Surface Modified Techniques and Emerging Functional Coating of Dental Implants
Abstract
:1. Introduction
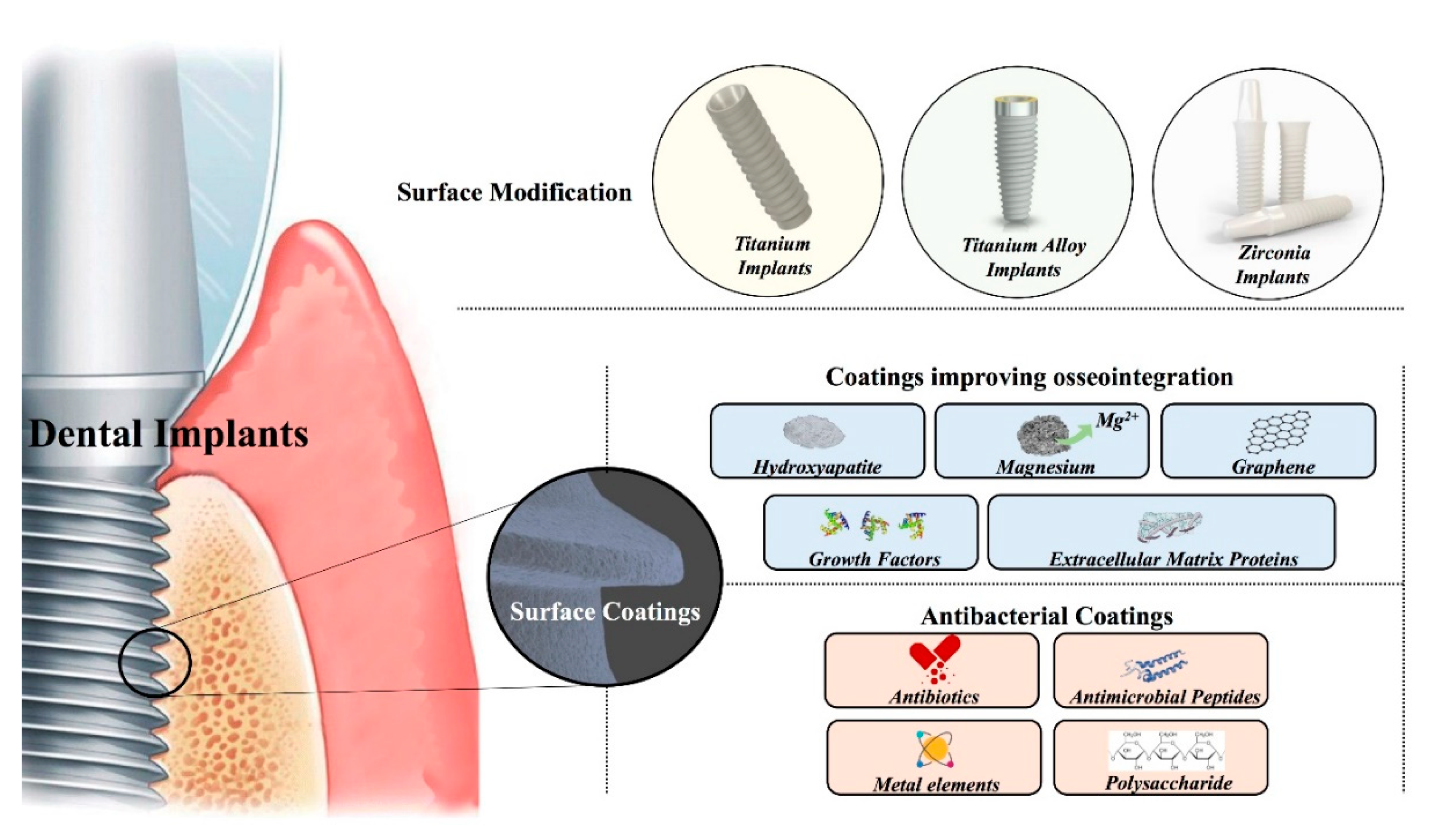
2. Surface Modification Technologies of Dental Implants
2.1. Surface Modifications of Titanium-Based Implants
2.1.1. Physical Modifications of Titanium Implants
2.1.2. Chemical Treatments
2.1.3. Multi-Step Modified Methodologies
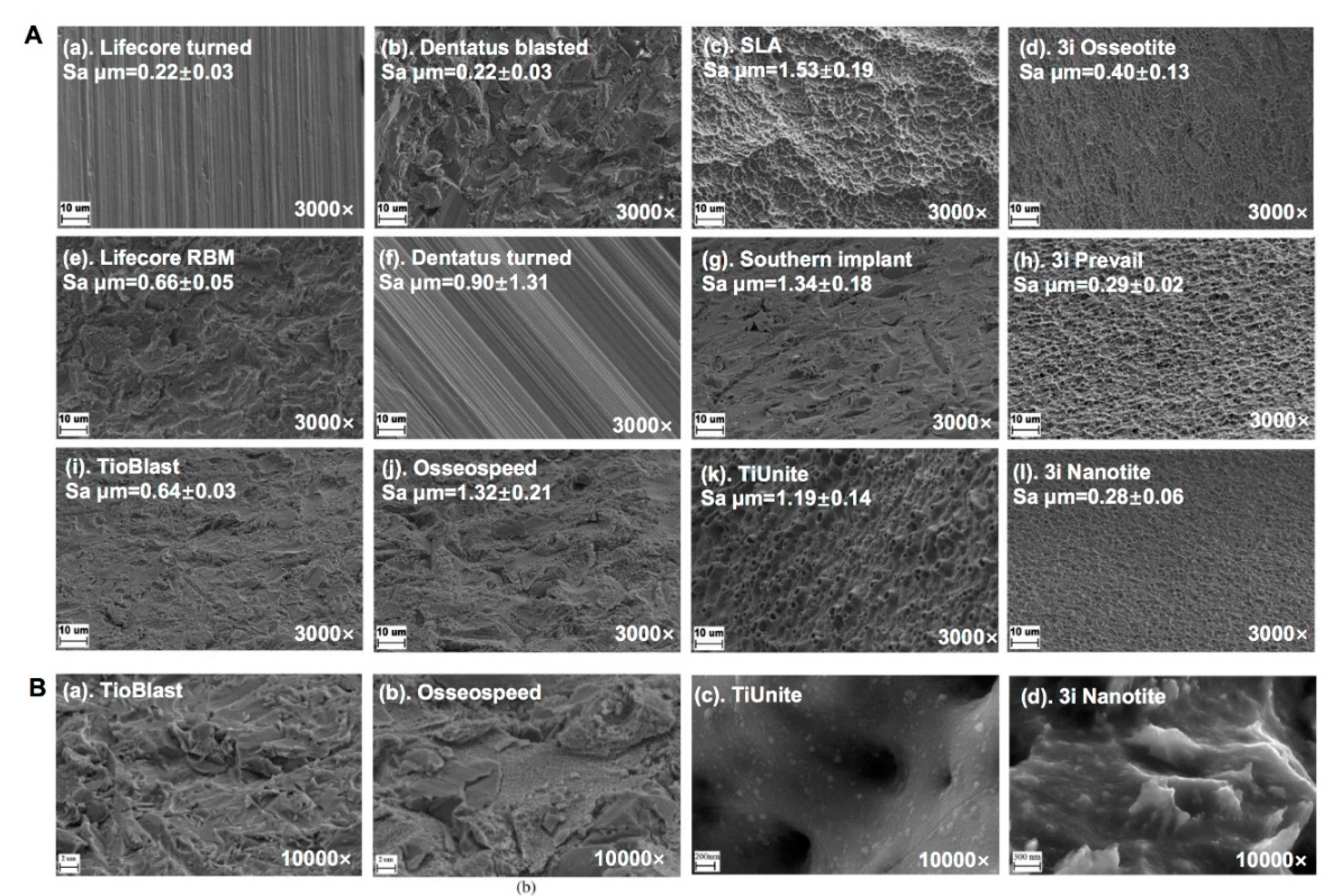
2.1.4. Surface Microstructure and Topography of Commercially Titanium Implants
2.2. Titanium Alloy Implants Surface Modifications
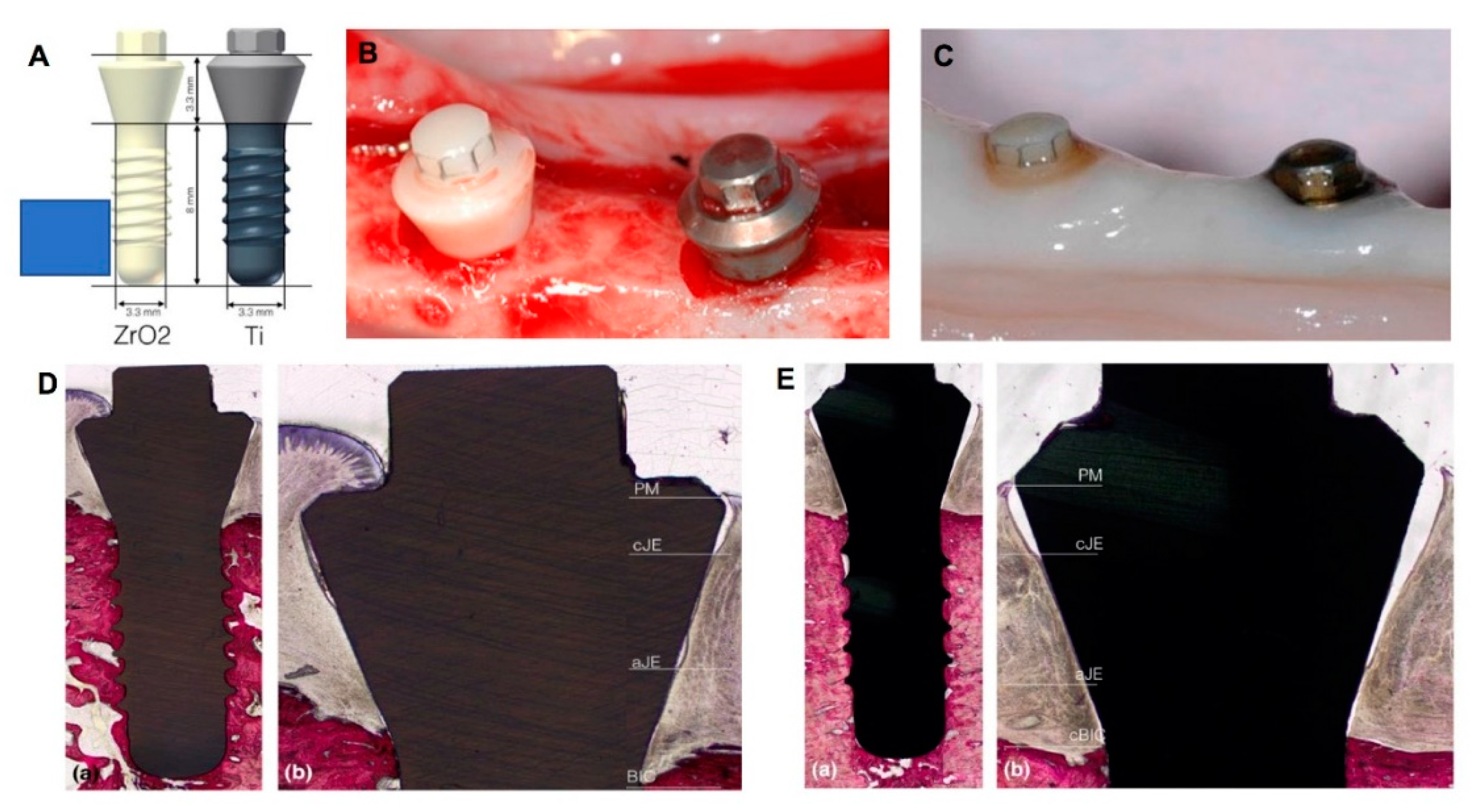
2.3. Zirconia Implant Surface Modifications
3. Coatings on Dental Implants
3.1. Coatings Improving Osseointegration
3.1.1. HA Layer and Nanocomposites
3.1.2. Magnesium
3.1.3. Graphene
3.1.4. Growth Factor Coatings
3.1.5. Extracellular Matrix Proteins
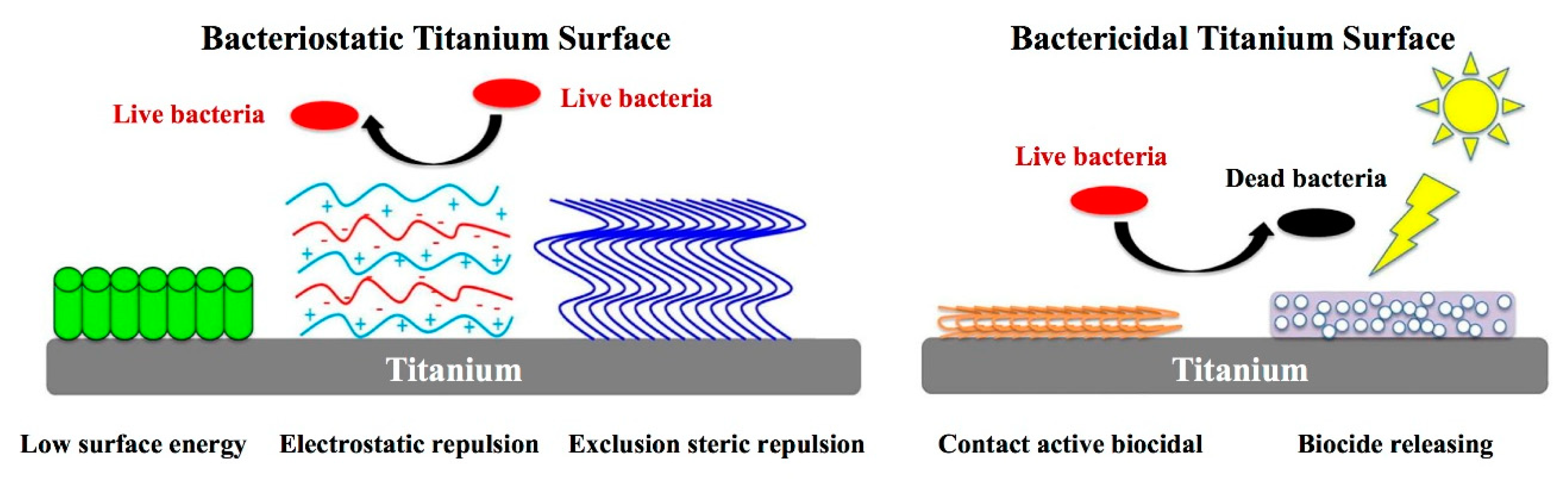
3.2. The Antibacterial Performances of Coating
3.2.1. Antibiotic Components of Implant Coating
3.2.2. The Antimicrobial Properties of Metal Element Components
3.2.3. The Antimicrobial Peptides (AMPs) of Coating Components
3.2.4. Polysaccharide Antibacterial Coatings
4. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chrcanovic, B.R.; Kisch, J.; Albrektsson, T.; Wennerberg, A. A retrospective study on clinical and radiological outcomes of oral implants in patients followed up for a minimum of 20 years. Clin. Implant Dent. Relat. Res. 2018, 20, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Niedermaier, R.; Stelzle, F.; Riemann, M.; Bolz, W.; Schuh, P.; Wachtel, H. Implant-Supported Immediately Loaded Fixed Full-Arch Dentures: Evaluation of Implant Survival Rates in a Case Cohort of up to 7 Years. Clin. Implant Dent. Relat. Res. 2017, 19, 4–19. [Google Scholar] [CrossRef] [Green Version]
- Dong, H.; Zhou, N.; Liu, H.; Huang, H.; Yang, G.; Chen, L.; Ding, M.; Mou, Y. Satisfaction analysis of patients with single implant treatments based on a questionnaire survey. Patient Prefer. Adherence 2019, 13, 695–704. [Google Scholar] [CrossRef] [Green Version]
- Zhou, N.; Dong, H.; Zhu, Y.X.; Liu, H.; Zhou, N.; Mou, Y.B. Analysis of implant loss risk factors especially in maxillary molar location: A retrospective study of 6977 implants in Chinese individuals. Clin. Implant Dent. Relat. Res. 2019, 21, 138–144. [Google Scholar] [CrossRef] [Green Version]
- Albrektsson, T.; Buser, D.; Sennerby, L. Crestal bone loss and oral implants. Clin. Implant Dent. Relat. Res. 2012, 14, 783–791. [Google Scholar] [CrossRef]
- Nguyen-Hieu, T.; Borghetti, A.; Aboudharam, G. Peri-implantitis: From diagnosis to therapeutics. J. Investig. Clin. Dent. 2012, 3, 79–94. [Google Scholar] [CrossRef]
- Stanford, C.M.; Keller, J.C. The concept of osseointegration and bone matrix expression. Crit. Rev. Oral Biol. Med. 1991, 2, 83–101. [Google Scholar] [CrossRef]
- Albrektsson, T.; Wennerberg, A. On osseointegration in relation to implant surfaces. Clin. Implant Dent. Relat. Res. 2019, 21 (Suppl. 1), 4–7. [Google Scholar] [CrossRef] [Green Version]
- Buser, D.; Sennerby, L.; Bruyn, H.D. Modern implant dentistry based on osseointegration: 50 years of progress, current trends and open questions. Periodontology 2000 2017, 73, 7–21. [Google Scholar] [CrossRef]
- Buser, D.; Schenk, R.K.; Steinemann, S.; Fiorellini, J.P.; Fox, C.H.; Stich, H. Influence of surface characteristics on bone integration of titanium implants. A histomorphometric study in miniature pigs. J. Biomed. Mater. Res. 1991, 25, 889–902. [Google Scholar] [CrossRef]
- Kasemo, B.; Gold, J. Implant surfaces and interface processes. Adv. Dent. Res. 1999, 13, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Tuna, T.; Wein, M.; Swain, M.; Fischer, J.; Att, W. Influence of ultraviolet photofunctionalization on the surface characteristics of zirconia-based dental implant materials. Dent. Mater. 2015, 31, e14–e24. [Google Scholar] [CrossRef] [PubMed]
- Asensio, G.; Vazquez-Lasa, B.; Rojo, L. Achievements in the Topographic Design of Commercial Titanium Dental Implants: Towards Anti-Peri-Implantitis Surfaces. J. Clin. Med. 2019, 8, 1982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subramani, K.; Jung, R.E.; Molenberg, A.; Hammerle, C.H. Biofilm on dental implants: A review of the literature. Int. J. Oral Maxillofac. Implants 2009, 24, 616–626. [Google Scholar]
- Liu, L.; Chen, G.; Chao, T.; Ratner, B.D.; Sage, E.H.; Jiang, S. Reduced foreign body reaction to implanted biomaterials by surface treatment with oriented osteopontin. J. Biomater. Sci. Polym. Ed. 2008, 19, 821–835. [Google Scholar] [CrossRef]
- Jemat, A.; Ghazali, M.J.; Razali, M.; Otsuka, Y. Surface Modifications and Their Effects on Titanium Dental Implants. Biomed Res. Int. 2015, 2015, 791725. [Google Scholar] [CrossRef] [Green Version]
- Linez-Bataillon, P.; Monchau, F.; Bigerelle, M.; Hildebrand, H.F. In vitro MC3T3 osteoblast adhesion with respect to surface roughness of Ti6Al4V substrates. Biomol. Eng. 2002, 19, 133–141. [Google Scholar] [CrossRef]
- Bosshardt, D.D.; Chappuis, V.; Buser, D. Osseointegration of titanium, titanium alloy and zirconia dental implants: Current knowledge and open questions. Periodontology 2000 2017, 73, 22–40. [Google Scholar] [CrossRef]
- Cooper, L.F. A role for surface topography in creating and maintaining bone at titanium endosseous implants. J. Prosthet. Dent. 2000, 84, 522–534. [Google Scholar] [CrossRef]
- Yurttutan, M.E.; Keskin, A. Evaluation of the effects of different sand particles that used in dental implant roughened for osseointegration. BMC Oral Health 2018, 18, 47. [Google Scholar] [CrossRef] [Green Version]
- Piattelli, M.; Scarano, A.; Paolantonio, M.; Iezzi, G.; Petrone, G.; Piattelli, A. Bone response to machined and resorbable blast material titanium implants: An experimental study in rabbits. J. Oral Implantol. 2002, 28, 2–8. [Google Scholar] [CrossRef] [Green Version]
- Li, H.F.; Wang, Y.B.; Zheng, Y.F.; Lin, J.P. Osteoblast response on Ti- and Zr-based bulk metallic glass surfaces after sand blasting modification. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 1721–1728. [Google Scholar] [CrossRef] [PubMed]
- Gonshor, A.; Goveia, G.; Sotirakis, E. A prospective, multicenter, 4-year study of the ACE Surgical resorbable blast media implant. J. Oral Implantol. 2003, 29, 174–180. [Google Scholar] [CrossRef]
- Cha, S.; Park, Y.S. Plasma in dentistry. Clin. Plasma Med. 2014, 2, 4–10. [Google Scholar] [CrossRef] [Green Version]
- Cunha, A.; Renz, R.P.; Blando, E.; de Oliveira, R.B.; Hubler, R. Osseointegration of atmospheric plasma-sprayed titanium implants: Influence of the native oxide layer. J. Biomed. Mater. Res. A 2014, 102, 30–36. [Google Scholar] [CrossRef]
- Andersen, O.Z.; Offermanns, V.; Sillassen, M.; Almtoft, K.P.; Andersen, I.H.; Sorensen, S.; Jeppesen, C.S.; Kraft, D.C.; Bottiger, J.; Rasse, M.; et al. Accelerated bone ingrowth by local delivery of strontium from surface functionalized titanium implants. Biomaterials 2013, 34, 5883–5890. [Google Scholar] [CrossRef]
- Yamaki, K.; Kataoka, Y.; Ohtsuka, F.; Miyazaki, T. Micro-CT evaluation of in vivo osteogenesis at implants processed by wire-type electric discharge machining. Dent. Mater. J. 2012, 31, 427–432. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.J.; Cui, D.Z.; Jeon, H.R.; Chung, H.J.; Park, Y.J.; Kim, O.S.; Kim, Y.J. Surface characteristics of thermally treated titanium surfaces. J. Periodontal Implant Sci. 2012, 42, 81–87. [Google Scholar] [CrossRef] [Green Version]
- Shi, X.; Xu, L.; Munar, M.L.; Ishikawa, K. Hydrothermal treatment for TiN as abrasion resistant dental implant coating and its fibroblast response. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 49, 1–6. [Google Scholar] [CrossRef]
- Hindy, A.; Farahmand, F.; Tabatabaei, F.A.-O. In vitro biological outcome of laser application for modification or processing of titanium dental implants. Lasers Med. Sci. 2017, 32, 1197–1206. [Google Scholar] [CrossRef]
- Yamazaki, M.; Yamada, M.; Ishizaki, K.; Sakurai, K. Ultraviolet-C irradiation to titanium implants increases peri-implant bone formation without impeding mineralization in a rabbit femur model. Acta Odontol. Scand. 2015, 73, 302–311. [Google Scholar] [CrossRef]
- Park, K.H.; Koak, J.Y.; Kim, S.K.; Han, C.H.; Heo, S.J. The effect of ultraviolet-C irradiation via a bactericidal ultraviolet sterilizer on an anodized titanium implant: A study in rabbits. Int. J. Oral Maxillofac. Implants 2013, 28, 57–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandracci, P.; Mussano, F.; Rivolo, P.; Carossa, S. Surface Treatments and Functional Coatings for Biocompatibility Improvement and Bacterial Adhesion Reduction in Dental Implantology. Coatings 2016, 6, 7. [Google Scholar] [CrossRef] [Green Version]
- Roach, M.D.; Williamson, R.S.; Blakely, I.P.; Didier, L.M. Tuning anatase and rutile phase ratios and nanoscale surface features by anodization processing onto titanium substrate surfaces. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 58, 213–223. [Google Scholar] [CrossRef]
- Mangano, F.G.; Pires, J.T.; Shibli, J.A.; Mijiritsky, E.; Iezzi, G.; Piattelli, A.; Mangano, C. Early Bone Response to Dual Acid-Etched and Machined Dental Implants Placed in the Posterior Maxilla: A Histologic and Histomorphometric Human Study. Implant Dent. 2017, 26, 24–29. [Google Scholar] [CrossRef]
- Kato, E.; Sakurai, K.; Yamada, M. Periodontal-like gingival connective tissue attachment on titanium surface with nano-ordered spikes and pores created by alkali-heat treatment. Dent. Mater. 2015, 31, e116–e130. [Google Scholar] [CrossRef]
- Ribeiro, A.R.; Oliveira, F.; Boldrini, L.C.; Leite, P.E.; Falagan-Lotsch, P.; Linhares, A.B.R.; Zambuzzi, W.F.; Fragneaud, B.; Campos, A.P.C.; Gouvea, C.P.; et al. Micro-arc oxidation as a tool to develop multifunctional calcium-rich surfaces for dental implant applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 54, 196–206. [Google Scholar] [CrossRef]
- Chung, C.J.; Su, R.T.; Chu, H.J.; Chen, H.T.; Tsou, H.K.; He, J.L. Plasma electrolytic oxidation of titanium and improvement in osseointegration. J. Biomed. Mater. Res. B Appl. Biomater. 2013, 101, 1023–1030. [Google Scholar] [CrossRef]
- Cervino, G.A.-O.; Fiorillo, L.A.-O.; Iannello, G.; Santonocito, D.A.-O.; Risitano, G.A.-O.; Cicciù, M.A.-O. Sandblasted and Acid Etched Titanium Dental Implant Surfaces Systematic Review and Confocal Microscopy Evaluation. Materials 2019, 12, 1763. [Google Scholar] [CrossRef] [Green Version]
- Yeo, I.A.-O. Modifications of Dental Implant Surfaces at the Micro- and Nano-Level for Enhanced Osseointegration. Materials 2019, 13, 89. [Google Scholar] [CrossRef] [Green Version]
- Cochran, D.L.; Buser, D.; ten Bruggenkate, C.M.; Weingart, D.; Taylor, T.M.; Bernard, J.P.; Peters, F.; Simpson, J.P. The use of reduced healing times on ITI implants with a sandblasted and acid-etched (SLA) surface: Early results from clinical trials on ITI SLA implants. Clin. Oral Implants Res. 2002, 13, 144–153. [Google Scholar] [CrossRef]
- Buser, D.; Broggini, N.; Wieland, M.; Schenk, R.K.; Denzer, A.J.; Cochran, D.L.; Hoffmann, B.; Lussi, A.; Steinemann, S.G. Enhanced bone apposition to a chemically modified SLA titanium surface. J. Dent. Res. 2004, 83, 529–533. [Google Scholar] [CrossRef]
- Wennerberg, A.; Galli, S.; Albrektsson, T. Current knowledge about the hydrophilic and nanostructured SLActive surface. Clin. Cosmet. Investig. Dent. 2011, 3, 59–67. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Cao, H.; Zhang, W.; Ding, X.; Yang, G.; Qiao, Y.; Liu, X.; Jiang, X. Enhanced Osseointegration of Hierarchical Micro/Nanotopographic Titanium Fabricated by Microarc Oxidation and Electrochemical Treatment. ACS Appl. Mater. Interfaces 2016, 8, 3840–3852. [Google Scholar] [CrossRef]
- Tack, L.; Schickle, K.; Boke, F.; Fischer, H. Immobilization of specific proteins to titanium surface using self-assembled monolayer technique. Dent. Mater. 2015, 31, 1169–1179. [Google Scholar] [CrossRef]
- Song, W.; Song, X.; Yang, C.; Gao, S.; Klausen, L.H.; Zhang, Y.; Dong, M.; Kjems, J. Chitosan/siRNA functionalized titanium surface via a layer-by-layer approach for in vitro sustained gene silencing and osteogenic promotion. Int. J. Nanomed. 2015, 10, 2335–2346. [Google Scholar] [CrossRef] [Green Version]
- Santander, S.; Alcaine, C.; Lyahyai, J.; Pérez, M.A.; Rodellar, C.; Doblaré, M.; Ochoa, I. In vitro osteoinduction of human mesenchymal stem cells in biomimetic surface modified titanium alloy implants. Dent. Mater. J. 2014, 33, 305–312. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.; Wang, S.; He, F.; Guo, Z.; Hu, P.; Zhao, R.; Huang, Y.; Chen, Q.; Ji, P.; Chu, L.; et al. Promotion of Osseointegration Using Protamine/Alginate/Bone Morphogenic Protein 2 Biofunctionalized Composite Coating on Nanopolymorphic Titanium Surfaces. J. Biomed. Nanotechnol. 2018, 14, 933–945. [Google Scholar] [CrossRef] [PubMed]
- Wennerberg, A.; Albrektsson, T. On implant surfaces: A review of current knowledge and opinions. Int. J. Oral Maxillofac. Implants 2010, 25, 63–74. [Google Scholar]
- Le Guéhennec, L.; Soueidan, A.; Layrolle, P.; Amouriq, Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent. Mater. 2007, 23, 844–854. [Google Scholar] [CrossRef]
- Svanborg, L.M.; Andersson, M.; Wennerberg, A. Surface characterization of commercial oral implants on the nanometer level. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 92, 462–469. [Google Scholar] [CrossRef]
- Yang, G.; Chen, L.; Gao, Y.; Liu, H.; Dong, H.; Mou, Y. Risk factors and reoperative survival rate of failed narrow-diameter implants in the maxillary anterior region. Clin. Implant Dent. Relat. Res. 2020, 22, 29–41. [Google Scholar] [CrossRef]
- Davidson, J.A.; Mishra, A.K.; Kovacs, P.; Poggie, R.A. New surface-hardened, low-modulus, corrosion-resistant Ti-13Nb-13Zr alloy for total hip arthroplasty. Biomed. Mater. Eng. 1994, 4, 231–243. [Google Scholar] [CrossRef]
- Khan, M.A.; Williams, R.L.; Williams, D.F. The corrosion behaviour of Ti-6Al-4V, Ti-6Al-7Nb and Ti-13Nb-13Zr in protein solutions. Biomaterials 1999, 20, 631–637. [Google Scholar] [CrossRef]
- Kaur, M.; Singh, K. Review on titanium and titanium based alloys as biomaterials for orthopaedic applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 102, 844–862. [Google Scholar] [CrossRef]
- Chikarakara, E.; Fitzpatrick, P.; Moore, E.; Levingstone, T.; Grehan, L.; Higginbotham, C.; Vazquez, M.; Bagga, K.; Naher, S.; Brabazon, D. In vitro fibroblast and pre-osteoblastic cellular responses on laser surface modified Ti-6Al-4V. Biomed. Mater. 2014, 10, 015007. [Google Scholar] [CrossRef] [Green Version]
- Gottlow, J.; Dard, M.; Kjellson, F.; Obrecht, M.; Sennerby, L. Evaluation of a new titanium-zirconium dental implant: A biomechanical and histological comparative study in the mini pig. Clin. Implant Dent. Relat. Res. 2012, 14, 538–545. [Google Scholar] [CrossRef]
- Saulacic, N.; Bosshardt, D.D.; Bornstein, M.M.; Berner, S.; Buser, D. Bone Apposition to a Titanium-Zirconium Alloy Implant, as Compared to Two Other Titanium-Containing Implants. Eur. Cells Mater. 2012, 23, 273–288. [Google Scholar] [CrossRef]
- Sharma, A.; McQuillan, A.J.; Sharma, L.A.; Waddell, J.N.; Shibata, Y.; Duncan, W.J. Spark anodization of titanium-zirconium alloy: Surface characterization and bioactivity assessment. J. Mater. Sci. Mater. Med. 2015, 26, 221. [Google Scholar] [CrossRef]
- Egusa, H.; Ko, N.; Shimazu, T.; Yatani, H. Suspected association of an allergic reaction with titanium dental implants: A clinical report. J. Prosthet. Dent. 2008, 100, 344–347. [Google Scholar] [CrossRef]
- Oliveira, V.M.C.A.; Aguiar, C.; Vazquez, A.M.; Robin, A.; Barboza, M.J.R. Improving corrosion resistance of Ti–6Al–4V alloy through plasma-assisted PVD deposited nitride coatings. Corros. Sci. 2014, 88, 317–327. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Y.; Zhang, C.; Liao, Z.; Liu, W. The improvement of wettability, biotribological behavior and corrosion resistance of titanium alloy pretreated by thermal oxidation. Tribol. Int. 2014, 79, 174–182. [Google Scholar] [CrossRef]
- Xu, G.; Shen, X.; Hu, Y.; Ma, P.; Cai, K. Fabrication of tantalum oxide layers onto titanium substrates for improved corrosion resistance and cytocompatibility. Surf. Coat. Technol. 2015, 272, 58–65. [Google Scholar] [CrossRef]
- Mokgalaka, M.N.; Popoola, A.P.I.; Pityana, S.L. In situ laser deposition of NiTi intermetallics for corrosion improvement of Ti–6Al–4V alloy. Trans. Nonferrous Metals Soc. China 2015, 25, 3315–3322. [Google Scholar] [CrossRef]
- Bartolomeu, F.; Buciumeanu, M.; Pinto, E.; Alves, N.; Silva, F.S.; Carvalho, O.; Miranda, G. Wear behavior of Ti6Al4V biomedical alloys processed by selective laser melting, hot pressing and conventional casting. Trans. Nonferrous Metals Soc. China 2017, 27, 829–838. [Google Scholar] [CrossRef]
- Zhang, J.; Gan, X.; Tang, H.; Zhan, Y. Enhancement of wear and corrosion resistance of low modulus β-type Zr-20Nb-xTi (x=0, 3) dental alloys through thermal oxidation treatment. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 76, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Hashim, D.; Cionca, N.; Courvoisier, D.S.; Mombelli, A. A systematic review of the clinical survival of zirconia implants. Clin. Oral Investig. 2016, 20, 1403–1417. [Google Scholar] [CrossRef] [Green Version]
- Stadlinger, B.; Hennig, M.; Eckelt, U.; Kuhlisch, E.; Mai, R. Comparison of zirconia and titanium implants after a short healing period. A pilot study in minipigs. Int. J. Oral Maxillofac. Surg. 2010, 39, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Scarano, A.; Di Carlo, F.; Quaranta, M.; Piattelli, A. Bone response to zirconia ceramic implants: An experimental study in rabbits. J. Oral Implantol. 2003, 29, 8–12. [Google Scholar] [CrossRef]
- Kohal, R.J.; Schwindling, F.S.; Bachle, M.; Spies, B.C. Peri-implant bone response to retrieved human zirconia oral implants after a 4-year loading period: A histologic and histomorphometric evaluation of 22 cases. J. Biomed. Mater. Res. B Appl. Biomater. 2016, 104, 1622–1631. [Google Scholar] [CrossRef]
- Liñares, A.; Grize, L.; Muñoz, F.A.-O.; Pippenger, B.E.; Dard, M.; Domken, O.; Blanco-Carrión, J. Histological assessment of hard and soft tissues surrounding a novel ceramic implant: A pilot study in the minipig. J. Clin. Periodontol. 2016, 43, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Gahlert, M.; Gudehus, T.; Eichhorn, S.; Steinhauser, E.; Kniha, H.; Erhardt, W. Biomechanical and histomorphometric comparison between zirconia implants with varying surface textures and a titanium implant in the maxilla of miniature pigs. Clin. Oral Implant. Res. 2007, 18, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Gahlert, M.; Rohling, S.; Wieland, M.; Eichhorn, S.; Kuchenhoff, H.; Kniha, H. A comparison study of the osseointegration of zirconia and titanium dental implants. A biomechanical evaluation in the maxilla of pigs. Clin. Implant Dent. Relat. Res. 2010, 12, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Shen, S.; Qian, M.; Zhang, F.; Chen, C.; Tay, F.R. Effects of Acid Treatment on Dental Zirconia: An in Vitro Study. PLoS ONE 2015, 10, e0136263. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, H.; Saito, K.; Kokubun, K.; Sasaki, H.; Yoshinari, M. Change in surface properties of zirconia and initial attachment of osteoblastlike cells with hydrophilic treatment. Dent. Mater. J. 2012, 31, 806–814. [Google Scholar] [CrossRef] [Green Version]
- Henningsen, A.; Smeets, R.; Heuberger, R.; Jung, O.T.; Hanken, H.; Heiland, M.; Cacaci, C.; Precht, C. Changes in surface characteristics of titanium and zirconia after surface treatment with ultraviolet light or non-thermal plasma. Eur. J. Oral Sci. 2018, 126, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Brezavšček, M.; Fawzy, A.; Bächle, M.; Tuna, T.; Fischer, J.; Att, W. The Effect of UV Treatment on the Osteoconductive Capacity of Zirconia-Based Materials. Materials 2016, 9, 958. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Matinlinna, J.P.; Tsoi, J.K.; Pow, E.H.; Miyazaki, T.; Shibata, Y.; Kan, C.W. A new modified laser pretreatment for porcelain zirconia bonding. Dent. Mater. 2013, 29, 559–565. [Google Scholar] [CrossRef]
- Hao, L.; Lawrence, J.; Chian, K.S. Osteoblast cell adhesion on a laser modified zirconia based bioceramic. J. Mater. Sci. Mater. Med. 2005, 16, 719–726. [Google Scholar] [CrossRef]
- Delgado-Ruiz, R.A.; Abboud, M.; Romanos, G.; Aguilar-Salvatierra, A.; Gomez-Moreno, G.; Calvo-Guirado, J.L. Peri-implant bone organization surrounding zirconia-microgrooved surfaces circularly polarized light and confocal laser scanning microscopy study. Clin. Oral Implants Res. 2015, 26, 1328–1337. [Google Scholar] [CrossRef]
- Cicciù, M.; Fiorillo, L.; Herford, A.S.; Crimi, S.; Bianchi, A.; D’Amico, C.; Laino, L.; Cervino, G. Bioactive Titanium Surfaces: Interactions of Eukaryotic and Prokaryotic Cells of Nano Devices Applied to Dental Practice. Biomedicines 2019, 7, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-Valverde, N.; Flores-Fraile, J.; Ramírez, J.; Sousa, B.; Herrero-Hernández, S.; López-Valverde, A. Bioactive Surfaces vs. Conventional Surfaces in Titanium Dental Implants: A Comparative Systematic Review. J. Clin. Med. 2020, 9, 2047. [Google Scholar] [CrossRef]
- Qadir, M.; Li, Y.; Wen, C.J.A.B. Ion-substituted calcium phosphate coatings by physical vapor deposition magnetron sputtering for biomedical applications: A review. Acta Biomater. 2019, 89, 14–32. [Google Scholar] [CrossRef] [PubMed]
- Jing, W.S.; Zhang, M.H.; Jin, L.; Zhao, J.; Gao, Q.; Ren, M.; Fan, Q.Y. Assessment of osteoinduction using a porous hydroxyapatite coating prepared by micro-arc oxidation on a new titanium alloy. Int. J. Surg. 2015, 24, 51–56. [Google Scholar] [CrossRef]
- Choi, A.; Ben-Nissan, B.; Matinlinna, J.; Conway, R.C. Current perspectives: Calcium phosphate nanocoatings and nanocomposite coatings in dentistry. J. Dent. Res. 2013, 92, 853–859. [Google Scholar] [CrossRef]
- Bergamo, E.; Pessoa, P.; Jimbo, R.; Neiva, R.; Coelho, P. The synergetic effect of implant macrogeometry and surface physicochemical modifications on osseointegration: An in vivo experimental study in sheep. J. Long-Term Eff. Med. Implant. 2020, 29, 295–302. [Google Scholar] [CrossRef]
- Fang, C.-H.; Lin, Y.-W.; Lin, F.-H.; Sun, J.-S.; Chao, Y.-H.; Lin, H.-Y.; Chang, Z.-C. Biomimetic Synthesis of Nanocrystalline Hydroxyapatite Composites: Therapeutic Potential and Effects on Bone Regeneration. Int. J. Mol. Sci. 2019, 20, 6002. [Google Scholar] [CrossRef] [Green Version]
- Hu, Z.; Wang, X.; Xia, W.; Wang, Z.; Zhang, P.; Xia, L.; Lin, K.; Zhu, M. Nano-Structure Designing Promotion Osseointegration of Hydroxyapatite Coated Ti–6Al–4V Alloy Implants in Diabetic Model. J. Biomed. Nanotechnol. 2019, 15, 1701–1713. [Google Scholar] [CrossRef]
- Ewald, A.; Kreczy, D.; Brückner, T.; Gbureck, U.; Bengel, M.; Hoess, A.; Nies, B.; Bator, J.; Klammert, U.; Fuchs, A.J.M. Development and Bone Regeneration Capacity of Premixed Magnesium Phosphate Cement Pastes. Materials 2019, 12, 2119. [Google Scholar] [CrossRef] [Green Version]
- Nabiyouni, M.; Ren, Y.; Bhaduri, S.B. Magnesium substitution in the structure of orthopedic nanoparticles: A comparison between amorphous magnesium phosphates, calcium magnesium phosphates, and hydroxyapatites. Mater. Sci. Eng. C 2015, 52, 11–17. [Google Scholar] [CrossRef]
- Pardun, K.; Treccani, L.; Volkmann, E.; Streckbein, P.; Heiss, C.; Gerlach, J.W.; Maendl, S.; Rezwan, K. Magnesium-containing mixed coatings on zirconia for dental implants: Mechanical characterization and in vitro behavior. J. Biomater. Appl. 2015, 30, 104–118. [Google Scholar] [CrossRef] [Green Version]
- Sikder, P.; Bhaduri, S.B. Microwave assisted synthesis and characterization of single-phase tabular hexagonal newberyite, an important bioceramic. J. Am. Ceram. Soc. 2018, 101, 2537–2544. [Google Scholar] [CrossRef]
- Lee, S.; Chang, Y.-Y.; Lee, J.; Perikamana, S.K.M.; Kim, E.M.; Jung, Y.-H.; Yun, J.-H.; Shin, H. Surface engineering of titanium alloy using metal-polyphenol network coating with magnesium ions for improved osseointegration. Biomater. Sci. 2020, 8, 3404–3417. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, R.; Han, Y.; Morin, J.; Luong-Van, E.; Chew, R.; Neto, A.C.; Nijhuis, C.; Rosa, V. Inhibiting Corrosion of Biomedical-Grade Ti-6Al-4V Alloys with Graphene Nanocoating. J. Dent. Res. 2020, 99, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.R.; HaeLin, J.; Jang, H.L.; Khoshakhlagh, P.; Akbari, M.; Nasajpour, A.; Zhang, Y.S.; Tamayol, A.; Khademhosseini, A. Graphene-based materials for tissue engineering. Adv. Drug Deliv. Rev. 2016, 105, 255–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Wang, Z. Involvement of FAK/P38 Signaling Pathways in Mediating the Enhanced Osteogenesis Induced by Nano-Graphene Oxide Modification on Titanium Implant Surface. Int. J. Nanomed. 2020, 15, 4659–4676. [Google Scholar] [CrossRef]
- Guang, M.; Huang, B.; Yao, Y.; Zhang, L.; Yang, B.; Gong, P. Effects of vascular endothelial growth factor on osteoblasts around dental implants in vitro and in vivo. J. Oral Sci. 2017, 59, 215–223. [Google Scholar] [CrossRef] [Green Version]
- Izquierdo-Barba, I.; Santos-Ruiz, L.; Becerra, J.; Feito, M.; Fernández-Villa, D.; Serrano, M.; Díaz-Güemes, I.; Fernández-Tomé, B.; Enciso, S.; Sánchez-Margallo, F.; et al. Synergistic effect of Si-hydroxyapatite coating and VEGF adsorption on Ti6Al4V-ELI scaffolds for bone regeneration in an osteoporotic bone environment. Acta Biomater. 2019, 83, 456–466. [Google Scholar] [CrossRef]
- Katagiri, T.; Watabe, T. Bone Morphogenetic Proteins. Cold Spring Harb. Perspect. Biol. 2016, 8, a021899. [Google Scholar] [CrossRef] [Green Version]
- Carreira, A.C.; Lojudice, F.H.; Halcsik, E.; Navarro, R.D.; Sogayar, M.C.; Granjeiro, J.M. Bone Morphogenetic Proteins Facts, Challenges, and Future Perspectives. J. Dent. Res. 2014, 93, 335. [Google Scholar] [CrossRef]
- Kim, J.-E.; Kang, S.-S.; Choi, K.-H.; Shim, J.-S.; Jeong, C.-M.; Shin, S.-W.; Huh, J.-B. The effect of anodized implants coated with combined rhBMP-2 and recombinant human vascular endothelial growth factors on vertical bone regeneration in the marginal portion of the peri-implant. Oral Surgery Oral Med. Oral Pathol. Oral Radiol. 2013, 115, e24–e31. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Eb, H.; Liu, T.; Hu, Q.; Liu, Y. Enhanced biocompatibility and improved osteogenesis of coralline hydroxyapatite modified by bone morphogenetic protein 2 incorporated into a biomimetic coating. Mater. Sci. Eng. C 2019, 96, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Guillot, R.; Pignot-Paintrand, I.; Lavaud, J.; Decambron, A.; Bourgeois, E.; Josserand, V.; Logeart-Avramoglou, D.; Viguier, E.; Picart, C. Assessment of a polyelectrolyte multilayer film coating loaded with BMP-2 on titanium and PEEK implants in the rabbit femoral condyle. Acta Biomater. 2016, 36, 310–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santo, V.E.; Gomes, M.E.; Mano, J.F.; Reis, R.L. Controlled release strategies for bone, cartilage, and osteochondral engineering—Part I: Recapitulation of native tissue healing and variables for the design of delivery systems. Tissue Eng. Part B Rev. 2013, 19, 308–326. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.H.; Moon, S.W.; Lee, D.W. Surface Modification of Titanium with BMP-2/GDF-5 by a Heparin Linker and Its Efficacy as a Dental Implant. Int. J. Mol. Sci. 2017, 18, 229. [Google Scholar] [CrossRef] [Green Version]
- Al-Jarsha, M.; Moulisova, V.; Leal-Egana, A.; Connell, A.; Naudi, K.B.; Ayoub, A.F.; Dalby, M.J.; Salmerón-Sánchez, M. Engineered Coatings for Titanium Implants to Present Ultralow Doses of BMP-7. ACS Biomater. Sci. Eng. 2018, 4, 1812–1819. [Google Scholar] [CrossRef]
- Terheyden, H.; Lang, N.P.; Bierbaum, S.; Stadlinger, B. Osseointegration—Communication of cells. Clin. Oral Implant. Res. 2012, 23, 1127–1135. [Google Scholar] [CrossRef]
- Kellesarian, S.V.; Malignaggi, V.; Kellesarian, T.; Ahmed, H.B.; Javed, F. Does incorporating collagen and chondroitin sulfate matrix in implant surfaces enhance osseointegration? A systematic review and meta-analysis. Int. J. Oral Maxillofac. Surg. 2018, 47, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Yin, D.; Komasa, S.; Yoshimine, S.; Sekino, T.; Okazaki, J. Effect of mussel adhesive protein coating on osteogenesis in vitro and osteointegration in vivo to alkali-treated titanium with nanonetwork structures. Int. J. Nanomed. 2019, 14, 3831–3843. [Google Scholar] [CrossRef] [Green Version]
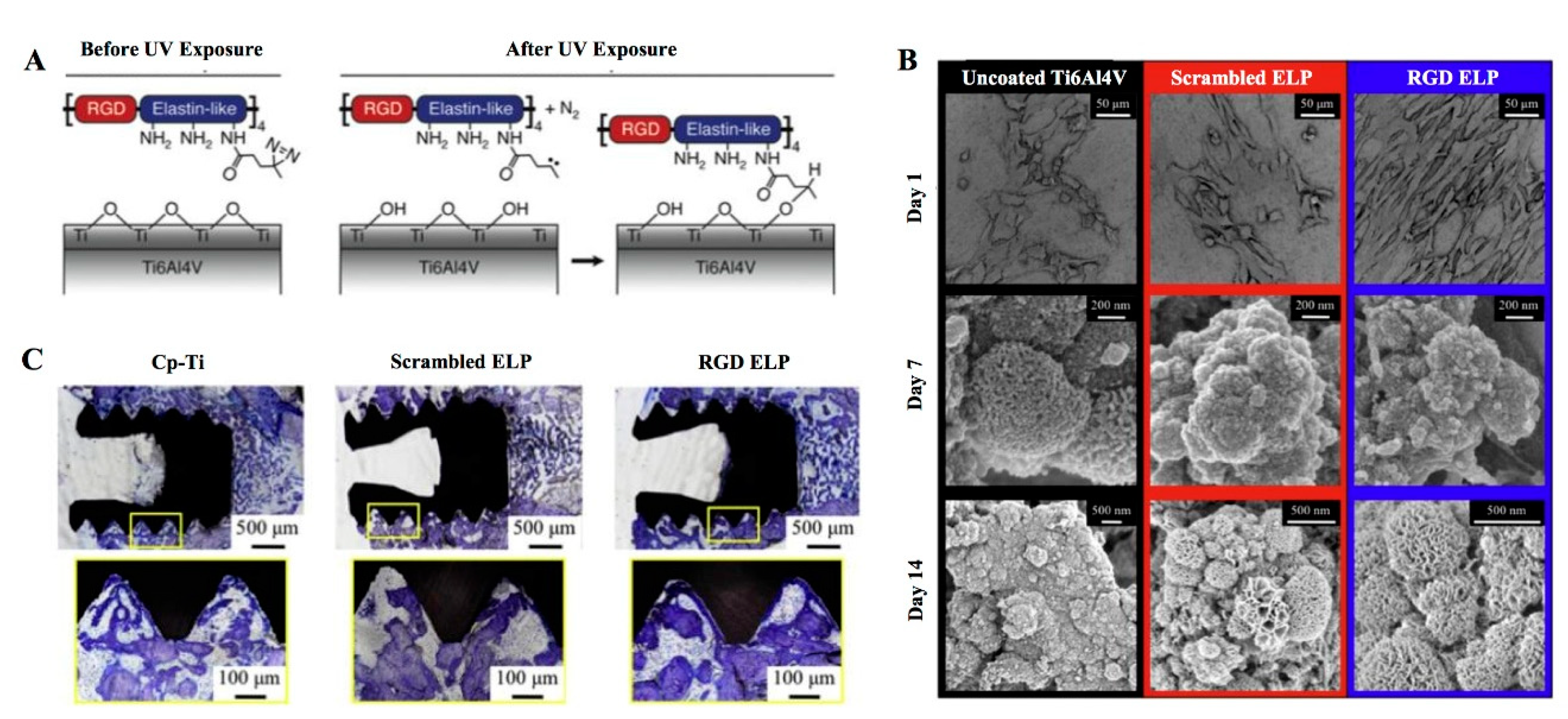
- Raphel, J.; Karlsson, J.; Galli, S.; Wennerberg, A.; Lindsay, C.; Haugh, M.G.; Pajarinen, J.; Goodman, S.B.; Jimbo, R.; Andersson, M.; et al. Engineered protein coatings to improve the osseointegration of dental and orthopaedic implants. Biomaterials 2016, 83, 269–282. [Google Scholar] [CrossRef] [Green Version]
- Franci, G.; Falanga, A.; Galdiero, S.; Palomba, L.; Rai, M.; Morelli, G.; Galdiero, M. Silver Nanoparticles as Potential Antibacterial Agents. Molecules 2015, 20, 8856–8874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chouirfa, H.; Bouloussa, H.; Migonney, V.; Falentin-Daudré, C. Review of titanium surface modification techniques and coatings for antibacterial applications. Acta Biomater. 2019, 83, 37–54. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Zhang, P.; Wang, X.; Kasugai, S. A doxycycline-treated hydroxyapatite implant surface attenuates the progression of peri-implantitis: A radiographic and histological study in mice. Clin. Implant Dent. Relat. Res. 2019, 21, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Alecio, A.B.W.; Ferreira, C.F.; Babu, J.; Shokuhfar, T.; Jo, S.; Magini, R.; Garcia-Godoy, F. Doxycycline Release of Dental Implants with Nanotube Surface, Coated with Poly Lactic-Co-Glycolic Acid for Extended pH-controlled Drug Delivery. J. Oral Implantol. 2019, 45, 267–273. [Google Scholar] [CrossRef]
- Kazek-Kesik, A.; Nosol, A.; Plonka, J.; Smiga-Matuszowicz, M.; Golda-Cepa, M.; Krok-Borkowicz, M.; Brzychczy-Wloch, M.; Pamula, E.; Simka, W. PLGA-amoxicillin-loaded layer formed on anodized Ti alloy as a hybrid material for dental implant applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 94, 998–1008. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Zhou, P.; Wang, L.X.; Xiong, X.L.; Zhang, Y.F.; Deng, Y.; Wei, S.C. Antibiotic-decorated titanium with enhanced antibacterial activity through adhesive polydopamine for dental/bone implant. J. R. Soc. Interface 2014, 11, 20140169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahi, R.G.; Albuquerque, M.T.P.; Munchow, E.A.; Blanchard, S.B.; Gregory, R.L.; Bottino, M.C. Novel bioactive tetracycline-containing electrospun polymer fibers as a potential antibacterial dental implant coating. Odontology 2017, 105, 354–363. [Google Scholar] [CrossRef]
- Bottino, M.C.; Munchow, E.A.; Albuquerque, M.T.P.; Kamocki, K.; Shahi, R.; Gregory, R.L.; Chu, T.M.G.; Pankajakshan, D. Tetracycline-incorporated polymer nanofibers as a potential dental implant surface modifier. J. Biomed. Mater. Res. Part B-Appl. Biomater. 2017, 105, 2085–2092. [Google Scholar] [CrossRef]
- Spriano, S.; Yamaguchi, S.; Baino, F.; Ferraris, S. A critical review of multifunctional titanium surfaces: New frontiers for improving osseointegration and host response, avoiding bacteria contamination. Acta Biomater. 2018, 79, 1–22. [Google Scholar] [CrossRef]
- Hickok, N.J.; Shapiro, I.M. Immobilized antibiotics to prevent orthopaedic implant infections. Adv. Drug Deliv. Rev. 2012, 64, 1165–1176. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Sun, Y.; Tian, A.; Xue, X.X.; Wang, L.; Alquhali, A.; Bai, X. Improved antibacterial activity and biocompatibility on vancomycin-loaded TiO2 nanotubes: In vivo and in vitro studies. Int. J. Nanomed. 2013, 8, 4379–4389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Ao, H.Y.; Yang, S.B.; Wang, Y.G.; Lin, W.T.; Yu, Z.F.; Tang, T.T. In vivo evaluation of the anti-infection potential of gentamicin-loaded nanotubes on titania implants. Int. J. Nanomed. 2016, 11, 2223–2234. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Wu, G.; Liu, X.; Sun, G.; Li, D.; Wei, H. A decomposable silica-based antibacterial coating for percutaneous titanium implant. Int. J. Nanomed. 2017, 12, 371–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, D.W.; Yun, Y.P.; Park, K.; Kim, S.E. Gentamicin and bone morphogenic protein-2 (BMP-2)-delivering heparinized-titanium implant with enhanced antibacterial activity and osteointegration. Bone 2012, 50, 974–982. [Google Scholar] [CrossRef] [PubMed]
- Lampe, I.; Beke, D.; Biri, S.; Csarnovics, I.; Csik, A.; Dombradi, Z.; Hajdu, P.; Hegedus, V.; Racz, R.; Varga, I.; et al. Investigation of silver nanoparticles on titanium surface created by ion implantation technology. Int. J. Nanomed. 2019, 14, 4709–4721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, I.X.; Zhang, J.; Zhao, I.S.; Mei, M.L.; Li, Q.; Chu, C.H. The Antibacterial Mechanism of Silver Nanoparticles and Its Application in Dentistry. Int. J. Nanomed. 2020, 15, 2555–2562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gunputh, U.F.; Le, H.; Lawton, K.; Besinis, A.; Tredwin, C.; Handy, R.D. Antibacterial properties of silver nanoparticles grown in situ and anchored to titanium dioxide nanotubes on titanium implant against Staphylococcus aureus. Nanotoxicology 2020, 14, 97–110. [Google Scholar] [CrossRef]
- Choi, S.H.; Jang, Y.S.; Jang, J.H.; Bae, T.S.; Lee, S.J.; Lee, M.H. Enhanced antibacterial activity of titanium by surface modification with polydopamine and silver for dental implant application. J. Appl. Biomater. Funct. Mater. 2019, 17, 2280800019847067. [Google Scholar] [CrossRef]
- Li, M.; Liu, Q.; Jia, Z.; Xu, X.; Shi, Y.; Cheng, Y.; Zheng, Y. Polydopamine-induced nanocomposite Ag/CaP coatings on the surface of titania nanotubes for antibacterial and osteointegration functions. J. Mater. Chem. B 2015, 3, 8796–8805. [Google Scholar] [CrossRef]
- Shang, B.; Xu, M.; Zhi, Z.; Xi, Y.; Wang, Y.; Peng, B.; Li, P.; Deng, Z. Synthesis of sandwich-structured silver@polydopamine@silver shells with enhanced antibacterial activities. J. Colloid Interface Sci. 2020, 558, 47–54. [Google Scholar] [CrossRef]
- Guan, M.; Chen, Y.; Wei, Y.; Song, H.; Gao, C.; Cheng, H.; Li, Y.; Huo, K.; Fu, J.; Xiong, W. Long-lasting bactericidal activity through selective physical puncture and controlled ions release of polydopamine and silver nanoparticles-loaded TiO(2) nanorods in vitro and in vivo. Int. J. Nanomed. 2019, 14, 2903–2914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chou, A.H.; LeGeros, R.Z.; Chen, Z.; Li, Y. Antibacterial effect of zinc phosphate mineralized guided bone regeneration membranes. Implant Dent. 2007, 16, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Hu, Y.; Xu, G.; Chen, W.; Xu, K.; Ran, Q.; Ma, P.; Zhang, Y.; Li, J.; Cai, K. Regulation of the biological functions of osteoblasts and bone formation by Zn-incorporated coating on microrough titanium. ACS Appl. Mater. Interfaces 2014, 6, 16426–16440. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Zhang, W.; Qiao, Y.; Jiang, X.; Liu, X.; Ding, C. Antibacterial activity and increased bone marrow stem cell functions of Zn-incorporated TiO2 coatings on titanium. Acta Biomater. 2012, 8, 904–915. [Google Scholar] [CrossRef]
- Luo, Q.; Cao, H.; Wang, L.; Ma, X.; Liu, X. ZnO@ZnS nanorod-array coated titanium: Good to fibroblasts but bad to bacteria. J. Colloid Interface Sci. 2020, 579, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni Aranya, A.; Pushalkar, S.; Zhao, M.; LeGeros, R.Z.; Zhang, Y.; Saxena, D. Antibacterial and bioactive coatings on titanium implant surfaces. J. Biomed. Mater. Res. A 2017, 105, 2218–2227. [Google Scholar] [CrossRef] [Green Version]
- Kranz, S.; Guellmar, A.; Voelpel, A.; Lesser, T.; Tonndorf-Martini, S.; Schmidt, J.; Schrader, C.; Faucon, M.; Finger, U.; Pfister, W.; et al. Bactericidal and Biocompatible Properties of Plasma Chemical Oxidized Titanium (TiOB(®)) with Antimicrobial Surface Functionalization. Materials 2019, 12, 866. [Google Scholar] [CrossRef] [Green Version]
- Mahamuni-Badiger, P.P.; Patil, P.M.; Badiger, M.V.; Patel, P.R.; Thorat-Gadgil, B.S.; Pandit, A.; Bohara, R.A. Biofilm formation to inhibition: Role of zinc oxide-based nanoparticles. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 108, 110319. [Google Scholar] [CrossRef]
- Grenho, L.; Salgado, C.L.; Fernandes, M.H.; Monteiro, F.J.; Ferraz, M.P. Antibacterial activity and biocompatibility of three-dimensional nanostructured porous granules of hydroxyapatite and zinc oxide nanoparticles—An in vitro and in vivo study. Nanotechnology 2015, 26, 315101. [Google Scholar] [CrossRef]
- Fröber, K.; Bergs, C.; Pich, A.; Conrads, G. Biofunctionalized zinc peroxide nanoparticles inhibit peri-implantitis associated anaerobes and Aggregatibacter actinomycetemcomitans pH-dependent. Anaerobe 2020, 62, 102153. [Google Scholar] [CrossRef]
- Rosenbaum, J.; Versace, D.L.; Abbad-Andallousi, S.; Pires, R.; Azevedo, C.; Cénédese, P.; Dubot, P.A.-O. Antibacterial properties of nanostructured Cu-TiO(2) surfaces for dental implants. Biomater. Sci. 2017, 5, 455–462. [Google Scholar] [CrossRef]
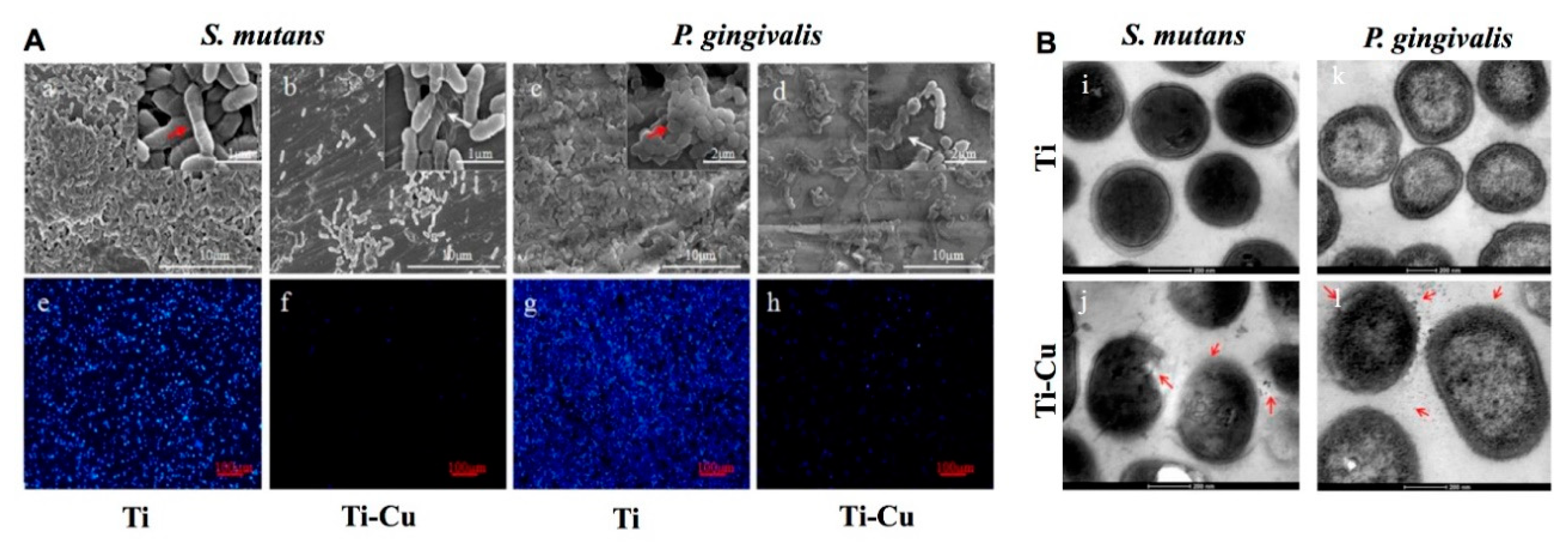
- Wang, X.; Dong, H.; Liu, J.; Qin, G.; Chen, D.; Zhang, E. In vivo antibacterial property of Ti-Cu sintered alloy implant. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 100, 38–47. [Google Scholar] [CrossRef]
- Liu, R.; Memarzadeh, K.; Chang, B.; Zhang, Y.; Ma, Z.; Allaker, R.P.; Ren, L.; Yang, K. Antibacterial effect of copper-bearing titanium alloy (Ti-Cu) against Streptococcus mutans and Porphyromonas gingivalis. Sci. Rep. 2016, 6, 29985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Astasov-Frauenhoffer, M.; Koegel, S.; Waltimo, T.; Zimmermann, A.; Walker, C.; Hauser-Gerspach, I.; Jung, C. Antimicrobial efficacy of copper-doped titanium surfaces for dental implants. J. Mater. Sci. Mater. Med. 2019, 30, 84. [Google Scholar] [CrossRef] [PubMed]
- Fowler, L.; Masia, N.; Cornish, L.A.; Chown, L.H.; Engqvist, H.; Norgren, S.; Öhman-Mägi, C.A.-O. Development of Antibacterial Ti-Cu(x) Alloys for Dental Applications: Effects of Ageing for Alloys with up to 10 wt% Cu. Materials 2019, 12, 4017. [Google Scholar] [CrossRef] [Green Version]
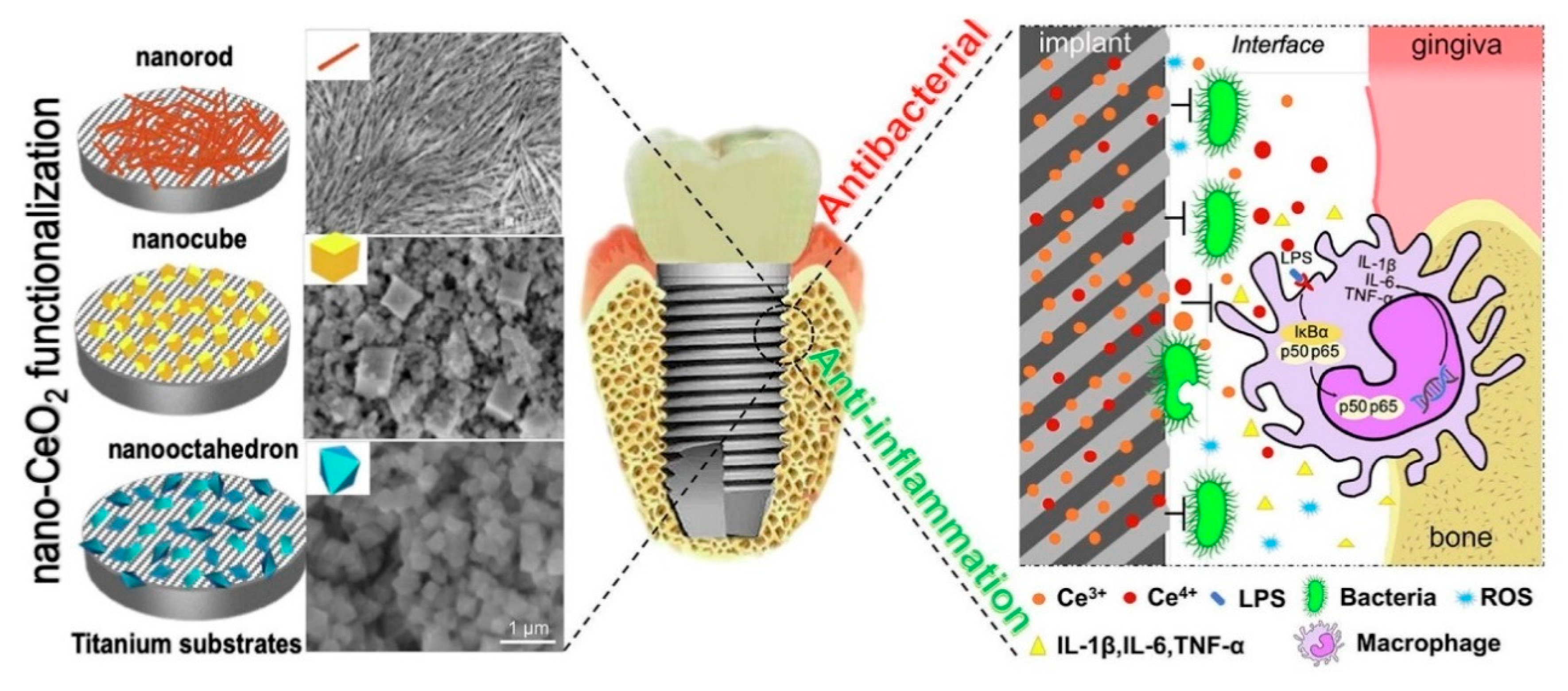
- Li, K.; Xie, Y.; You, M.; Huang, L.; Zheng, X. Plasma sprayed cerium oxide coating inhibits H2O2-induced oxidative stress and supports cell viability. J. Mater. Sci. Mater. Med. 2016, 27, 100. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.; Wu, J.; Xu, Y.; Zhang, Y.; Wang, R.; Li, K.; Xu, Y. Chemical Stability and Antimicrobial Activity of Plasma-Sprayed Cerium Oxide-Incorporated Calcium Silicate Coating in Dental Implants. Implant Dent. 2019, 28, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Vassie, J.A.; Whitelock, J.M.; Lord, M.S. Endocytosis of cerium oxide nanoparticles and modulation of reactive oxygen species in human ovarian and colon cancer cells. Acta Biomater. 2017, 50, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Naganuma, T. Shape design of cerium oxide nanoparticles for enhancement of enzyme mimetic activity in therapeutic applications. Nano Res. 2016, 10, 199–217. [Google Scholar] [CrossRef]
- Li, X.; Qi, M.; Sun, X.; Weir, M.D.; Tay, F.R.; Oates, T.W.; Dong, B.; Zhou, Y.; Wang, L.; Xu, H.H.K. Surface treatments on titanium implants via nanostructured ceria for antibacterial and anti-inflammatory capabilities. Acta Biomater. 2019, 94, 627–643. [Google Scholar] [CrossRef]
- Levine, B.R.; Sporer, S.; Poggie, R.A.; Della Valle, C.J.; Jacobs, J.J. Experimental and clinical performance of porous tantalum in orthopedic surgery. Biomaterials 2006, 27, 4671–4681. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.M.; Li, Y.; Gu, Y.X.; Zhang, C.N.; Lai, H.C.; Shi, J.Y. Ta-Coated Titanium Surface with Superior Bacteriostasis and Osseointegration. Int. J. Nanomed. 2019, 14, 8693–8706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiang, Y.; Li, J.; Liu, X.; Cui, Z.; Yang, X.; Yeung, K.W.K.; Pan, H.; Wu, S. Construction of poly(lactic-co-glycolic acid)/ZnO nanorods/Ag nanoparticles hybrid coating on Ti implants for enhanced antibacterial activity and biocompatibility. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 79, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Vu, A.A.; Robertson, S.F.; Ke, D.; Bandyopadhyay, A.; Bose, S. Mechanical and biological properties of ZnO, SiO(2), and Ag(2)O doped plasma sprayed hydroxyapatite coating for orthopaedic and dental applications. Acta Biomater. 2019, 92, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, L.; Zhao, M.; Dong, L.; Wu, J.; Li, D. Biological actions of Cu/Zn coimplanted TiN on Ti-6Al-4V alloy. Biointerphases 2019, 14, 051008. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Feng, T.; Huang, D.; Liu, P.; Lin, P.; Wu, Y.; Ye, Z.; Ji, J.; Li, P.; Huang, W. Antibacterial and hydroxyapatite-forming coating for biomedical implants based on polypeptide-functionalized titania nanospikes. Biomater. Sci. 2019, 8, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.; Carvalho, I.F.; Montelaro, R.C.; Gomes, P.; Martins, M.C. Covalent immobilization of antimicrobial peptides (AMPs) onto biomaterial surfaces. Acta Biomater. 2011, 7, 1431–1440. [Google Scholar] [CrossRef] [Green Version]
- Stallmann, H.P.; Faber, C.; Bronckers, A.L.; de Blieck-Hogervorst, J.M.; Brouwer, C.P.; Amerongen, A.V.; Wuisman, P.I. Histatin and lactoferrin derived peptides: Antimicrobial properties and effects on mammalian cells. Peptides 2005, 26, 2355–2359. [Google Scholar] [CrossRef]
- Godoy-Gallardo, M.; Mas-Moruno, C.; Fernandez-Calderon, M.C.; Perez-Giraldo, C.; Manero, J.M.; Albericio, F.; Gil, F.J.; Rodriguez, D. Covalent immobilization of hLf1-11 peptide on a titanium surface reduces bacterial adhesion and biofilm formation. Acta Biomater. 2014, 10, 3522–3534. [Google Scholar] [CrossRef]
- Godoy-Gallardo, M.; Mas-Moruno, C.; Yu, K.; Manero, J.M.; Gil Mur, F.J.; Kizhakkedathu, J.N.; Rodríguez, D. Antibacterial properties of hLf1-11 peptide onto titanium surfaces: A comparison study between silanization and surface initiated polymerization. Biomacromolecules 2015, 16, 483–496. [Google Scholar] [CrossRef] [Green Version]
- Warnke, P.H.; Voss, E.; Russo, P.A.; Stephens, S.; Kleine, M.; Terheyden, H.; Liu, Q. Antimicrobial peptide coating of dental implants: Biocompatibility assessment of recombinant human beta defensin-2 for human cells. Int. J. Oral Maxillofac. Implants 2013, 28, 982–988. [Google Scholar] [CrossRef] [Green Version]
- Gorr, S.U.; Abdolhosseini, M.; Shelar, A.; Sotsky, J. Dual host-defence functions of SPLUNC2/PSP and synthetic peptides derived from the protein. Biochem. Soc. Trans. 2011, 39, 1028–1032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, L.; Lai, Y.; Huang, W.; Huang, S.; Xu, Z.; Chen, J.; Wu, D. Biofunctionalization of microgroove titanium surfaces with an antimicrobial peptide to enhance their bactericidal activity and cytocompatibility. Colloids Surf. B Biointerfaces 2015, 128, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Holmberg, K.V.; Abdolhosseini, M.; Li, Y.; Chen, X.; Gorr, S.U.; Aparicio, C. Bio-inspired stable antimicrobial peptide coatings for dental applications. Acta Biomater. 2013, 9, 8224–8231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Hirt, H.; Li, Y.; Gorr, S.U.; Aparicio, C. Antimicrobial GL13K peptide coatings killed and ruptured the wall of Streptococcus gordonii and prevented formation and growth of biofilms. PLoS ONE 2014, 9, e111579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.Y.; Wang, X.J.; Wang, L.N.; Ying, X.X.; Ren, X.; Liu, H.Y.; Xu, L.; Ma, G.W. High in vitro antibacterial activity of Pac-525 against Porphyromonas gingivalis biofilms cultured on titanium. Biomed Res. Int. 2015, 2015, 909870. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Zhang, Y.; Shen, X.; Tao, B.; Liu, J.; Yuan, Z.; Cai, K. The fabrication and in vitro properties of antibacterial polydopamine-LL-37-POPC coatings on micro-arc oxidized titanium. Colloids Surf. B Biointerfaces 2018, 170, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Zhou, L.; Deng, Y.; Chen, X.; Xiong, X.; Deng, F.; Wei, S. A carboxymethyl chitosan and peptide-decorated polyetheretherketone ternary biocomposite with enhanced antibacterial activity and osseointegration as orthopedic/dental implants. J. Mater. Chem. B 2016, 4, 1878–1890. [Google Scholar] [CrossRef]
- Campos, D.M.; Toury, B.; D’Almeida, M.; Attik, G.N.; Ferrand, A.; Renoud, P.; Grosgogeat, B. Acidic pH resistance of grafted chitosan on dental implant. Odontology 2015, 103, 210–217. [Google Scholar] [CrossRef]
- Palla-Rubio, B.; Araújo-Gomes, N.; Fernández-Gutiérrez, M.; Rojo, L.; Suay, J.; Gurruchaga, M.; Goñi, I. Synthesis and characterization of silica-chitosan hybrid materials as antibacterial coatings for titanium implants. Carbohydr. Polym. 2019, 203, 331–341. [Google Scholar] [CrossRef]
- Valverde, A.; Perez-Alvarez, L.; Ruiz-Rubio, L.; Pacha Olivenza, M.A.; Garcia Blanco, M.B.; Diaz-Fuentes, M.; Vilas-Vilela, J.L. Antibacterial hyaluronic acid/chitosan multilayers onto smooth and micropatterned titanium surfaces. Carbohydr. Polym. 2019, 207, 824–833. [Google Scholar] [CrossRef] [PubMed]
- Divakar, D.D.; Jastaniyah, N.T.; Altamimi, H.G.; Alnakhli, Y.O.; Muzaheed; Alkheraif, A.A.; Haleem, S. Enhanced antimicrobial activity of naturally derived bioactive molecule chitosan conjugated silver nanoparticle against dental implant pathogens. Int. J. Biol. Macromol. 2018, 108, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yang, Y.; Zhang, H.; Xu, Z.; Zhao, L.; Wang, J.; Qiu, Y.; Liu, B. Improvements on biological and antimicrobial properties of titanium modified by AgNPs-loaded chitosan-heparin polyelectrolyte multilayers. J. Mater. Sci. Mater. Med. 2019, 30, 52. [Google Scholar] [CrossRef] [PubMed]
- Norowski, P.A.; Courtney, H.S.; Babu, J.; Haggard, W.O.; Bumgardner, J.D. Chitosan coatings deliver antimicrobials from titanium implants: A preliminary study. Implant Dent. 2011, 20, 56–67. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, H.; Liu, H.; Zhou, N.; Li, Q.; Yang, G.; Chen, L.; Mou, Y. Surface Modified Techniques and Emerging Functional Coating of Dental Implants. Coatings 2020, 10, 1012. https://doi.org/10.3390/coatings10111012
Dong H, Liu H, Zhou N, Li Q, Yang G, Chen L, Mou Y. Surface Modified Techniques and Emerging Functional Coating of Dental Implants. Coatings. 2020; 10(11):1012. https://doi.org/10.3390/coatings10111012
Chicago/Turabian StyleDong, Heng, Hui Liu, Na Zhou, Qiang Li, Guangwen Yang, Li Chen, and Yongbin Mou. 2020. "Surface Modified Techniques and Emerging Functional Coating of Dental Implants" Coatings 10, no. 11: 1012. https://doi.org/10.3390/coatings10111012






