Anticorrosion Properties of the Low-Temperature Glow Plasma Nitriding Layer on AISI 904L Austenitic Stainless Steel in Hydrofluoric Acid Obtained at Various NH3 Pressures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Nitriding
2.3. Morphological Characterization
2.4. Microhardness
2.5. X-ray Diffraction
2.6. Electrochemical Tests
2.7. Immersed Corrosion Test
2.8. Scanning Kelvin Probe (SKP)
3. Results
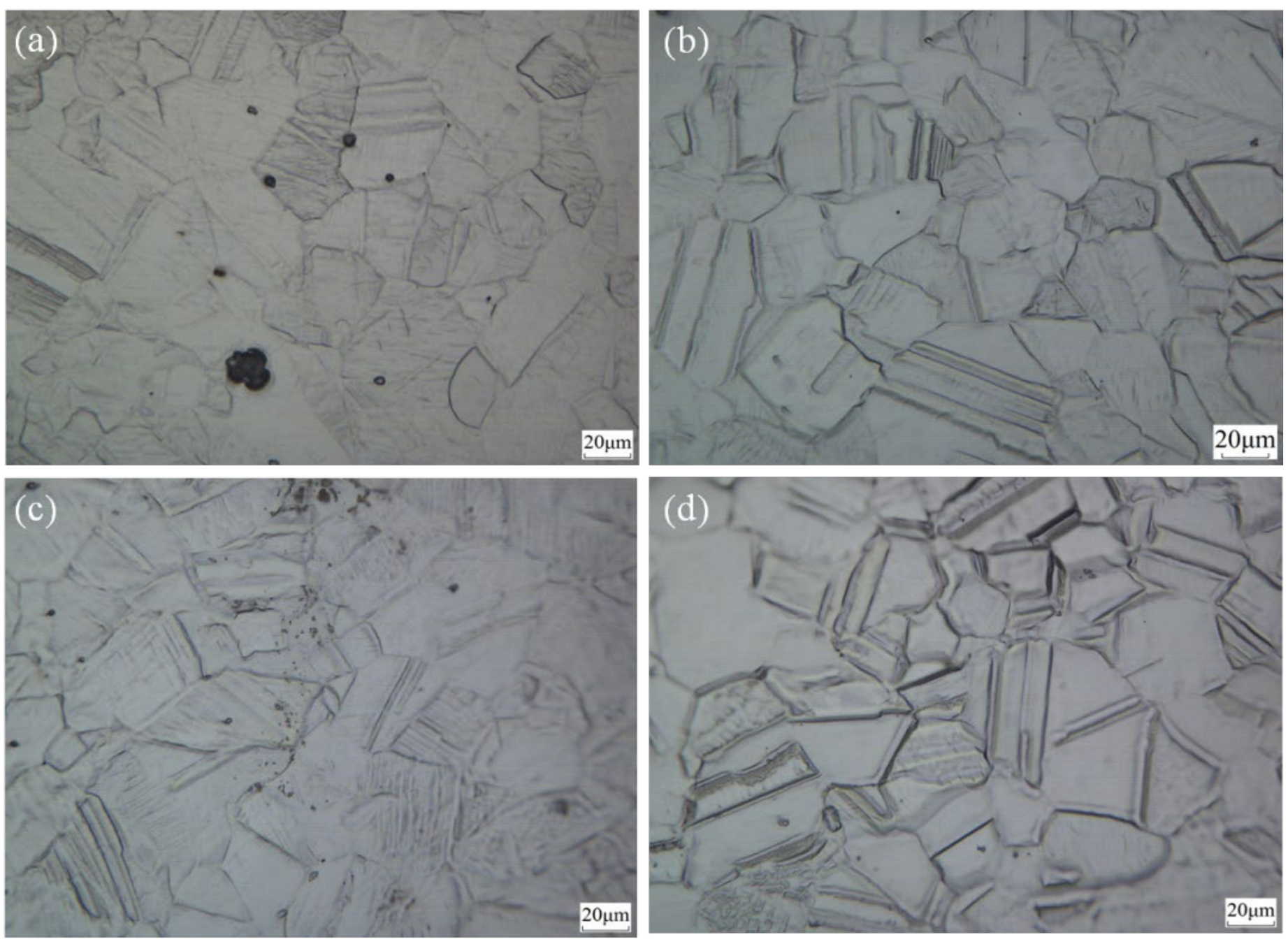
3.1. Optical Micrograph and Microhardness
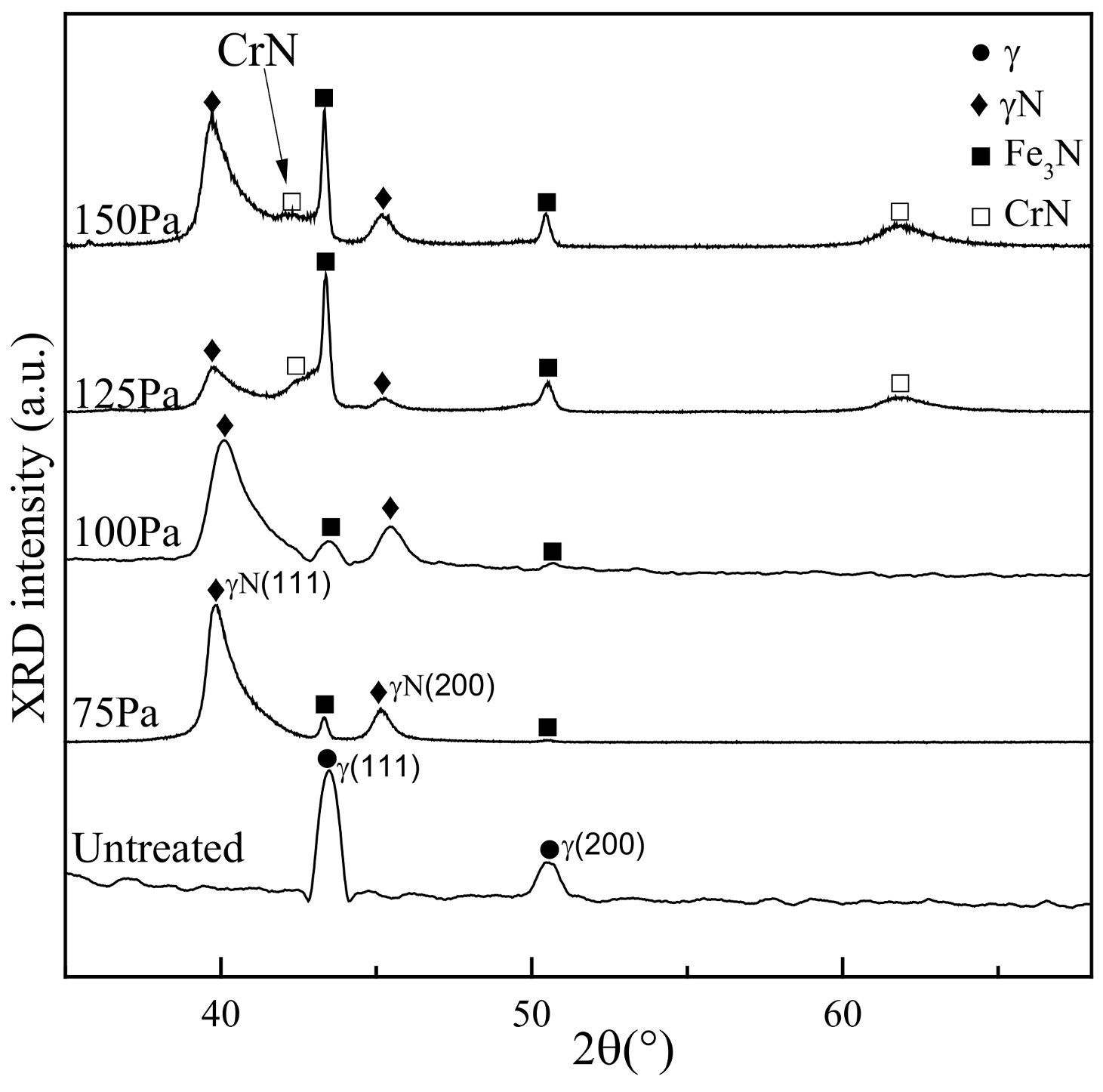
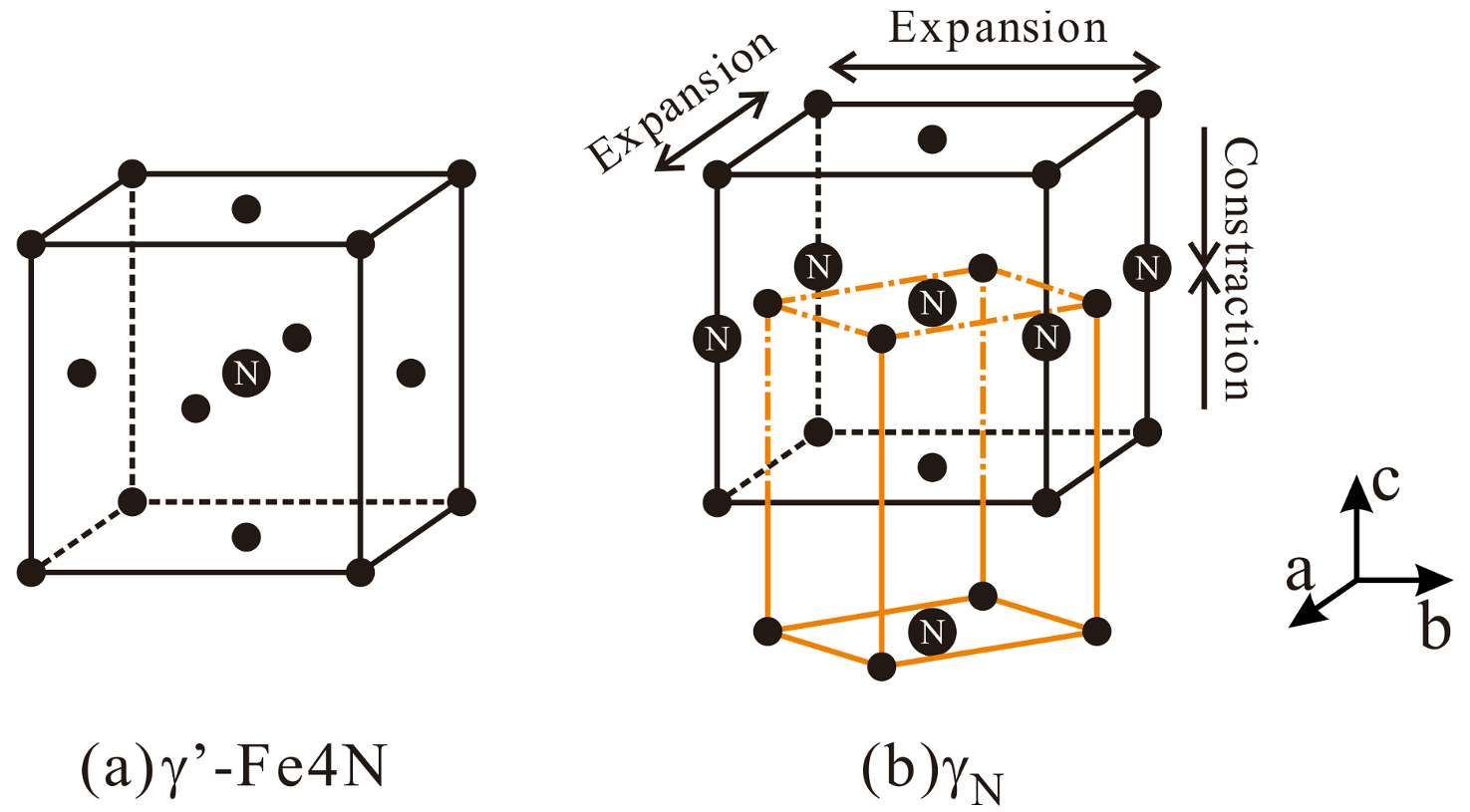
3.2. X-ray Diffractometer (XRD) Spectrum Analysis
3.3. Electrochemical Behavior
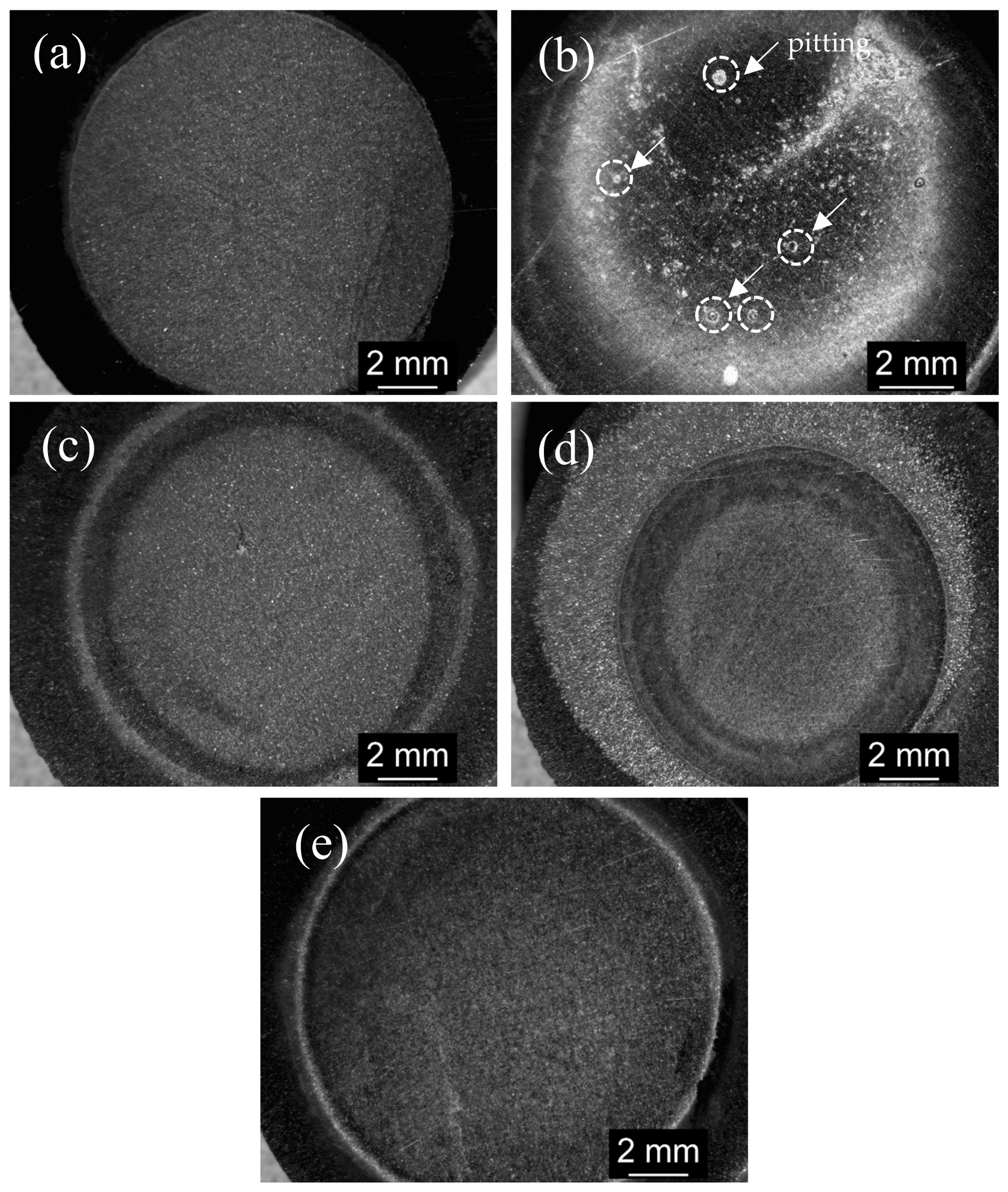
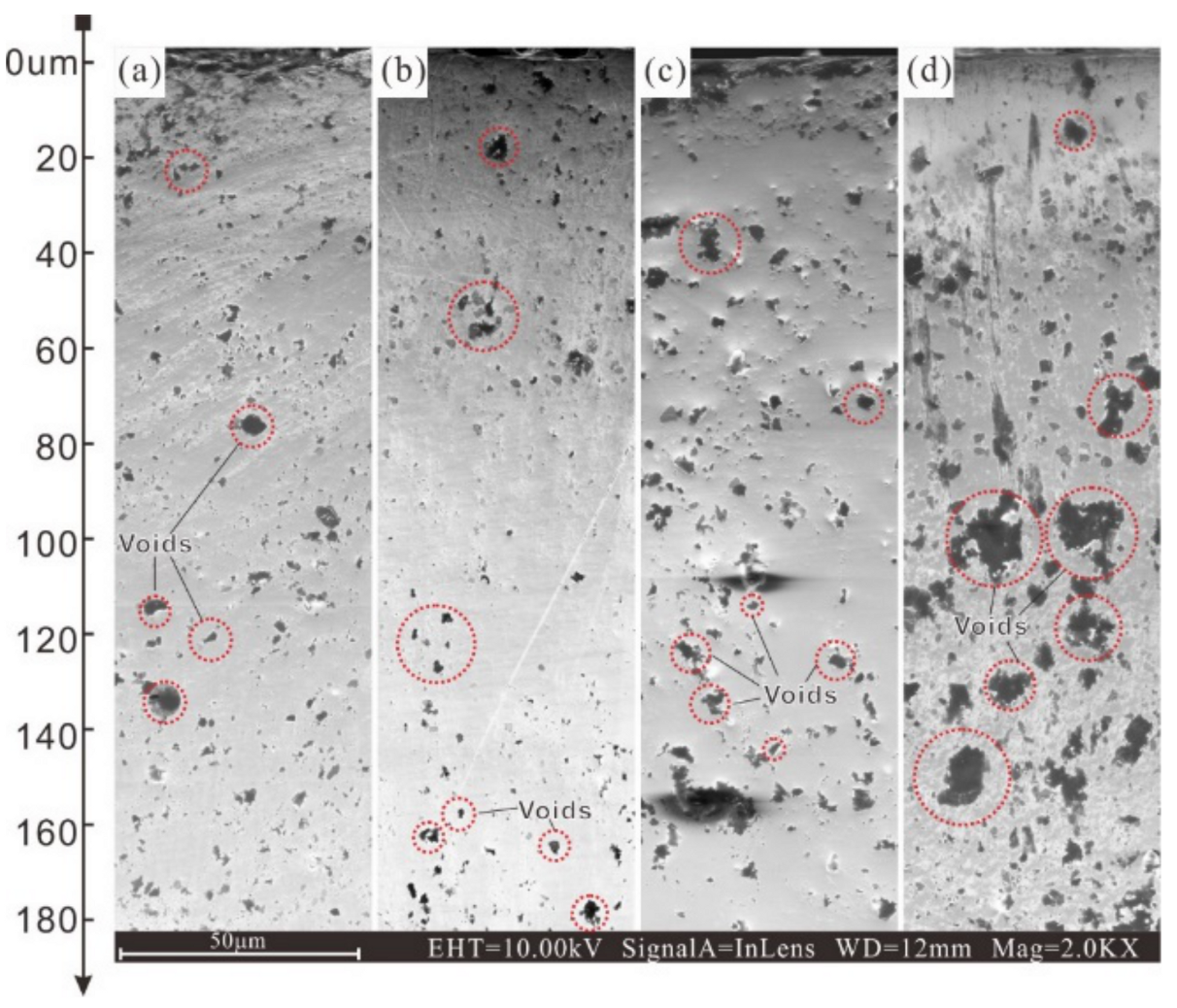
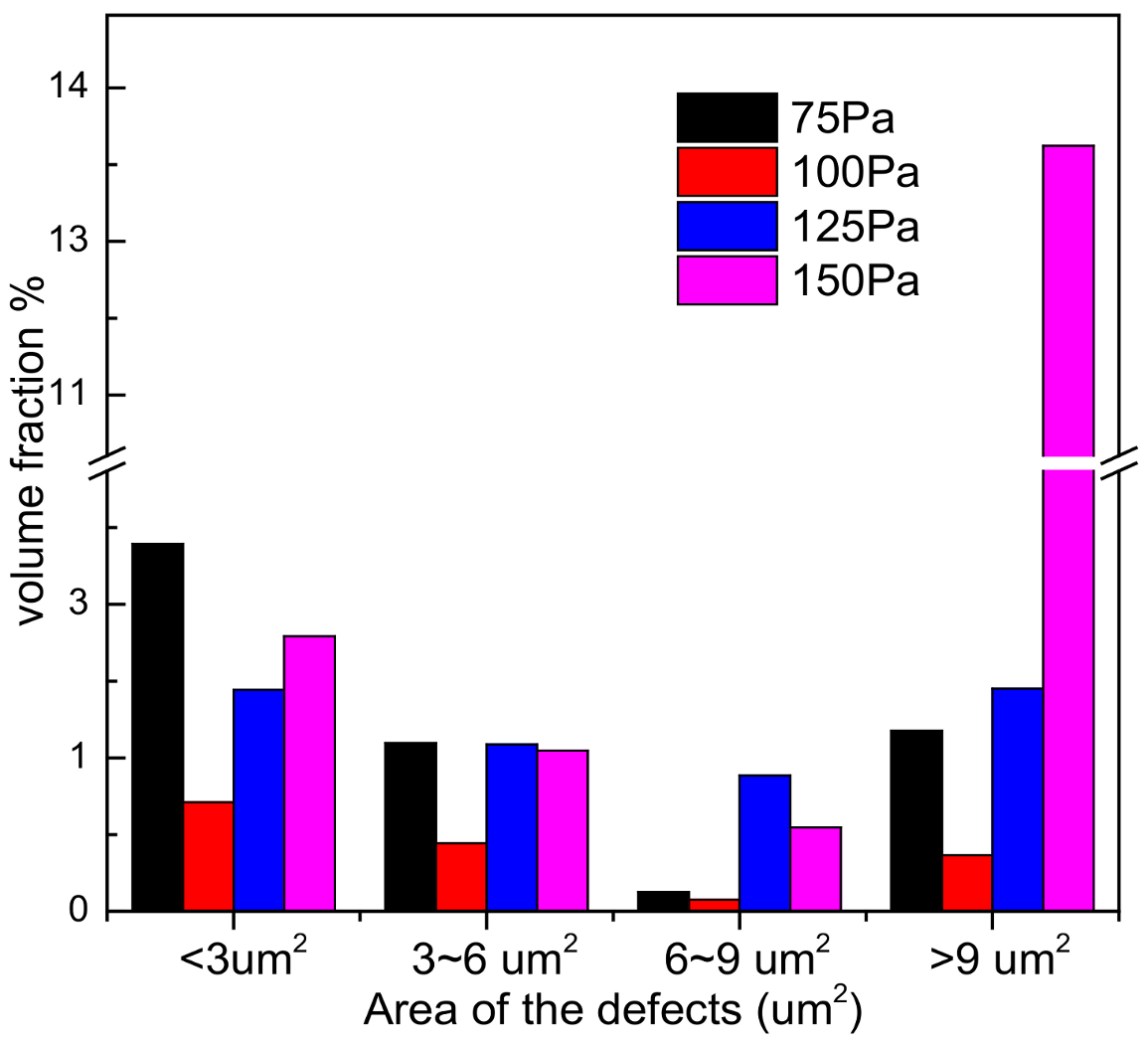
3.4. Corrosion Morphology Analysis
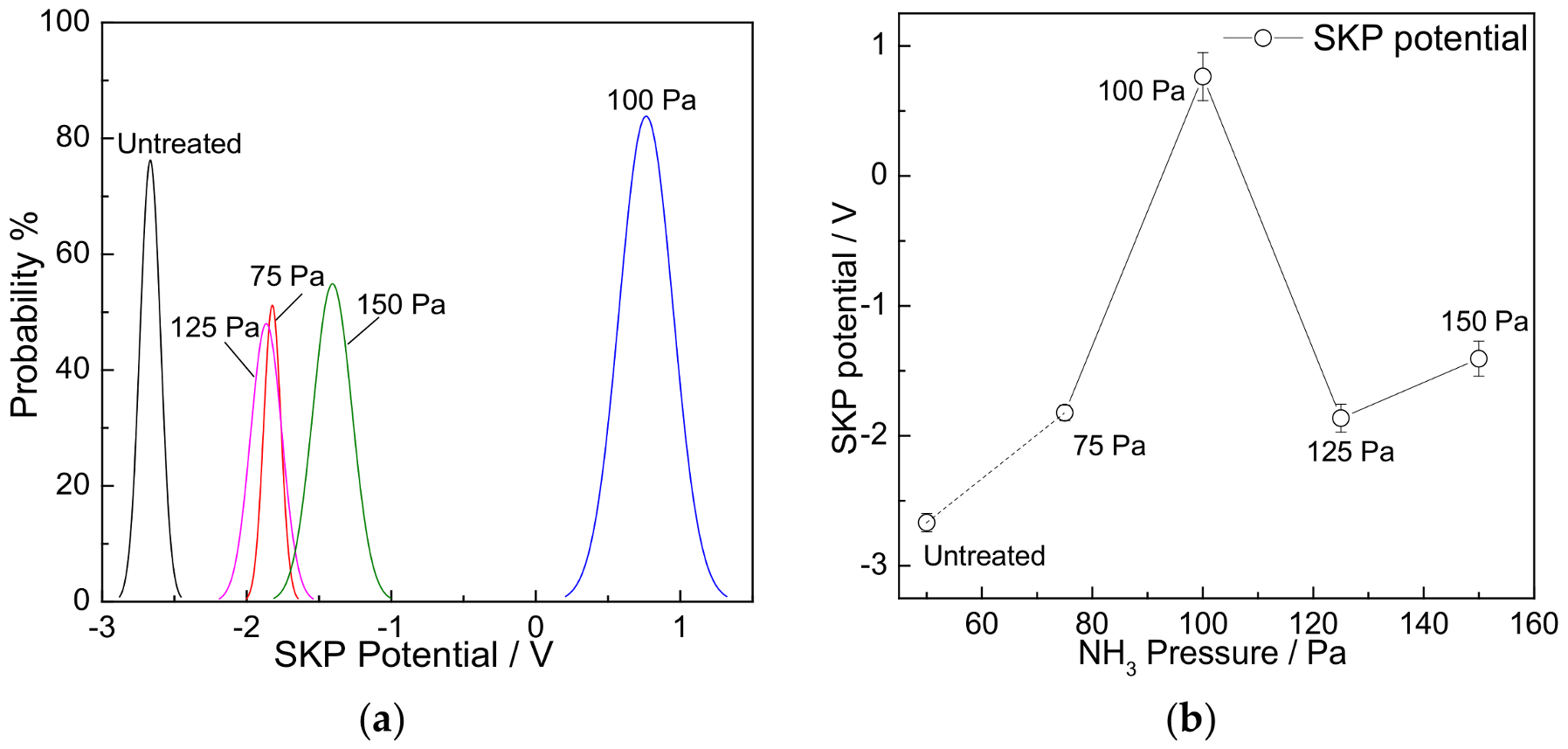
3.5. SKP
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jones, R.; Randle, V.; Owen, G. Carbide precipitation and grain boundary plane selection in overaged type 316 austenitic stainless steel. Mater. Sci. Eng. A 2008, 496, 256–261. [Google Scholar] [CrossRef]
- Schwind, M.; Källqvist, J.; Nilsson, J.O. σ-phase precipitation in stabilized austenitic stainless steels. J. Acta Mater. 2000, 48, 2473–2481. [Google Scholar] [CrossRef]
- Zou, G.; Shi, W.; Xiang, S.; Ji, X.; Ma, G.; Ballinger, R.G. Corrosion behavior of 904L austenitic stainless steel in hydrofluoric acid. RSC Adv. 2018, 8, 2811–2817. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, F.A.P.; Heck, S.; Pereira, R.; Picon, C.; Nascente, P.; Casteletti, L.C. Ion nitriding of a superaustenitic stainless steel: Wear and corrosion characterization. Surf. Coat. Technol. 2010, 204, 3087–3090. [Google Scholar] [CrossRef]
- Fossati, A.; Galvanetto, E.; Bacci, T.; Borgioli, F. Improvement of corrosion resistance of austenitic stainless steels by means of glow-discharge nitriding. Corros. Rev. 2011, 29, 209–221. [Google Scholar] [CrossRef]
- Li, X.; Thaiwatthana, S.; Dong, H.; Bell, T. Thermal stability of carbon S phase in 316 stainless steel. Surf. Eng. 2002, 18, 448–451. [Google Scholar] [CrossRef]
- Zhang, Z.L.; Bell, T. Structure and corrosion resistance of plasma nitrided stainless steel. Surf. Eng. 1985, 1, 131–136. [Google Scholar] [CrossRef]
- D’Avico, L.; Beltrami, R.; Pargoletti, E.; Trasatti, S.P.; Cappelletti, G. Insight into the release agents/PVD coatings interaction for plastic mold technology. Coatings 2020, 10, 281. [Google Scholar] [CrossRef] [Green Version]
- Gaona-Tiburcio, C.; Montoya-Rangel, M.; Cabral-Miramontes, J.A.; Estupiñan-López, F.; Zambrano-Robledo, P.; Orozco-Cruz, R.; Chacon-Nava, J.; Baltazar-Zamora, M.A.; Almeraya-Calderón, F. Corrosion resistance of multilayer coatings deposited by PVD on inconel 718 using electrochemical impedance spectroscopy technique. Coatings 2020, 10, 521. [Google Scholar] [CrossRef]
- Dinu, M.; Mouele, E.S.; Parau, A.C.; Vladescu, A.; Petrik, L.F.; Braic, M. Enhancement of the corrosion resistance of 304 stainless steel by Cr–N and Cr(N,O) coatings. Coatings 2018, 8, 132. [Google Scholar]
- Lo, K.; Shek, C.; Lai, J. Recent developments in stainless steels. Mater. Sci. Eng. R Rep. 2009, 65, 39–104. [Google Scholar] [CrossRef]
- Borgioli, F.; Galvanetto, E.; Bacci, T. Low temperature nitriding of AISI 300 and 200 series austenitic stainless steels. Vacuum 2016, 127, 51–60. [Google Scholar] [CrossRef]
- Collins, G.A.; Hutchings, R.; Short, K.T.; Tendys, J.; Li, X.; Samandi, M. Nitriding of austenitic stainless steel by plasma immersion ion implantation. Surf. Coat. Technol. 1995, 74, 417–424. [Google Scholar] [CrossRef]
- Christiansen, T.; Somers, M.A.J. On the crystallographic structure of S-phase. Scr. Mater. 2004, 50, 35–37. [Google Scholar] [CrossRef]
- Maistro, G. Microstructural Characterization of Expanded Austenite in 304L and 904L Austenitic Stainless Steels; Chalmers University of Technology: Gothenburg, Sweden, 2015. [Google Scholar]
- Ramkumar, K.D.; Chandrasekhar, A.; Srivastava, A.; Preyas, H.; Chandra, S.; Dev, S.; Arivazhagan, N. Effects of filler metals on the segregation, mechanical properties and hot corrosion behaviour of pulsed current gas tungsten arc welded super-austenitic stainless steel. J. Manuf. Process. 2016, 24, 46–61. [Google Scholar] [CrossRef]
- Bellezze, T.; Giuliani, G.; Viceré, A.; Roventi, G. Study of stainless steels corrosion in a strong acid mixture. Part 2: Anodic selective dissolution, weight loss and electrochemical impedance spectroscopy tests. Corros. Sci. 2018, 130, 12–21. [Google Scholar] [CrossRef]
- Bellezze, T.; Giuliani, G.; Roventi, G. Study of stainless steels corrosion in a strong acid mixture. Part 1: Cyclic potentiodynamic polarization curves examined by means of an analytical method. Corros. Sci. 2018, 130, 113–125. [Google Scholar] [CrossRef]
- Haruman, E.; Sun, Y.; Adenan, M.S. A comparative study of the tribocorrosion behaviour of low temperature nitrided austenitic and duplex stainless steels in NaCl solution. Tribol. Int. 2020, 151, 106412. [Google Scholar] [CrossRef]
- Dearnley, P.A.; Aldrich-Smith, G. Corrosion—wear mechanisms of hard coated austenitic 316L stainless steels. Wear 2004, 256, 491–499. [Google Scholar] [CrossRef]
- Nakanishi, T.; Tsuchiyama, T.; Mitsuyasu, H.; Iwamoto, Y.; Takaki, S. Effect of partial solution nitriding on mechanical properties and corrosion resistance in a type 316L austenitic stainless steel plate. Mater. Sci. Eng. A 2007, 460, 186–194. [Google Scholar] [CrossRef]
- Yasumaru, N. Low-temperature ion nitriding of austenitic stainless steels. Mater. Trans. JIM 1998, 39, 1046–1052. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Zhang, M.; Zhao, Y.; Shen, M.; Lei, H.; Xu, L.; Xiao, J.; Gong, J.; Yu, B.; Sun, C. Enhancement of mechanical property and corrosion resistance of 316L stainless steels by low temperature arc plasma nitriding. Surf. Coat. Technol. 2016, 298, 64–72. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, W.; Wang, J.; Jiang, R.; Xiang, S. Anticorrosion Properties of the Low-Temperature Glow Plasma Nitriding Layer on AISI 904L Austenitic Stainless Steel in Hydrofluoric Acid Obtained at Various NH3 Pressures. Coatings 2020, 10, 1156. https://doi.org/10.3390/coatings10121156
Shi W, Wang J, Jiang R, Xiang S. Anticorrosion Properties of the Low-Temperature Glow Plasma Nitriding Layer on AISI 904L Austenitic Stainless Steel in Hydrofluoric Acid Obtained at Various NH3 Pressures. Coatings. 2020; 10(12):1156. https://doi.org/10.3390/coatings10121156
Chicago/Turabian StyleShi, Wei, Jiaxu Wang, Ruyi Jiang, and Song Xiang. 2020. "Anticorrosion Properties of the Low-Temperature Glow Plasma Nitriding Layer on AISI 904L Austenitic Stainless Steel in Hydrofluoric Acid Obtained at Various NH3 Pressures" Coatings 10, no. 12: 1156. https://doi.org/10.3390/coatings10121156




