar]
- Raynaud, S.; Champion, E.; Bernache-Assollant, D.; Laval, J.-P. Determination of Calcium/Phosphorus Atomic Ratio of Calcium Phosphate Apatites Using X-ray Diffractometry. J. Am. Ceram. Soc. 2004, 84, 359–366. [Google Scholar] [CrossRef]
- AFNOR. (French Standardization Association) Détermination Qualitative et Quantitative des Phases Étrangères Présentes Dans les Poudres, Dépôts et Céramiques à Base de Phosphates de Calcium, Désignation NFS94–067; AFNOR: Paris, France, 1993. [Google Scholar]



| Pellets (n = 3 × 2) | Phage Concentration (±standard deviation) (PFU/mL) |
|---|---|
| Control without alginate | 1.3 × 106 (±0.2%) |
| Coated with 0.5% alginate | 2.3 × 106 (±0.5%) |
| Coated with 1.0% alginate | 1.3 × 107 (±0.6%) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ismail, R.; Dorighello Carareto, N.D.; Hornez, J.-C.; Bouchart, F. A Localized Phage-Based Antimicrobial System: Effect of Alginate on Phage Desorption from β-TCP Ceramic Bone Substitutes. Antibiotics 2020, 9, 560. https://doi.org/10.3390/antibiotics9090560
Ismail R, Dorighello Carareto ND, Hornez J-C, Bouchart F. A Localized Phage-Based Antimicrobial System: Effect of Alginate on Phage Desorption from β-TCP Ceramic Bone Substitutes. Antibiotics. 2020; 9(9):560. https://doi.org/10.3390/antibiotics9090560
Chicago/Turabian StyleIsmail, Rached, Natalia D. Dorighello Carareto, Jean-Christophe Hornez, and Franck Bouchart. 2020. "A Localized Phage-Based Antimicrobial System: Effect of Alginate on Phage Desorption from β-TCP Ceramic Bone Substitutes" Antibiotics 9, no. 9: 560. https://doi.org/10.3390/antibiotics9090560
APA StyleIsmail, R., Dorighello Carareto, N. D., Hornez, J.-C., & Bouchart, F. (2020). A Localized Phage-Based Antimicrobial System: Effect of Alginate on Phage Desorption from β-TCP Ceramic Bone Substitutes. Antibiotics, 9(9), 560. https://doi.org/10.3390/antibiotics9090560





