Microfibrillated Cellulose Based Barrier Coatings for Abrasive Paper Products
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Coating Formulations
2.3. Rheology Measurements
2.4. Coating Application
2.5. Coating Characterization
2.5.1. Coat Weight, Air Permeance, and Surface Roughness Measurements
2.5.2. Water Contact Angle Measurements
2.5.3. Oil Cobb Test with Special Monomer
2.5.4. KIT Test
2.5.5. Oil Drop Test
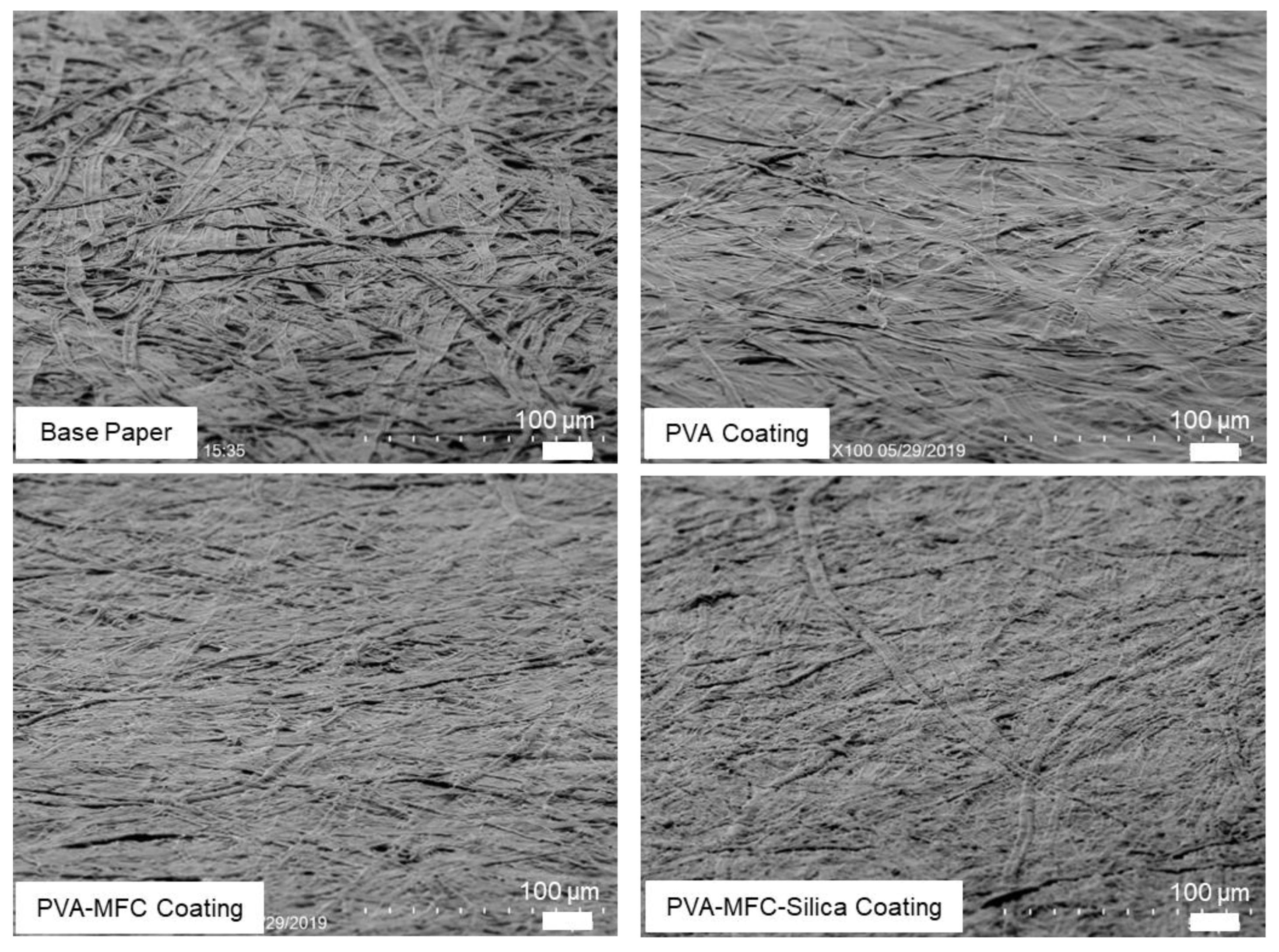
2.5.6. Scanning Electron Microscopy (SEM)
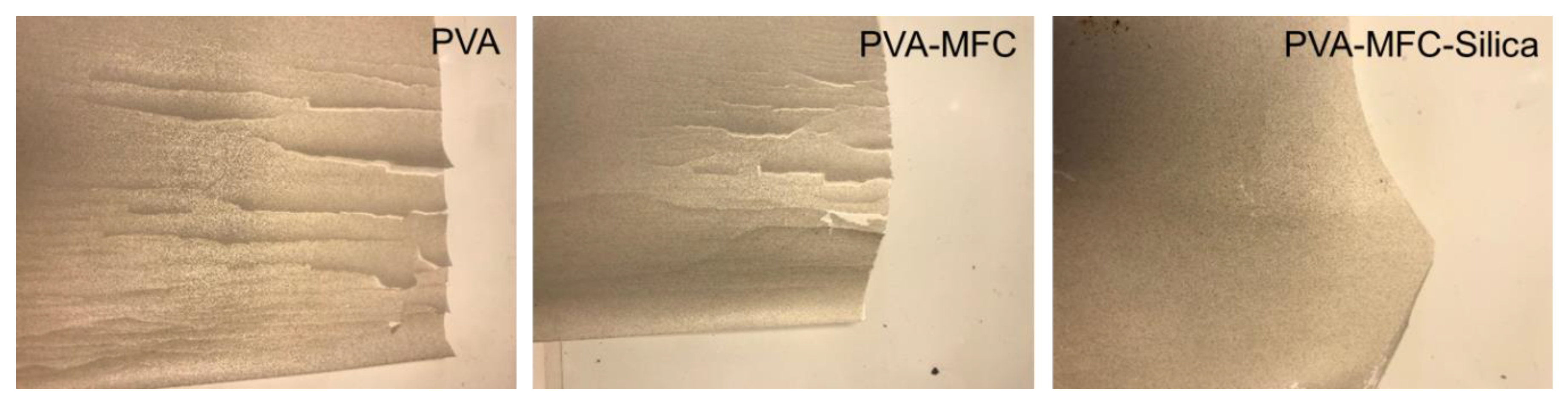
2.5.7. Adhesion Tests
3. Results and Discussion
3.1. Rheology and Coat Weights
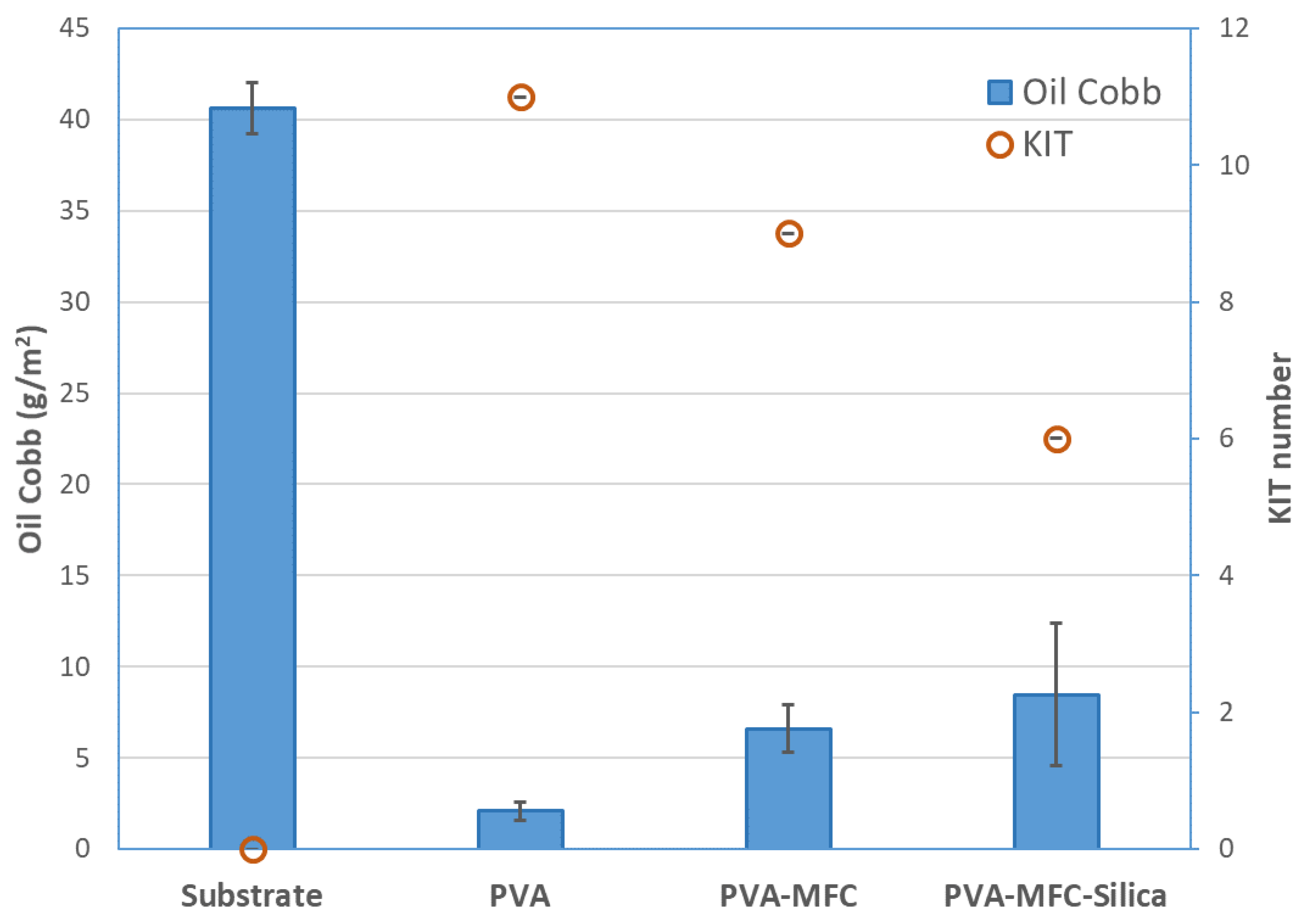
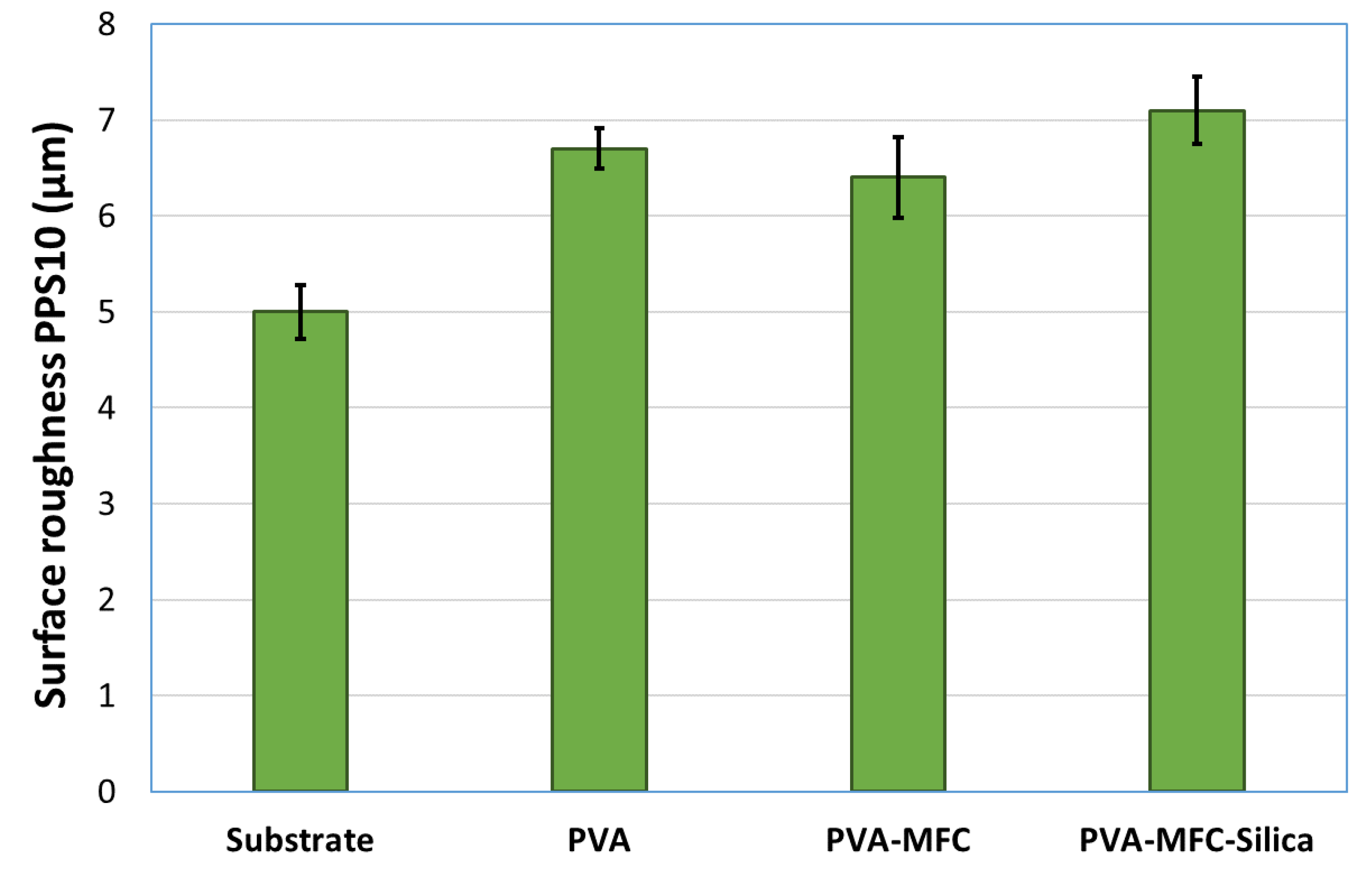
3.2. Barrier Performance
3.3. Adhesion Performance
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cohen, M.A.; Kronzer, F.J. Substrate for Supporting Abrasive Grit Adhesives, Method for Forming Same and Abrasive Material. European Patent No. EP0237784B1, 23 September 1987. [Google Scholar]
- Lindquist, G.M.; Boyak, S.M. Fine Abrasive Paper Backing Material and Method of Making Thereof. WIPO (PCT). Patent No. WO2006073795A2, 13 July 2006. [Google Scholar]
- Knop, A.; Pilato, L.A. Phenolic Resins: Chemistry, Applications and Performance; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Kronzer, F.J. Substrate Having a Thermoplastic Binder Coating for Use in Fabricating Abrasive Sheets and Abrasive Sheets Manufactured Therewith. U.S. Patent No. US4240807A, 23 December 1980. [Google Scholar]
- Azeredo, H.M.; Rosa, M.F.; Mattoso, L.H.C. Nanocellulose in bio-based food packaging applications. Ind. Crop. Prod. 2017, 97, 664–671. [Google Scholar] [CrossRef]
- Rastogi, V.K.; Samyn, P. Bio-Based Coatings for Paper Applications. Coatings 2015, 5, 887–930. [Google Scholar] [CrossRef]
- Hubbe, M.A.; Ferrer, A.; Tyagi, P.; Yin, Y.; Salas, C.; Pal, L.; Rojas, O.J. Nanocellulose in Thin Films, Coatings, and Plies for Packaging Applications: A Review. Bioresource 2017, 12, 2143–2233. [Google Scholar] [CrossRef]
- Kumar, V.; Elfving, A.; Koivula, H.M.; Bousfield, D.W.; Toivakka, M. Roll-to-Roll Processed Cellulose Nanofiber Coatings. Ind. Eng. Chem. Res. 2016, 55, 3603–3613. [Google Scholar] [CrossRef]
- Aulin, C.; Gällstedt, M.; Lindström, T. Oxygen and oil barrier properties of microfibrillated cellulose films and coatings. Cellulose 2010, 17, 559–574. [Google Scholar] [CrossRef]
- Syverud, K.; Stenius, P. Strength and barrier properties of MFC films. Cellulose 2008, 16, 75–85. [Google Scholar] [CrossRef]
- Kumar, V.; Nazari, B.; Bousfield, D.; Toivakka, M. Rheology of Microfibrillated Cellulose Suspensions in Pressure-Driven Flow. Appl. Rheol. 2016, 26. [Google Scholar] [CrossRef]
- Nazari, B.; Kumar, V.; Bousfield, D.W.; Toivakka, M. Rheology of cellulose nanofibers suspensions: Boundary driven flow. J. Rheol. 2016, 60, 1151–1159. [Google Scholar] [CrossRef]
- Ottesen, V.; Kumar, V.; Toivakka, M.; Carrasco, G.C.; Syverud, K.; Gregersen, Ø.W. Viability and properties of roll-to-roll coating of cellulose nanofibrils on recycled paperboard. Nord. Pulp Pap. Res. J. 2017, 32, 2. [Google Scholar] [CrossRef]
- Kumar, V.; Ottesen, V.; Syverud, K.; Gregersen, Ø.W.; Toivakka, M. Coatability of Cellulose Nanofibril Suspensions: Role of Rheology and Water Retention. BioResources 2017, 12. [Google Scholar] [CrossRef]
- Molki, B.; Behzad, T.; Aframehr, W.M.; Nasri-Nasrabadi, B.; Bahrami, B.; Ahmadi, M.; Komeily-Nia, Z.; Bagheri, R. Properties investigation of polyvinyl alcohol barrier films reinforced by calcium carbonate nanoparticles. Mater. Res. Express 2019, 6, 055311. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Mohan, D.J.; Kang, I.-A.; Doh, G.-H.; Lee, S.; Han, S.O. Nanocellulose reinforced PVA composite films: Effects of acid treatment and filler loading. Fibers Polym. 2009, 10, 77–82. [Google Scholar] [CrossRef]
- Panaitescu, D.M.; Frone, A.N.; Ghiurea, M.; Chiulan, I. Influence of storage conditions on starch/PVA films containing cellulose nanofibers. Ind. Crop. Prod. 2015, 70, 170–177. [Google Scholar] [CrossRef]
- Uchida, T.; Iwaguro, F.; Yanai, R.; Dodo, H. Preparation of cellulose nanofibers coated with poly(vinyl alcohol) crystals and their application in composite films. RSC Adv. 2017, 7, 19828–19832. [Google Scholar] [CrossRef]
- Huang, J.; Lyu, S.; Fu, F.; Wu, Y.; Wang, S. Green preparation of a cellulose nanocrystals/polyvinyl alcohol composite superhydrophobic coating. RSC Adv. 2017, 7, 20152–20159. [Google Scholar] [CrossRef]
- Virtanen, S.; Vartianen, J.; Setälä, H.; Tammelin, T.; Vuoti, S. Modified nanofibrillated cellulose–polyvinyl alcohol films with improved mechanical performance. RSC Adv. 2014, 4, 11343–11350. [Google Scholar] [CrossRef]
- Baheti, V.; Militký, J. Reinforcement of wet milled jute nano/micro particles in polyvinyl alcohol films. Fibers Polym. 2013, 14, 133–137. [Google Scholar] [CrossRef]
- Bai, H.; Li, Y.; Wang, W.; Chen, G.; Rojas, O.J.; Dong, W.; Liu, X. Interpenetrated polymer networks in composites with poly(vinyl alcohol), micro- and nano-fibrillated cellulose (M/NFC) and polyHEMA to develop packaging materials. Cellulose 2015, 22, 3877–3894. [Google Scholar] [CrossRef]
- Cheng, Q.; Wang, S.; Rials, T.G. Poly(vinyl alcohol) nanocomposites reinforced with cellulose fibrils isolated by high intensity ultrasonication. Compos. Part A Appl. Sci. Manuf. 2009, 40, 218–224. [Google Scholar] [CrossRef]
- Hu, D.; Wang, L. Physical and antibacterial properties of polyvinyl alcohol films reinforced with quaternized cellulose. J. Appl. Polym. Sci. 2016, 133, 43552. [Google Scholar] [CrossRef]
- Pereira, A.L.S.; Nascimento, D.M.D.; Filho, M.D.S.; Morais, J.; Vasconcelos, N.F.; Feitosa, J.P.D.A.; Brígida, A.; Rosa, M.D.F. Improvement of polyvinyl alcohol properties by adding nanocrystalline cellulose isolated from banana pseudostems. Carbohydr. Polym. 2014, 112, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Heiskanen, I.; Backfolk, K.; Axrup, L. A Coated Substrate, a Process for Production of a Coated Substrate, a Package and a Dispersion Coating. WIPO (PCT). Patent No. WO2011056130A1, 12 May 2011. [Google Scholar]
- Guezennec, C. Développement de Nouveaux Matériaux d’ Emballage à Partir de Micro- et Nano- Fibrilles de Cellulose Development of New Packaging Materials Based on Micro- and Nano-Fibrillated Cellulose; Université de Grenoble: Saint-Martin-d’Hères, France, 2012. [Google Scholar]
- Hamada, H.; Beckvermit, J.; Bousfield, D.W. Nanofibrillated Cellulose with Fine Clay as a Coating Agent to Improve Print Quality. In TAPPI Papercon; TAPPI Press: Atlanta, GA, USA, 2010. [Google Scholar]
- TAPPI. Grammage of Paper and Paperboard (Weight per Unit Area). Available online: https://www.tappi.org/content/tag/sarg/t410.pdf (accessed on 19 November 2020).
- TAPPI. Air Permeance of Paper and Paperboard. Available online: https://www.complianceonline.com/images/supportpages/501050/sample_T547.pdf (accessed on 19 November 2020).
- TAPPI. Roughness of Paper and Paperboard (Print-Surf Method). Tappi Stand. T 555 om-15; TAPPI Press: Atlanta, GA, USA, 2015. [Google Scholar]



| Formulation | PVA | PVA-MFC | PVA-MFC-Silica |
|---|---|---|---|
| Weight ratio | 100 | 90:10 | (90:10)70:30 |
| Solids content (%) | 10 | 10 | 10 |
| Sample | Reference | Substrate | PVA | PVA-MFC | PVA-MFC-Silica |
|---|---|---|---|---|---|
| Coat weight (g/m2) | 8–10 | - | 4 | 2.5 | 3 |
| Air permeance (µm/Pa·s) | 0.003 * | 1.69 ± 0.09 | 0.003 * | 0.003 * | 0.006 ± 0.002 |
| Surface roughness (µm) | 5.5 ± 0.3 | 5 ± 0.3 | 6.7 ± 0.2 | 6.4 ± 0.4 | 7.1 ± 0.3 |
| Oil Cobb (g/m2) | 3.8 ± 2 | 40.6 ± 1.4 | 2.1 ± 0.5 | 6.6 ± 1.3 | 8.5 ± 3.9 |
| KIT number | 9 | 0 | 11 | 9 | 6 |
| Sample | Water Contact Angle at 0.5 s | Adhesion to the Next Layer |
|---|---|---|
| (°) | (Qualitative Estimate) | |
| Substrate | 112 | - |
| PVA | 58 | Poor/No adhesion |
| PVA-MFC | 46 | Poor |
| PVA-MFC-Silica | 55 | Good |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, V.; Kenttä, E.; Andersson, P.; Forsström, U. Microfibrillated Cellulose Based Barrier Coatings for Abrasive Paper Products. Coatings 2020, 10, 1108. https://doi.org/10.3390/coatings10111108
Kumar V, Kenttä E, Andersson P, Forsström U. Microfibrillated Cellulose Based Barrier Coatings for Abrasive Paper Products. Coatings. 2020; 10(11):1108. https://doi.org/10.3390/coatings10111108
Chicago/Turabian StyleKumar, Vinay, Eija Kenttä, Petter Andersson, and Ulla Forsström. 2020. "Microfibrillated Cellulose Based Barrier Coatings for Abrasive Paper Products" Coatings 10, no. 11: 1108. https://doi.org/10.3390/coatings10111108
APA StyleKumar, V., Kenttä, E., Andersson, P., & Forsström, U. (2020). Microfibrillated Cellulose Based Barrier Coatings for Abrasive Paper Products. Coatings, 10(11), 1108. https://doi.org/10.3390/coatings10111108





