Preparation and Anti-frost Performance of PDMS-SiO2/SS Superhydrophobic Coating
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of PDMS-SiO2 Hybrid
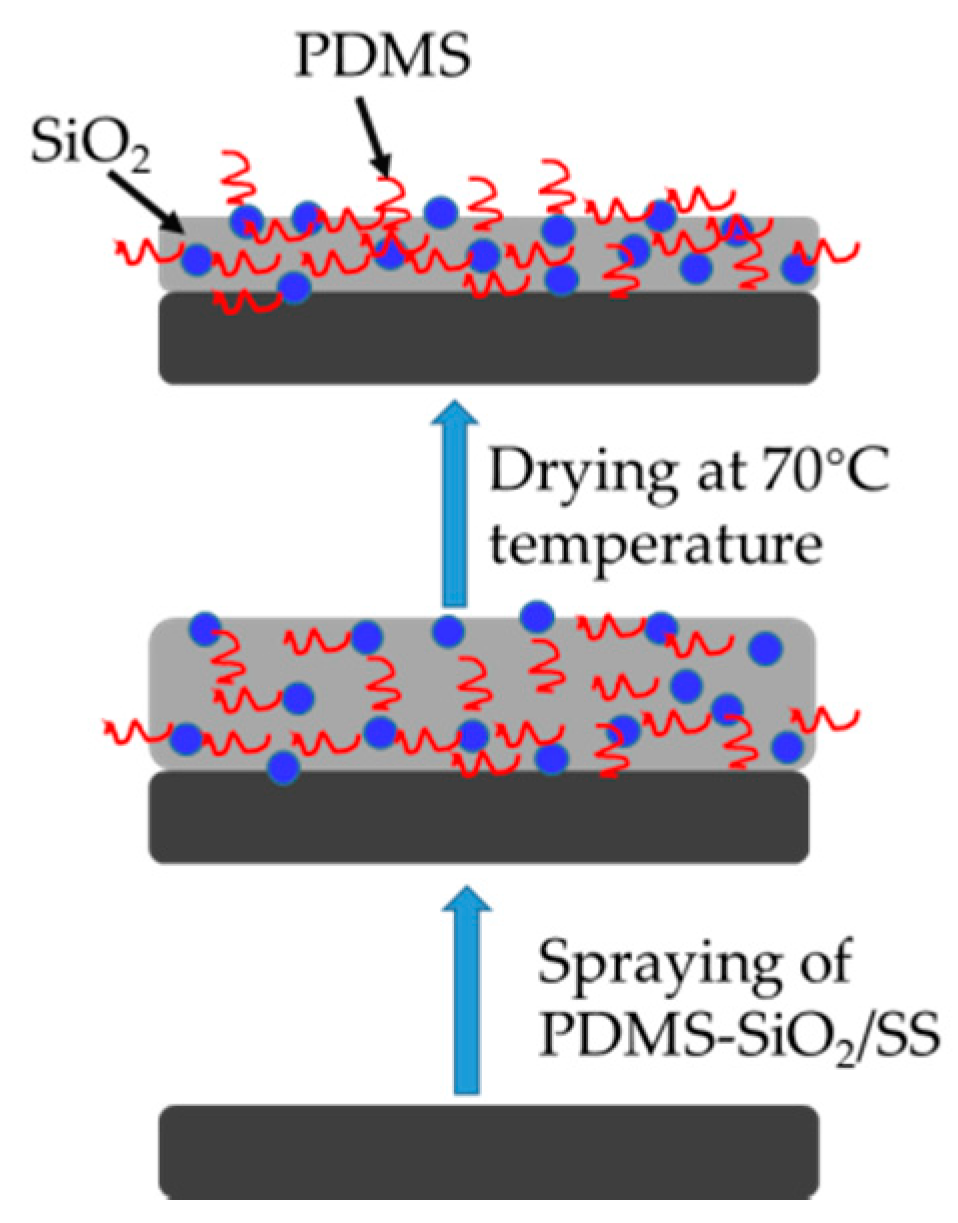
2.3. Synthesis of PDMS-SiO2/SS Hybrid Coating
2.4. Frosting Test
2.5. Characterization
3. Results and Discussion
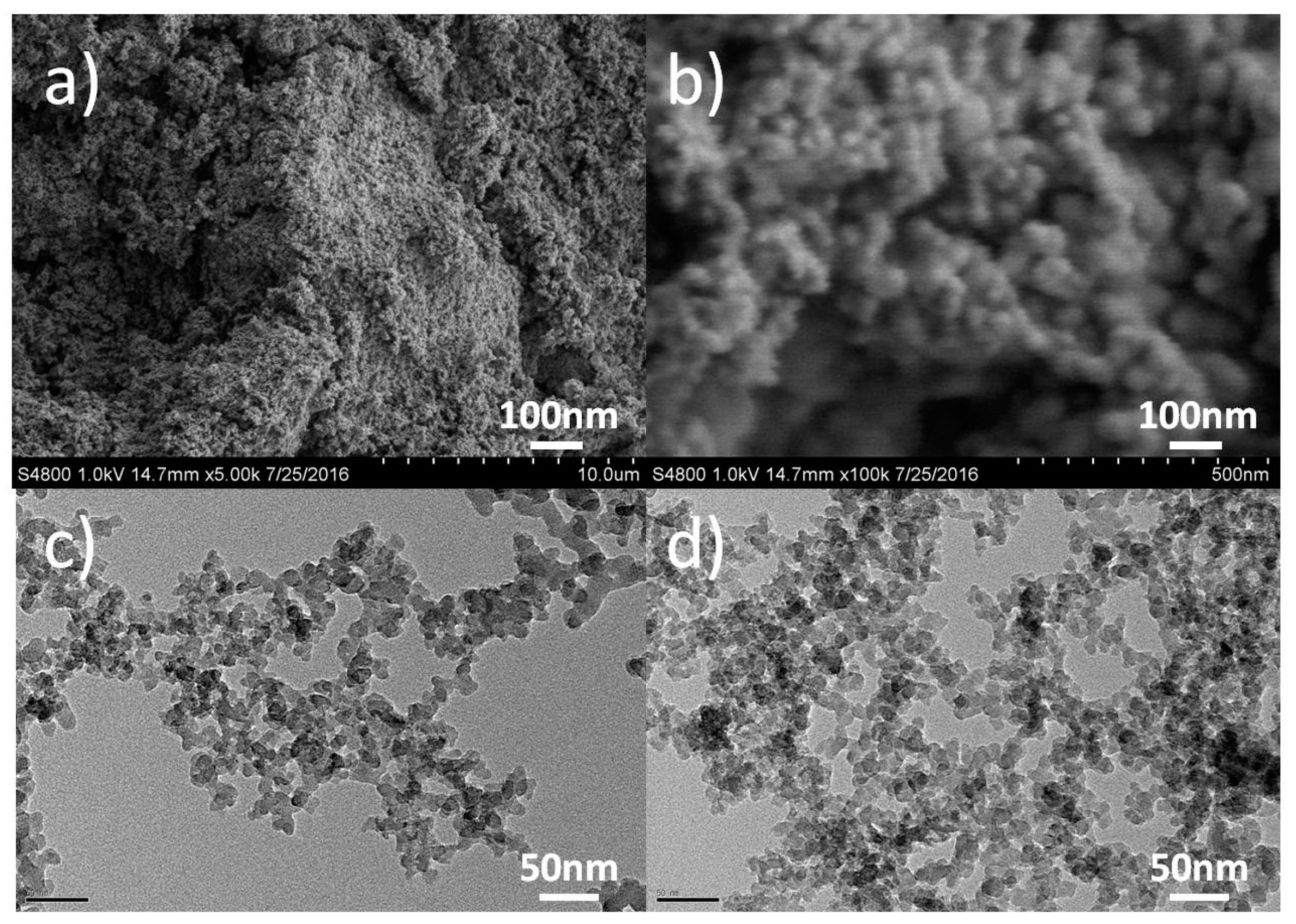
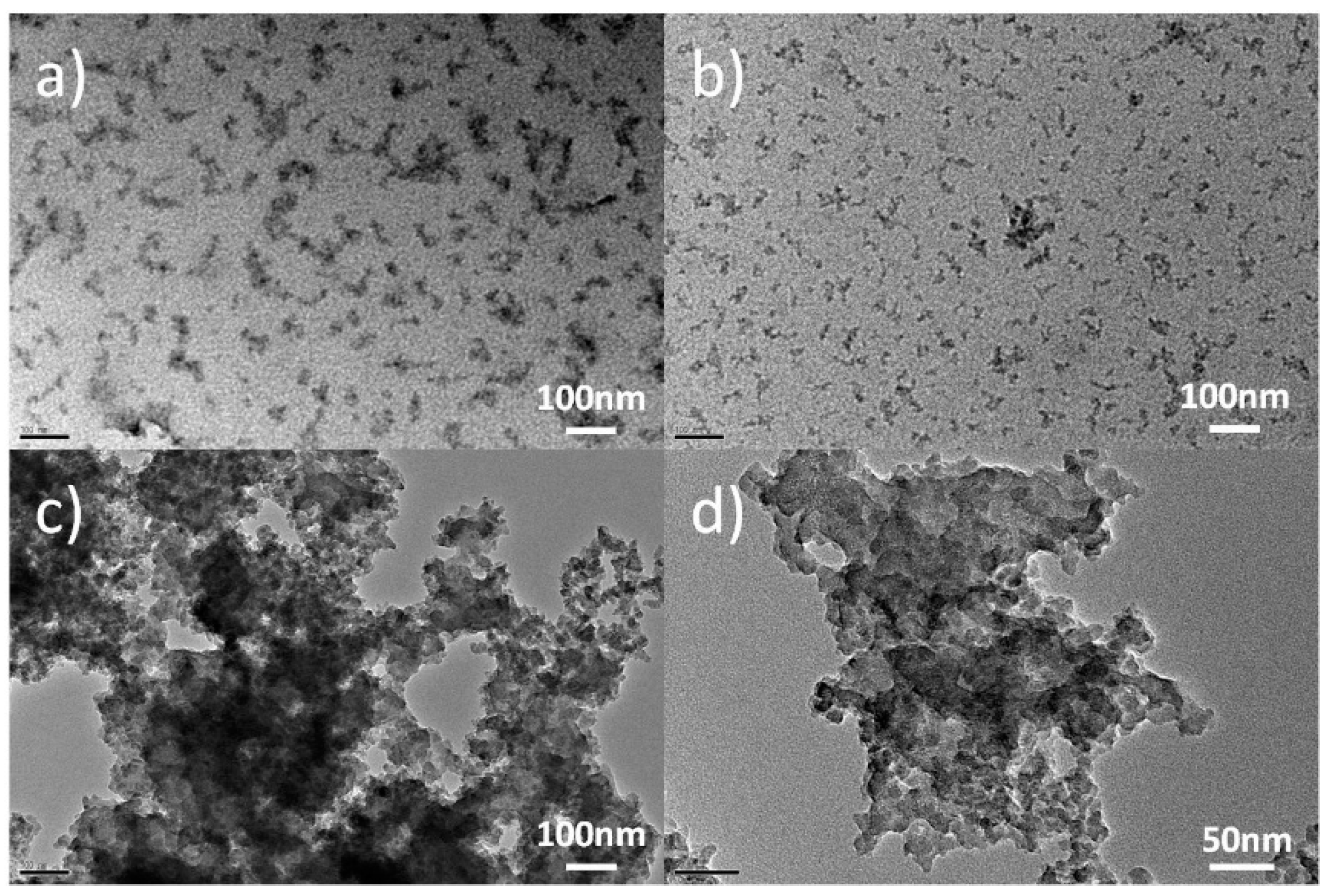
3.1. Surface Morphology and Compositions
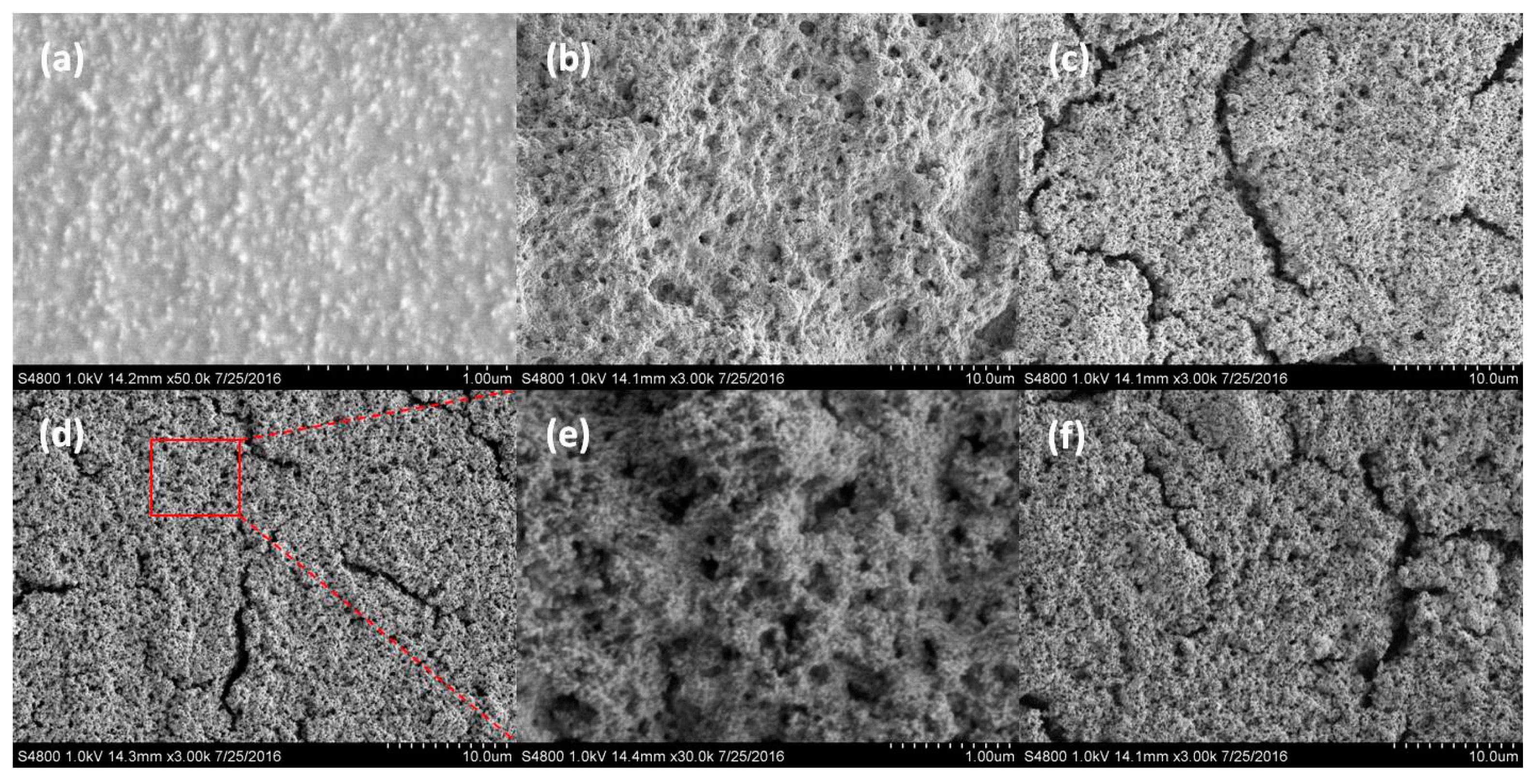
3.2. Surface Morphology and Wettability of PDMS-SiO2/SS Hybrid Coatings
3.3. Frosting Characteristics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, K.; Luo, S.X.; Yu, J.; Tang, Q.T.; Su, L.; Fang, Y.D. An experimental investigation on frosting characteristics of a microchannel outdoor heat exchanger in an air conditioning heat pump system for electric vehicles. Int. J. Energ. Res. 2020, 44, 7807–7819. [Google Scholar] [CrossRef]
- Laforte, J.L.; Allaire, M.A.; Laflamme, J. State-of-the-art on power line de-icing. Atmos. Res. 1998, 46, 143–158. [Google Scholar] [CrossRef]
- Bragg, M.B.; Heinrich, D.C.; Valarezo, W.O. Effect of under-wing frost on a transport aircraft airfoil at flight Reynolds number. J. Aircr. 1994, 31, 1372–1378. [Google Scholar] [CrossRef]
- Stone, H.A. Ice-Phobic surfaces that are wet. ACS Nano 2012, 6, 6536–6540. [Google Scholar] [CrossRef]
- Boutar, Y.; Naïmi, S.; Mezlini, S.; Silva, L.F.M.d.; Hamdaoui, M.; Ali, M.B.M. Effect of adhesive thickness and surface roughness on the shear strength of aluminium one-component polyurethane adhesive single-lap joints for automotive applications. J. Adhes. Sci. Technol. 2016, 30, 1913–1929. [Google Scholar] [CrossRef]
- Frankenstein, S.; Tuthill, A.M. Ice adhesion to locks and dams: Past work; future directions? J. Cold Reg. Eng. 2002, 16, 83–96. [Google Scholar] [CrossRef]
- Ayres, J.; Simendinger, W.H.; Balik, C.M. Characterization of titanium alkoxidesol-gel systems designed for anti-icing coatings. J. Coat. Technol. Res. 2007, 4, 463–471. [Google Scholar] [CrossRef]
- Kondepudi, S.N.; O’Neal, D.L. Effects of different fin configurations on the performance of finned-tube heat exchangers under frosting conditions. ASHRAE Trans. 1990, 96, 439–444. [Google Scholar]
- Adachi, K.; Saiki, K.; Sato, H. Suppression of frosting on a metal surface using ultrasonic vibrations. ICE Tech. Rep. Ultrason. 1998, 98, 9–12. [Google Scholar]
- Bharathidasan, T.; Vijay Kumar, S.; Bobji, M.S.; Chakradhar, R.P.S.; Basu, B.J. Effect of wettability and surface roughness on ice-adhesion strength of hydrophilic, hydrophobic, and superhydrophobic surfaces. Appl. Surf. Sci. 2014, 314, 241–250. [Google Scholar] [CrossRef]
- Highgate, D.; Knight, C.; Probert, S.D. Anomalous ‘Fresszing’ of water in hydrophilic polymeric structures. Appl. Energy 1989, 34, 243–259. [Google Scholar] [CrossRef]
- Okoroafor, E.U.; Newborough, M. Minimizing frost growth on cold surfaces exposed to humid air by means of crosslinked hydrophilic polymeric coatings. Appl. Therm. Eng. 2000, 20, 737–758. [Google Scholar] [CrossRef]
- Gong, J.Y.; Hou, J.Q.; Yang, L.W.; Wu, W.F.; Li, G.J.; Gao, T.Y. Mesoscopic investigation of frost crystal nucleation on cold surface based on the lattice-Boltzmann method. J. Mech. Sci. Technol. 2019, 33, 1925–1935. [Google Scholar] [CrossRef]
- Liang, C.H.; Wang, F.; Lü, Y.; Yang, M.T.; Zhang, X.S. Experimental and theoretical study of frost melting water retention on fin surfaces with different surface characteristics. Exp. Therm. Fluid Sci. 2016, 71, 70–76. [Google Scholar] [CrossRef]
- Li, L.T.; Wang, W.; Sun, Y.Y.; Feng, Y.C.; Lu, W.P.; Zhu, J.H.; Ge, Y.J. Investigation of defrosting water retention on the surface of evaporator impacting the performance of air source heat pump during periodic frosting-defrosting cycles. Appl. Energy 2014, 135, 98–107. [Google Scholar] [CrossRef]
- Moallem, E.; Cremaschi, L.; Fisher, D.E.; Padhmanabhan, S. Experimental measurements of the surface coating and water retention effects on frosting performance of microchannel heat exchangers for heat pump systems. Exp. Therm. Fluid Sci. 2012, 39, 176–188. [Google Scholar] [CrossRef]
- Wang, F.C.; Li, C.R.; Lv, Y.Z.; Lv, F.C.; Du, Y.F. Ice accretion on superhydrophobic aluminum surfaces under low-temperature conditions. Cold Reg. Sci. Technol. 2010, 62, 29–33. [Google Scholar] [CrossRef]
- Liu, Z.L.; Gou, Y.J.; Wang, J.T.; Cheng, S.Y. Frost formation on a super-hydrophobic surface under natural convection conditions. Int. J. Heat Mass Transf. 2008, 51, 5975–5982. [Google Scholar] [CrossRef]
- Zheng, J.F.; He, A.H.; Li, J.X.; Xu, J.; Han, C.C. Studies on the controlled morphology and wettability of polystyrene surfaces by electrospinning or electrospraying. Polymer 2006, 47, 7095–7102. [Google Scholar] [CrossRef]
- Xu, B.; Han, Q.; Chen, J.P.; Li, F.; Wang, N.J.; Li, D.; Pan, X.Y. Experimental investigation of frost and defrost performance of microchannel heat exchangers for heat pump systems. Appl. Engery 2013, 103, 180–188. [Google Scholar] [CrossRef]
- Xia, Y.; Zhong, Y.; Hrnjak, P.S.; Jacobi, A.M. Frost, defrost, and refrost and its impact on the air-side thermal-hydraulic performance of louvered-fin, flat-tube heat exchangers. Int. J. Refrig. 2006, 29, 1066–1079. [Google Scholar] [CrossRef]
- Padhmanabhan, S.; Cremaschi, L.; Fisher, D. Comparison of frost and defrost performance between microchannel coil and fin-and-tube coil for heat pump systems. Int. J. Air-Cond. Refrig. 2011, 19, 273–284. [Google Scholar] [CrossRef]
- Zhong, Y.F.; Joardar, A.; Gu, Z.P.; Park, Y.-G.; Jacobi, A.M. Dynamic dip testing as a method to assess the condensate drainage behavior from the air-side surface of compact heat exchangers. Exp. Therm. Fluid Sci. 2005, 29, 957–970. [Google Scholar] [CrossRef]
- Joardar, A.; Gu, Z.; Jacobi, A.M. Off-Cycle Condensate Drainage Behavior of Compact Heat Exchangers: Assessment and Enhancement. In Proceedings of the International Refrigeration and Air Conditioning Conference at Purdue, West Lafayette, IN, USA, 17–20 July 2006. [Google Scholar]
- Liu, L.P.; Jacobi, A.M. Issues affecting the reliability of dynamic dip testing as a method to assess the condensate drainage behavior from the air-side surface of dehumidifying heat exchangers. Exp. Therm. Fluid Sci. 2008, 32, 1512–1522. [Google Scholar] [CrossRef]
- Boreyko, J.B.; Srijanto, B.R.; Nguyen, T.D.; Vega, C.; Fuentes-Cabrera, M.; Collier, C.P. Dynamic defrosting on nanostructured superhydrophobic Surfaces. Langmuir 2013, 29, 9516–9524. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.L.; Jones, A.K.; Sikka, V.K.; Wu, J.Z.; Gao, D. Anti-icing superhydrophobic coatings. Langmuir 2009, 25, 12444–12448. [Google Scholar] [CrossRef] [PubMed]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Cassie, A.B.D. Contact angles. Discuss. Faraday Soc. 1948, 3, 11–16. [Google Scholar] [CrossRef]
- Hobbs, P.V.; Rangno, A.L. Ice particle concentrations in clouds. J. Atmos. Sci. 1985, 42, 2523–2549. [Google Scholar] [CrossRef]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Trans. Faraday Soc. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Wenzel, R.N. Surface roughness and contact angle. J. Phys. Colloid Chem. 1949, 53, 1466–1467. [Google Scholar] [CrossRef]
- Yoshimura, Y.; Fukiba, K.; Sato, S. Heat transfer improvement of a cooling tube using a splitter plate under frosting condition. Trans. JSRAE 2016, 33, 221–230. [Google Scholar]
- Miljkovic, N.; Wang, E.N. Condensation heat transfer on superhydrophobic surfaces. MRS Bull. Mater. Res. Soc. 2013, 38, 397–406. [Google Scholar] [CrossRef]
- Wang, L.; Gong, Q.H.; Zhan, S.H.; Jiang, L.; Zheng, Y.M. Robust anti-icing performance of a flexible superhydrophobic surface. Adv. Mater. 2016, 28, 7729–7735. [Google Scholar] [CrossRef]
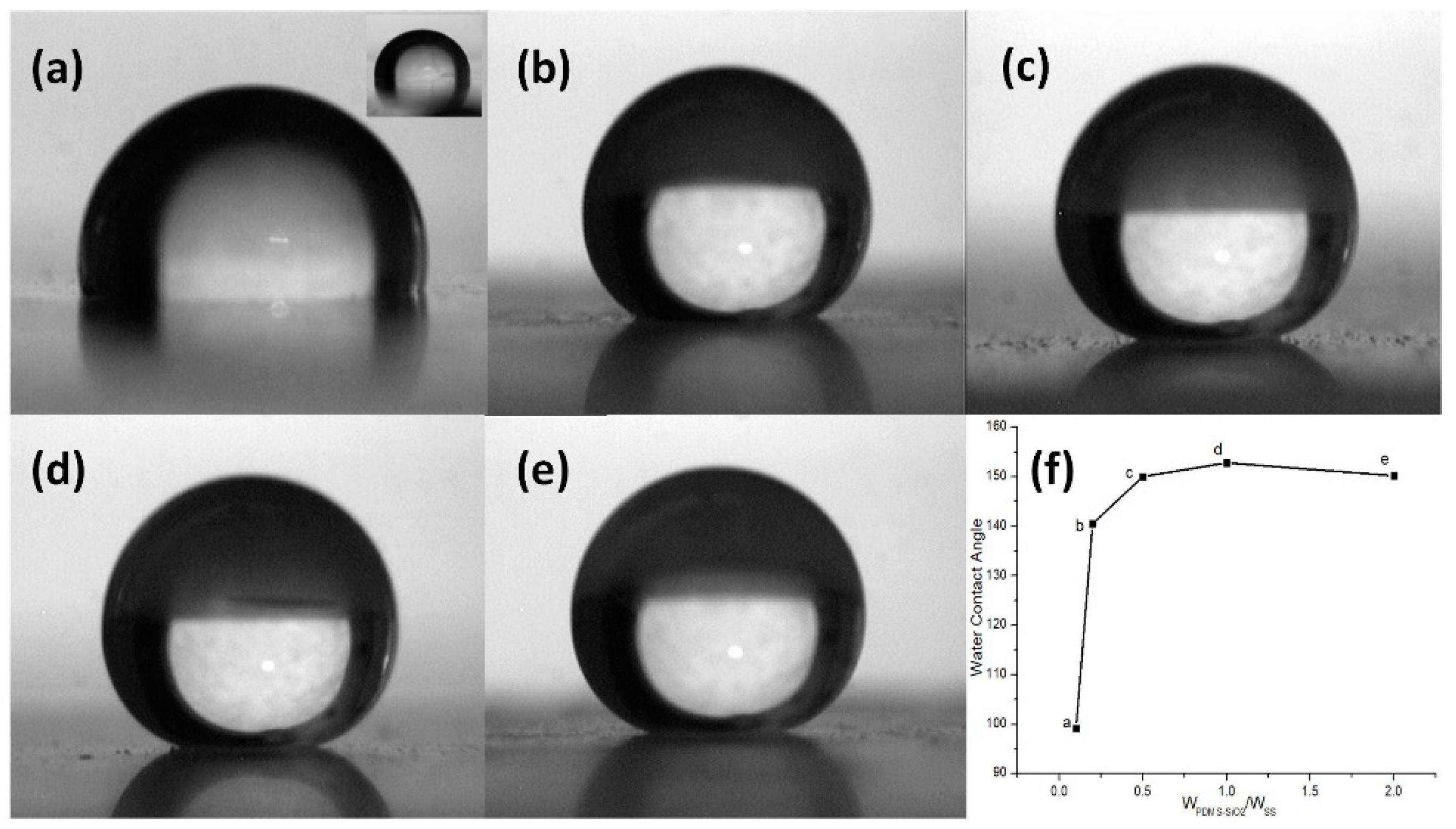
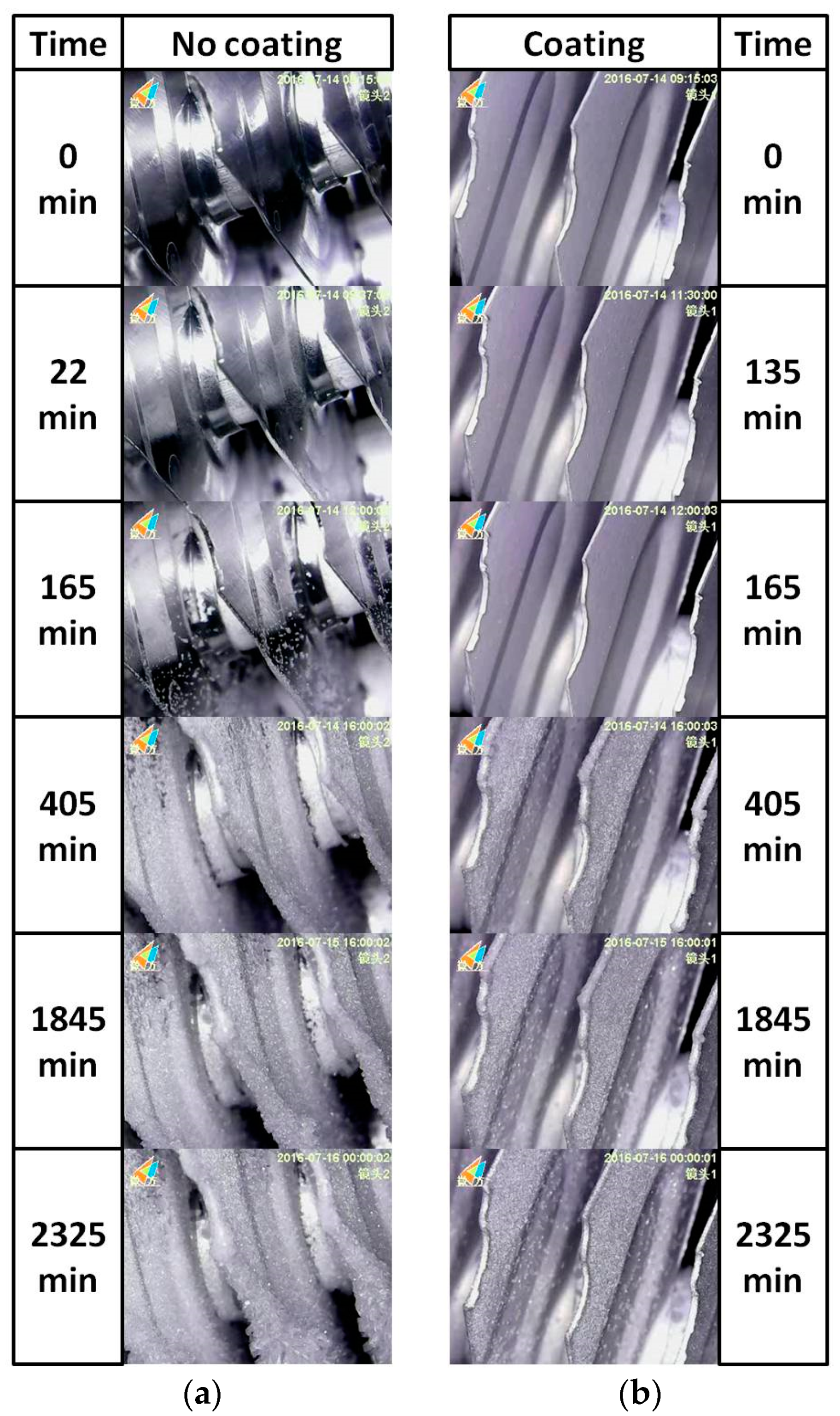
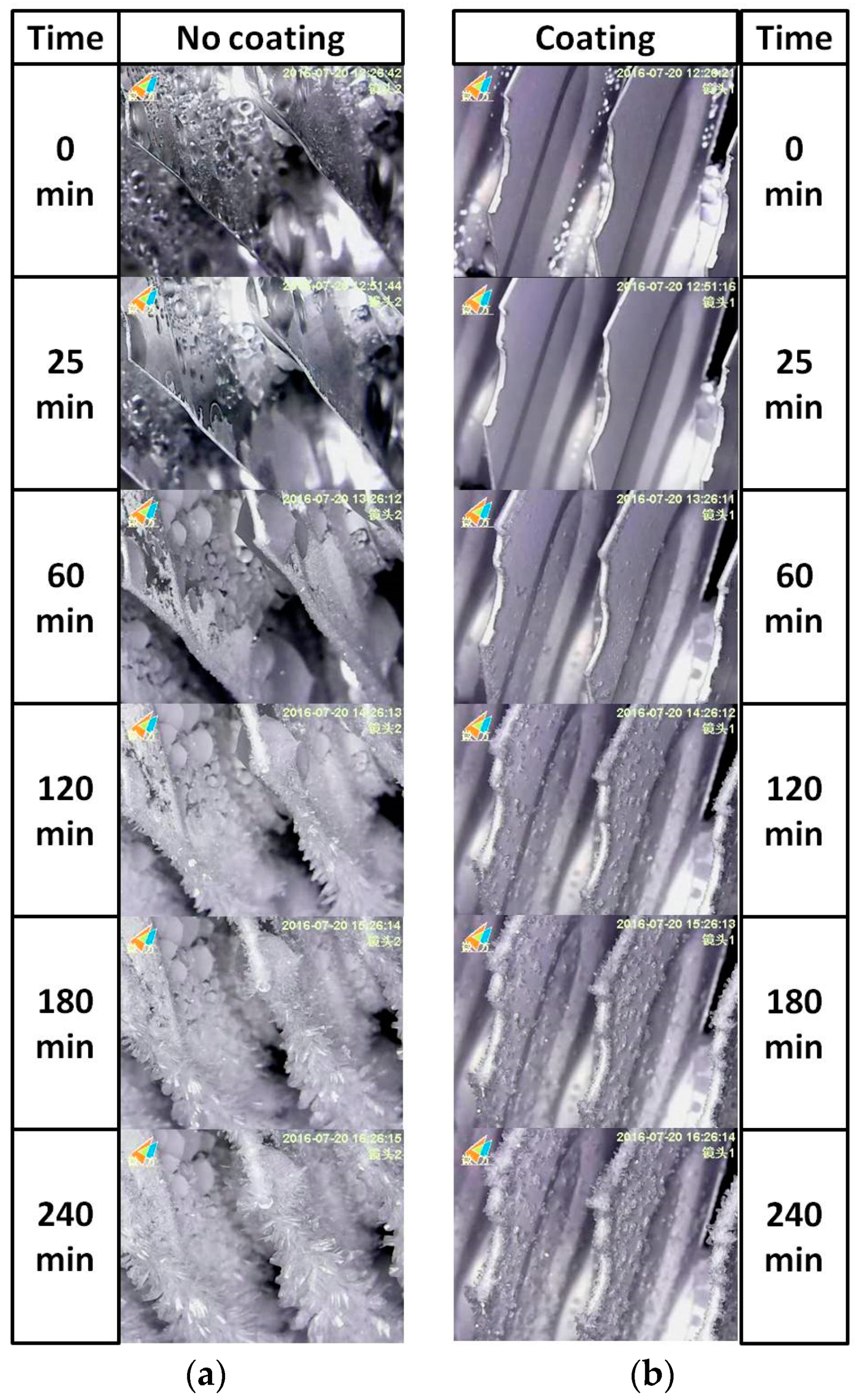
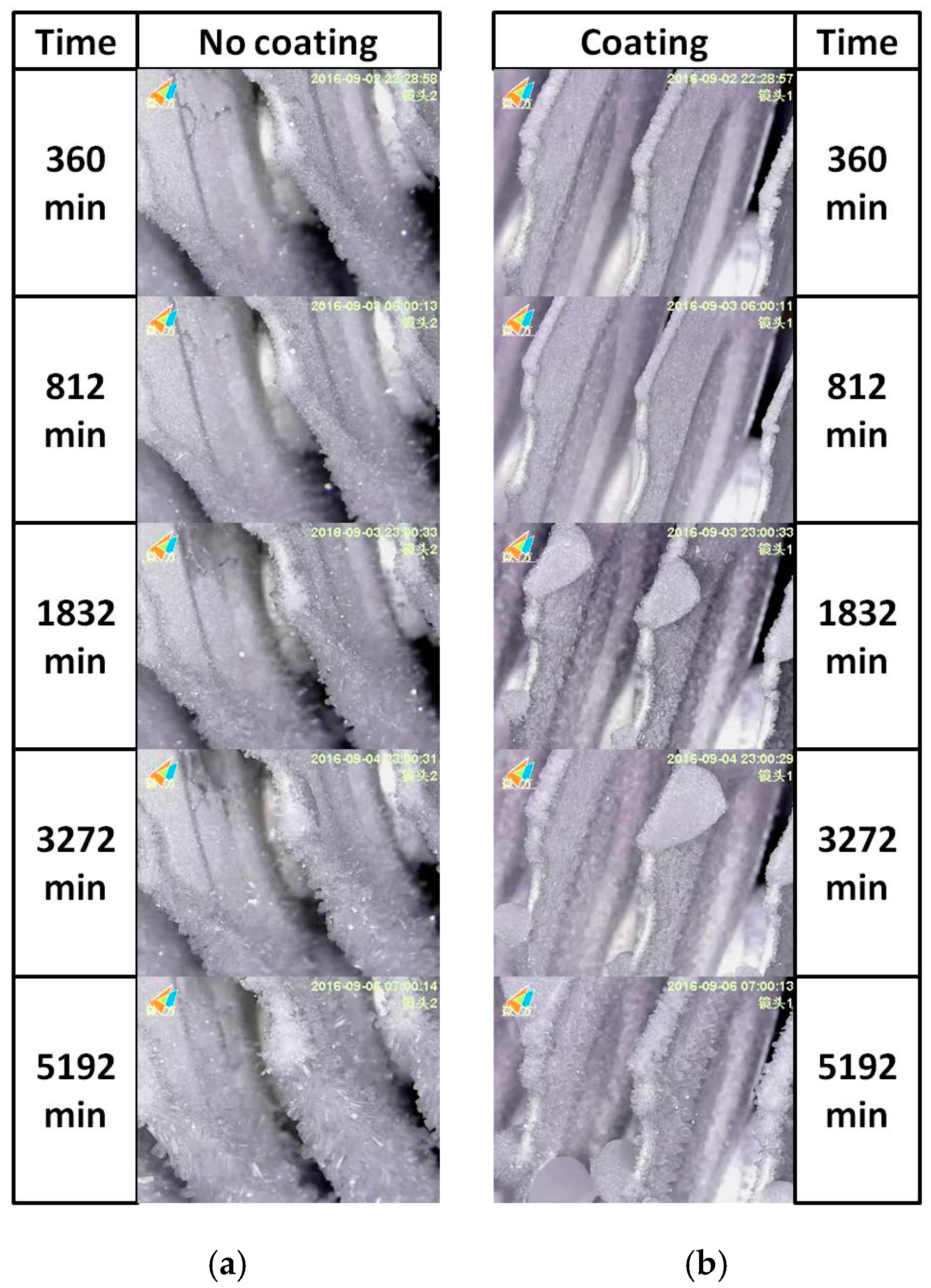
| Sample Designation | Atomic Percentage (%) | Contact Angle (°) | PDMS-SiO2:SS (wt %) | ||||
|---|---|---|---|---|---|---|---|
| C | O | Si | Na | Al | |||
| PDMS-SiO2 | 26.78 | 55.90 | 17.32 | – | – | – | – |
| PDMS-SiO2/SS-1 | 30.38 | 51.21 | 14.59 | 2.18 | 1.64 | 152.82 | 1:1 |
| PDMS-SiO2/SS-2 | 31.28 | 38.45 | 29.50 | – | 0.77 | 150.22 | 2:1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, L.; Sun, J.; Li, X.; Zhang, X.; Chen, L.; Tian, X. Preparation and Anti-frost Performance of PDMS-SiO2/SS Superhydrophobic Coating. Coatings 2020, 10, 1051. https://doi.org/10.3390/coatings10111051
Jia L, Sun J, Li X, Zhang X, Chen L, Tian X. Preparation and Anti-frost Performance of PDMS-SiO2/SS Superhydrophobic Coating. Coatings. 2020; 10(11):1051. https://doi.org/10.3390/coatings10111051
Chicago/Turabian StyleJia, Li, Jun Sun, Xiaoxiao Li, Xian Zhang, Lin Chen, and Xinyou Tian. 2020. "Preparation and Anti-frost Performance of PDMS-SiO2/SS Superhydrophobic Coating" Coatings 10, no. 11: 1051. https://doi.org/10.3390/coatings10111051
APA StyleJia, L., Sun, J., Li, X., Zhang, X., Chen, L., & Tian, X. (2020). Preparation and Anti-frost Performance of PDMS-SiO2/SS Superhydrophobic Coating. Coatings, 10(11), 1051. https://doi.org/10.3390/coatings10111051






