Abstract
Graphitic carbon nitride (g-C3N4) was supported on SrAl2O4:Eu,Dy-SiO2 by a colloidal-sol coating method to improve its light absorption property. Transmission electron microscopy (TEM) revealed that the nanoparticles of g-C3N4 were coated on sub-micron phosphor particles and nanoscale surface roughness was imparted by the SiO2-binder. Photoluminescence (PL) spectrum of the g-C3N4 supported on SrAl2O4:Eu,Dy exhibited a broadband emission from 400 to 650 nm. Increasing silica-binder in the g-C3N4/SrAl2O4:Eu,Dy composites suppressed the PL emission peak at 525 nm for SrAl2O4:Eu,Dy. Photocatalytic degradation activity was evaluated with 5 ppm methylene blue (MB) solutions under germicidal ultraviolet (UV) and visible (Vis) solar light illuminations. The UV/Vis photocatalytic efficiency was improved by supporting g-C3N4 on the SrAl2O4:Eu,Dy phosphor and with the addition of SiO2 as a binder. In addition, low silica addition effectively improved the adhesiveness of the g-C3N4 coating on the SrAl2O4:Eu,Dy surface. Recyclability tests of photocatalysis for the SrAl2O4:Eu,Dy-0.01M SiO2/50wt% g-C3N4 composites exhibited a remarkable stability by maintaining the degradation efficiencies above 90% in four cycles. Therefore, the composite of g-C3N4-supported SrAl2O4:Eu,Dy-SiO2 is a prospective photocatalyst activating under UV/Vis light irradiation for the elimination of environmental pollutants.
1. Introduction
Nano-based materials technologies offer promising methods for harnessing solar energy to tackle air and water pollution problems. In the past decades, the trending researches were based on employing TiO2 semiconductors in photocatalysis applications [1,2]. Apart from this UV-activated material, the graphitic carbon nitride (g-C3N4) organic semiconductor is reportedly a versatile material for efficiently utilizing a broader visible light solar spectrum. A band gap of 2.7 eV insinuates the g-C3N4 non-metallic compound to absorb visible light of ~460 nm and facilitate photocatalytic reactions [3]. Additionally, significant research attention has been focused on g-C3N4 attributable to its easy, low-cost fabrication from carbon-nitrogen (C-N) rich precursors, such as urea [4], dicyandiamide [5], and melamine [6]. However, the bulk g-C3N4 metal-free photocatalyst provides small specific surface areas to volume ratio, slow reaction kinetics and fast charge recombination [7,8].
In an effort to reduce the bulkiness of g-C3N4, researchers have conducted experiments through mechanical ball milling, chemical, ultrasonic-assisted, and thermal exfoliation methods [9,10,11,12]. Coupling the exfoliated g-C3N4 with other compounds remarkably forms hybrid materials with unique heterojunctions. For instance, mesoporous g-C3N4/TiO2 [13], g-C3N4/N-TiO2 [14], amorphous SiO2 with g-C3N4 [15], YVO4/g-C3N4 [16], g-C3N4/SiO2-SnO2 [17], and MoS2/Al2O3/g-C3N4 [18] hybrid/ternary composites showed improvements in photoactivity. It is emphasized that heterojunctions existing at the interfacial periphery of g-C3N4 and the coupled compounds play an important role in light sensitization and providing a pathway for electron-hole separation [19,20,21,22]. Therefore, hybrid/ternary composites with g-C3N4 have great potential in solving pollution related problems [23,24,25].
One compound which has received tremendous attention in phosphorescent display materials and as a thermally stable catalyst support is a rare-earth doped phosphor [26,27]. Precisely, strontium aluminate phosphor materials such as Sr4Al14O25:Eu,Dy [26,28,29] and SrAl2O4:Eu,Dy produce an aesthetic visible light (blue or green, respectively) after UV-light excitation [30]. Recent studies revealed that solution combustion method is a robust method for synthesizing strontium aluminate phosphor with nanoparticle size distribution [31]. Although strontium aluminate phosphors are referred to as a physicochemical stable support for improved catalytic efficiencies [29,32], the effects of supporting g-C3N4 on SrAl2O4:Eu,Dy have not been extensively studied. Additionally, g-C3N4 catalyst is allegedly a futuristic material for solving pollution related problems at a large scale with minimal cost-constraint [33,34,35]. Therefore, it is necessary to further investigate on the effect of coupling the strontium aluminate phosphor with g-C3N4. Another notable advantage of coupling g-C3N4 nanoparticles with a 20–100 μm phosphor support is the possible improvement in density of nanocomposites [30]. This way, the recyclability of nanocomposites by sedimentation significantly reduces secondary polluting problems related to nanoparticles application [36,37]. In consequence, nanocomposites photocatalyst applications are broadened in solving organic pollutants in aquatic systems.
SrAl2O4:Eu,Dy phosphors have unique light scattering property which is beneficial for energy harvesting [38]. Moreover, SrAl2O4:Eu,Dy typically possess a long after-glow which has been reported to even sustain photocatalytic activity in visible light and in dark state [39,40]. Since high temperature sintering of phosphor reduces porosity, a buffer binding layer such as SiO2 is necessary to improve adhesion of the g-C3N4 catalyst. Additionally, SiO2 coatings on SrAl2O4:Eu,Dy prevents the degradation of O-Sr-O bonds in an aqueous environment [41]. SiO2 is popularly known for high surface areas and has been coupled with other compounds at weight percent ratios (wt.%) showing good photocatalytic performance [15]. However, adding SiO2 in molar ratio to SrAl2O4:Eu,Dy/g-C3N4 have not been studied.
In this study, the g-C3N4 was supported on SrAl2O4:Eu,Dy phosphor with aid of SiO2 as a binding agent. The photocatalytic performance was evaluated with respect to amount of g-C3N4 on SrAl2O4:Eu,Dy phosphor with or without silica binder under ultraviolet (UV) and visible light activation. Photocatalyst reusability studies were also conducted to evaluate the practicality of repeatedly using the SrAl2O4:Eu,Dy-SiO2/g-C3N4 composite in mitigating pollutants.
2. Materials and Methods
SrAl2O4:Eu,Dy long-lasting phosphor for supporting graphitic carbon nitride (g-C3N4) catalyst was synthesized by solution combustion method. Strontium aluminate-sol was prepared by mixing Sr(NO3)2 (97.0%, Junsei Chemical Co., Ltd., Tokyo, Japan) and Al(NO3)2•9H2O at 0.95/1 molar ratio (98.0% Yakuri Pure Chemicals Co., Ltd., Kyoto, Japan) in 20 mL distilled water (D.I = H2O) at 60 °C for 30 min. At the same time, Eu/Dy rare earth-sol was prepared in a separate 50-mL beaker from a 0.02/0.03 molar ratio of Eu2O3/Dy2O3 (99.99%, Sigma-Aldrich, St. Louis, MO, USA), respectively, and nitric acid (70%, Daejung Chemicals & Metals Co., Ltd., Siheung-si, Gyeonggi-do, Korea). After a 10-min stirring procedure at 60 °C, 4 wt.% B2O3 (Sigma-Aldrich, St. Louis, MO, USA) and 2 wt.% urea (99.0%, Samchun Pure Chemicals Co., Ltd., Pyeongtaek, Gyeonggi-do, Korea) were promptly added with continuous mixing for 20 min. The Sr-Al-nitrate sol and Eu/Dy rare earth-sol were further mixed in a beaker at 120 °C to form a thick multi-element solution. A 5-min electric muffle furnace (SK1700-B30, Thermotechno Co., Siheung-si, Gyeonggi-do, Korea) combustion process was performed at 600 °C in a closed crucible to obtain yellowish-fluffy SrAl2O4:Eu,Dy powders. Additionally, obtained powders were ground with pestle-mortar and finally sintered in an electric tube furnace (AH Jeon Industrial Co., Namyangju-si, Gyeonggi-do, Korea) with N2-H2 (95% N2, 5% H2, Union gas, Yongin-si, Gyeonggi-do, Korea) reducing gas atmosphere at 1100 °C for 1 h. Dried SrAl2O4:Eu,Dy powders were further pulverized by high-energy grinding in a Planetary Mono Mill (Fritsch Pulverisette-6, Idar-Oberstein, Germany) for 1 h at 450 rpm to reduce agglomeration prior to catalyst coating.
The SiO2-binding-sol was prepared from a tetraethyl orthosilicate (TEOS) precursor (98%, Sigma-Aldrich, St. Louis, MO, USA). In this process, 16 mL ethanol (Duksan Pure Chemicals Co., Ansan-si, Kyunggi-do, Korea), 4 mL D.I water, and 0.8 mL NH4OH (30%, Duksan Pure Chemicals Co., Ansan-si, Kyunggi-do, Korea) were magnetically stirred in a 50-mL beaker at 30 °C. After 10-min stirring, 0.16 mL TEOS was added drop-wise to the alkaline solution. Then, SiO2-sol was thoroughly mixed with ultra-sonic agitation (Powersonic 510, 10L, DH-D250H, Daihan Scientific, Wonju-si, Gangwon-do, Korea). Batches of 0.25 g SrAl2O4:Eu,Dy phosphor were coated with varying silica-sol (0.005, 0.01, 0.02, 0.1, and 0.2) molar concentration by dip coating for 10 min with ultrasonic-treatment. This was followed by centrifuging at 5000 rpm for 5 min to remove excess ethanol solution. The obtained SrAl2O4:Eu,Dy-SiO2 samples were dried at 100 °C for 6 h and calcined at 400 °C for 2 h.
For the synthesis of carbon nitride (g-C3N4) catalyst, 5 g of melamine (99.0%, Duksan Pure Chemicals Co., Ansan-si, Kyunggi-do, Korea) was thermally decomposed in a muffle furnace at 550 °C for 2 h. The yellow bulky g-C3N4 product was pulverized for 1 h in a pulveriser at 450 rpm (Fritsch, DE/pulverisette 23 mini mill) to increase surface area. The g-C3N4 was exfoliated by ultrasonic treatment for 30-min in glass vials with 10 mL ethanol. The g-C3N4 impregnation-sol was prepared for coating comparison batches with varying SiO2-binder coating on SrAl2O4:Eu,Dy phosphor. The g-C3N4 impregnation on SrAl2O4:Eu,Dy proceeded as a wet coating step, where 2 g of phosphor was dispersed with ultrasonic treatment in 10 mL g-C3N4 sol were coated with the colloidal g-C3N4 solution and calcined at 450 °C for 2 h.
SrAl2O4:Eu,Dy-SiO2/g-C3N4 composites were analyzed for crystallinity and morphology by X-ray diffraction (XRD) with Cu Kα radiation (Bruker AXS8 Advanced, D8 Discover, Bruker AXS GmbH, Karlsruhe, Germany) and Scanning Electron Microscopy (SEM, Hitachi S-4300, Hitachi Ltd., Tokyo, Japan), respectively. High resolution textural properties and elemental mappings were evaluated by transmission electron microscopy (TEM) model JEM-2010/JEOL JP with inbuilt Elemental Dispersive Spectroscopy (EDS). UV-Vis diffuse reflectance absorption characteristics of solid photocatalyst samples were analyzed by UV-VIS-NIR Spectrophotometer (UV-3150 Shimadzu, Kyoto, Japan). The photoluminescence (PL) spectra were obtained from a fluorescence spectrophotometer (Hitachi F-4500, Tokyo, Japan) under 350 nm Xe excitation wavelengths at 2.5 nm emission slit width. The specific surface area and porosity of the nano crystalline g-C3N4, SrAl2O4:Eu,Dy/g-C3N4 and SrAl2O4:Eu,Dy-0.01M SiO2/g-C3N4 were determined by Brunauer–Emmett–Teller (BET) and Barrett–Joyner–Halenda (BJH) methods on a Micropore Physisorption/Chemisorption Analyzer (AUTOSORB-IQ-MP).
UV-light photocatalytic activity was evaluated on 2 mg/mL catalyst to methylene blue (MB) solution. The 5 ppm methylene blue dye, (MB, 95%, Duksan Pure Chemical Co., Ansan-si, Kyunggi-do, Korea) organic pollutant was placed in a beaker with photocatalyst composite and stirred in the dark to attain adsorption equilibrium. The photo-reaction was initiated under 8 W UV germicidal lamp (Sankyo Denki Co, Kanagawa, Japan) illuminations and at 1-h interval 2 mL samples were pipetted for absorption measurements. Figure S1 shows spectra for UV light. The photocatalyst reaction mixture was exposed to 254 nm UV illuminations until MB solution was decolorized. UV-VIS-NIR spectrophotometer (UV-3150 Shimadzu, Kyoto, Japan) was used to measure the MB absorption variation for the photodegradation cycles for different photocatalyst samples.
Visible light photoactivity was evaluated on reaction mixtures prepared from 1 mg/mL of catalyst/MB solution (5 ppm). Then, the photoreaction mixture was placed in a stainless steel Luzchem Solar Simulator (SOLSIM2, Ottawa, Ontario, Canada) with a 300 W Xe lamp. Figure S2 shows the Xe lamp spectra (Solsim 395). The UV-light photons were cut-off with a 410 nm filter (UV L41 (W) 82 mm, Kenko Zeta, Tokina Co., Tokyo, Japan) and the light intensity was fixed at 6000 lux. The solar simulator shutter was closed and 2 mL sample was pipetted at 15-min intervals until the MB solution decolorized. The UV-Vis measurements were analyzed as explained in UV-light photocatalyst tests.
Photocatalytic reusability tests were performed to evaluate the extent of catalyst photostability and suitability for long-term use in pollutants mitigation. The recycling tests were investigated on 50 wt.% g-C3N4 supported on SrAl2O4:Eu,Dy-0.01M SiO2 samples in 5 ppm MB solution at 2 mg/mL of catalyst/MB loading ratio. Procedures explained in UV-photocatalysis were followed to photo activate and collect reaction mixture samples. However, after 240 min of photoactivity, the catalyst was centrifuge collected, washed in ethanoic solution, rinsed with distilled water and dried in an electric oven. At 2 mg/mL catalyst/MB dye ratio, the catalyst was reused again in the photo-reaction mixture to degrade MB-dye solutions for 3-cycles (240-min per cycle) with after cycle wash-centrifuge steps. Finally, the 2-mL aliquot samples for four cycles were measured by a UV-VIS-NIR spectrophotometer (UV-3150, Shimadzu, Kyoto, Japan) for methylene blue absorbance variation.
3. Results
Figure 1 exhibits the XRD patterns for bulk g-C3N4 and nano crystalline g-C3N4 supported on the SrAl2O4:Eu,Dy phosphor. Bulk graphitic carbon nitride has crystallinity as shown by two peaks indexed (100) at 13.3° and (002) at 27.5° in reference to JCPDS 87-1526. SrAl2O4:Eu,Dy is crystalline with characteristic peaks labelled in reference to JCPDS 34-0379. The SrAl2O4:Eu,Dy phosphor peaks at (211), (031) and (222) weakened with additional g-C3N4 and both g-C3N4-SiO2 coating. This confirms the amorphous nature of SiO2 as well as the overall decrease of phosphor host matrix compared to pure phosphor sample. The nano crystalline g-C3N4/SrAl2O4:Eu,Dy with or without silica only contained phosphor peaks. The absence of nano crystalline g-C3N4 confirms the bulk structure changed to amorphous during the pulverization milling and ultrasonic exfoliation to reduce agglomeration. Additionally, the nano crystalline g-C3N4 was beyond the XRD detection limit as supported on sub-micron phosphor matrix and also with the amorphous nature of silica. The disappearance of g-C3N4 peaks in composite materials has been observed in other researches, with an increase in the amount of the crystalline [16,42] or amorphous SiO2 [43].
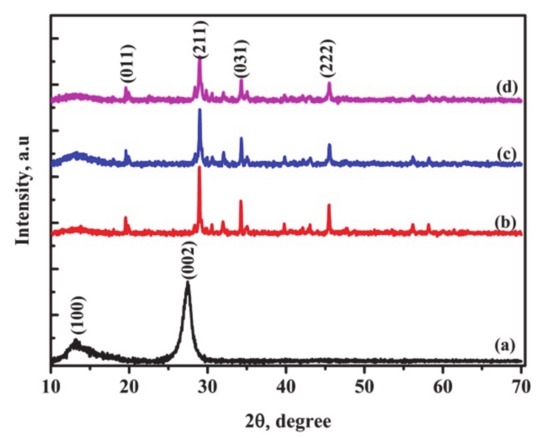
Figure 1.
XRD patterns for (a) bulk g-C3N4 (b) SrAl2O4:Eu,Dy (c) SrAl2O4:Eu,Dy/50 wt.% g-C3N4 and (d) 50 wt.% g-C3N4 supported on SrAl2O4:Eu,Dy-0.01M SiO2.
Figure 2 shows SEM images of (a) bulk g-C3N4, (b) SrAl2O4:Eu,Dy, and (c) SrAl2O4:Eu,Dy- 15 wt.% g-C3N4. The bulk g-C3N4 in Figure 2a are aggregates of several micrometers while SrAl2O4:Eu,Dy phosphor powders in Figure 2b show agglomerated particles of around few micrometers due to 1100 °C sintering temperature. However, Figure 2c shows the composite of g-C3N4/SrAl2O4:Eu,Dy in which the nano crystalline g-C3N4 particles are coated well partly on the SrAl2O4:Eu,Dy.
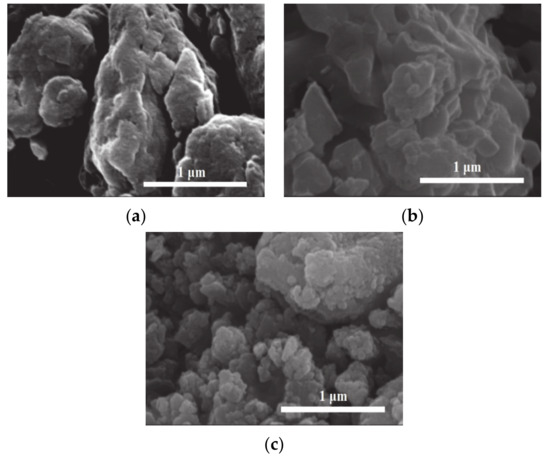
Figure 2.
SEM images (a) g-C3N4 (b) SrAl2O4:Eu,Dy and (c) SrAl2O4:Eu,Dy/15 wt.% g-C3N4.
Figure 3 shows TEM, EDS spectra and elemental composition of the g-C3N4 supported on phosphor. Figure 3a shows TEM image of phosphor particles encapsulated with g-C3N4, where the black-contrast micro-particle is phosphor whilst the grey voluminous encapsulate with light-contrast is g-C3N4. The EDS spectra in Figure 3b confirm that all chemical elements are present in respective element compositions in Table 1. However, the composite of g-C3N4-coated SrAl2O4:Eu,Dy/SiO2 in Figure 3c has nanoscale surface roughness as compared to g-C3N4-supported on SrAl2O4:Eu,Dy without silica. This surface roughness is accredited to SiO2-binding which improves the stability of SrAl2O4:Eu,Dy/g-C3N4 adhesion. Surface-surface interaction of compounds is further indication of improvement of physiochemical properties which is major advantage of coupling materials. Figure 3d and Table 2 confirm that all chemical elements in SrAl2O4:Eu,Dy-SiO2/g-C3N4 were present.
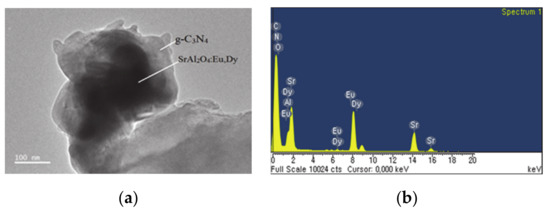
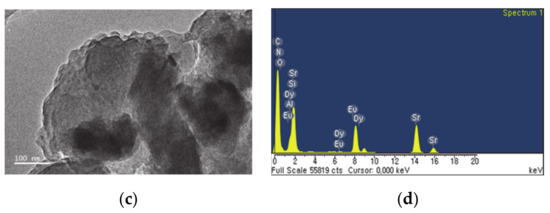
Figure 3.
TEM images (a,c) and EDS spectra (b,d) where (a,b) is SrAl2O4:Eu,Dy/50 wt.% g-C3N4 and (c,d) is 50 wt.% g-C3N4 supported on SrAl2O4:Eu,Dy-0.01M SiO2.

Table 1.
Elemental analysis for SrAl2O4:Eu,Dy/50 wt.% g-C3N4.

Table 2.
Elemental analysis for 50 wt.% g-C3N4 supported on SrAl2O4:Eu,Dy-0.01M SiO2.
Figure 4 shows the nitrogen adsorption-desorption isotherms for (a) nano crystalline g-C3N4, (b) SrAl2O4:Eu,Dy and (c) 50 wt.% g-C3N4-supported on SrAl2O4:Eu,Dy-0.01M SiO2. Figure 4 adsorption-desorption graphs are similar to type (III) hysteresis loop. As shown in the figures, the g-C3N4, SrAl2O4:Eu,Dy and SrAl2O4:Eu,Dy-0.01M SiO2/50 wt.% g-C3N4 samples are mesoporous since average pore diameter is in a range of 2 to 50 nm. The invariant pore diameter further confirms that the phosphor is a pore-free crystalline supporter and a silica binder insignificantly affects the composite porosity. Table 3 exhibits the BET specific surface area, BJH pore volume, and pore diameter for g-C3N4, SrAl2O4:Eu,Dy and 50 wt.% g-C3N4-supported on SrAl2O4:Eu,Dy-0.01M SiO2. Accordingly, BET specific surface areas for nano crystalline g-C3N4, SrAl2O4:Eu,Dy and SrAl2O4:Eu,Dy-0.01M SiO2/50 wt.% g-C3N4 were 14.9 m2/g, 13.2 m2/g and 11.7 m2/g, respectively. The specific surface area and pore volume decreased on samples coupled with crystalline phosphor and silica binder. This is justified to the volumetric bulkiness of phosphor-support and calcination procedure which causes particle coalescence as confirmed SEM morphology in Figure 2b. The BJH pore diameters for g-C3N4, SrAl2O4:Eu,Dy, and SrAl2O4:Eu,Dy-0.01M SiO2/50 wt.% g-C3N4 were 3.81 nm, 3.80 nm, and 3.82 nm, respectively.
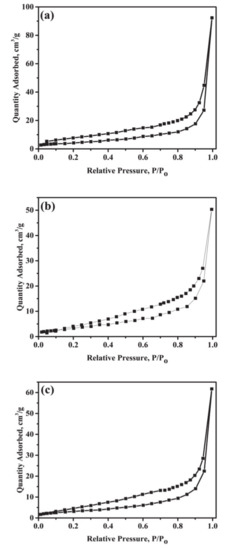
Figure 4.
Nitrogen adsorption-desorption isotherms for (a) g-C3N4 (b) SrAl2O4:Eu,Dy and (c) 50 wt.% g-C3N4 supported on SrAl2O4:Eu,Dy-0.01M SiO2.

Table 3.
BET specific surface area, BJH pore volume and pore diameter for the catalysts.
Figure 5 shows the diffuse reflectance absorption spectra for g-C3N4 in comparison with various amounts of g-C3N4 supported on SrAl2O4:Eu,Dy. The bulk g-C3N4 sample exhibits a strong UV optical absorption edge at 460 nm corresponding to the band gap of 2.7 eV as referenced by other researchers [24,44]. SrAl2O4:Eu,Dy phosphor has strong absorption peak in the UV-Vis region. However, with increasing amount of g-C3N4 on phosphor surface, the peak absorption intensity is enhanced. Moreover, a shift towards the visible light region increased with increase in g-C3N4 on phosphor. The SrAl2O4:Eu,Dy/50 wt.% g-C3N4 exhibits the largest shift as compared to other samples. This is a typical result of coupling compound materials, since each compound incorporates individual characteristic to the hybrid system.
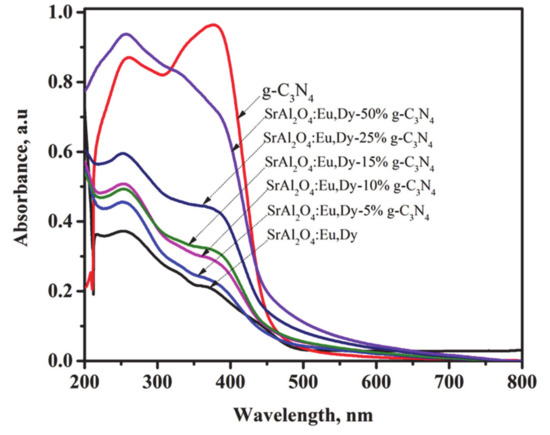
Figure 5.
Diffuse reflectance absorption spectra for g-C3N4 and SrAl2O4:Eu,Dy hybrid composites.
Figure 6a shows photoluminescence emission spectra for g-C3N4 supported on SrAl2O4:Eu,Dy phosphor. The g-C3N4 and SrAl2O4:Eu,Dy emission peak is maximum at 455 nm and 525 nm, respectively. Supporting g-C3N4 on SrAl2O4:Eu,Dy suppresses the 525 nm-peak owing to energy transfers as observed in CuO/phosphor [45]; or dissipation as well as emission light blockage effect similar to phosphor/TiO2 [46]. This also further confirms the successful encapsulation of phosphor with g-C3N4. As the amount of g-C3N4 on SrAl2O4:Eu,Dy increases, the overall phosphor amount in the SrAl2O4:Eu,Dy/g-C3N4 composite decreases. As a consequence, the overall light absorption capacity by SrAl2O4:Eu,Dy/g-C3N4 composite is simultaneously reduced in SrAl2O4:Eu,Dy and increased in g-C3N4.
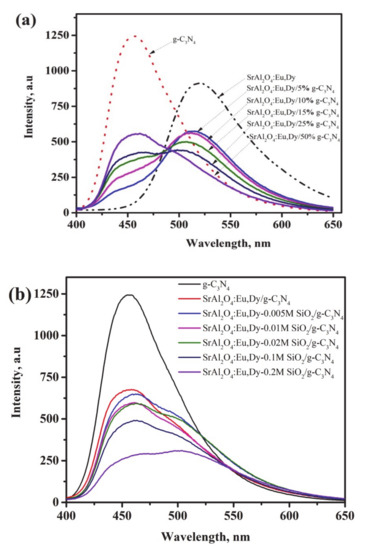
Figure 6.
Photoluminescence spectra for (a) the SrAl2O4:Eu,Dy/g-C3N4 and (b) for SrAl2O4:Eu,Dy/50 wt.% g-C3N4 with varying SiO2-binder concentration.
Figure 6b shows the photoluminescence spectra of the SrAl2O4:Eu,Dy-50 wt.% g-C3N4 with varying an amount of SiO2 as a binding reagent. Additional SiO2-binder results in suppression the 455 nm g-C3N4 peak. Precisely, the g-C3N4 peak decreased to 50% in 0.005M SiO2 and to 20% in 0.2M SiO2 samples. The variation in g-C3N4 emission peak with increase in silica exhibits the extent of energy suppression or dissipation in the SiO2-layer. The emission light dissipation is observed in non-luminescent YVO4 coupled with g-C3N4 was also linked to charge transfer [16]. Similarly, where light emission is to be preserved, the lowest SiO2-binder is required for sustaining the practical application.
Figure 7 shows the visible light photocatalytic efficiencies for the g-C3N4/SrAl2O4:Eu,Dy composites. The physically mixed SrAl2O4:Eu,Dy and g-C3N4 (SrAl2O4:Eu,Dy + g-C3N4) at 1:1 wt% ratio show an improvement in photocatalytic activity as compared to only g-C3N4 sample. However, the 60-min cycle is exhibiting completion of reaction in the thermally treated SrAl2O4:Eu,Dy/10 wt.% g-C3N4, SrAl2O4:Eu,Dy/15 wt.%, SrAl2O4:Eu,Dy/25 wt.% g-C3N4 and the SrAl2O4:Eu,Dy/50 wt.% g-C3N4 with higher efficiencies than the physically mixed SrAl2O4:Eu,Dy and g-C3N4. Clearly, the coupling of nano crystalline g-C3N4 with SrAl2O4:Eu,Dy phosphor with thermal treatment improves photocatalytic efficiency. This aspect of photocatalysis improvement in heterogeneous composites emanates from the high electron-hole separation efficiency. Interestingly in the first 15 min, the SrAl2O4:Eu,Dy/50 wt.% g-C3N4 degraded almost 60% of the MB solutions. Therefore, further work on the effect of SiO2-binder on photocatalytic activity was investigated on this composite under UV and visible light illumination.
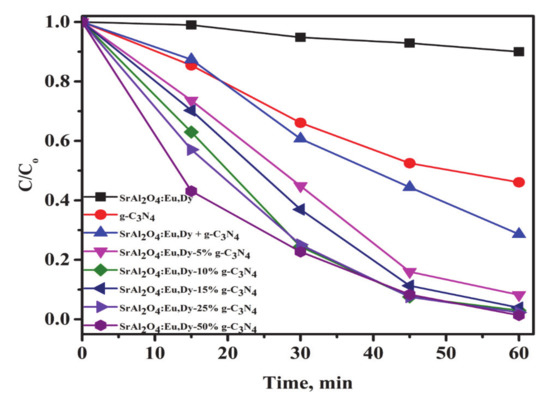
Figure 7.
The photodegradation efficiency of MB solution for SrAl2O4:Eu,Dy/g-C3N4 hybrid composites under visible light solar illumination. Where (SrAl2O4:Eu,Dy + g-C3N4) is physically mixed at 1:1 wt.% ratio).
Figure 8 shows the photodegradation efficiencies (a) and rate of reaction (b) for the g-C3N4 supported on SrAl2O4:Eu,Dy/SiO2 composites in a MB solution under UV-light irradiation. At a glance, pure nano crystalline g-C3N4 degrades only 40% of MB solution while photocatalytic efficiencies for the composites of g-C3N4-supported SrAl2O4:Eu,Dy with and without SiO2 are enhanced to 90% in a 240-min cycle. The photocatalytic degradation efficiency was significantly improved with additional silica-binder of concentration of as low as 0.01M. In contrast, an amount higher than 0.01M SiO2 binder impedes the photocatalytic activity. In other words, excess silica layer possibly acts as a slight barrier for photocatalysis progression in SrAl2O4:Eu,Dy/g-C3N4 composites. The UV-photocatalytic reaction proceeds as the 1st order, where in Figure 8b the 0.01M SiO2 phosphor/g-C3N4 composite has the fastest reaction. Table 4 shows the rate constants for the UV photocatalytic reaction. The experimental errors data were inserted in the Supplementary File (Table S1). The silica content of 0.02M, 0.1M and 0.2M shows lower rate constants than the only SrAl2O4:Eu,Dy/g-C3N4 without SiO2 binder. Hence, a low silica addition of 0.005–0.01M is the optimum concentration to promote photocatalytic performance. Regardless of the lower specific surface area or pore volume as shown in Table 3, the SrAl2O4:Eu,Dy/g-C3N4 composite with/without silica binder merely exhibited higher photoactivity than only nano crystalline g-C3N4. Therefore, photocatalytic activity in the composites is dependent on the chemical crystallinity and UV-Vis absorption properties.
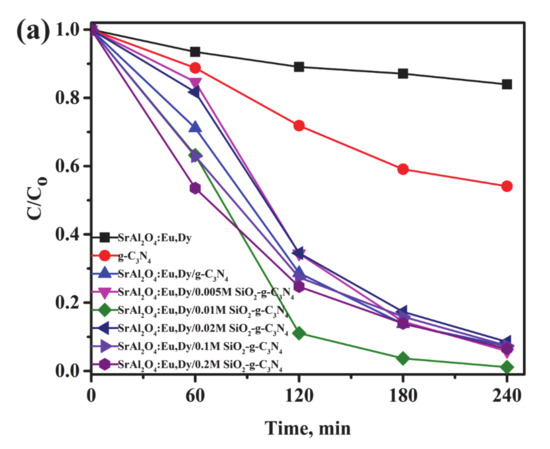
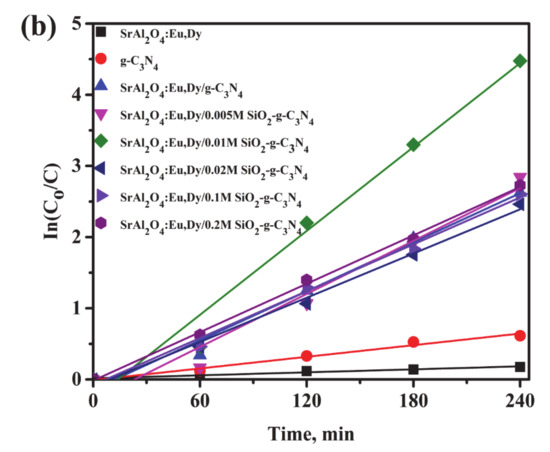
Figure 8.
Photo-degradation efficiency (a) and rate of reaction (b) for 50 wt.% g-C3N4 supported on SrAl2O4:Eu,Dy/SiO2 in MB solutions under UV-light illumination.

Table 4.
Rate constants for UV-light photoactivity.
Figure 9 shows the photo-degradation efficiency (a) and rate of reaction (b) for 50 wt.% g-C3N4 supported on SrAl2O4:Eu,Dy/SiO2 in an MB solution under visible light illumination. The photodegradation reaction proceeded to completion within a 60-min cycle under visible light irradiation. The performance of the g-C3N4-phosphor-SiO2 dominates the nano crystalline g-C3N4 sample. This is due to the overall improvement in electron-hole separation efficiencies in hybrid composites. The extent of visible light performance reaching to above 90% in a 60-min cycle implies that the hybrid composite is a promising candidate for environmental pollutants. The rates of reaction constant shown in Table 5 show that photocatalytic response kinetics are improved with supporting g-C3N4 on SrAl2O4:Eu,Dy phosphor together with additional SiO2 binder. The experimental errors data were inserted in the Supplementary File (Table S2).
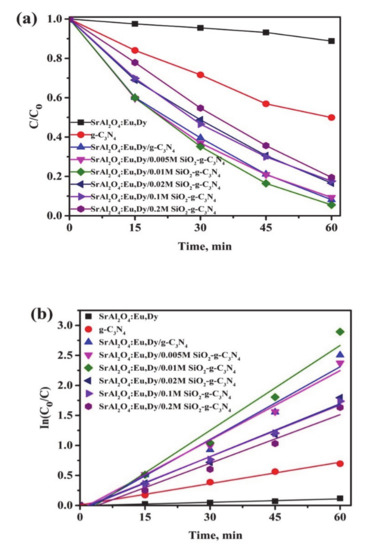
Figure 9.
Photo-degradation efficiency (a) and rate of reaction (b) for 50 wt.% g-C3N4 supported on SrAl2O4:Eu,Dy/SiO2 in MB solutions under visible light illuminations.

Table 5.
Rate constants for visible light photoactivity.
Figure 10 shows MB degradation efficiency by reusing 50% g-C3N4-supported on SrAl2O4:Eu,Dy-0.01M SiO2 composite. The photocatalytic recyclability test was performed in 4 cycles (240-min per cycle) and showed that the g-C3N4-coated SrAl2O4:Eu,Dy/SiO2 composite has exceptional photostability with a degradation efficiency above 90% in successive cycles. The g-C3N4-coated SrAl2O4:Eu,Dy/SiO2 composite exhibits stability in the four cycles which is an important property for catalysts.
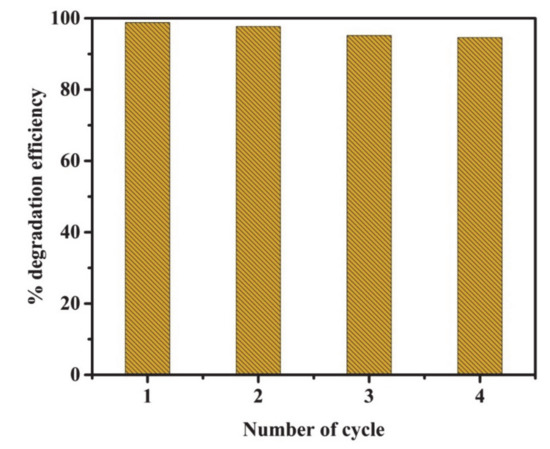
Figure 10.
Photocatalytic recyclability test of 50 wt.% g-C3N4 supported on SrAl2O4:Eu,Dy-0.01M SiO2.
Figure 11 shows a schematic diagram for the photocatalysis mechanism in the MB-SrAl2O4:Eu, Dy-SiO2/g-C3N4 photoreaction mixture. Methylene blue has negligible effects on photocatalytic activity [47]. On the same note, SrAl2O4:Eu,Dy phosphor is reported to exhibit negligible photocatalytic activity in aqueous RhB dye solutions. Moreover, its unique energy levels absorb even visible light and emit a blue fluorescence which sustains a photocatalytic reaction [40]. Silica was added at a molar ratio to perform a binding role between phosphor and g-C3N4. Since heterojunction interfaces exist in composite materials [48], even visible light would be sensitized by the SrAl2O4:Eu,Dy/g-C3N4. As observed in Figure 7, the thermally treated SrAl2O4:Eu,Dy/g-C3N4 composites exhibited higher efficiencies that physically mixed SrAl2O4:Eu,Dy and g-C3N4. Therefore, thermal treatment is beneficial in the formation of heterojunctions.
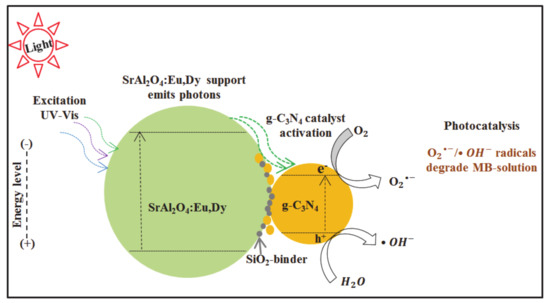
Figure 11.
Schematic diagram for photocatalysis mechanism for the MB-SrAl2O4:Eu,Dy-SiO2/g-C3N4 photoreaction mixture.
SrAl2O4:Eu,Dy phosphor performs the role of sensitizing UV or partial visible light photons and releasing visible light photons. Then, the g-C3N4 catalyst is activated by photons λ < 460 nm or even visible light photons from phosphor through the SrAl2O4:Eu,Dy/g-C3N4 heterojunction. Then, the activated g-C3N4 releases electrons which are adsorbed to oxygen whilst holes are adsorbed to water molecules forming superoxide and hydroxide radicals which attack and decompose the MB-dye structure.
Photocatalytic reactions of SrAl2O4:Eu,Dy phosphor-g-C3N4 composite supported with thin silica-binding (0.01M SiO2) layer proceeds faster than the only g-C3N4. With SiO2 in large amounts of 0.02M, 0.1M, and 0.2M, the photocatalytic activity was reduced, which means that the interfacial (contact) junction between SrAl2O4:Eu,Dy and g-C3N4 was disrupted. Thus, the SrAl2O4:Eu,Dy support forms a heterojunction with the g-C3N4 catalyst (with or without SiO2-binder layer).
4. Discussion
The phosphor SrAl2O4:Eu,Dy support synthesized by solution combustion and sintering at 1100 °C for 1 h exhibited broad photoluminescence at 525 nm. In this research, sintering at 1100 °C for 1 h produce stable support at lower energy consumption than the solid-state sintering at 1300–1500 °C for 2–6 h dwelling time [30]. Particle agglomeration is unavoidable since sintering promotes grain growth. Therefore, to improve particle dispersion on agglomerated SrAl2O4:Eu,Dy phosphor a pulverizing or mixing step is necessary.
SrAl2O4:Eu,Dy support and g-C3N4 catalyst have strong absorption in the UV-region. Interestingly, by coupling these two materials unique optical properties were observed through shift of UV-Vis diffuse reflectance absorption spectra towards the visible light region in Figure 5. Additional SiO2 between SrAl2O4:Eu,Dy phosphor and g-C3N4 also weakened the g-C3N4 peak. Thus, Silica act as energy dissipation centres. This phenomenon of energy dissipation was also observed in Al2O3 coupled with CaAl2O4:Eu,Nd/TiO2 long lasting phosphor [49].
According to one research, when g-C3N4 and SiO2 were coupled, higher (50 wt.%) silica resulted in the lowest PL emission intensities but with most superior catalytic property [15]. Moreover, these composites were reported to fortunately possess exceptionally large surface areas of more than 100 m2/g. These results contradict with our result in Figure 6, where g-C3N4/SiO2 supported on light emitting phosphor suppressed both the PL intensities and photocatalytic properties. Therefore, it is possible to tune the photocatalytic property by combining with long-lasting phosphor to observe enhanced activity at low silica addition.
In our work, we focused on the photocatalytic effects of SrAl2O4:Eu,Dy-0.01M SiO2/50 wt.% g-C3N4 which exhibited a red-shift in the diffuse reflectance absorption. Therefore, we recommend further investigations in the evaluation of quantum efficiencies in relation to photocatalytic efficiencies.
5. Conclusions
Graphitic carbon nitride (g-C3N4) was supported on SrAl2O4:Eu,Dy with or without a SiO2-binding agent. The g-C3N4-SrAl2O4:Eu,Dy composites exhibit unique broadened photoluminescence emission spectra with their respective dominant emission peaks at 450 nm and 525 nm. Silica additions as a binder between g-C3N4 and SrAl2O4:Eu,Dy act as energy dissipation centers for light emitted from phosphor. The UV-Vis light photocatalytic performances are improved several times in the composite of g-C3N4-supported SrAl2O4:Eu,Dy as compared to pure g-C3N4. The photocatalytic efficiency was improved with an addition of low SiO2 amount as a binding reagent. However, a higher SiO2-coating layer acts as a barrier to the photodegradation efficiencies. Thermally treating SrAl2O4:Eu,Dy-g-C3N4 composites is beneficial for the improved photodegradation of methylene blue pollutants.
Supplementary Materials
The following are available online at https://www.mdpi.com/2079-6412/10/10/917/s1, Figure S1: Spectra for UV-light, Figure S2: The Xe lamp spectra (Solsim 395), Table S1: Rate constants for UV-light photoactivity, Table S2: Rate constants for visible light photoactivity.
Author Contributions
Supervision, J.-S.K.; writing—original draft preparation, S.M. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by a grant (20CTAP-C157721-01) from Infrastructure and transportation technology promotion research program funded by Ministry of Land, Infrastructure and Transport of Korean government.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gupta, S.M.; Tripathi, M. A review of TiO2 nanoparticles. Chin. Sci. Bull. 2011, 56, 1639–1657. [Google Scholar] [CrossRef]
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium dioxide photocatalysis. J. Photochem. Photobiol. C Photochem. Rev. 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Wu, P.; Wang, J.; Zhao, J.; Guo, L.; Osterloh, F.E. Structure defects in g-C3N4 limit visible light driven hydrogen evolution and photovoltage. J. Mater. Chem. A 2014, 2, 20338–20344. [Google Scholar] [CrossRef]
- He, Y.; Wang, Y.; Zhang, L.; Teng, B.; Fan, M. High-efficiency conversion of CO2 to fuel over ZnO/g-C3N4 photocatalyst. Appl. Catal. B Environ. 2015, 168, 1–8. [Google Scholar] [CrossRef]
- Li, W.; Wang, L.; Zhang, Q.; Chen, Z.; Deng, X.; Feng, C.; Xu, L.; Sun, M. Fabrication of an ultrathin 2D/2D C3N4/MoS2 heterojunction photocatalyst with enhanced photocatalytic performance. J. Alloys Compd. 2019, 808, 151681. [Google Scholar] [CrossRef]
- Ruan, D.; Kim, S.; Fujitsuka, M.; Majima, T. Defects rich g-C3N4 with mesoporous structure for efficient photocatalytic H2 production under visible light irradiation. Appl. Catal. B Environ. 2018, 238, 638–646. [Google Scholar] [CrossRef]
- Jin, Z.; Zhang, Q.; Yuan, S.; Ohno, T. Synthesis high specific surface area nanotube g-C3N4 with two-step condensation treatment of melamine to enhance photocatalysis properties. RSC Adv. 2015, 5, 4026–4029. [Google Scholar] [CrossRef]
- Wang, X.L.; Yang, H.G. Facile fabrication of high-yield graphitic carbon nitride with a large surface area using bifunctional urea for enhanced photocatalytic performance. Appl. Catal. B Environ. 2017, 205, 624–630. [Google Scholar] [CrossRef]
- Chen, Q.; Hou, H.; Zhang, D.; Hu, S.; Min, T.; Liu, B.; Yang, C.; Pu, W.; Hu, J.; Yang, J. Enhanced visible-light driven photocatalytic activity of hybrid ZnO/g-C3N4 by high performance ball milling. J. Photochem. Photobiol. A Chem. 2018, 350, 1–9. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, L.; Shi, R.; Zhu, Y. Chemical exfoliation of graphitic carbon nitride for efficient heterogeneous photocatalysis. J. Mater. Chem. A 2013, 1, 14766–14772. [Google Scholar] [CrossRef]
- Liao, G.; Gong, Y.; Zhang, L.; Gao, H.; Yang, G.-J.; Fang, B. Semiconductor polymeric graphitic carbon nitride photocatalysts: The “holy grail” for the photocatalytic hydrogen evolution reaction under visible light. Energy Environ. Sci. 2019, 12, 2080–2147. [Google Scholar] [CrossRef]
- Li, Y.; Sun, Y.; Ho, W.; Zhang, L.L.; Huang, H.; Cai, Q.; Dong, F. Highly enhanced visible-light photocatalytic NOx purification and conversion pathway on self-structurally modified g-C3N4 nanosheets. Sci. Bull. 2018, 63, 609–620. [Google Scholar] [CrossRef]
- Wang, S.; Hua, X.; Ji, J.; Cai, Z.; Zhao, Y. Facile synthesis of highly efficient mpg-C3N4/TiO2 visible-light-induced photocatalyst and its formaldehyde removal performance in coating application. J. Nanopart. Res. 2019, 21, 187. [Google Scholar] [CrossRef]
- Zhou, S.; Liu, Y.; Li, J.; Wang, Y.; Jiang, G.; Zhao, Z.; Wang, D.; Duan, A.; Liu, J.; Wei, Y. Facile in situ synthesis of graphitic carbon nitride (g-C3N4)-N-TiO2 heterojunction as an efficient photocatalyst for the selective photoreduction of CO2 to CO. Appl. Catal. B Environ. 2014, 158, 20–29. [Google Scholar] [CrossRef]
- Peng, L.; Li, Z.-W.; Zheng, R.-R.; Yu, H.; Dong, X.-T. Preparation and characterization of mesoporous g-C3N4/SiO2 material with enhanced photocatalytic activity. J. Mater. Res. 2019, 34, 1785–1794. [Google Scholar] [CrossRef]
- Cai, J.; He, Y.; Wang, X.; Zhang, L.; Dong, L.; Lin, H.; Zhao, L.; Yi, X.; Weng, W.; Wan, H. Photodegradation of RhB over YVO4/g-C3N4 composites under visible light irradiation. RSC Adv. 2013, 3, 20862. [Google Scholar] [CrossRef]
- Peng, L.; Zheng, R.-R.; Feng, D.-W.; Yu, H.; Dong, X. Synthesis of eco-friendly porous g-C3N4/SiO2/SnO2 composite with excellent visible-light responsive photocatalysis. Arab. J. Chem. 2020, 13, 4275–4285. [Google Scholar] [CrossRef]
- Vattikuti, S.P.; Byon, C. Hydrothermally synthesized ternary heterostructured MoS2/Al2O3/g-C3N4 photocatalyst. Mater. Res. Bull. 2017, 96, 233–245. [Google Scholar] [CrossRef]
- Di, L.; Yang, H.; Xian, T.; Chen, X. Construction of Z-Scheme g-C3N4/CNT/Bi2Fe4O9 Composites with Improved Simulated-Sunlight Photocatalytic Activity for the Dye Degradation. Micromachines 2018, 9, 613. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Y.; Shuai, D.; Shen, Y.; Xiong, W.; Wang, L. Graphitic carbon nitride (g-C3N4)-based photocatalysts for water disinfection and microbial control: A review. Chemosphere 2019, 214, 462–479. [Google Scholar] [CrossRef]
- Zhang, W.; Zhou, L.; Shi, J.; Deng, H. Synthesis of Ag3PO4/G-C3N4 Composite with Enhanced Photocatalytic Performance for the Photodegradation of Diclofenac under Visible Light Irradiation. Catalysts 2018, 8, 45. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, X.; Fan, J.; Xue, D.; Zhang, B.; Yin, S. N-TiO2/g-C3N4/Up-conversion phosphor composites for the full-spectrum light-responsive deNOx photocatalysis. J. Mater. Sci. 2018, 53, 7266–7278. [Google Scholar] [CrossRef]
- Ye, S.; Wang, R.; Wu, M.; Yuan, Y. A review on g-C3N4 for photocatalytic water splitting and CO2 reduction. Appl. Surf. Sci. 2015, 358, 15–27. [Google Scholar] [CrossRef]
- Mamba, G.; Mishra, A.K. Graphitic carbon nitride (g-C3N4) nanocomposites: A new and exciting generation of visible light driven photocatalysts for environmental pollution remediation. Appl. Catal. B Environ. 2016, 198, 347–377. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, X.; Zhang, K.; Lu, P.; Zhang, D. Facile fabrication of sandwich-like BiOI/AgI/g-C3N4 composites for efficient photocatalytic degradation of methyl orange and reduction of Cr(VI). J. Nanopart. Res. 2018, 20, 328. [Google Scholar] [CrossRef]
- Li, H.; Yin, S.; Wang, Y.; Sato, T. Persistent luminescence assisted photocatalytic properties of CaAl2O4:(Eu,Nd)/TiO2−xNy and Sr4Al14O25:(Eu,Dy)/TiO2−xNy. J. Mol. Catal. A: Chem. 2012, 129–133. [Google Scholar] [CrossRef]
- Zargoosh, K.; Aliabadi, H.M. SrAl2O4:Eu2+: Dy3+/WO3/polyester nanocomposite as a highly efficient and environmentally friendly photocatalyst for removal of dyes from industrial wastes. Environ. Nanotechnol. Monit. Manag. 2019, 12, 100273. [Google Scholar] [CrossRef]
- Demirci, S.; Gültekin, S.; Akalin, S.A.; Oter, O.; Ertekin, K.; Çelik, E. Synthesis and spectral characterization of Sr4Al14O25:Eu2+/Dy3+ blue–green phosphorous powders by sol–gel method. Mater. Sci. Semicond. Process. 2015, 31, 611–617. [Google Scholar] [CrossRef]
- Sung, H.-J.; Kim, B.-M.; Jung, S.-C.; Kim, J.-S. Photocatalytic Characteristics for The Nanocrystalline TiO2 Supported On Sr4Al14O25: Eu2+, Dy3+ Phosphor Beads. Adv. Mater. Lett. 2016, 7, 36–41. [Google Scholar] [CrossRef]
- Rojas-Hernandez, R.E.; Rubio-Marcos, F.; Rodríguez, M.A.; Fernández, J.F. Long lasting phosphors: SrAl2O4:Eu, Dy as the most studied material. Renew. Sustain. Energy Rev. 2018, 81, 2759–2770. [Google Scholar] [CrossRef]
- Rojas-Hernandez, R.E.; Rodríguez, M.A.; Rubio-Marcos, F.; Serrano, A.; Fernández, J.F. Designing nanostructured strontium aluminate particles with high luminescence properties. J. Mater. Chem. C 2015, 3, 1268–1276. [Google Scholar] [CrossRef]
- Havasi, V.; Vödrédi, B.; Kukovecz, Á. Photocatalytic performance of Sr4Al14O25: Eu,Dy phosphor assisted ZnO:Co+Ag nanocomposite under continuous and pulsed illumination. Catal. Today 2017, 284, 107–113. [Google Scholar] [CrossRef]
- Yuan, Y.; Cao, S.-W.; Liao, Y.; Yin, L.-S.; Xue, C. Red phosphor/g-C3N4 heterojunction with enhanced photocatalytic activities for solar fuels production. Appl. Catal. B Environ. 2013, 140, 164–168. [Google Scholar] [CrossRef]
- Lam, S.-M.; Sin, J.-C.; Mohamed, A.R. A review on photocatalytic application of g-C3N4/semiconductor (CNS) nanocomposites towards the erasure of dyeing wastewater. Mater. Sci. Semicond. Process. 2016, 47, 62–84. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, H.; Wu, Z.; Wang, L. g-C3N4 based composite photocatalysts for photocatalytic CO2 reduction. Catal. Today 2018, 300, 160–172. [Google Scholar] [CrossRef]
- Foye, C. The Relationship Between Size of Living Space and Subjective Well-Being. J. Happiness Stud. 2016, 18, 427–461. [Google Scholar] [CrossRef]
- Saiful Amran, S.N.B.; Wongso, V.; Abdul Halim, N.S.; Husni, M.K.; Sambudi, N.S.; Wirzal, M.D.H. Immobilized carbon-doped TiO2 in polyamide fibers for the degradation of methylene blue. J. Asian Ceram. Soc. 2019, 7, 321–330. [Google Scholar] [CrossRef]
- Sun, H.; Pan, L.; Piao, X.; Sun, Z. Long afterglow SrAl2O4: Eu,Dy phosphors for CdS quantum dot-sensitized solar cells with enhanced photovoltaic performance. J. Mater. Chem. A 2013, 1, 6388. [Google Scholar] [CrossRef]
- Yin, H.; Chen, X.; Hou, R.; Zhu, H.; Li, S.; Huo, Y.; Li, H. Ag/BiOBr Film in a Rotating-Disk Reactor Containing Long-Afterglow Phosphor for Round-the-Clock Photocatalysis. ACS Appl. Mater. Interfaces 2015, 7, 20076–20082. [Google Scholar] [CrossRef]
- Liu, X.; Chen, X.; Li, Y.; Wu, B.; Luo, X.; Ouyang, S.; Luo, S.; Al Kheraif, A.A.; Lin, J. A g-C3N4@Au@SrAl2O4:Eu2+,Dy3+ composite as an efficient plasmonic photocatalyst for round-the-clock environmental purification and hydrogen evolution. J. Mater. Chem. A 2019, 7, 19173–19186. [Google Scholar] [CrossRef]
- Sikandar, M.A.; Ahmad, W.; Khan, M.H.; Ali, F.; Waseem, M. Effect of water resistant SiO2 coated SrAl2O4: Eu2+ Dy3+ persistent luminescence phosphor on the properties of Portland cement pastes. Constr. Build. Mater. 2019, 228, 116823. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Sun, G.; Luo, N.; Zhang, B.; Zhang, Z. Synthesis of a Flower-Like g-C3N4/ZnO Hierarchical Structure with Improved CH4 Sensing Properties. Nanomaterials 2019, 9, 724. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, S.; Hu, W.; Cai, J.; Zhang, L.; Dong, L.; Zhao, L.; He, Y. Synthesis and photocatalytic activity of SiO2/g-C3N4 composite photocatalyst. Mater. Lett. 2014, 115, 53–56. [Google Scholar] [CrossRef]
- Sudhaik, A.; Raizada, P.; Shandilya, P.; Jeong, D.-Y.; Lim, J.-H.; Singh, P. Review on fabrication of graphitic carbon nitride based efficient nanocomposites for photodegradation of aqueous phase organic pollutants. J. Ind. Eng. Chem. 2018, 67, 28–51. [Google Scholar] [CrossRef]
- Mavengere, S.; Jung, S.-C.; Kim, J.-S. Visible Light Photocatalytic Activity of NaYF4:(Yb,Er)-CuO/TiO2 Composite. Catalysts 2018, 8, 521. [Google Scholar] [CrossRef]
- Kim, J.-S.; Kim, S.-W.; Jung, S.-C. Photocatalytic reaction characteristics of the titanium dioxide supported on the long phosphorescent phosphor by a low pressure chemical vapor deposition. J. Nanosci. Nanotechnol. 2014, 14, 7751–7755. [Google Scholar] [CrossRef] [PubMed]
- Mavengere, S.; Kim, J.-S. UV–visible light photocatalytic properties of NaYF4:(Gd, Si)/TiO2 composites. Appl. Surf. Sci. 2018, 444, 491–496. [Google Scholar] [CrossRef]
- Hu, M.; Xing, Z.; Cao, Y.; Li, Z.; Yan, X.; Xiu, Z.; Zhao, T.; Yang, S.; Zhou, W. Ti3+ self-doped mesoporous black TiO2/SiO2/g-C3N4 sheets heterojunctions as remarkable visible-lightdriven photocatalysts. Appl. Catal. B Environ. 2018, 226, 499–508. [Google Scholar] [CrossRef]
- Yoon, J.-H.; Jung, S.-C.; Kim, J.-S. Photocatalytic effects for the TiO2-coated phosphor materials. Mater. Chem. Phys. 2011, 125, 342–346. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).