Recent Development of Corrosion Factors and Coating Applications in Biomass Firing Plants
Abstract
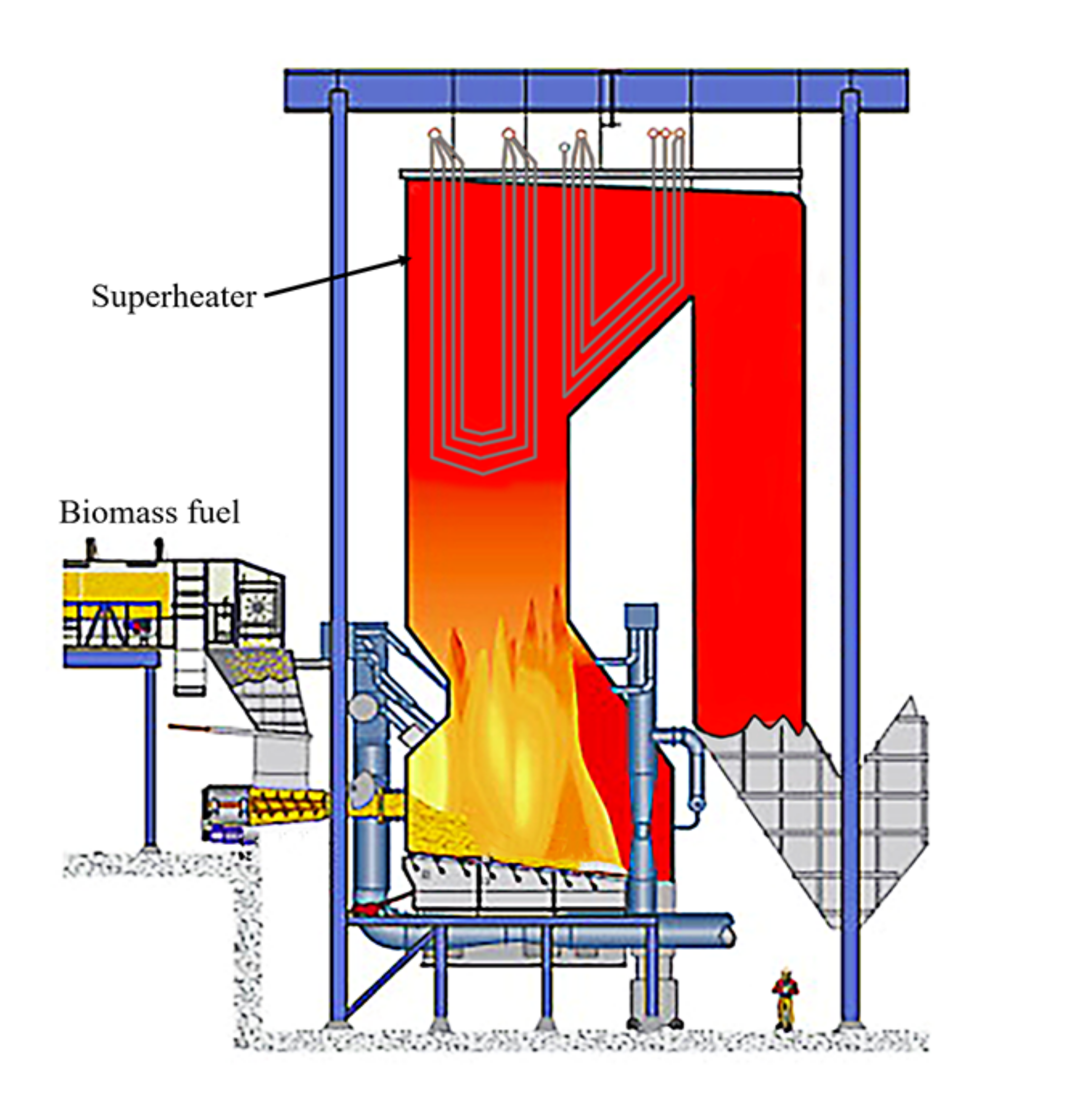
:1. Introduction
2. Corrosion-Related Factors
2.1. Biomass Composition
2.2. Operation Conditions
3. The Application of Corrosion-Resistant Coatings
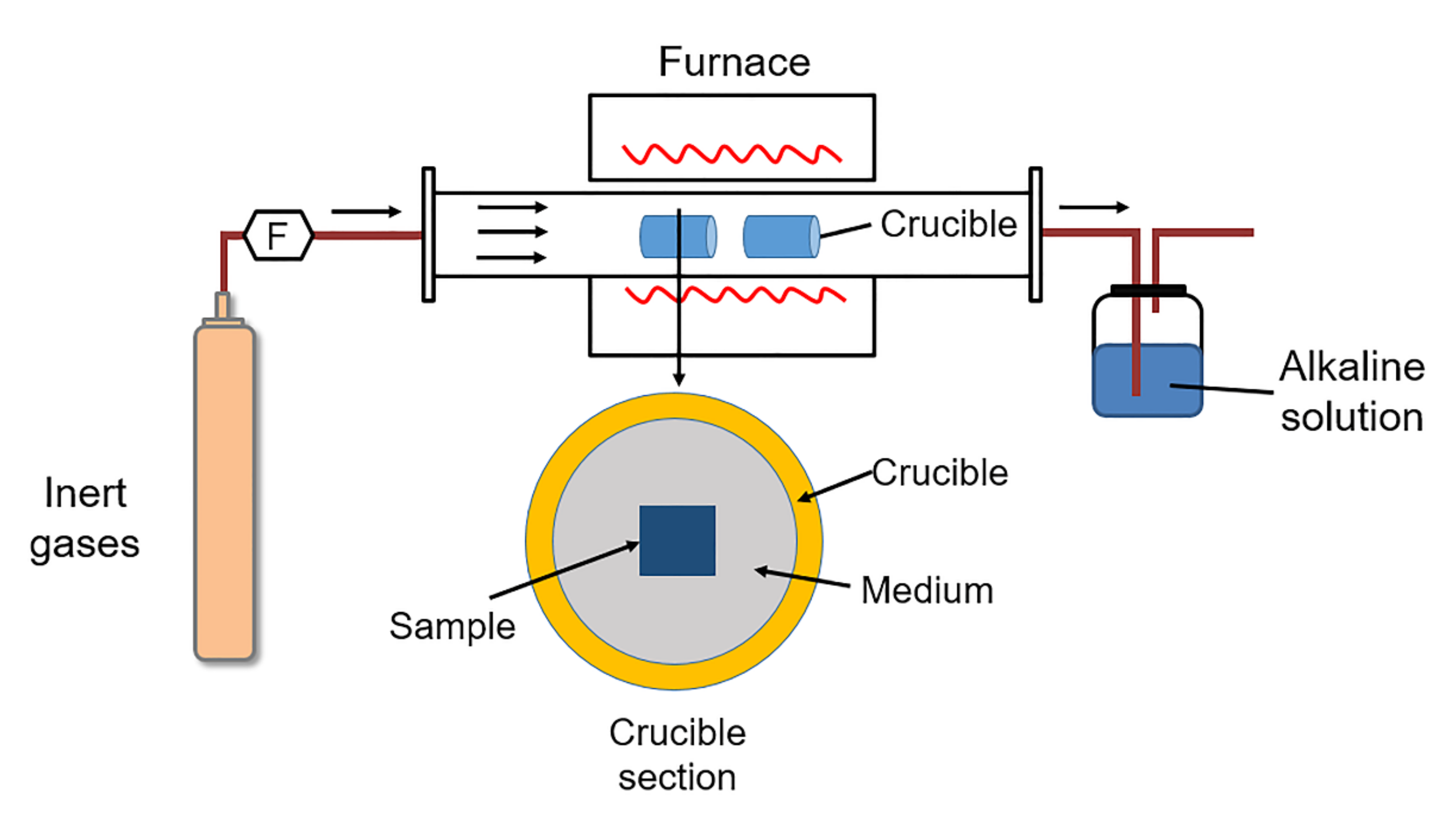
3.1. Thermal Spray
3.1.1. Ni-Based Coatings
3.1.2. Fe-Based Coatings
3.2. Aluminizing
3.3. Laser Cladding
3.4. Materials and Technique Selection Strategy
4. Conclusions
- Elements, such as K, Cl, S, in biomass fuels will seriously reduce the service life of superheater. In particular, Cl ions will penetrate the material and form loose oxides on the surface, which will destroy the material. However, these elements are unavoidable during the combustion process. As technology advances, the temperature inside the boiler will continue to increase, which will inevitably lead to more severe corrosion. Not only that but also the magnitude of the temperature changes and the concentration of the combustion oxidizer plays a very important role.
- Coatings formed by thermal spray technology (HVAF and HVOF), laser cladding technology, and aluminizing technology provide protection. Under high-temperature corrosion, chromium and aluminum in Ni-based or Fe-based coatings form an oxide film to retard corrosion, which has been verified in experimental and practical applications.
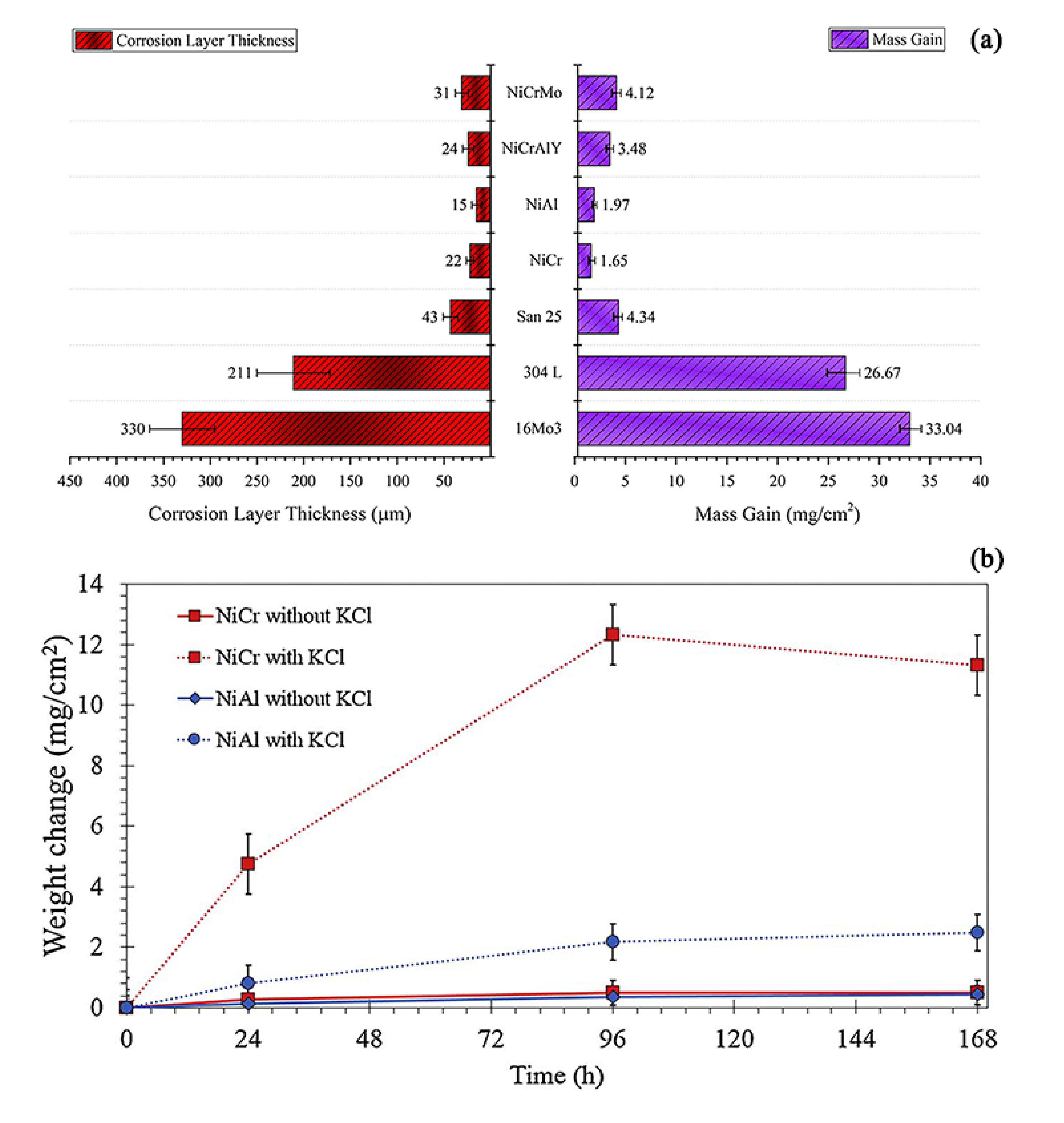
- The increase of chromium content can significantly enhance the corrosion resistance of the coating in a certain range. The high-temperature corrosion resistance of NiAl coating is better than that of NiCr coating formed by thermal spraying, but its long-term service performance and actual boiler service performance need to be further studied. The addition of other elements, such as Ti, Mo, and W, may accelerate the formation of oxide layers during high-temperature corrosion.
- The coating formed by aluminizing has low porosity and high substrate bonding strength. Depending on the substrate material and process parameters, aluminizing technology can form different coatings on the substrate surface, including Ni–Al and Fe–Al. Ni–Al coating exhibits better corrosion resistance than Fe–Al coating.
- Laser cladding technology can not only form a coating that is consistent with the density of the alloy but also refine the surface grains of the coating. However, there are few relevant experimental data, and many materials can be studied.
Funding
Conflicts of Interest
References
- Khan, A.A.; de Jong, W.; Jansens, P.J.; Spliethoff, H. Biomass combustion in fluidized bed boilers: Potential problems and remedies. Fuel Process. Technol. 2009, 90, 21–50. [Google Scholar] [CrossRef]
- Saidur, R.; Abdelaziz, E.A.; Demirbas, A.; Hossain, M.S.; Mekhilef, S. A review on biomass as a fuel for boilers. Renew. Sustain. Energy Rev. 2011, 15, 2262–2289. [Google Scholar] [CrossRef]
- Demirbas, A. Potential applications of renewable energy sources, biomass combustion problems in boiler power systems and combustion related environmental issues. Prog. Energy Combust. Sci. 2005, 31, 171–192. [Google Scholar] [CrossRef]
- Demirbas, A. Combustion characteristics of different biomass fuels. Prog. Energy Combust. Sci. 2004, 30, 219–230. [Google Scholar] [CrossRef]
- Montgomery, M.; Jensen, S.A.; Borg, U.; Biede, O.; Vilhelmsen, T. Experiences with high temperature corrosion at straw-firing power plants in Denmark. Mater. Corros. 2011, 62, 593–605. [Google Scholar] [CrossRef]
- Lim, S.; Lee, K.T. Leading global energy and environmental transformation: Unified ASEAN biomass-based bio-energy system incorporating the clean development mechanism. Biomass Bioenergy 2011, 35, 2479–2490. [Google Scholar] [CrossRef]
- Kirkels, A.F. Discursive shifts in energy from biomass: A 30 year European overview. Renew. Sustain. Energy Rev. 2012, 16, 4105–4115. [Google Scholar] [CrossRef]
- Nielsen, H.P.; Frandsen, F.J.; Dam-Johansen, K.; Baxter, L.L. The implications of chlorine-associated corrosion on the operation of biomass-fired boilers. Prog. Energy Combust. Sci. 2000, 26, 283–298. [Google Scholar] [CrossRef]
- Yu, C.; Qin, J.; Nie, H.; Fang, M.; Luo, Z. Experimental research on agglomeration in straw-fired fluidized beds. Appl. Energy 2011, 88, 4534–4543. [Google Scholar] [CrossRef]
- Jenkins, B.; Baxter, L.; Miles, T.; Miles, T. Combustion properties of biomass. Fuel Process. Technol. 1998, 54, 17–46. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G. An overview of the chemical composition of biomass. Fuel 2010, 89, 913–933. [Google Scholar] [CrossRef]
- Yin, C.; Rosendahl, L.; Kær, S.K.; Clausen, S.; Hvid, S.L.; Hille, T. Mathematical Modeling and Experimental Study of Biomass Combustion in a Thermal 108 MW Grate-Fired Boiler. Energy Fuels 2008, 22, 1380–1390. [Google Scholar] [CrossRef] [Green Version]
- Michelsen, H.P.; Frandsen, F.; Dam-Johansen, K.; Larsen, O.H. Deposition and high temperature corrosion in a 10 MW straw fired boiler. Fuel Process. Technol. 1998, 54, 95–108. [Google Scholar] [CrossRef]
- Montgomery, M.; Larsen, O.H. Field test corrosion experiments in Denmark with biomass fuels. Part 2: Co-firing of straw and coal. Mater. Corros. 2002, 53, 185–194. [Google Scholar] [CrossRef]
- Sharp, W.B.A. Superheater Corrosion in Biomass Boilers: Today’s Science and Technology; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 2011.
- Vokál, V.; Rohr, V.; Pomeroy, M.J.; Schütze, M. Corrosion of alloys and their diffusion aluminide coatings by KCl:K2SO4 deposits at 650 °C in air. Mater. Corros. 2008, 59, 374–379. [Google Scholar] [CrossRef]
- Dębowska, A.; Magdziarz, A.; Kopia, A.; Kalemba–Rec, I.; Petrzak, P. Influence of fuel ashes on corrosion of surface coatings cladded by CMT method. Energy Sources Part A Recover. Util. Environ. Eff. 2019, 41, 427–437. [Google Scholar] [CrossRef]
- Kawahara, Y. An Overview on Corrosion-Resistant Coating Technologies in Biomass/Waste-to-Energy Plants in Recent Decades. Coatings 2016, 6, 34. [Google Scholar] [CrossRef] [Green Version]
- Frandsen, F.J. Utilizing biomass and waste for power production—A decade of contributing to the understanding, interpretation and analysis of deposits and corrosion products. Fuel 2005, 84, 1277–1294. [Google Scholar] [CrossRef]
- Sadeghi, E.; Markocsan, N.; Joshi, S. Advances in Corrosion-Resistant Thermal Spray Coatings for Renewable Energy Power Plants. Part I: Effect of Composition and Microstructure. J. Therm. Spray Technol. 2019, 28, 1749–1788. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.L.; Dahl, K.V.; Christiansen, T.L.; Montgomery, M.; Hald, J. Corrosion behaviour of Ni and nickel aluminide coatings exposed in a biomass fired power plant for two years. Surf. Coat. Technol. 2019, 362, 355–365. [Google Scholar] [CrossRef]
- Wu, D.L.; Dahl, K.V.; Grumsen, F.B.; Christiansen, T.L.; Montgomery, M.; Hald, J. Breakdown mechanism of γ-Al2O3 on Ni2Al3 coatings exposed in a biomass fired power plant. Corros. Sci. 2020, 108583. [Google Scholar] [CrossRef]
- Sadeghi, E.; Markocsan, N.; Joshi, S. Advances in Corrosion-Resistant Thermal Spray Coatings for Renewable Energy Power Plants: Part II—Effect of Environment and Outlook. J. Therm. Spray Technol. 2019, 28, 1789–1850. [Google Scholar] [CrossRef] [Green Version]
- Latreche, H.; Doublet, S.; Tegeder, G.; Wolf, G.; Masset, P.; Weber, T.; Schütze, M. Behaviour of NiAl APS-coatings in chlorine-containing atmospheres. Mater. Corros. 2008, 59, 573–583. [Google Scholar] [CrossRef]
- Wu, D.; Dahl, K.V.; Madsen, J.L.; Christiansen, T.L.; Montgomery, M.; Hald, J. Effects of Different Fuel Specifications and Operation Conditions on the Performance of Coated and Uncoated Superheater Tubes in Two Different Biomass-Fired Boilers. ACS Appl. Energy Mater. 2018, 1, 1463–1475. [Google Scholar] [CrossRef]
- Lehmusto, J.; Skrifvars, B.J.; Yrjas, P.; Hupa, M. High temperature oxidation of metallic chromium exposed to eight different metal chlorides. Corros. Sci. 2011, 53, 3315–3323. [Google Scholar] [CrossRef]
- Bankiewicz, D.; Yrjas, P.; Lindberg, D.; Hupa, M. Determination of the corrosivity of Pb-containing salt mixtures. Corros. Sci. 2013, 66, 225–232. [Google Scholar] [CrossRef]
- Sorell, G. The role of chlorine in high temperature corrosion in waste-to-energy plants. Mater. High Temp. 1997, 14, 207–220. [Google Scholar] [CrossRef]
- Zahs, A.; Spiegel, M.; Grabke, H. The influence of alloying elements on the chlorine-induced high temperature corrosion of Fe-Cr alloys in oxidizing atmospheres. Mater. Corros. 1999, 50, 561–578. [Google Scholar] [CrossRef]
- Kawahara, Y. Role of molten phase content of deposits for high-temperature corrosion in waste incineration environment. Mater. High Temp. 1997, 14, 269–276. [Google Scholar] [CrossRef]
- Andersson, S.; Blomqvist, E.W.; Bäfver, L.; Jones, F.; Davidsson, K.; Froitzheim, J.; Karlsson, M.; Larsson, E.; Liske, J. Sulfur recirculation for increased electricity production in Waste-to-Energy plants. Waste Manag. 2014, 34, 67–78. [Google Scholar] [CrossRef] [Green Version]
- Oksa, M.; Varis, T.; Ruusuvuori, K. Performance testing of iron based thermally sprayed HVOF coatings in a biomass-fired fluidised bed boiler. Surf. Coat. Technol. 2014, 251, 191–200. [Google Scholar] [CrossRef]
- Liu, S.; Liu, Z.; Wang, Y.; Tang, J. A comparative study on the high temperature corrosion of TP347H stainless steel, C22 alloy and laser-cladding C22 coating in molten chloride salts. Corros. Sci. 2014, 83, 396–408. [Google Scholar] [CrossRef]
- Otsuka, N. A thermodynamic approach on vapor-condensation of corrosive salts from flue gas on boiler tubes in waste incinerators. Corros. Sci. 2008, 50, 1627–1636. [Google Scholar] [CrossRef]
- Engin, B.; Kayahan, U.; Atakül, H. A comparative study on the air, the oxygen-enriched air and the oxy-fuel combustion of lignites in CFB. Energy 2020, 196, 117021. [Google Scholar] [CrossRef]
- Guo, W.; Wu, Y.; Zhang, J.; Hong, S.; Chen, L.; Qin, Y. A Comparative Study of Cyclic Oxidation and Sulfates-Induced Hot Corrosion Behavior of Arc-Sprayed Ni-Cr-Ti Coatings at Moderate Temperatures. J. Therm. Spray Technol. 2015, 24, 789–797. [Google Scholar] [CrossRef]
- Fantozzi, D.; Matikainen, V.; Uusitalo, M.; Koivuluoto, H.; Vuoristo, P. Chlorine-induced high temperature corrosion of Inconel 625 sprayed coatings deposited with different thermal spray techniques. Surf. Coat. Technol. 2017, 318, 233–243. [Google Scholar] [CrossRef]
- Sadeghimeresht, E.; Markocsan, N.; Nylén, P.; Björklund, S. Corrosion performance of bi-layer Ni/Cr2C3 -NiCr HVAF thermal spray coating. Appl. Surf. Sci. 2016, 369, 470–481. [Google Scholar] [CrossRef]
- Szymański, K.; Hernas, A.; Moskal, G.; Myalska, H. Thermally sprayed coatings resistant to erosion and corrosion for power plant boilers—A review. Surf. Coat. Technol. 2015, 268, 153–164. [Google Scholar] [CrossRef]
- Oksa, M.; Auerkari, P.; Salonen, J.; Varis, T. Nickel-based HVOF coatings promoting high temperature corrosion resistance of biomass-fired power plant boilers. Fuel Process. Technol. 2014, 125, 236–245. [Google Scholar] [CrossRef]
- Torrell, M.; Dosta, S.; Miguel, J.R.; Guilemany, J.M. Optimisation of HVOF thermal spray coatings for their implementation as MSWI superheater protectors. Corros. Eng. Sci. Technol. 2010, 45, 84–93. [Google Scholar] [CrossRef]
- Tuurna, S.; Varis, T.; Penttilä, K.; Ruusuvuori, K.; Holmström, S.; Yli-Olli, S. Optimised selection of new protective coatings for biofuel boiler applications. Mater. Corros. 2011, 62, 642–649. [Google Scholar] [CrossRef]
- Kumar, M.; Singh, H.; Singh, N. Production of nanocrystalline Ni-20Cr coatings for high-temperature applications. J. Therm. Spray Technol. 2014, 23, 692–707. [Google Scholar] [CrossRef]
- Oksa, M.; Metsäjoki, J.; Kärki, J. Thermal Spray Coatings for High-Temperature Corrosion Protection in Biomass Co-Fired Boilers. J. Therm. Spray Technol. 2014, 24, 194–205. [Google Scholar] [CrossRef]
- Oksa, M.; Metsäjoki, J. Optimizing NiCr and FeCr HVOF Coating Structures for High Temperature Corrosion Protection Applications. J. Therm. Spray Technol. 2014, 24, 436–453. [Google Scholar] [CrossRef]
- Sadeghimeresht, E.; Reddy, L.; Hussain, T.; Huhtakangas, M.; Markocsan, N.; Joshi, S. Influence of KCl and HCl on high temperature corrosion of HVAF-sprayed NiCrAlY and NiCrMo coatings. Mater. Des. 2018, 148, 17–29. [Google Scholar] [CrossRef]
- Hussain, T.; Dudziak, T.; Simms, N.J.; Nicholls, J.R. Fireside corrosion behavior of HVOF and plasma-sprayed coatings in advanced coal/biomass Co-fired power plants. J. Therm. Spray Technol. 2013, 22, 797–807. [Google Scholar] [CrossRef]
- Hussain, T.; Simms, N.J.; Nicholls, J.R. Modelling fireside corrosion of thermal sprayed coatings in co-firing of coal/biomass. Mater. Corros. 2014, 65, 197–205. [Google Scholar] [CrossRef]
- Bai, M.; Reddy, L.; Hussain, T. Experimental and thermodynamic investigations on the chlorine-induced corrosion of HVOF thermal sprayed NiAl coatings and 304 stainless steels at 700 °C. Corros. Sci. 2018, 135, 147–157. [Google Scholar] [CrossRef]
- Oksa, M.; Tuurna, S.; Varis, T. Increased lifetime for biomass and waste to energy power plant boilers with HVOF coatings: High temperature corrosion testing under chlorine-containing molten salt. J. Therm. Spray Technol. 2013, 22, 783–796. [Google Scholar] [CrossRef]
- Varis, T.; Bankiewicz, D.; Yrjas, P.; Oksa, M.; Suhonen, T.; Tuurna, S.; Ruusuvuori, K.; Holmström, S. High temperature corrosion of thermally sprayed NiCr and FeCr coatings covered with a KCl-K2SO4 salt mixture. Surf. Coat. Technol. 2015, 265, 235–243. [Google Scholar] [CrossRef]
- Reddy, L.; Shipway, P.; Davis, C.; Hussain, T. HVOF and Laser-Cladded Fe–Cr–B Coating in Simulated Biomass Combustion: Microstructure and Fireside Corrosion. Oxid. Met. 2017, 87, 825–835. [Google Scholar] [CrossRef] [Green Version]
- Paul, S.; Harvey, M.D.F. Corrosion testing of Ni alloy HVOF coatings in high temperature environments for biomass applications. J. Therm. Spray Technol. 2013, 22, 316–327. [Google Scholar] [CrossRef]
- Song, B.; Voisey, K.T.; Hussain, T. High temperature chlorine-induced corrosion of Ni50Cr coating: HVOLF, HVOGF, cold spray and laser cladding. Surf. Coat. Technol. 2018, 337, 357–369. [Google Scholar] [CrossRef]
- Hussain, T.; Simms, N.J.; Nicholls, J.R.; Oakey, J.E. Fireside corrosion degradation of HVOF thermal sprayed FeCrAl coating at 700–800 °C. Surf. Coat. Technol. 2015, 268, 165–172. [Google Scholar] [CrossRef]
- Oksa, M.; Metsäjoki, J.; Kärki, J. Corrosion Testing of Thermal Spray Coatings in a Biomass Co-Firing Power Plant. Coatings 2016, 6, 65. [Google Scholar] [CrossRef] [Green Version]
- Matsubara, Y.; Sochi, Y.; Tanabe, M.; Takeya, A. Advanced coatings on furnace wall tubes. J. Therm. Spray Technol. 2007, 16, 195–201. [Google Scholar] [CrossRef]
- Sadeghimeresht, E.; Reddy, L.; Hussain, T.; Markocsan, N.; Joshi, S. Chlorine-induced high temperature corrosion of HVAF-sprayed Ni-based alumina and chromia forming coatings. Corros. Sci. 2018, 132, 170–184. [Google Scholar] [CrossRef]
- Sadeghimeresht, E.; Eklund, J.; Phother Simon, J.; Liske, J.; Markocsan, N.; Joshi, S. Effect of water vapor on the oxidation behavior of HVAF-sprayed NiCr and NiCrAlY coatings. Mater. Corros. 2018, 69, 1431–1440. [Google Scholar] [CrossRef]
- Agüero, A.; Baráibar, I.; Gutiérrez, M.; Hernández, M.; Muelas, R.; Rodríguez, S. Biomass corrosion behavior of steels and coatings in contact with KCl/K2SO4 at 550 °C under an oxy-fuel combustion atmosphere: A screening laboratory test. Surf. Coat. Technol. 2018, 350, 188–200. [Google Scholar] [CrossRef]
- Eklund, J.; Phother, J.; Sadeghi, E.; Joshi, S.; Liske, J. High-Temperature Corrosion of HVAF-Sprayed Ni-Based Coatings for Boiler Applications. Oxid. Met. 2019, 91, 729–747. [Google Scholar] [CrossRef] [Green Version]
- Jafari, R.; Sadeghimeresht, E.; Farahani, T.S.; Huhtakangas, M.; Markocsan, N.; Joshi, S. KCl-Induced High-Temperature Corrosion Behavior of HVAF-Sprayed Ni-Based Coatings in Ambient Air. J. Therm. Spray Technol. 2018, 27, 500–511. [Google Scholar] [CrossRef] [Green Version]
- Li, X.Z.; Li, H.C.; Wang, Y.T.; Li, B. Investigations on the behavior of laser cladding Ni-Cr-Mo alloy coating on TP347H stainless steel tube in HCl rich environment. Surf. Coat. Technol. 2013, 232, 627–639. [Google Scholar] [CrossRef]
- Bellucci, A.; Bellini, S.; Pileggi, R.; Stocchi, D.; Tuurna, S. Effect of Al Enrichment by Pack Cementation of FeCr Coatings Deposited by HVOF. J. Therm. Spray Technol. 2014, 24, 244–251. [Google Scholar] [CrossRef]
- Wu, D.L.; Dahl, K.V.; Christiansen, T.L.; Montgomery, M.; Hald, J. Microstructural investigations of Ni and Ni2Al3 coatings exposed in biomass power plants. Mater. High Temp. 2018, 35, 255–266. [Google Scholar] [CrossRef] [Green Version]
- Kiamehr, S.; Lomholt, T.N.; Dahl, K.V.; Christiansen, T.L.; Somers, M.A.J. Application of aluminum diffusion coatings to mitigate the KCl-induced high-temperature corrosion. Mater. Corros. 2017, 68, 82–94. [Google Scholar] [CrossRef] [Green Version]
- Orlicka, D.; Simms, N.J.; Hussain, T.; Nicholls, J.R. Comparison between oxidation of Fe-Cr-Al sputter coatings in air and air-HCl environments at 550 °C. Mater. High Temp. 2015, 32, 167–176. [Google Scholar] [CrossRef]
- Quinlan, F.B.; Grobel, L.P. Treatment of Metals. U.S. Patent 2,303,869, 1 December 1942. [Google Scholar]
- Jeurgens, L.P.; Sloof, W..; Tichelaar, F..; Mittemeijer, E. Structure and morphology of aluminium-oxide films formed by thermal oxidation of aluminium. Thin Solid Films 2002, 418, 89–101. [Google Scholar] [CrossRef]
- Ameer, M.A.; Fekry, A.M.; Heakal, F.E.T. Electrochemical behaviour of passive films on molybdenum-containing austenitic stainless steels in aqueous solutions. Electrochim. Acta 2004, 50, 43–49. [Google Scholar] [CrossRef]
- Kiamehr, S.; Dahl, K.V.; Montgomery, M.; Somers, M.A.J. KCl-induced high temperature corrosion of selected commercial alloys: Part I: Chromia-formers. Mater. Corros. 2015, 66, 1414–1429. [Google Scholar] [CrossRef]
- Kiamehr, S.; Dahl, K.V.; Montgomery, M.; Somers, M.A.J. KCl-induced high temperature corrosion of selected commercial alloys: Part II: Alumina and silica-formers. Mater. Corros. 2016, 67, 26–38. [Google Scholar] [CrossRef]
- Lehmusto, J.; Yrjas, P.; Skrifvars, B.J.; Hupa, M. High temperature corrosion of superheater steels by KCl and K2CO3 under dry and wet conditions. Fuel Process. Technol. 2012, 104, 253–264. [Google Scholar] [CrossRef]
- Pettersson, C.; Pettersson, J.; Asteman, H.; Svensson, J.E.; Johansson, L.G. KCl-induced high temperature corrosion of the austenitic Fe-Cr-Ni alloys 304L and Sanicro 28 at 600 °C. Corros. Sci. 2006, 48, 1368–1378. [Google Scholar] [CrossRef]
- Grabke, H.J.; Reese, E.; Spiegel, M. The effects of chlorides, hydrogen chloride, and sulfur dioxide in the oxidation of steels below deposits. Corros. Sci. 1995, 37, 1023–1043. [Google Scholar] [CrossRef]
- Paneru, M.; Stein-Brzozowska, G.; Maier, J.; Scheffknecht, G. Corrosion mechanism of alloy 310 austenitic steel beneath NaCl deposit under varying SO2 concentrations in an oxy-fuel combustion atmosphere. Energy Fuels 2013, 27, 5699–5705. [Google Scholar] [CrossRef]
- Van Lith, S.C.; Frandsen, F.J.; Montgomery, M.; Vilhelmsen, T.; Jensen, S.A. Lab-scale investigation of deposit-induced chlorine corrosion of superheater materials under simulated biomass-firing conditions. Part 1: Exposure at 560 °C. Energy Fuels 2009, 23, 3457–3468. [Google Scholar] [CrossRef]
- Pettersson, J.; Asteman, H.; Svensson, J.E.; Johansson, L.G. KCl Induced Corrosion of a 304-type Austenitic Stainless Steel at 600 °C; The Role of Potassium. Oxid. Met. 2005, 64, 23–41. [Google Scholar] [CrossRef]
- Jonsson, T.; Froitzheim, J.; Pettersson, J.; Svensson, J.E.; Johansson, L.G.; Halvarsson, M. The influence of KCl on the corrosion of an Austenitic stainless steel (304L) in oxidizing humid conditions at 600 °C: A microstructural study. Oxid. Met. 2009, 72, 213–239. [Google Scholar] [CrossRef]
- Enestam, S.; Bankiewicz, D.; Tuiremo, J.; Mäkelä, K.; Hupa, M. Are NaCl and KCl equally corrosive on superheater materials of steam boilers? Fuel 2013, 104, 294–306. [Google Scholar] [CrossRef]
- Pettersson, J.; Svensson, J.E.; Johansson, L.G. Alkali Induced Corrosion of 304-Type Austenitic Stainless Steel at 600 °C; Comparison between KCl, K2CO3 and K2SO4. Mater. Sci. Forum 2008, 595–598, 367–375. [Google Scholar] [CrossRef]
- Wang, W.; Wang, J.; Sun, Z.; Li, J.; Li, L.; Song, X.; Wen, X.; Xie, L.; Yang, X. Effect of Mo and aging temperature on corrosion behavior of (CoCrFeNi)100-xMox high-entropy alloys. J. Alloys Compd. 2020, 812, 152139. [Google Scholar] [CrossRef]





| Plant Description | Type | Steam Temp. °C | Pressure MPa | Size MWth | Commissioned |
|---|---|---|---|---|---|
| Haslev | Grate-fired | 440 | 6.7 | 20 | 1989 |
| Slagelse | Grate-fired | 450 | 6.7 | 32 | 1990 |
| Rudkøbing CHP 100% straw | Grate-fired | 450 | 6.1 | 10.7 | 1990 |
| Masnedø CHP-almost 100% straw | Grate-fired | 520 | 9.2 | 33 | 1998 |
| Ensted CHP-100% straw boiler 100% woodchip | Grate-fired | 470 470–540 | 20.1 20.1 | 80 15 | - |
| Maribo Sakskøbing CHP: 100% straw | Grate-fired | 540 | 9.3 | 33 | 2000 |
| Avedøre 2 Bioboiler: 100% straw | Grate-fired | 540 | 30 | 105 | 2001 |
| Fyn 8: 100% straw | Grate-fired | 540 | 12 | 117 | 2009 |
| Grenå 50% CFB straw, 50% coal | CFB | 505 | 9.2 | 80 | 1992 |
| Studstrup suspension fired coal plant modified for coal + 10% straw | Suspension fired | 540 | 25 | 830 | 2002 rebuilt Unit 4 |
| Avedøre II main boiler: oil + gas + wood | Suspension fired | 540-580 | 30 | 800 | 2005 rebuilt Unit 3 2001 |
| Herning Wood + gas | Grate-fired | 515 | 11.5 | 288 | 2002 rebuilt to biomass 2009 |
| Amager I multifuel straw pellets, coal, wood pellets, heavy fuel oil | Suspension fired | 560 | 18 | 350 |
| Kind of Biomass Fuels | Sewage Sludge | Wooden Chips | Wheat Straw | Palm Kennel Shell | Coal | Municipal Waste | |
|---|---|---|---|---|---|---|---|
| Contaminants of fuels | Ash | ● | △ | △ | ○ | ● | ● |
| Cl | ● | △ | △ | △ | ● | ○ | |
| S | ● | △ | ● | ○ | △ | ● | |
| Ash constituents | Alkaline metals (Na, K) | △ | △ | ● | △ | ○ | ● |
| Alkaline earth metals (Ca, Mg) | △ | ● | △ | ● | △ | △ | |
| Heavy metals (Zn, Pb) | ○ | ○ | ○ | ○ | ○ | ● | |
| Others (Fe, P) | ● | △ | △ | ○ | ● | △ | |
| Corrosivity of formed environment | Severe | Medium | Severe | Weak | Medium | Severe | |
| Preparation Technology | References |
|---|---|
| Thermal spraying | [24,32,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62] |
| Laser cladding | [33,52,54,63] |
| Aluminizing | [16,64,65,66] |
| Physical vapor deposition | [67] |
| Material | Composition (wt.%) | Tech | Temp (°C) | Time (h) | Atmosphere | Salt (mg/cm2) | Mass Change (mg/cm2) | Corrosion Layer Thickness (μm) | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Ni5Al | Ni- < 5.7Al | HVAF | 600 | 168 | 5O2 + Bal.N2 (vol.%) + 500 vppm HCl | 0.1 KCl | 2.49 | ~2 | [58] |
| Air | 0.1 KCl | 1.97 | ~15 | [62] | |||||
| 5O2 + 20H2O + Bal.N2 (vol.%) | 1 KCl | −0.25 | ~4 | [61] | |||||
| HVOF | 550 | 672 | 8O2 + 30H2O + 2N2 + 60CO2 (vol.%) + 400 vppm HCl + 2 vppm SO2 | 0.4~0.7 (45KCl + 55K2SO4 wt.%) | ~32 | Failure | [60] | ||
| Ni31Al | Ni-31Al | HVOF | 700 | 250 | 5O2 + Bal.N2 (vol.%) + 500ppm HCl | 30 (10KCl + 90Kaolinite wt.%) | - | ~3 | [49] |
| APS | 800 | 280 | 99.8Ar + 0.2Cl2(vol.%) + 3 ppm O2 | - | - | ~75 | [24] | ||
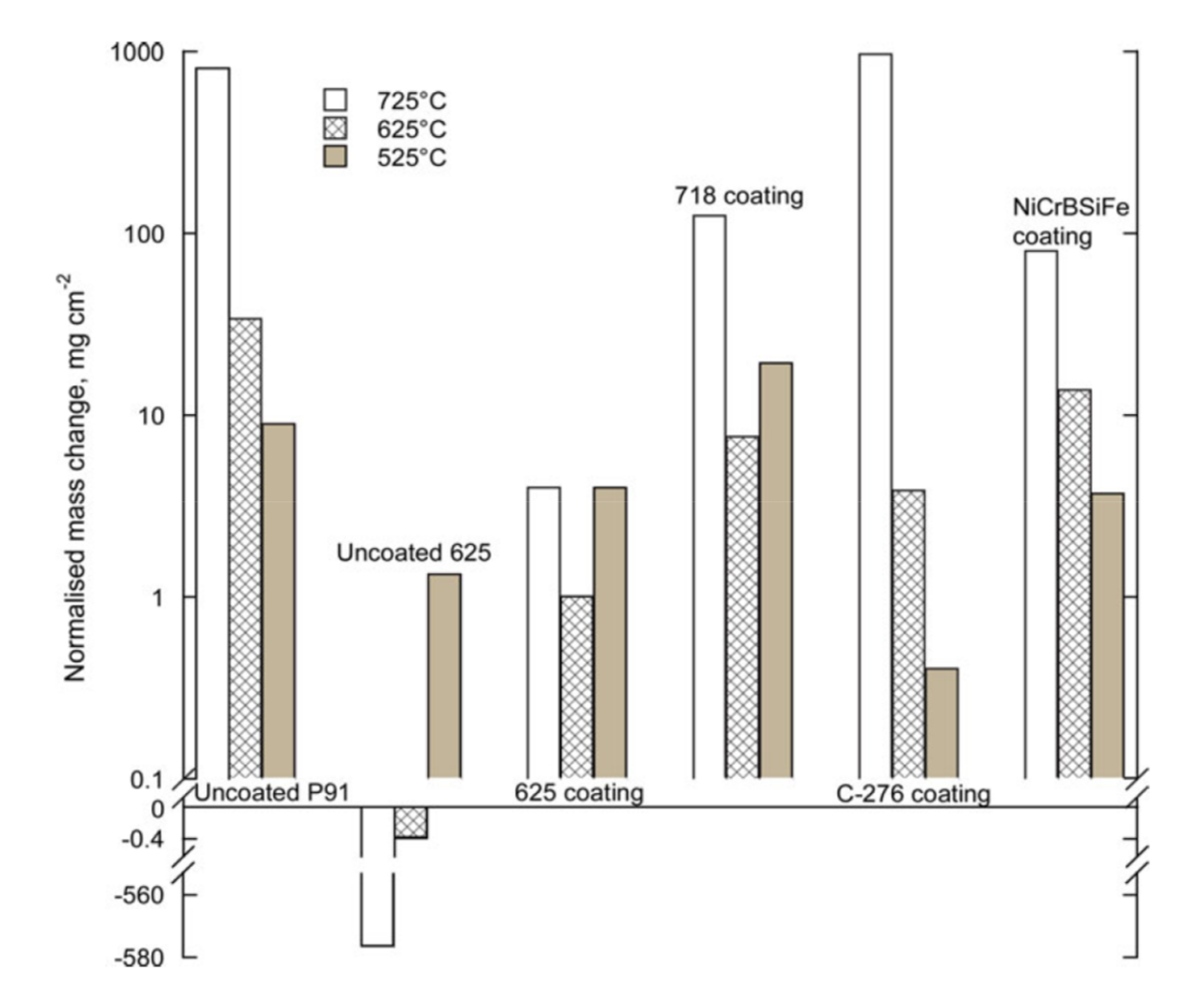
| C-276 | Ni-15.55Cr-16.48Mo-5.09Fe-3.81W | HVOF | 525 | 168 | 9O2 + 7H2O + 84N2(vol.%) + 175 vppm HCl | Embedded in salt mixture (45.1K2SO4 + 54.9KCl wt.%) | 0.4 | ~3 | [53] |
| 625 | 3.9 | ~40 | |||||||
| 725 | 972 | >10,000 | |||||||
| NiCrBSiFe | Ni-17.2Cr-3.7Si-3.1B-4.6Fe-0.8C | HVOF | 525 | 3.7 | ~10 | ||||
| 625 | 13.8 | - | |||||||
| 725 | 79.9 | ~250 | |||||||
| Alloy 718 | Ni-18.78Cr-17.9Fe-3.04Mo-5.26Nb-0.48Al | HVOF | 525 | 19.4 | ~50 | ||||
| 625 | 7.7 | ~25 | |||||||
| 725 | 124.8 | ~230 | |||||||
| Ni20Cr | Ni-20Cr | HVOF | 550 | 672 | 60CO2 + 30H2O + 8O2 + 2N2(vol.%) + 400 vppm HCl + 2 vppm SO2 | 0.4~0.7 (45KCl + 55K2SO4 wt.%) | ~3 | 1–5 (528 h) | [60] |
| Ni21Cr | Ni-21Cr | HVAF | 600 | 168 | 5O2 + Bal.N2 (vol.%) + 500 vppm HCl | 0.1 KCl | 11.32 | ~18.75 | [58] |
| Air | 0.1 KCl | 1.65 | ~22 | [62] | |||||
| 5O2 + 20H2O + Bal.N2 (vol.%) | 1 KCl | 2.5 | ~17 | [61] | |||||
| C22 | Ni-21.3Cr-13.2Mo-3W-2.93Fe-2Co | LC | 550 | 96 | Air | Embedded in salt mixture (98.6KCl + 1.4NaCl/95.5KCl + 4.5NaCl wt.%) | 17/10.5 | - | [33] |
| 600 | 68/148 | - | |||||||
| 650 | 177.8/177.7 | - | |||||||
| 700 | 160/56 | - | |||||||
| 750 | 50/46 | - | |||||||
| 650 | 168 | 5O2 + 12CO2 + 0.05HCl + Bal.N2 (vol.%) | - | 0.1 | - | [63] | |||
| 700 | 0.15 | - | |||||||
| Diamalloy 4006 | Ni- > 21.2Cr- > 10W- > 9.0Mo- > 4.2Cu- > 0.8C- > 0.75B | HVOF-CJS | 600 | 168 | Air | Embedded in salt mixture (50 K2SO4 + 50KCl mol.%) | - | 7 | [51] |
| Air + 30H2O (vol.%) | - | 7 | |||||||
| 575 | 168 | 5O2 + 10H2O + Bal.N2 (vol.%) | Spray deposition (6.5NaCl + 59Na2SO4 + 34.5KCl wt.%) | - | ~105 | [50] | |||
| 625 | - | ~25 | |||||||
| 550 | 1300 | Actual boiler | - | 1~2 | [56] | ||||
| 750 | - | Failure | |||||||
| HVOF-DJ | 575 | 168 | 5O2 + 10H2O + Bal.N2 (vol.%) | Spray deposition (6.5NaCl + 59Na2SO4 + 34.5KCl wt.%) | - | ~20 | [50] | ||
| 625 | - | ~25 | |||||||
| NiCrAlY | Ni-21Cr-7Al-1Y | HVAF | 600 | 168 | Air | 0.1 KCl | 3.48 | ~24 | [62] |
| 5O2 + 20H2O + Bal.N2 (vol.%) | 1 KCl | 0.4 | ~3 | [61] | |||||
| 5O2 + Bal.N2 (vol.%) + 500 vppm HCl | 0.1 KCl | 1.49 | ~10 | [46] | |||||
| HVOF | 200 | 17520 | Actual boiler | - | 0 | [40] | |||
| 650 | 1000 | 4O2 + 8H2O + 14CO2 + 73.8N2 (vol.%) + 400 vppm HCl + 1300 vppm SO2 | 20 (37.5Na2SO4 + 37.5K2SO4 + 25 Fe2O3 mol.%) | ~170 | >250 (50% probability) | [48] | |||
| 20(30Na2SO4 + 30K2SO4 + 20Fe2O3 + 20Kaolinite mol.%) | ~60 | 20 (50% probability) | |||||||
| 20(22.5Na2SO4 + 22.5K2SO4 + 15Fe2O3 + 40Kaolinite mol.%) | ~42 | 18 (50% probability) | |||||||
| 20(15Na2SO4 + 15K2SO4 + 10Fe2O3 + 60 Kaolinite mol.%) | ~20 | >7 (50% probability) | |||||||
| 20(7.5Na2SO4 + 7.5K2SO4 + 5Fe2O3 + 80Kaolinite mol.%) | ~12 | >7 (50% probability) | |||||||
| 20(37.5Na2SO4 + 37.5K2SO4 + 25Fe2O3 mol.%) | - | 180 (50% probability) | [47] | ||||||
| APS | - | 210 (50% probability) | |||||||
| Ni21Cr9Mo | Ni-21Cr-9Mo | HVAF | 600 | 168 | Air | 0.1 KCl | 4.12 | ~31 | [62] |
| Ni21Cr9Mo-SiO2 | SiO2-containg Ni21Cr9Mo | HVAF | 600 | 168 | 5O2 + Bal.N2 (vol.%) + 500 vppm HCl | 0.1 KCl | 0.67 | ~15 | [46] |
| IN625 | Ni- < 21.5Cr- < 9Mo- < 3.7Nb + Ta- < 2.5Fe- < 0.2Si- < 0.1Mn | HVOF-CJS | 575 | 168 | 5O2 + 10H2O + Bal.N2 (vol.%) | Spray deposition (6.5NaCl + 59Na2SO4 + 34.5KCl wt.%) | - | ~150 | [50] |
| 625 | - | ~40 | |||||||
| HVOF-DJ | 575 | - | ~75 | ||||||
| 625 | - | ~20 | |||||||
| 550 | 5900 | Actual boiler | - | 6~18 | [44,56] | ||||
| 750 | - | 323~434 | |||||||
| 110 | 17520 | - | 0 | [40] | |||||
| HVOF | 650 | 1000 | 4O2 + 8H2O + 14CO2 + 73.8N2 (vol.%) + 400 vppm HCl + 1300 vppm SO2 | 20 (37.5Na2SO4 + 37.5K2SO4 + 25Fe2O3 mol.%) | ~120 | 125 (50% probability) | [48] | ||
| 20(30Na2SO4 + 30K2SO4 + 20Fe2O3 + 20Kaolinite mol.%) | ~75 | 90 (50% probability) | |||||||
| 20(22.5Na2SO4 + 22.5K2SO4 + 15Fe2O3 + 40Kaolinite mol.%) | ~58 | 38 (50% probability) | |||||||
| 20(15Na2SO4 + 15K2SO4 + 10Fe2O3 + 60 Kaolinite mol.%) | ~48 | <10 (50% probability) | |||||||
| 20(7.5Na2SO4 + 7.5K2SO4 + 5Fe2O3 + 80Kaolinite mol.%) | ~23 | <10 (50% probability) | |||||||
| 20(37.5Na2SO4 + 37.5K2SO4 + 25Fe2O3 mol.%) | - | 175 (50% probability) | [47] | ||||||
| 525 | 168 | 9O2 + 7H2O + Bal.N2(vol.%) + 175 vppm HCl | Embedded in salt mixture (45.1K2SO4 + 54.9KCl wt.%) | 4 | - | [53] | |||
| 625 | 1 | ~3 | |||||||
| 725 | 4 | ~5 | |||||||
| 400 | 360 | Air | (52KCl + 48ZnCl2 wt.%) | - | ~10 | [41] | |||
| 550 | 168 | Air + 12H2O (vol.%) | KCl | - | 4.55 | [37] | |||
| TWAS | - | 1.19 | |||||||
| CS | - | 9.25 | |||||||
| HVAF | - | 11.58 | |||||||
| APS | 650 | 1000 | 4O2 + 8H2O + 14CO2 + 73.8N2 (vol.%) + 400 vppm HCl + 1300 vppm SO2 | 20(37.5Na2SO4 + 37.5K2SO4 + 25Fe2O3 mol.%) | - | 110 (50% probability) | [47] | ||
| Carpenter 6119 | Ni-22.58Cr-12.77Mo-4Fe-2.63W-0.26Si | HVOF | 400 | 360 | Air | 5(52KCl + 48ZnCl2 wt.%) | - | ~18 | [41] |
| NiCr16Mo | Ni-24Cr-16.5Mo-1.5Fe-0.5Al | HVOF | 110 | 17520 | Actual boiler | - | 1 | [40] | |
| Ni45Cr | Ni-43.3Cr-0.41Ti-0.02C | WAS | 550 | 168 | Air + 30H2O (vol.%) | Embedded in salt mixture (50K2SO4 + 50KCl mol.%) | - | 5 | [51] |
| Air | 2 | ||||||||
| 600 | Air + 30H2O (vol.%) | 12 | |||||||
| Air | 30 | ||||||||
| Ni45Cr | Ni- > 45Cr < 2.2Si < 1.1Fe | HVOF | 600 | 168 | Air + 30H2O (vol.%) | Embedded in salt mixture (50K2SO4 + 50KCl mol.%) | - | 9 | [51] |
| Air | 9 | ||||||||
| HVOF-CJS | 575 | 168 | 5O2 + 10H2O + Bal.N2 (vol.%) | Spray deposition (6.5NaCl + 59Na2SO4 + 34.5KCl wt.%) | - | ~110 | [50] | ||
| 625 | - | ~60 | |||||||
| 550 | 5900 | Actual boiler | - | 3~6 | [44,56] | ||||
| 750 | - | 6~31 | |||||||
| HVOF-DJ | 575 | 168 | 5O2 + 10H2O + Bal.N2 (vol.%) | Spray deposition (6.5NaCl + 59Na2SO4 + 34.5KCl wt.%) | - | ~55 | [50] | ||
| 625 | - | ~40 | |||||||
| HVOF | 650 | 1000 | 4O2 + 8H2O + 14CO2 + 73.8N2 (vol.%) + 400 HCl + 1300 SO2 (vppm) | 20(37.5Na2SO4 + 37.5K2SO4 + 25Fe2O3 mol.%) | - | ~87 (50% probability) | [47] | ||
| APS | - | 13 (50% probability) | |||||||
| Ni50Cr | Ni-46Cr-1.1Fe-0.5Si-0.1C (cold) | HVOF-DJ | 550 | 168 | Air + 30H2O (vol.%) | Embedded in salt mixture (50K2SO4 + 50KCl mol.%) | - | 16 | [51] |
| Air | 5 | ||||||||
| Ni-46Cr-1.1Fe-0.5Si-0.1C (hot) | Air + 30H2O (vol.%) | 39 | |||||||
| Air | 16 | ||||||||
| Ni-46Cr-1.1Fe-0.5Si-0.1C (cold) | 600 | Air + 30H2O (vol.%) | Embedded in salt mixture (50K2SO4 + 50KCl mol.%) | - | 52 | ||||
| Air | 21 | ||||||||
| Ni-46Cr-1.1Fe-0.5Si-0.1C (hot) | Air + 30H2O (vol.%) | 56 | |||||||
| Air | 25 | ||||||||
| Ni- > 45Cr-2.1Si-1.1Fe | 550 | 5900 | Actual boiler | - | 4~8 | [44] | |||
| 750 | - | 20~65 | |||||||
| Ni50Cr | Ni-46Cr-2Si-1Fe-0.1C | HVOF-Liquid | 700 | 250 | 5O2 + Bal.N2 (vol.%) + 500 ppm HCl | 14 ± 1 KCl | - | ~40 | [54] |
| 650 | 1000 | 4O2 + 8H2O + 14CO2 + 73.8N2 (vol.%) + 400 vppm HCl + 1300 vppm SO2 | 20 (37.5Na2SO4 + 37.5K2SO4 + 25Fe2O3 mol.%) | ~130 | ~120 (50% probability) | [48] | |||
| 20(30Na2SO4 + 30K2SO4 + 20Fe2O3 + 20Kaolinite mol.%) | ~78 | <15 (50% probability) | |||||||
| 20(22.5Na2SO4 + 22.5K2SO4 + 15Fe2O3 + 40Kaolinite mol.%) | ~55 | <15 (50% probability) | |||||||
| 20(15Na2SO4 + 15K2SO4 + 10Fe2O3 + 60 Kaolinite mol.%) | ~38 | <15 (50% probability) | |||||||
| 20(7.5Na2SO4 + 7.5K2SO4 + 5Fe2O3 + 80Kaolinite mol.%) | ~22 | <15 (50% probability) | |||||||
| 575 | 168 | 5O2 + 10H2O + Bal.N2 (vol.%) | Spray deposition (6.5NaCl + 59Na2SO4 + 34.5KCl wt.%) | - | 2.71 (mm/year) | [45] | |||
| 625 | - | 1.56 (mm/year) | |||||||
| 550 | 5900 | Actual boiler | - | 0.01 (mm/year) | |||||
| 750 | - | 0.1 (mm/year) | |||||||
| HVOF-Gas | 700 | 250 | 5O2 + Bal.N2 (vol.%) + 500 ppm HCl | 14 ± 1 KCl | - | ~45 | [54] | ||
| LC | - | ~9 | |||||||
| NiCrTi | Ni- < 46Cr- < 1Ti | AS | 550 | 5900 | Actual boiler | - | 10~22 | [44,56] | |
| 750 | - | 16~65 | |||||||
| Ni50Cr | Ni-50Cr-0.3Si-0.2C | CS | 700 | 250 | 5O2 + Bal.N2 (vol.%) + 500 ppm HCl | 14 ± 1 KCl | - | ~57.5 | [54] |
| Fe13Cr | Fe-13.2Cr-0.39Si-0.25Ni-0.46Mn-0.41C | WAS | 550 | 168 | Air + 30H2O (vol.%) | Embedded in salt mixture (50K2SO4 + 50KCl mol.%) | - | 48 | [51] |
| Air | 57 | ||||||||
| 600 | Air + 30H2O (vol.%) | 72 | |||||||
| Air | 146 | ||||||||
| Diamalloy 1003 | Fe-16.06Cr-9.88Ni-2.04Mo-1.21Mn | HVOF | 400 | 360 | Air | 5(52KCl + 48ZnCl2 wt.%) | - | ~13 | [41] |
| SHS 7574 | Fe-17.78Cr-14.24Mo-5.9W-2.96B-2.1Mn-1.36Si-0.88C | HVOF | 700 | 250 | 5O2 + Bal.N2 (vol.%) + 500 ppm HCl | 14 ± 1 KCl | - | 276 (Failure) | [52] |
| LC | - | 557 ± 33 | [52] | ||||||
| FeCr | Fe- < 20Cr | HVOF | 650 | 300 | 2O2 + 29H2O + 44CO2 + 0.6SO2 + 0.2HCl + N2 (vol.%) | Embedded in (84CaCO3 + 15CaSO4 + 1KCl wt.%) | - | ~800 | [64] |
| Fe- > 20Cr | - | ~180 | |||||||
| FeCrAl | Fe-21.7Cr-5.9Al-0.83Si-0.76Mn | HVOF | 700 | 1000 | 4O2 + 8H2O + 14CO2 + 73.8N2 (vol.%) + 400 vppm HCl + 1300 vppm SO2 | 20(37.5Na2SO4 + 37.5K2SO4 + 25Fe2O3 mol.%) | ~70 | ~85 (50% probability) | [55] |
| 750 | ~125 | >250 (50% probability) | |||||||
| 800 | ~170 | ~300 (50% probability) | |||||||
| 650 | - | 140 (50% probability) | [47] | ||||||
| APS | 650 | - | 70 (50% probability) | ||||||
| SHS9172 | Fe- < 25Cr- < 15W- < 12Nb- < 6Mo- < 4C- < 3Mn- < 2Si | HVOF-CJS | 600 | 168 | Air + 30H2O (vol.%) | Embedded in salt mixture (50K2SO4 + 50KCl mol.%) | - | 22 | [51] |
| Air | 6 | ||||||||
| 575 | 5O2 + 10H2O + Bal.N2 (vol.%) | Spray deposition (6.5NaCl + 59Na2SO4 + 34.5KCl wt.%) | - | ~30 | [50] | ||||
| 625 | - | ~35 | |||||||
| HVOF-DJ | 575 | - | ~25 | ||||||
| 625 | - | ~25 | |||||||
| 575 | - | 1.30 (mm/year) | [45] | ||||||
| 625 | - | 1.25 (mm/year) | |||||||
| 550 | 5900 | Actual boiler | - | 23~80 | [44,45,56] | ||||
| 750 | - | 55~111 | |||||||
| SHS7174wire | >45Fe- < 30Cr- < 10Mo- < 5B- < 4W- < 2C- < 2Mn- < 2Si | WAS | 550 | 168 | Air + 30H2O (vol.%) | Embedded in salt mixture (50K2SO4 + 50KCl mol.%) | - | 2 | [51] |
| Air | 3 | ||||||||
| 600 | 168 | Air + 30H2O (vol.%) | 10 | ||||||
| Air | 5 | ||||||||
| Fe50Cr | Fe-50Cr | HVOF | 550 | 672 | 60CO2 + 30H2O + 8O2 + 2N2 (vol.%) + 400 vppm HCl + 2 vppm SO2 | 0.4~0.7 (45KCl + 55K2SO4 wt.%) | 6 | 4–10 (528 h) | [60] |
| Fe-Cr-Al | 0.4~59.4Fe-1.4~99.4Cr-0.3~43.2Al | PVD | 550 | 150 | Air + 315 vppm HCl | - | −0.88~0.65 | <1 | [67] |
| Slurry aluminide | Al + inorganic compounds | D | 550 | 672 | 8O2 + 30H2O + 60CO2 + 2N2 (vol.%) + 400 vppm HCl + 2 vppm SO2 | 0.4~0.7 (45KCl + 55K2SO4 wt.%) | 3 | 1–2 (528 h) | [60] |
| Cr enriched aluminide | Cr + Al slurry | 7 | ~6 (528 h) | ||||||
| FeCr + Al | Fe- < 20Cr + overaluminized | HVOF + D | 650 | 300 | 2O2 + 29H2O + 44CO2 + 0.6SO2 + 0.2HCl + Bal.N2 (vol%) | Embedded in (84CaCO3 + 15CaSO4 + 1KCl wt.%) | - | ~10 | [64] |
| Fe- > 20Cr + overaluminized | - | ~8 | |||||||
| P91 | 10% Al, 1% NH4Cl, 89% Al2O3 | D | 650 | 300 | Air | Embedded in salt mixture (50K2SO4 + 50KCl mol.%) | - | 20 | [16] |
| 10Al + 10Fe + 6AlCl3 + 74Al2O3 mol.% | 600 | 168 | 1 mm KCl | - | ~1 | [66] | |||
| 10Al + 6AlCl3 + 84Al2O3 mol.% | - | >40 | |||||||
| 17Cr alloy | 5% Al, 0,5% NH4Cl, 94,5% Al2O3 | D | 650 | 300 | Embedded in salt mixture (50K2SO4 + 50KCl mol.%) | - | 30 | [16] | |
| Alloy 800 | - | 30 | |||||||
| In 617 | - | 60 | |||||||
| Ni | 10Al + 6AlCl3 + 84Al2O3 mol.% | D | 600 | 168 | 1 mm KCl | - | <1 | [66] | |
| TP347H | 10Al + 8AlCl3 + 82Al2O3 mol.% | D | 540 | 6757 | Actual boiler | - | Failure | [65] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, D.; Yuan, Z.; Liu, S.; Zheng, J.; Wei, X.; Zhang, C. Recent Development of Corrosion Factors and Coating Applications in Biomass Firing Plants. Coatings 2020, 10, 1001. https://doi.org/10.3390/coatings10101001
Wu D, Yuan Z, Liu S, Zheng J, Wei X, Zhang C. Recent Development of Corrosion Factors and Coating Applications in Biomass Firing Plants. Coatings. 2020; 10(10):1001. https://doi.org/10.3390/coatings10101001
Chicago/Turabian StyleWu, Duoli, Ziyi Yuan, Su Liu, Jiayin Zheng, Xinlong Wei, and Chao Zhang. 2020. "Recent Development of Corrosion Factors and Coating Applications in Biomass Firing Plants" Coatings 10, no. 10: 1001. https://doi.org/10.3390/coatings10101001




