Colored Microbial Coatings in Show Caves from the Galapagos Islands (Ecuador): First Microbiological Approach
Abstract
:1. Introduction
2. Materials and Methods
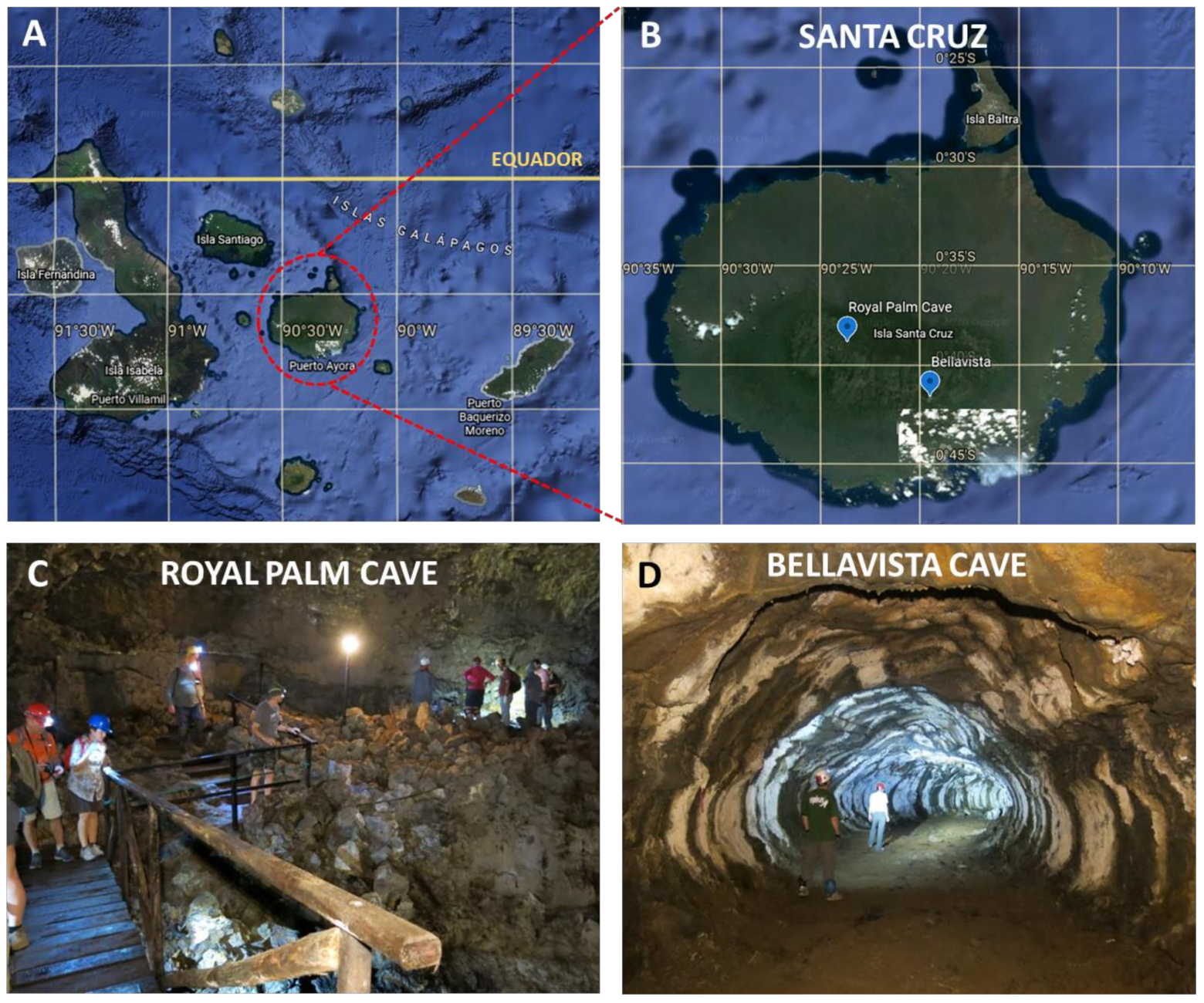
2.1. Studied Site and Sampling
2.2. Field Emission Scanning Electron Microscopy
2.3. Molecular Analysis of Microbial Communities
3. Results and Discussion
3.1. Microscopy Observations
3.2. Microbial Communities
- (i)
- Methanotrophs/methylotrophs
- (ii)
- Predatory bacteria
- (iii)
- Plant/soil bacteria
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Galápagos Islands—UNESCO World Heritage Centre. Available online: https://whc.unesco.org/en/list/1/ (accessed on 15 October 2020).
- Satre, D. Using the Ultimate Natural Laboratory to Teach Evolution, Natural Selection and Biodiversity. Procedia Soc. Behav. Sci. 2015, 177, 289–294. [Google Scholar] [CrossRef] [Green Version]
- Elias, S.A. Galápagos Island Biodiversity. In Encyclopedia of the World’s Biomes; Elsevier: Amsterdam, The Netherlands, 2020; pp. 198–216. [Google Scholar]
- Carvajal Barriga, E.J.; Barahona, P.P.; Tufiño, C.; Bastidas, B.; Guamán-Burneo, C.; Freitas, L.; Rosa, C. An Overview of the Yeast Biodiversity in the Galápagos Islands and Other Ecuadorian Regions. In Biodiversity —The Dynamic Balance of the Planet; InTech: London, UK, 2014. [Google Scholar]
- Dal Forno, M.; Bungartz, F.; Yánez-Ayabaca, A.; Lücking, R.; Lawrey, J.D. High levels of endemism among Galapagos basidiolichens. Fungal Divers. 2017, 85, 45–73. [Google Scholar] [CrossRef]
- Mayhew, L.E.; Geist, D.J.; Childers, S.; Pierson, J. Microbial community comparisons as a function of the physical and geochemical conditions of Galápagos Island fumaroles. Geomicrobiol. J. 2007, 24, 615–625. [Google Scholar] [CrossRef]
- Campoverde, N.C.G.; Hassenrück, C.; Buttigieg, P.L.; Gärdes, A.; Carolina, N.; Campoverde, G.; Buttigieg, P.L.; Gärdes, A. Characterization of bacterioplankton communities and quantification of organic carbon pools off the Galapagos Archipelago under contrasting environmental conditions. PeerJ 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.J.; Addison, A.; Toulkeridis, T. Biological potential of under-studied cave fauna of the Galapagos Islands. Rev. Geoespacial 2011, 8, 13–22. [Google Scholar]
- Constantin, S.; Toulkeridis, T.; Moldovan, O.T.; Villacís, M.; Addison, A. Caves and karst of Ecuador–state-of-the-art and research perspectives. Phys. Geogr. 2019, 40, 28–51. [Google Scholar] [CrossRef]
- Gallardo, G.; Toulkeridis, T. Volcanic Caves and Other Speleological Attractions. Santa Cruz, Galápagos; San Francisco University Press: San Francisco, CA, USA, 2008. [Google Scholar]
- Simkin, T. Geology of Galapagos. Biol. J. Linn. Soc. 1984, 21, 61–75. [Google Scholar] [CrossRef]
- Northup, D.E.; Melim, L.A.; Spilde, M.N.; Hathaway, J.J.M.; Garcia, M.G.; Moya, M.; Stone, F.D.; Boston, P.J.; Dapkevicius, M.L.N.E.; Riquelme, C. Lava cave microbial communities within mats and secondary mineral deposits: Implications for life detection on other planets. Astrobiology 2011, 11, 601–618. [Google Scholar] [CrossRef] [Green Version]
- Riquelme, C.; Hathaway, J.J.M.; Dapkevicius, M.L.N.E.; Miller, A.Z.; Kooser, A.; Northup, D.E.; Jurado, V.; Fernandez, O.; Saiz-Jimenez, C.; Cheeptham, N. Actinobacterial diversity in volcanic caves and associated geomicrobiological interactions. Front. Microbiol. 2015, 6. [Google Scholar] [CrossRef] [Green Version]
- Lavoie, K.H.; Winter, A.S.; Read, K.J.H.; Hughes, E.M.; Spilde, M.N.; Northup, D.E. Comparison of bacterial communities from lava cave microbial mats to overlying surface soils from Lava Beds National Monument, USA. PLoS ONE 2017, 12, e0169339. [Google Scholar] [CrossRef]
- Gonzalez-Pimentel, J.L.; Miller, A.Z.; Jurado, V.; Laiz, L.; Pereira, M.F.C.; Saiz-Jimenez, C. Yellow coloured mats from lava tubes of La Palma (Canary Islands, Spain) are dominated by metabolically active Actinobacteria. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, A.Z.; Pereira, M.F.C.; Calaforra, J.M.; Forti, P.; Dionísio, A.; Saiz-Jimenez, C. Siliceous Speleothems and Associated Microbe-Mineral Interactions from Ana Heva Lava Tube in Easter Island (Chile). Geomicrobiol. J. 2014, 31, 236–245. [Google Scholar] [CrossRef] [Green Version]
- Hathaway, J.J.M.; Garcia, M.G.; Balasch, M.M.; Spilde, M.N.; Stone, F.D.; Dapkevicius, M.D.L.N.E.; Amorim, I.R.; Gabriel, R.; Borges, P.A.V.; Northup, D.E. Comparison of Bacterial Diversity in Azorean and Hawai’ian Lava Cave Microbial Mats. Geomicrobiol. J. 2014, 31, 205–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riquelme, C.; de Dapkevicius, M.L.E.; Miller, A.Z.; Charlop-Powers, Z.; Brady, S.; Mason, C.; Cheeptham, N. Biotechnological potential of Actinobacteria from Canadian and Azorean volcanic caves. Appl. Microbiol. Biotechnol. 2017, 101, 843–857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antić, A.; Peppoloni, S.; Di Capua, G. Applying the Values of Geoethics for Sustainable Speleotourism Development. Geoheritage 2020, 12, 1–9. [Google Scholar] [CrossRef]
- Léveillé, R.J.; Datta, S. Lava tubes and basaltic caves as astrobiological targets on Earth and Mars: A review. Planet. Space Sci. 2010, 58, 592–598. [Google Scholar] [CrossRef]
- Lane, D. 16S/23S rRNA sequencing. In Nucleic Acid Techniques in Bacterial Systematic; Stackenbrandt, E., Goodfellow, M., Eds.; John Wiley: Chichester, UK, 1991; pp. 115–175. [Google Scholar]
- Weisburg, W.G.; Barns, S.M.; Pelletier, D.A.; Lane, D.J. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 1991, 173, 697–703. [Google Scholar] [CrossRef] [Green Version]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [Green Version]
- Hill, C.; Forti, P. Cave Minerals of the World; National Speleological Society: Huntsville, Al, USA, 1997; ISBN 1-879961-07-5. [Google Scholar]
- Melim, L.A.; Northup, D.E.; Spilde, M.N.; Jones, B.; Boston, P.J.; Bixby, R.J. Reticulated filaments in cave pool speleothems: Microbe or mineral? J. Cave Karst Stud. 2008, 70, 135–141. [Google Scholar] [CrossRef]
- Miller, A.Z.; Hernández-Mariné, M.; Jurado, V.; Dionísio, A.; Barquinha, P.; Fortunato, E.; Afonso, M.J.; Chaminé, H.I.; Saiz-Jimenez, C. Enigmatic reticulated filaments in subsurface granite. Environ. Microbiol. Rep. 2012, 4, 596–603. [Google Scholar] [CrossRef]
- Melim, L.A.; Northup, D.E.; Spilde, M.N.; Boston, P.J. Update: Living reticulated filaments from Herbstlabyrinth-Adventhöhle Cave System, Germany. J. Cave Karst Stud. 2015, 77, 87–90. [Google Scholar] [CrossRef]
- Riquelme, C.; Rigal, F.; Hathaway, J.J.; Northup, D.E.; Spilde, M.N.; Borges, P.A.V.; Gabriel, R.; Amorim, I.R.; Dapkevicius, M.L.N.E. Cave microbial community composition in oceanic islands: Disentangling the effect of different colored mats in diversity patterns of Azorean lava caves. FEMS Microbiol. Ecol. 2015, 91, fiv141. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.Z.; Garcia-Sanchez, A.M.; Martin-Sanchez, P.M.; Pereira, M.F.C.; Spangenberg, J.E.; Jurado, V.; Dionísio, A.; Afonso, M.J.; Iglésias Chaminé, H.I.; Hermosin, B.; et al. Origin of abundant moonmilk deposits in a subsurface granitic environment. Sedimentology 2018, 65. [Google Scholar] [CrossRef] [Green Version]
- Cañaveras, J.C.; Cuezva, S.; Sanchez-Moral, S.; Lario, J.; Laiz, L.; Gonzalez, J.M.; Saiz-Jimenez, C. On the origin of fiber calcite crystals in moonmilk deposits. Naturwissenschaften 2006, 93, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Maciejewska, M.; Całusińska, M.; Cornet, L.; Adam, D.; Pessi, I.S.; Malchair, S.; Delfosse, P.; Baurain, D.; Barton, H.A.; Carnol, M.; et al. High-throughput sequencing analysis of the actinobacterial spatial diversity in moonmilk deposits. Antibiotics 2018, 7, 27. [Google Scholar] [CrossRef] [Green Version]
- Yarza, P.; Yilmaz, P.; Pruesse, E.; Glöckner, F.O.; Ludwig, W.; Schleifer, K.H.; Whitman, W.B.; Euzéby, J.; Amann, R.; Rosselló-Móra, R. Uniting the classification of cultured and uncultured bacteria and archaea using 16S rRNA gene sequences. Nat. Rev. Microbiol. 2014, 12, 635–645. [Google Scholar] [CrossRef]
- Huber, K.J.; Overmann, J. Vicinamibacteraceae fam. Nov., the first described family within the subdivision 6 Acidobacteria. Int. J. Syst. Evol. Microbiol. 2018, 68, 2331–2334. [Google Scholar] [CrossRef]
- Jones, R.T.; Robeson, M.S.; Lauber, C.L.; Hamady, M.; Knight, R.; Fierer, N. A comprehensive survey of soil acidobacterial diversity using pyrosequencing and clone library analyses. ISME J. 2009, 3, 442–453. [Google Scholar] [CrossRef]
- Schabereiter-Gurtner, C.; Saiz-Jimenez, C.; Piñar, G.; Lubitz, W.; Rölleke, S. Altamira cave Paleolithic paintings harbor partly unknown bacterial communities. FEMS Microbiol. Lett. 2002, 211, 7–11. [Google Scholar] [CrossRef] [Green Version]
- Zimmermann, J.; Gonzalez, J.M.; Saiz-Jimenez, C.; Ludwig, W. Detection and phylogenetic relationships of highly diverse uncultured acidobacterial communities in Altamira Cave using 23S rRNA sequence analyses. Geomicrobiol. J. 2005, 22, 379–388. [Google Scholar] [CrossRef]
- González Pimentel, J.L. Microorganismos de las Cuevas Volcánicas de La Palma (Islas Canarias). Diversidad y Potencial uso Biotecnológico. Ph.D. Thesis, Universidad Pablo de Olavide, Sevilla, Spain, 2019. [Google Scholar]
- Wang, J.; Wang, J.; Zhang, Z.; Li, Z.; Zhang, Z.; Zhao, D.; Wang, L.; Lu, F.; Li, Y. Shifts in the Bacterial Population and Ecosystem Functions in Response to Vegetation in the Yellow River Delta Wetlands. mSystems 2020, 5. [Google Scholar] [CrossRef]
- DeBruyn, J.M.; Nixon, L.T.; Fawaz, M.N.; Johnson, A.M.; Radosevich, M. Global biogeography and quantitative seasonal dynamics of Gemmatimonadetes in soil. Appl. Environ. Microbiol. 2011, 77, 6295–6300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yun, Y.; Wang, H.; Man, B.; Xiang, X.; Zhou, J.; Qiu, X.; Duan, Y.; Engel, A.S. The relationship between pH and bacterial communities in a single karst ecosystem and its implication for soil acidification. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Cho, Y.-J.; Jung, D.; Jo, K.; Lee, E.-J.; Lee, J.-S. Microbial Diversity in Moonmilk of Baeg-nyong Cave, Korean CZO. Front. Microbiol. 2020, 11, 613. [Google Scholar] [CrossRef] [PubMed]
- Saiz-Jimenez, C. The Microbiology of Show Caves, Mines, Tunnels, and Tombs: Implications for Management and Conservation. In Microbial Life of Cave Systems; DE GRUYTER: Berlin, Germany, 2015. [Google Scholar]
- Jurado, V.; del Rosal, Y.; Gonzalez-Pimentel, J.L.; Hermosin, B.; Saiz-Jimenez, C. Biological control of phototrophic biofilms in a show cave: The case of nerja cave. Appl. Sci. 2020, 10, 3448. [Google Scholar] [CrossRef]
- Garcia, M.G.; Moya, M.; Spilde, M.N. Discovering new diversity in Hawaiian lava tube microbial mats. In Proceedings of the 15th International Congress of Speleology, Kerrvile, TX, USA, 19–26 July 2009; pp. 364–369. [Google Scholar]
- Hathaway, J.J.M. Molecular Phylogenetic Investigation of Microbial Diversity and Nitrogen Cycling in Lava Tubes. Master’s Thesis, The University of New Mexico, Albuquerque, NM, USA, 2010. [Google Scholar]
- Busse, H.J. Review of the taxonomy of the genus Arthrobacter, emendation of the genus arthrobacter sensu lato, proposal to reclassify selected species of the genus Arthrobacter in the novel genera Glutamicibacter gen. Nov., Paeniglutamicibacter gen. nov., Pseudogluta. Int. J. Syst. Evol. Microbiol. 2016, 66, 9–37. [Google Scholar] [CrossRef] [Green Version]
- Borodina, E.; Kelly, D.P.; Schumann, P.; Rainey, F.A.; Ward-Rainey, N.L.; Wood, A.P. Enzymes of dimethylsulfone metabolism and the phylogenetic characterization of the facultative methylotrophs Arthrobacter sulfonivorans sp. nov., Arthrobacter methylotrophus sp. nov., and Hyphomicrobium sulfonivorans sp. nov. Arch. Microbiol. 2002, 177, 173–183. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, H.; Yang, R.; Li, S.; Zhou, M.; Gao, T.; An, L.; Chen, X.; Dyson, P. Complete genome sequence of a psychotrophic Pseudarthrobacter sulfonivorans strain Ar51 (CGMCC 4.7316), a novel crude oil and multi benzene compounds degradation strain. J. Biotechnol. 2016, 231, 81–82. [Google Scholar] [CrossRef] [Green Version]
- Dedysh, S.N.; Didriksen, A.; Danilova, O.V.; Belova, S.E.; Liebner, S.; Svenning, M.M. Methylocapsa palsarum sp. nov., a methanotroph isolated from a subarctic discontinuous permafrost ecosystem. Int. J. Syst. Evol. Microbiol. 2015, 65, 3618–3624. [Google Scholar] [CrossRef]
- Fernandez-Cortes, A.; Cuezva, S.; Alvarez-Gallego, M.; Garcia-Anton, E.; Pla, C.; Benavente, D.; Jurado, V.; Saiz-Jimenez, C.; Sanchez-Moral, S. Subterranean atmospheres may act as daily methane sinks. Nat. Commun. 2015, 6, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Webster, K.; Schimmelmann, A.; Drobniak, A.; Mastalerz, M.; Lagarde, L.R.; Boston, P.; Lennon, J. Diversity and composition of cave methanotrophic communities. bioRxiv 2018, 412213. [Google Scholar] [CrossRef] [Green Version]
- Martineau, C.; Mauffrey, F.; Villemur, R. Comparative analysis of denitrifying activities of Hyphomicrobium nitrativorans, Hyphomicrobium denitrificans, and Hyphomicrobium zavarzinii. Appl. Environ. Microbiol. 2015, 81, 5003–5014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manolache, E.; Onac, B. Geomicrobiology of Black Sediments in Vântului Cave (Romania): Preliminary Results. Cave Karst Sci. 2000, 27, 109–112. [Google Scholar]
- De Bruijn, I.; Cheng, X.; de Jager, V.; Expósito, R.G.; Watrous, J.; Patel, N.; Postma, J.; Dorrestein, P.C.; Kobayashi, D.; Raaijmakers, J.M. Comparative genomics and metabolic profiling of the genus Lysobacter. BMC Genom. 2015, 16, 991. [Google Scholar] [CrossRef]
- Kumar, H.K.S.; Gan, H.M.; Tan, M.H.; Eng, W.W.H.; Barton, H.A.; Hudson, A.O.; Savka, M.A. Genomic characterization of eight Ensifer strains isolated from pristine caves and a whole genome phylogeny of Ensifer (Sinorhizobium). J. Genom. 2017, 5, 12–15. [Google Scholar] [CrossRef] [Green Version]
- Hatayama, K.; Saito, K. Calcite formation induced by Ensifer adhaerens, Microbacterium testaceum, Paeniglutamicibacter kerguelensis, Pseudomonas protegens and Rheinheimera texasensis. Antonie Leeuwenhoek 2019, 112, 711–721. [Google Scholar] [CrossRef]
- Zhao, C.T.; Wang, E.T.; Zhang, Y.M.; Chen, W.F.; Sui, X.H.; Chen, W.X.; Liu, H.C.; Zhang, X.X. Mesorhizobium silamurunense sp. nov., isolated from root nodules of astragalus species. Int. J. Syst. Evol. Microbiol. 2012, 62, 2180–2186. [Google Scholar] [CrossRef] [PubMed]
- Díaz Herráiz, M. Caracterización de Comunidades Microbianas en Tumbas Etruscas y Romanas. Ph.D. Thesis, Universidad de Sevilla, Sevilla, Spain, 2015. [Google Scholar]
- Yao, R.; Wang, R.; Wang, D.; Su, J.; Zheng, S.; Wang, G. Paenibacillus selenitireducens sp. nov., a selenite-reducing bacterium isolated from a selenium mineral soil. Int. J. Syst. Evol. Microbiol. 2014, 64, 805–811. [Google Scholar] [CrossRef]
- Floor, G.H. Selenium Cycling in Volcanic Environments: The Role of Soils as Reactive Interfaces. Ph.D. Thesis, University of Girona, Girona, Spain, 2011. [Google Scholar]
- Lin, S.Y.; Wu, Y.H.; Hameed, A.; Liu, Y.C.; Young, C.C. Ammoniphilus resinae sp. Nov., an endosporeforming bacterium isolated from resin fragments. Int. J. Syst. Evol. Microbiol. 2016, 66, 3010–3016. [Google Scholar] [CrossRef] [Green Version]
- Domínguez Moñino, I. Evaluación y Control de Comunidades Microbianas en Cuevas Turísticas. Ph.D. Thesis, Universidad de Sevilla, Sevilla, Spain, 2015. [Google Scholar]
- Martin-Sanchez, P.M.; Jurado, V.; Porca, E.; Bastian, F.; Lacanette, D.; Alabouvette, C.; Saiz-Jimenez, C. Airborne microorganisms in Lascaux Cave (France). Int. J. Speleol. 2014, 43, 295–303. [Google Scholar] [CrossRef]
- Bastian, F.; Alabouvette, C.; Saiz-Jimenez, C. Bacteria and free-living amoeba in the Lascaux Cave. Res. Microbiol. 2009, 160, 38–40. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Cortes, A.; Cuezva, S.; Sanchez-Moral, S.; Cañaveras, J.C.; Porca, E.; Jurado, V.; Martin-Sanchez, P.M.; Saiz-Jimenez, C. Detection of human-induced environmental disturbances in a show cave. Environ. Sci. Pollut. Res. 2011, 18, 1037–1045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michel, A.J.; Ward, L.M.; Goffredi, S.K.; Dawson, K.S.; Baldassarre, D.T.; Brenner, A.; Gotanda, K.M.; McCormack, J.E.; Mullin, S.W.; O’Neill, A.; et al. The gut of the finch: Uniqueness of the gut microbiome of the Galápagos vampire finch. Microbiome 2018, 6, 167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh, W.; Bagchi, A.; Mandal, S.; Dam, B.; Roy, P. Tetrathiobacter kashmirensis gen. nov., sp. nov., a novel mesophilic, neutrophilic, tetrathionate-oxidizing, facultatively chemolithotrophic betaproteobacterium isolated from soil from a temperate orchard in Jammu and Kashmir, India. Int. J. Syst. Evol. Microbiol. 2005, 55, 1779–1787. [Google Scholar] [CrossRef]
- Wang, X.; Jin, D.; Zhou, L.; Wu, L.; An, W.; Zhao, L. Draft Genome Sequence of Advenella kashmirensis Strain W13003, a Polycyclic Aromatic Hydrocarbon-Degrading Bacterium. Genome Announc. 2014, 2, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Cortés-Lorenzo, C.; del Mar Sánchez-Peinado, M.; Oliver-Rodríguez, B.; Vílchez, J.L.; González-López, J.J.; Rodríguez-Díaz, M. Two novel strains within the family Caulobacteraceae capable of degradation of linear alkylbenzene sulfonates as pure cultures. Int. Biodeterior. Biodegrad. 2013, 85, 62–65. [Google Scholar] [CrossRef]
- Huber, K.J.; Geppert, A.M.; Wanner, G.; Fösel, B.U.; Wüst, P.K.; Overmann, J. The first representative of the globally widespread subdivision 6 Acidobacteria, Vicinamibacter silvestris gen. nov., sp. nov., isolated from subtropical savannah soil. Int. J. Syst. Evol. Microbiol. 2016, 66, 2971–2979. [Google Scholar] [CrossRef]
- Vieira, S.; Luckner, M.; Wanner, G.; Overmann, J. Luteitalea pratensis gen. nov., sp. nov. a new member of subdivision 6 Acidobacteria isolated from temperate grassland soil. Int. J. Syst. Evol. Microbiol. 2017, 67, 1408–1414. [Google Scholar] [CrossRef]
- Bárta, J.; Tahovská, K.; Šantrůčková, H.; Oulehle, F. Microbial communities with distinct denitrification potential in spruce and beech soils differing in nitrate leaching. Sci. Rep. 2017, 7, 9738. [Google Scholar] [CrossRef]
- Eichorst, S.A.; Trojan, D.; Roux, S.; Herbold, C.; Rattei, T.; Woebken, D. Genomic insights into the Acidobacteria reveal strategies for their success in terrestrial environments. Environ. Microbiol. 2018, 20, 1041–1063. [Google Scholar] [CrossRef] [Green Version]
- Hu, D.; Zang, Y.; Mao, Y.; Gao, B. Identification of Molecular Markers That Are Specific to the Class Thermoleophilia. Front. Microbiol. 2019, 10, 1185. [Google Scholar] [CrossRef] [PubMed]
- Perry, J.J. Thermoleophilum. In Bergey’s Manual of Systematics of Archaea and Bacteria; Wiley: Hoboken, NJ, USA, 2015; pp. 1–6. [Google Scholar]
- Yakimov, M.M.; Lünsdorf, H.; Golyshin, P.N. Thermoleophilum album and Thermoleophilum minutum are culturable representations of group 2 of the Rubrobacteridae (Actinobacteria). Int. J. Syst. Evol. Microbiol. 2003, 53, 377–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velasco, A.G.V.; Kowalchuk, G.A.; Mañero, F.J.G.; Ramos, B.; Yergeau, E.; García, J.A.L. Increased microbial activity and nitrogen mineralization coupled to changes in microbial community structure in the rhizosphere of Bt corn. Appl. Soil Ecol. 2013, 68, 46–56. [Google Scholar] [CrossRef]
- Bartelme, R.P.; Custer, J.M.; Dupont, C.L.; Espinoza, J.L.; Torralba, M.; Khalili, B.; Carini, P. Influence of Substrate Concentration on the Culturability of Heterotrophic Soil Microbes Isolated by High-Throughput Dilution-to-Extinction Cultivation. mSphere 2020, 5. [Google Scholar] [CrossRef] [Green Version]
- Yu, B.; Liu, C.; Wang, S.; Wang, W.; Zhao, S.; Zhu, G. Applying constructed wetland-microbial electrochemical system to enhance NH4+ removal at low temperature. Sci. Total Environ. 2020, 724, 138017. [Google Scholar] [CrossRef]
- Kushkevych, I.; Kobzová, E.; Vítězová, M.; Vítěz, T.; Dordević, D.; Bartoš, M. Acetogenic microorganisms in operating biogas plants depending on substrate combinations. Biologia 2019, 74, 1229–1236. [Google Scholar] [CrossRef]
- Brzeszcz, J.; Kapusta, P.; Steliga, T.; Turkiewicz, A. Hydrocarbon removal by two differently developed microbial inoculants and comparing their actions with biostimulation treatment. Molecules 2020, 25, 661. [Google Scholar] [CrossRef] [Green Version]
- Itoh, T.; Yamanoi, K.; Kudo, T.; Ohkuma, M.; Takashina, T. Aciditerrimonas ferrireducens gen. nov., sp. nov., an iron-reducing thermoacidophilic actinobacterium isolated from a solfataric field. Int. J. Syst. Evol. Microbiol. 2011, 61, 1281–1285. [Google Scholar] [CrossRef] [Green Version]
- Honeker, L.K.; Gullo, C.F.; Neilson, J.W.; Chorover, J.; Maier, R.M. Effect of Re-acidification on Buffalo Grass Rhizosphere and Bulk Microbial Communities during Phytostabilization of Metalliferous Mine Tailings. Front. Microbiol. 2019, 10, 1209. [Google Scholar] [CrossRef]
- Huang, S.; Jaffé, P.R. Isolation and characterization of an ammonium-oxidizing iron reducer: Acidimicrobiaceae sp. A6. PLoS ONE 2018. [Google Scholar] [CrossRef] [Green Version]
- Zhu, H.Z.; Zhang, Z.F.; Zhou, N.; Jiang, C.Y.; Wang, B.J.; Cai, L.; Liu, S.J. Diversity, distribution and co-occurrence patterns of bacterial communities in a karst cave system. Front. Microbiol. 2019, 10, 1726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Tayyab, M.; Abubakar, A.Y.; Yang, Z.; Pang, Z.; Islam, W.; Lin, Z.; Li, S.; Luo, J.; Fan, X.; et al. Bacteria with Different Assemblages in the Soil Profile Drive the Diverse Nutrient Cycles in the Sugarcane Straw Retention Ecosystem. Diversity 2019, 11, 194. [Google Scholar] [CrossRef] [Green Version]
- Fawaz, M. Revealing the Ecological Role of Gemmatimonadetes Through Cultivation and Molecular Analysis of Agricultural Soils. Master’s Thesis, University of Tennessee, Knoxville, TN, USA, May 2013. [Google Scholar]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miller, A.Z.; García-Sánchez, A.M.; L. Coutinho, M.; Costa Pereira, M.F.; Gázquez, F.; Calaforra, J.M.; Forti, P.; Martínez-Frías, J.; Toulkeridis, T.; Caldeira, A.T.; et al. Colored Microbial Coatings in Show Caves from the Galapagos Islands (Ecuador): First Microbiological Approach. Coatings 2020, 10, 1134. https://doi.org/10.3390/coatings10111134
Miller AZ, García-Sánchez AM, L. Coutinho M, Costa Pereira MF, Gázquez F, Calaforra JM, Forti P, Martínez-Frías J, Toulkeridis T, Caldeira AT, et al. Colored Microbial Coatings in Show Caves from the Galapagos Islands (Ecuador): First Microbiological Approach. Coatings. 2020; 10(11):1134. https://doi.org/10.3390/coatings10111134
Chicago/Turabian StyleMiller, Ana Z., Angela M. García-Sánchez, Mathilda L. Coutinho, Manuel F. Costa Pereira, Fernando Gázquez, José M. Calaforra, Paolo Forti, Jesús Martínez-Frías, Theofilos Toulkeridis, Ana T. Caldeira, and et al. 2020. "Colored Microbial Coatings in Show Caves from the Galapagos Islands (Ecuador): First Microbiological Approach" Coatings 10, no. 11: 1134. https://doi.org/10.3390/coatings10111134
APA StyleMiller, A. Z., García-Sánchez, A. M., L. Coutinho, M., Costa Pereira, M. F., Gázquez, F., Calaforra, J. M., Forti, P., Martínez-Frías, J., Toulkeridis, T., Caldeira, A. T., & Saiz-Jimenez, C. (2020). Colored Microbial Coatings in Show Caves from the Galapagos Islands (Ecuador): First Microbiological Approach. Coatings, 10(11), 1134. https://doi.org/10.3390/coatings10111134






