Conformal Functionalization of Cotton Fibers via Isoreticular Expansion of UiO-66 Metal-Organic Frameworks
Abstract
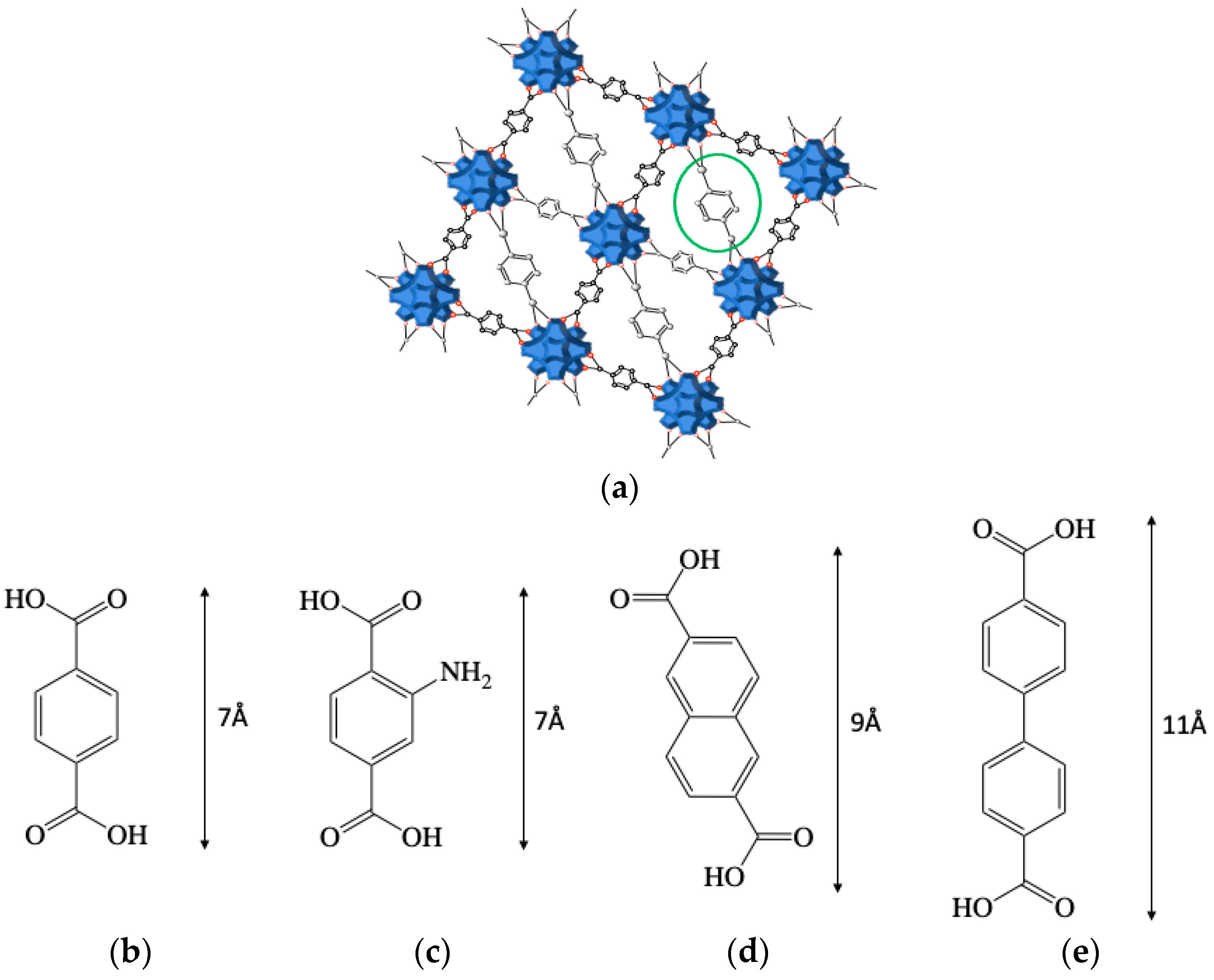
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
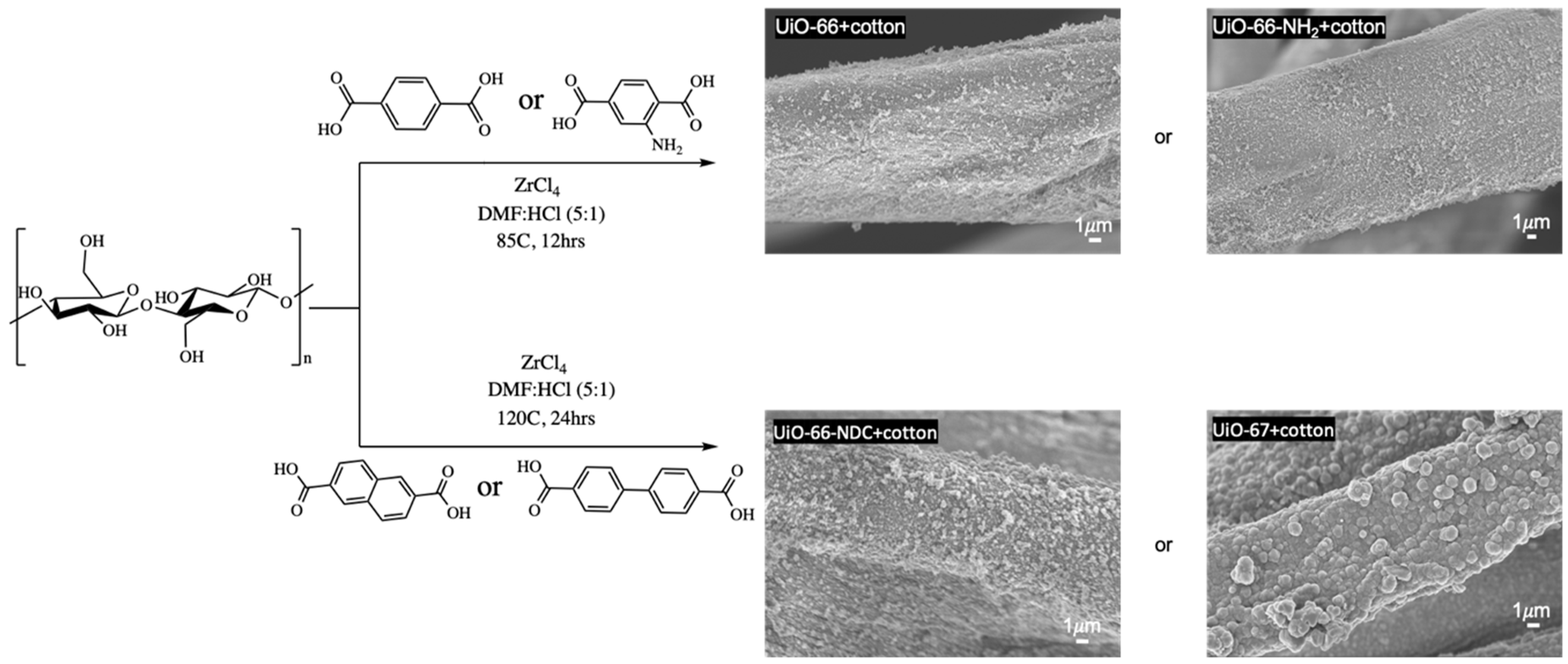
2.2.1. Cotton+UiO-66 and Cotton+UiO-66-NH2
2.2.2. Cotton+UiO-66-NDC and Cotton+UiO-67
2.2.3. Characterization of the Samples
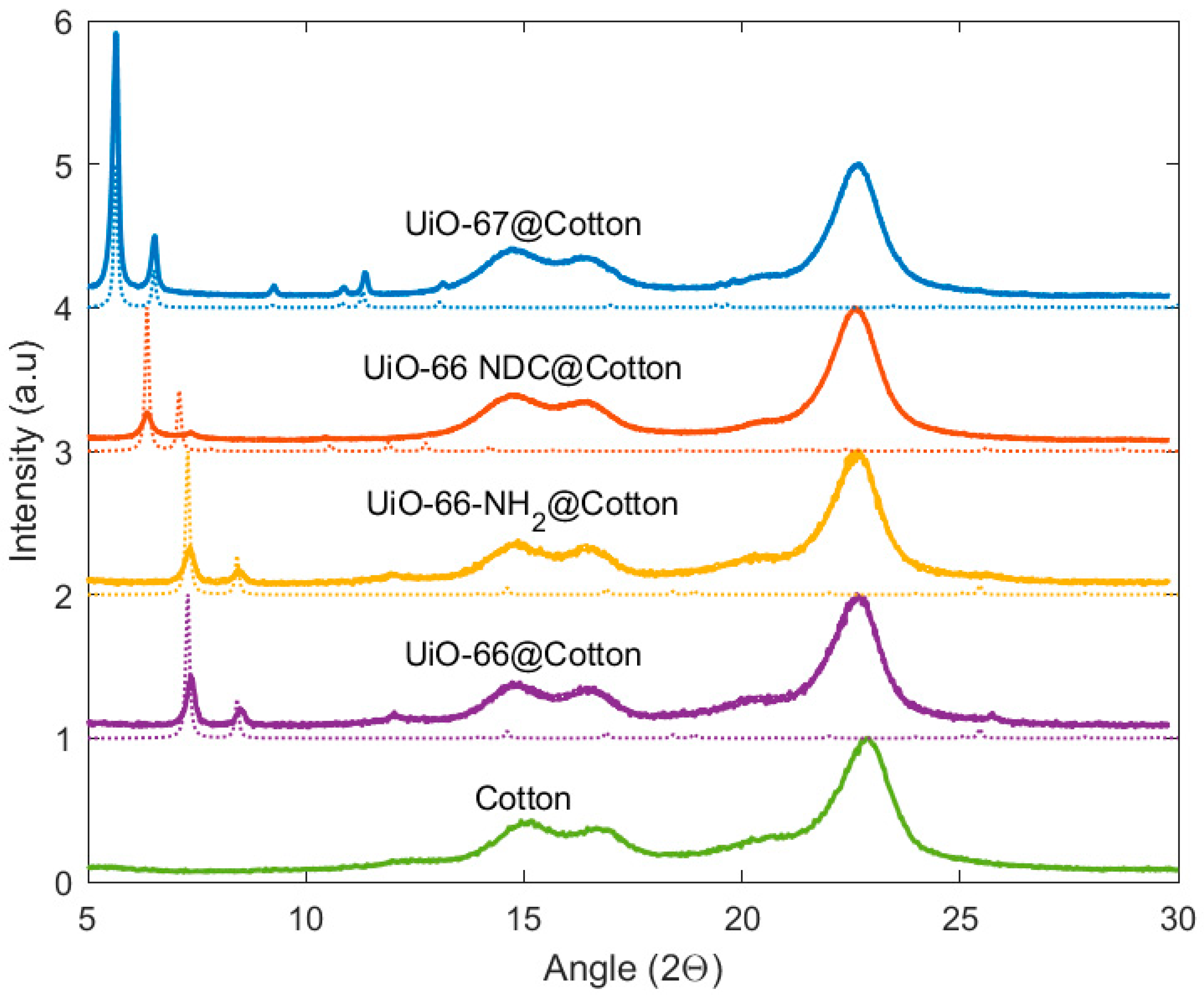
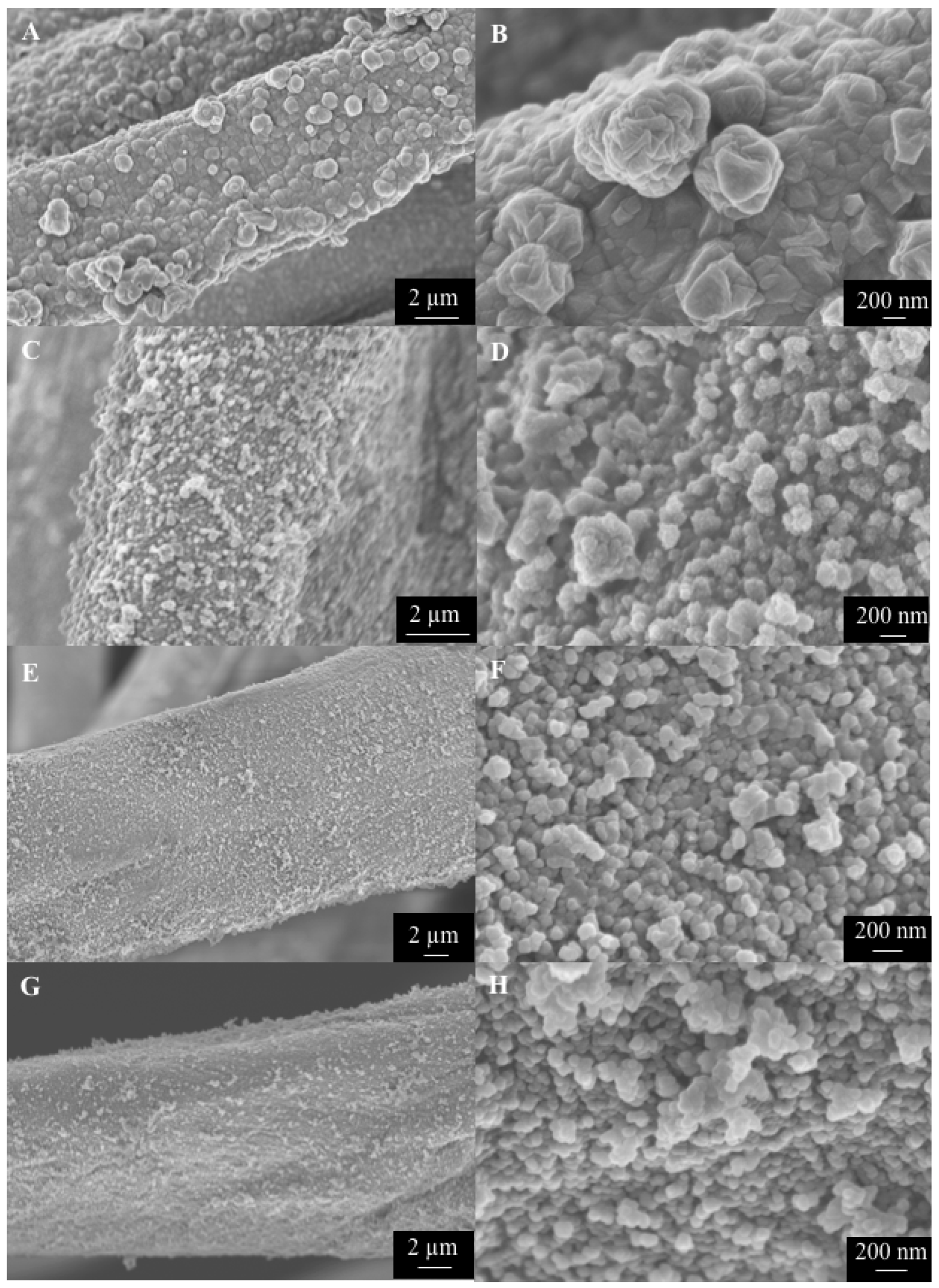
3. Results
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Caro, J. Quo vadis, MOF? Chem. Ing. Tech. 2018, 90, 1759–1768. [Google Scholar] [CrossRef]
- Kaskel, S. The Chemistry of Metal–Organic Frameworks: Synthesis, Characterization, and Applications, 1st ed.; Wiley-VCH: Weinheim, Germany, 2016; Volume 1, pp. 1–3. [Google Scholar] [CrossRef]
- Alezi, D.; Belmabkhout, Y.; Eddaoudi, M. MOF Crystal Chemistry Paving the Way to Gas Storage Needs: Aluminum-Based soc-MOF for CH4, O2, and CO2 Storage. J. Am. Chem. Soc. 2015, 137, 11308–13318. [Google Scholar] [CrossRef] [PubMed]
- Bonneau, M.; Lavenn, C.; Kitagawa, S. Upscale synthesis of a binary pillared layered MOF for hydrocarbon gas storage and separation. Green Chem. 2020, 22, 718–724. [Google Scholar] [CrossRef]
- Connolly, B.; Madden, D.; Fairen-Jimenez, D. Shaping the Future of Fuel: Monolithic Metal–Organic Frameworks for High-Density Gas Storage. J. Am. Chem. Soc. 2020, 142, 8541–8549. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, L.; Chen, B. Porous metal-organic frameworks for gas storage and separation: Status and challenges. Energy Chem. 2019, 1, 100006. [Google Scholar] [CrossRef]
- Xue, D.-X.; Wang, Q.; Bai, J. Amide-functionalized metal–organic frameworks: Syntheses, structures and improved gas storage and separation properties. Coord. Chem. Rev. 2019, 378, 2–16. [Google Scholar] [CrossRef]
- Kang, Y.-S.; Lu, Y.; Sun, W.-Y. Metal–organic frameworks with catalytic centers: From synthesis to catalytic application. Coord. Chem. Rev. 2019, 378, 262–280. [Google Scholar] [CrossRef]
- Sharma, V.K.; Feng, M. Water depollution using metal-organic frameworks-catalyzed advanced oxidation processes: A review. J. Hazard. Mater. 2019, 372, 3–16. [Google Scholar] [CrossRef]
- Konnerth, H.; Matsagar, B.; Wu, K.C.-W. Metal-organic framework (MOF)-derived catalysts for fine chemical production. Coord. Chem. Rev. 2020, 416, 213319. [Google Scholar] [CrossRef]
- Xu, C.; Fang, R.; Li, Y. Functional metal–organic frameworks for catalytic applications. Coord. Chem. Rev. 2019, 388, 268–292. [Google Scholar] [CrossRef]
- Li, F.; Wang, D.; Zou, J.-P. Design and syntheses of MOF/COF hybrid materials via postsynthetic covalent modification: An efficient strategy to boost the visible-light-driven photocatalytic performance. Appl. Catal. B Environ. 2019, 243, 621–628. [Google Scholar] [CrossRef]
- Dong, D.; Yan, C.; Zhang, Z. An electron-donating strategy to guide the construction of MOF photocatalysts toward co-catalyst-free highly efficient photocatalytic H2 evolution. J. Mater. Chem. A 2019, 7, 24180–24185. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Abdi, J. Nanoporous metal-organic framework (MOF-199): Synthesis, characterization and photocatalytic degradation of Basic Blue 41. Microchem. J. 2019, 144, 436–442. [Google Scholar] [CrossRef]
- Cavka, J.H.; Jakobsen, S.; Olsbye, U.; Guillou, N.; Lamberti, C.; Bordiga, S.; Lillerud, K.P. A New Zirconium Inorganic Building Brick Forming Metal Organic Frameworks with Exceptional Stability. J. Am. Chem. Soc. 2008, 130, 13850–13851. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.-L.; Feng, D.; Wang, K.; Gu, Z.-Y.; Wei, Z.; Chen, Y.-P.; Zhou, H.-C. An Exceptionally Stable, Porphyrinic Zr Metal–Organic Framework Exhibiting pH-Dependent Fluorescence. J. Am. Chem. Soc. 2013, 135, 13934–13938. [Google Scholar] [CrossRef] [PubMed]
- Schelling, M.; Kim, M.; Otal, E.; Hinestroza, J.-P. Decoration of Cotton Fibers with a Water-Stable Metal–Organic Framework (UiO-66) for the Decomposition and Enhanced Adsorption of Micropollutants in Water. Bioengineering 2018, 5, 14. [Google Scholar] [CrossRef] [Green Version]
- Ockwig, N.W.; Allendorf, M.D. Cobalt based isoreticular metal-organic frameworks (IRMOFs): A theoretical study on viability and stability. In Proceedings of the 211th ECS Meeting, Chicago, IL, USA, 6–10 May 2007. [Google Scholar]
- Furukawa, H.; Go, Y.B.; Ko, N.; Park, Y.K.; Uribe-Romo, F.J.; Kim, J.; O’Keeffe, M.; Yaghi, O.M. Isoreticular Expansion of Metal–Organic Frameworks with Triangular and Square Building Units and the Lowest Calculated Density for Porous Crystals. Inorg. Chem. 2011, 50, 9147–9152. [Google Scholar] [CrossRef]
- Zhou, H.-C.; Kitagawa, S. Metal–Organic Frameworks (MOFs). Chem. Soc. Rev. 2014, 43, 5415–5418. [Google Scholar] [CrossRef] [Green Version]
- Eddaoudi, M.; Kim, J.; Rosi, N.; Vodak, D. Systematic design of pore size and functionality in isoreticular MOFs and their application in methane storage. Science 2002, 295, 469–472. [Google Scholar] [CrossRef] [Green Version]
- Razavi, S.A.A.; Masoomi, M.Y.; Islamoglu, T.; Morsali, A.; Xu, Y.; Hupp, J.T.; Farha, O.K.; Wang, J.; Junk, P.C. Improvement of Methane–Framework Interaction by Controlling Pore Size and Functionality of Pillared MOFs. Inorg. Chem. 2017, 56, 2581–2588. [Google Scholar] [CrossRef]
- Chen, X.; Peng, Y.; Cui, Y. Sixteen isostructural phosphonate metal-organic frameworks with controlled Lewis acidity and chemical stability for asymmetric catalysis. Nat. Commun. 2017, 8, 2171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katz, M.J.; Brown, Z.J.; Colon, Y.J.; Siu, P.W.; Scheidt, K.A.; Snurr, R.Q.; Hupp, J.T.; Farha, O.K. A facile synthesis of UiO-66, UiO-67 and their derivatives. Chem. Commun. 2013, 49, 9449. [Google Scholar] [CrossRef] [PubMed]
- Garibay, S.J.; Cohen, S.M. Isoreticular synthesis and modification of frameworks with the UiO-66 topology. Chem. Commun. 2010, 46, 7700–7702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cmarik, G.E.; Kim, M.; Cohen, S.M.; Walton, K.S. Tuning the Adsorption Properties of UiO-66 via Ligand Functionalization. Langmuir 2012, 28, 15606–15613. [Google Scholar] [CrossRef] [PubMed]
- Gomes-Silva, C.; Luz, I.; Llabres i Xamena, F.X.; Corma, A.; Garcia, H. Water Stable Zr–Benzenedicarboxylate Metal–Organic Frameworks as Photocatalysts for Hydrogen Generation. Chem. Eur. J. 2010, 16, 11133–11138. [Google Scholar] [CrossRef]
- Yuan, S.; Feng, L.; Zhou, H.-C. Stable Metal-Organic Frameworks: Design, Synthesis, and Applications. Adv. Mater. 2018, 30. [Google Scholar] [CrossRef] [Green Version]
- Ragon, F.; Chevreau, H.; Devic, T.; Serre, C.; Horcajada, P. Impact of the Nature of the Organic Spacer on the Crystallization Kinetics of UiO-66(Zr)-Type MOFs. Chem. Eur. J. 2015, 21, 7135–7143. [Google Scholar] [CrossRef]
- Lopez-Maya, E.; Montoro, C.; Rodriguez-Albelo, L.M.; Aznar Cervantes, S.D.; Lozano-Perez, A.A.; Cenis, J.L.; Barea, E.; Navarro, J.A.R. Textile/Metal–Organic-Framework Composites as Self-Detoxifying Filters for Chemical-Warfare Agents. Angew. Chem. Int. Ed. 2015, 54, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Rauf, S.; Vijjapu, M.T.; Andres, M.A.; Gascon, I.; Roubeau, O.; Eddaoudi, M.; Salama, K.N. A Highly Selective Metal-Organic Framework Textile Humidity Sensor. Acs Appl. Mater. Interfaces 2020, 26, 29999–30006. [Google Scholar] [CrossRef]
- Lee, D.T.; Jamir, J.D.; Peterson, G.W.; Parsons, G.N. Protective Fabrics: Metal-Organic Framework Textiles for Rapid Photocatalytic Sulfur Mustard Simulant Detoxification. Matter 2020, 2, 404–415. [Google Scholar] [CrossRef] [Green Version]
- Bunge, M.A.; Davis, A.B.; West, K.N.; West, C.W.; Glover, T.G. Synthesis and Characterization of UiO-66-NH2 Metal–Organic Framework Cotton Composite Textiles. Ind. Eng. Chem. Res. 2018, 57, 9151–9161. [Google Scholar] [CrossRef]
- Butova, V.V.; Budnyk, A.P.; Lamberti, C. Modulator effect in UiO-66-NDC (1,4-naphthalenedicarboxilic acid) synthesis and comparison with UiO-67-NDC isoreticular MOFs. Cryst. Growth Des. 2017, 17, 5422–5431. [Google Scholar] [CrossRef]
- El Osta, R.; Feyand, M.; Walton, R.I. Crystallisation Kinetics of Metal Organic Frameworks from in situ Time-Resolved X-ray Diffraction. Powder Diffr. 2013, 28, S256. [Google Scholar] [CrossRef]
- French, A.D. Idealized powder diffraction patterns for cellulose polymorphs. Cellulose 2014, 21, 885–896. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.J.; Martin, A.E.; Conrad, C.M. An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-Ray Diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- David, J.; Trolliard, G.; Volkringer, C.; Loiseau, T.; Maitre, A. Synthesis of zirconium oxycarbide powders using metal–organic framework (MOF) compounds as precursors. RSC Adv. 2015, 51650–51661. [Google Scholar] [CrossRef]
- Van Vleet, M.J.; Weng, T.; Schmidt, J.R. In Situ, Time-Resolved, and Mechanistic Studies of Metal−Organic Framework Nucleation and Growth. Chem. Rev. 2018, 3681. [Google Scholar] [CrossRef]
- Otal, E.H.; Kim, M.L.; Calvo, M.E.; Karvonen, L.; Fabregas, I.O.; Sierra, C.A.; Hinestroza, J.P. A panchromatic modification of the light absorption spectra of metal–organic frameworks. Chem. Commun. 2016, 52, 6665–6668. [Google Scholar] [CrossRef] [Green Version]
- Loof, D.; Hiller, M.; Oschkinat, H.; Koschek, K. Quantitative and Qualitative Analysis of Surface Modified Cellulose Utilizing TGA-MS. Materials 2016, 9, 415. [Google Scholar] [CrossRef] [Green Version]
- Zhu, P.; Sui, S.; Wang, B.; Sun, K.; Sun, G. A study of pyrolysis and pyrolysis products of flame-retardant cotton fabrics by DSC, TGA, and PY–GC–MS. J. Anal. Appl. Pyrolysis 2004, 71, 645–655. [Google Scholar] [CrossRef]

| Sample Name | Mof Formula | Residue % M/M | MOF % M/M |
|---|---|---|---|
| cotton | – | 0.20 | – |
| Cotton+UIO-66 | Zr24C192H112O128 | 0.91 | 1.6 |
| Cotton+UIO-66-NH2 | Zr24C192H136O128N24 | 1.19 | 2.3 |
| Cotton+UIO-66-NDC | Zr24C288H160O128 | 0.73 | 1.4 |
| Cotton+UIO-67 | Zr24C336H208O128 | 0.45 | 0.72 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schelling, M.; Otal, E.; Kim, M.; Hinestroza, J.P. Conformal Functionalization of Cotton Fibers via Isoreticular Expansion of UiO-66 Metal-Organic Frameworks. Coatings 2020, 10, 1172. https://doi.org/10.3390/coatings10121172
Schelling M, Otal E, Kim M, Hinestroza JP. Conformal Functionalization of Cotton Fibers via Isoreticular Expansion of UiO-66 Metal-Organic Frameworks. Coatings. 2020; 10(12):1172. https://doi.org/10.3390/coatings10121172
Chicago/Turabian StyleSchelling, Marion, Eugenio Otal, Manuela Kim, and Juan P. Hinestroza. 2020. "Conformal Functionalization of Cotton Fibers via Isoreticular Expansion of UiO-66 Metal-Organic Frameworks" Coatings 10, no. 12: 1172. https://doi.org/10.3390/coatings10121172
APA StyleSchelling, M., Otal, E., Kim, M., & Hinestroza, J. P. (2020). Conformal Functionalization of Cotton Fibers via Isoreticular Expansion of UiO-66 Metal-Organic Frameworks. Coatings, 10(12), 1172. https://doi.org/10.3390/coatings10121172








