The Bioactive Polypyrrole/Polydopamine Nanowire Coating with Enhanced Osteogenic Differentiation Ability with Electrical Stimulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
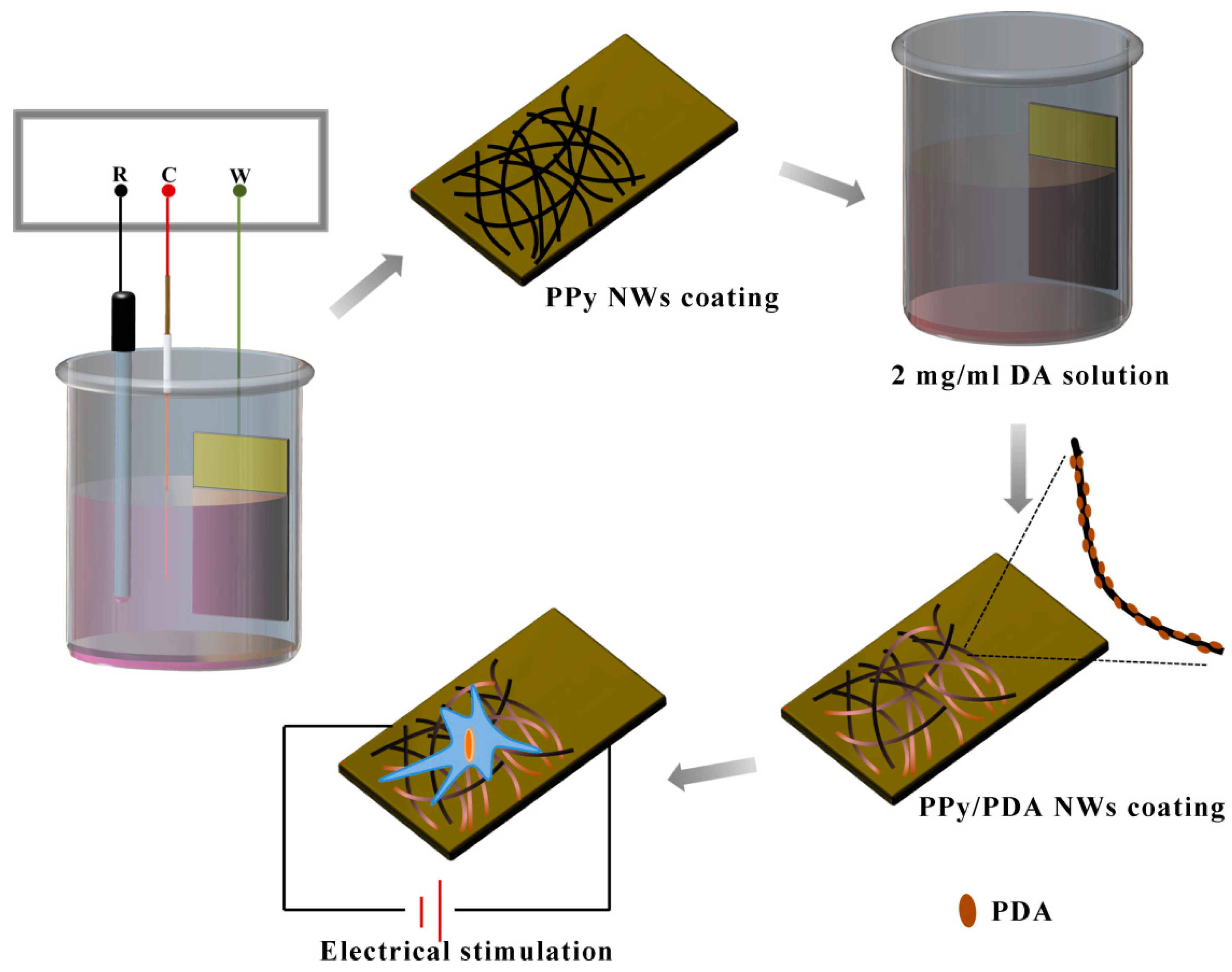
2.2. Synthesis of PPy/PDA Nanowires (NWs) Coating
2.3. Characterization
2.4. Cell Culture
2.5. Effect of Surface Morphology on MC3T3-E1 Adhesion and Proliferation and Differentiation
2.5.1. MTT Test
2.5.2. Cell Morphology
2.5.3. Total Protein Content and Alkaline Phosphatase Activity
2.6. Effect of Electrical Stimulation on MC3T3-E1 Adhesion and Proliferation and Differentiation
2.7. Statistical Analysis
3. Results and Discussion
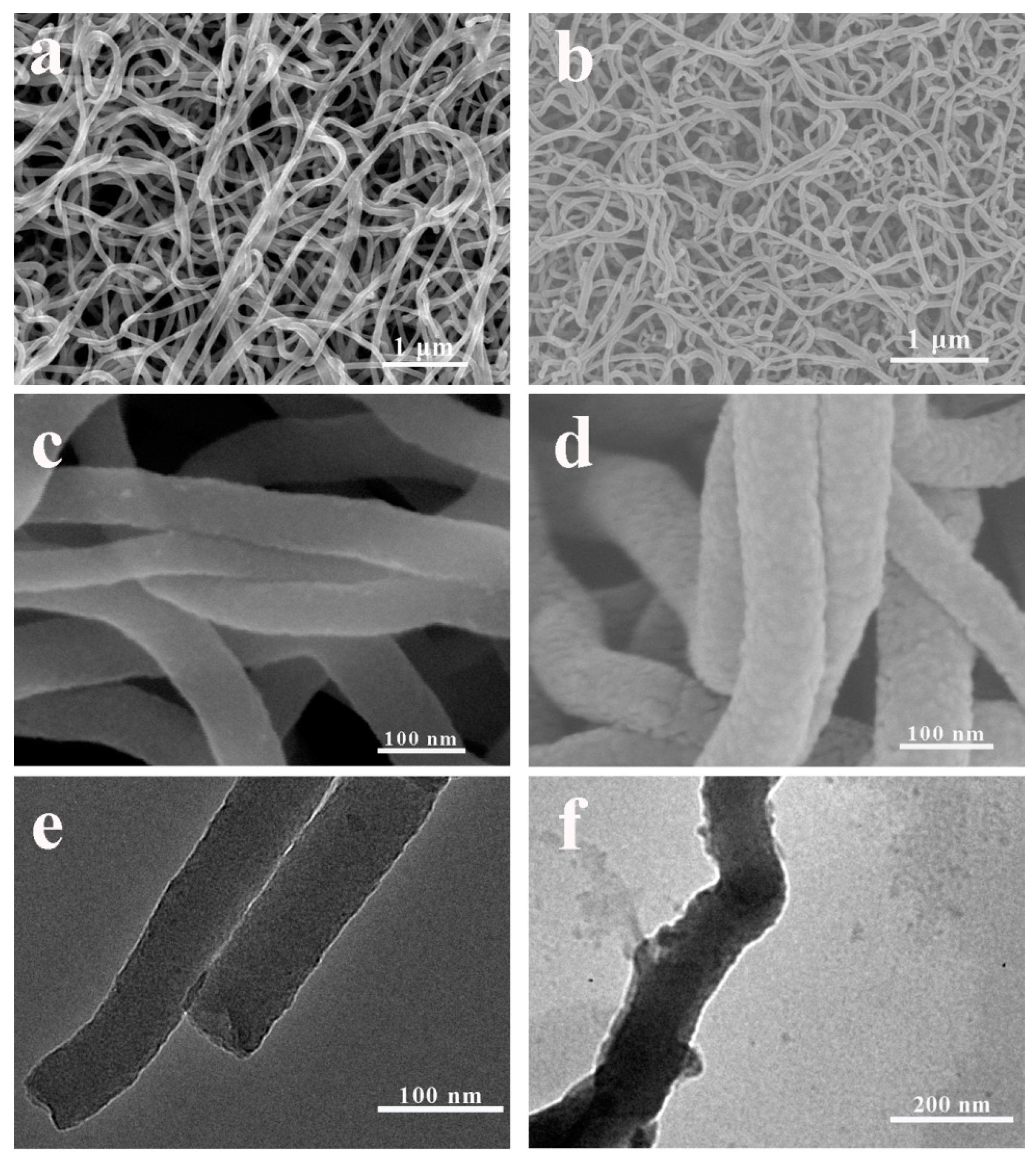
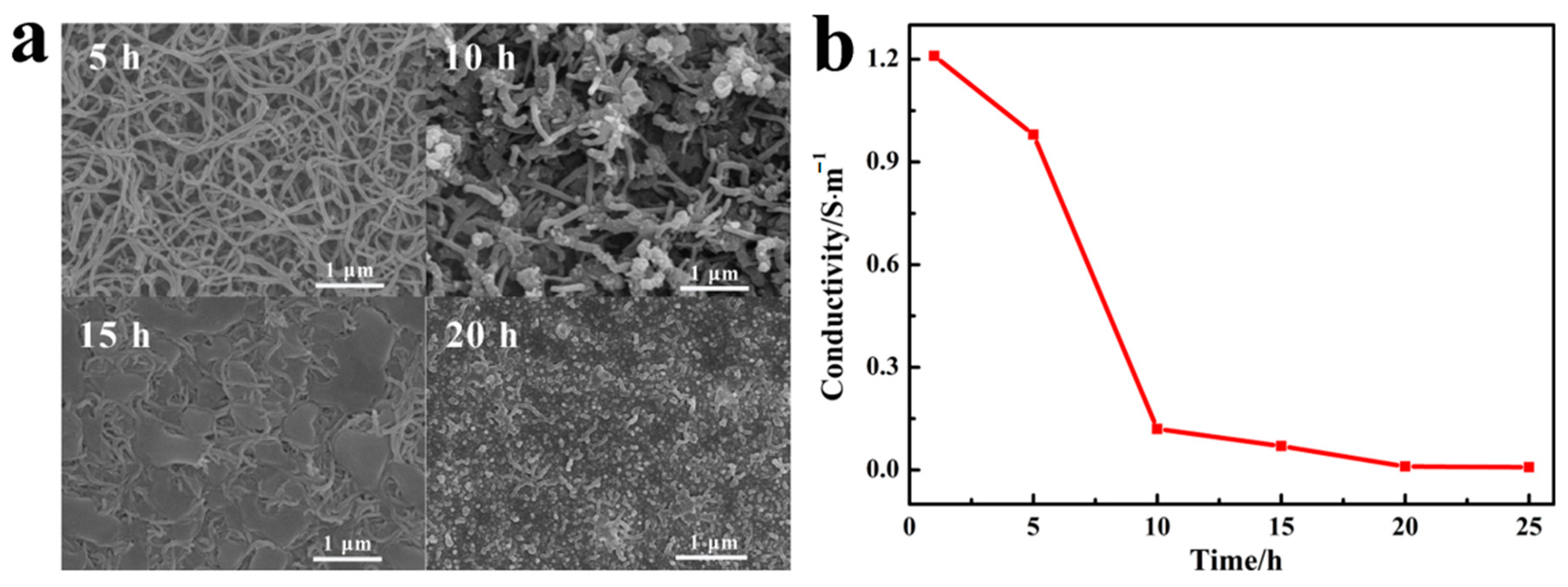
3.1. Characterization of PPy/PDA NWs
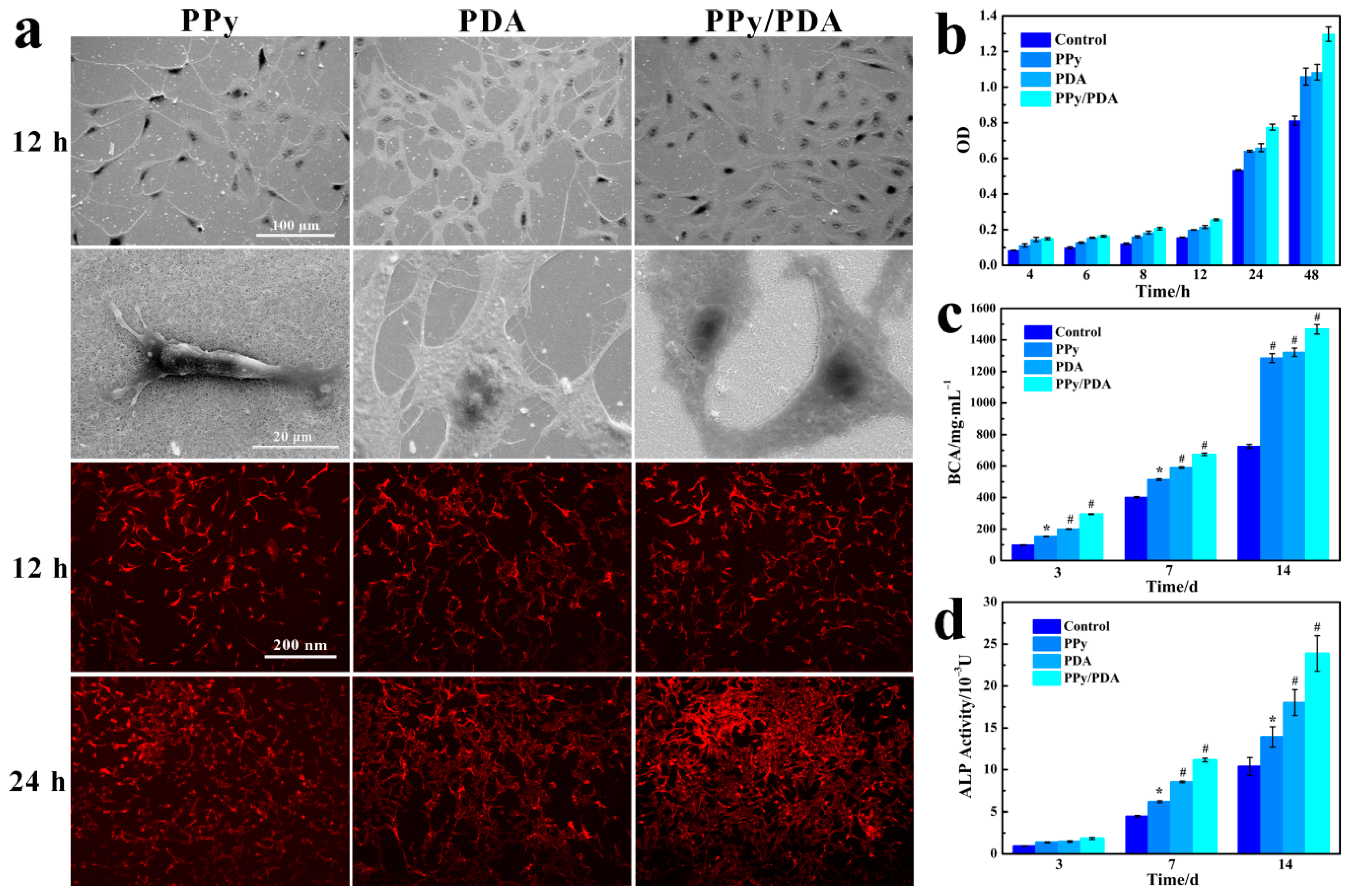
3.2. Effect of PPy/PDA NWs Coating on MC3T3-E1 Activity
3.3. Synergistic Effect of PPy/PDA NWs Coating with Electrical Stimulation on MC3T3-E1 Activity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wu, J.-G.; Chen, J.-H.; Liu, K.-T.; Luo, S.-C. Engineering antifouling conducting polymers for modern biomedical applications. ACS Appl. Mater. Interfaces 2019, 11, 21294–21307. [Google Scholar] [CrossRef] [PubMed]
- Hackett, A.J.; Malmström, J.; Travas-Sejdic, J. Functionalization of conducting polymers for biointerface applications. Prog. Polym. Sci. 2017, 70, 18–33. [Google Scholar] [CrossRef]
- Hardy, J.G.; Lee, J.Y.; Schmidt, C.E. Biomimetic conducting polymer-based tissue scaffolds. Curr. Opin. Biotechnol. 2013, 24, 847–854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, L.; Wei, Z.; Wan, M. Conducting polymer nanostructures and their application in biosensors. J. Colloid Interface Sci. 2010, 341, 1–11. [Google Scholar] [CrossRef]
- Boehler, C.; Oberueber, F.; Asplund, M. Tuning drug delivery from conducting polymer films for accurately controlled release of charged molecules. J. Control Release 2019, 304, 173–180. [Google Scholar] [CrossRef]
- Jiang, S.; Sun, Y.; Cui, X.; Huang, X.; He, Y.; Ji, S.; Shi, W.; Ge, D. Enhanced drug loading capacity of polypyrrole nanowire network for controlled drug release. Synth. Met. 2013, 163, 19–23. [Google Scholar] [CrossRef] [Green Version]
- Mihardja, S.S.; Sievers, R.E.; Lee, R.J. The effect of polypyrrole on arteriogenesis in an acute rat infarct model. Biomaterials 2008, 29, 4205–4210. [Google Scholar] [CrossRef] [Green Version]
- Bu, Y.; Xu, H.; Li, X.; Xu, W.; Yin, Y.-X.; Dai, H.; Wang, X.-B.; Huang, Z.-J.; Xu, P.-H. A conductive sodium alginate and carboxymethyl chitosan hydrogel doped with polypyrrole for peripheral nerve regeneration. RSC Adv. 2018, 8, 10806–10817. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.Y.; Bashur, C.A.; Goldstein, A.S.; Schmidt, C.E. Polypyrrole-coated electrospun PLGA nanofibers for neural tissue applications. Biomaterials 2009, 30, 4325–4335. [Google Scholar] [CrossRef] [Green Version]
- Harjo, M.; Torop, J.; Järvekülg, M.; Tamm, T.; Kiefer, R. Electrochemomechanical behavior of polypyrrole-coated nanofiber scaffolds in cell culture medium. Polymers 2019, 11, 1043. [Google Scholar] [CrossRef] [Green Version]
- Hu, W.-W.; Hsu, Y.-T.; Cheng, Y.-C.; Li, C.; Ruaan, R.-C.; Chien, C.-C.; Chung, C.-A.; Tsao, C.-W. Electrical stimulation to promote osteogenesis using conductive polypyrrole films. Mater. Sci. Eng. C 2014, 37, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Chen, J.; Zhou, L.; Tu, L.; Fan, L.; Zhang, F.; Dai, C.; Liu, Y.; Ning, C.; Du, J.; et al. Polypyrrole nanocones and dynamic piezoelectric stimulation-induced stem cell osteogenic differentiation. ACS Biomater. Sci. Eng. 2019, 5, 4386–4392. [Google Scholar] [CrossRef]
- Hardy, J.G.; Sukhavasi, R.C.; Aguilar, D.; Villancio-Wolter, M.K.; Mouser, D.J.; Geissler, S.A.; Nguy, L.; Chow, J.K.; Kaplan, D.L.; Schmidt, C.E. Electrical stimulation of human mesenchymal stem cells on biomineralized conducting polymers enhances their differentiation towards osteogenic outcomes. J. Mater. Chem. B 2015, 3, 8059–8064. [Google Scholar] [CrossRef] [Green Version]
- Zhou, T.; Yan, L.; Xie, C.; Li, P.; Jiang, L.; Fang, J.; Zhao, C.; Ren, F.; Wang, K.; Wang, Y.; et al. A mussel-inspired persistent ROS-scavenging, electroactive, and osteoinductive scaffold based on electrochemical-driven in situ nanoassembly. Small 2019, 15, 1805440. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Wang, D.; Ke, J.; Ma, L.; Zhou, J.; Shao, H.; Zhu, H.; Liu, L.; Zhang, Y.; Peng, F.; et al. Improved in vitro angiogenic behavior of human umbilical vein endothelial cells with oxidized polydopamine coating. Colloids Surf. B 2020, 194, 111176. [Google Scholar] [CrossRef]
- Waite, J.H. The adhesive proteins secreted by mussels are the inspiration behind a versatile approach to the surface modification of a wide range of inorganic and organic materials, resulting in the fabrication of multifunctional coatings for a variety of applications. Nat. Mater. 2008, 7, 8–9. [Google Scholar] [CrossRef]
- Zhang, W.; Pan, Z.; Yang, F.K.; Zhao, B. A facile in situ approach to polypyrrole functionalization through bioinspired catechols. Adv. Funct. Mater. 2015, 25, 1588–1597. [Google Scholar] [CrossRef]
- Zeng, X.; Luo, M.; Liu, G.; Wang, X.; Tao, W.; Lin, Y.; Ji, X.; Nie, L.; Mei, L. Polydopamine-modified black phosphorous nanocapsule with enhanced stability and photothermal performance for tumor multimodal treatments. Adv. Sci. 2018, 5, 1800510. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.-B.; Hu, W.; Wu, M.; Gong, L.; Tang, A.; Xiang, L.; Zhu, B.; Zhu, L.-P.; Zeng, H. Cost-effective strategy for surface modification via complexation of disassembled polydopamine with Fe(III) ions. Langmuir 2019, 35, 4101–4109. [Google Scholar] [CrossRef]
- Ponzio, F.; Bertani, P.; Ball, V. Role of surfactants in the control of dopamine–eumelanin particle size and in the inhibition of film deposition at solid–liquid interfaces. J. Colloid Interface Sci. 2014, 431, 176–179. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, F.K.; Pan, Z.; Zhang, J.; Zhao, B. Bio-inspired dopamine functionalization of polypyrrole for improved adhesion and conductivity. Macromol. Rapid Commun. 2014, 35, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Jang, L.K.; Park, H.S.; Lee, J.Y. Electrochemical deposition of conductive and adhesive polypyrrole-dopamine films. Sci. Rep. 2016, 6, 30475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, J.; Yang, M.; Luo, L.; Xu, A.; Tang, B.; Cheng, D.; Cai, G.; Wang, X. Stretchable and highly sensitive braided composite yarn@polydopamine@polypyrrole for wearable applications. ACS Appl. Mater. Interfaces 2019, 11, 7338–7348. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Jang, L.K.; Jang, M.; Lee, S.; Hardy, J.G.; Lee, J.Y. Electrically conductive polydopamine-polypyrrole as high performance biomaterials for cell stimulation in vitro and electrical signal recording in vivo. ACS Appl. Mater. Interfaces 2018, 10, 33032–33042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Zhou, Y.; Feng, K.; Trinidad, J.; Yu, A.; Zhao, B. Morphologically controlled bioinspired dopamine-polypyrrole nanostructures with tunable electrical properties. Adv. Electron. Mater. 2015, 1, 1500205. [Google Scholar] [CrossRef]
- Xie, C.; Li, P.; Han, L.; Wang, Z.; Zhou, T.; Deng, W.; Wang, K.; Lu, X. Electroresponsive and cell-affinitive polydopamine/polypyrrole composite microcapsules with a dual-function of on-demand drug delivery and cell stimulation for electrical therapy. NPG Asia Mater. 2017, 9, 358. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Zhou, L.; Yu, P.; Liu, Y.; Chen, J.; Liao, J.; Li, W.; Chen, W.; Zhou, W.; Yi, X.; et al. Polydopamine-assisted electrochemical fabrication of polypyrrole nanofibers on bone implants to improve bioactivity. Macromol. Mater. Eng. 2016, 301, 1288–1294. [Google Scholar] [CrossRef]
- Tan, J.; Zhang, Z.; He, Y.; Yue, Q.; Xie, Z.; Ji, H.; Sun, Y.; Shi, W.; Ge, D. Electrochemical synthesis of conductive. superhydrophobic and adhesive polypyrrole-polydopamine nanowires. Synth. Met. 2017, 234, 86–94. [Google Scholar] [CrossRef]
- Huang, J.; Wang, K.; Wei, Z. Conducting polymernanowire arrays with enhanced electrochemical performance. J. Mater. Chem. 2010, 20, 1117–1121. [Google Scholar] [CrossRef]
- Debiemme-Chouvy, C. Template-free one-step electrochemical formation of polypyrrole nanowire array. Electrochem. Commun. 2009, 11, 298–301. [Google Scholar] [CrossRef]
- Ma, F.-F.; Zhang, D.; Zhang, N.; Huang, T.; Wang, Y. Polydopamine-assisted deposition of polypyrrole on electrospun poly(vinylidene fluoride) nanofibers for bidirectional removal of cation and anion dyes. Chem. Eng. J. 2018, 354, 432–444. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.; Pan, T.; Wang, R.; Yue, Y.; Shen, J. Anti-corrosion and conductivity of the electrodeposited graphene/polypyrrole composite coating for metallic bipolar plates. Prog. Org. Coat 2019, 136, 105237. [Google Scholar] [CrossRef]
- Luo, R.; Tang, L.; Wang, J.; Zhao, Y.; Tu, Q.; Weng, Y.; Shen, R.; Huang, N. Improved immobilization of biomolecules to quinone-rich polydopamine for efficient surface functionalization. Colloids Surf. B 2013, 106, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Zangmeister, R.A.; Morris, T.A.; Tarlov, M.J. Characterization of polydopamine thin films deposited at short times by autoxidation of dopamine. Langmuir 2013, 29, 8619–8628. [Google Scholar] [CrossRef]
- Qqarles, L.D.; Yohay, D.A.; Lever, L.W.; Caton, R.; Wenstrup, R.J. Distinct proliferative and differentiated stages of murine MC3T3-El cells in culture: An in vitro model of osteoblast development. J. Bone Miner. Res. 1992, 7, 683–692. [Google Scholar] [CrossRef]
- Lirani-Galvão, A.P.R.; Chavassieux, P.; Portero-Muzy, N.; Bergamaschi, C.T.; Silva, O.L.; Carvalho, A.B.; Lazaretti-Castro, M.; Delmas, P.D. Low-intensity electrical stimulation counteracts the effects of ovariectomy on bone tissue of rats: Effects on bone microarchitecture, viability of osteocytes, and nitric oxide expression. Calcif. Tissue Int. 2009, 84, 502–509. [Google Scholar] [CrossRef]
- Lirani-Galvão, A.P.R.; Lazaretti-Castro, M.; Portero-Muzy, N.; Bergamaschi, C.T.; Silva, O.L.; Carvalho, A.B.; Delmas, P.D.; Chavassieux, P. Is nitric oxide a mediator of the effects of low-intensity electrical stimulation on bone in ovariectomized rats? Calcif. Tissue Int. 2010, 87, 52–59. [Google Scholar] [CrossRef]
- He, Y.; Wang, S.; Mu, J.; Dai, L.; Zhang, Z.; Sun, Y.; Shi, W.; Ge, D. Synthesis of polypyrrole nanowires with positive effect on MC3T3-E1 cell functions through electrical stimulation. Mater. Sci. Eng. C 2017, 71, 43–50. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Y.; Dai, L.; Zhang, X.; Sun, Y.; Shi, W.; Ge, D. The Bioactive Polypyrrole/Polydopamine Nanowire Coating with Enhanced Osteogenic Differentiation Ability with Electrical Stimulation. Coatings 2020, 10, 1189. https://doi.org/10.3390/coatings10121189
He Y, Dai L, Zhang X, Sun Y, Shi W, Ge D. The Bioactive Polypyrrole/Polydopamine Nanowire Coating with Enhanced Osteogenic Differentiation Ability with Electrical Stimulation. Coatings. 2020; 10(12):1189. https://doi.org/10.3390/coatings10121189
Chicago/Turabian StyleHe, Yuan, Lingfeng Dai, Xiuming Zhang, Yanan Sun, Wei Shi, and Dongtao Ge. 2020. "The Bioactive Polypyrrole/Polydopamine Nanowire Coating with Enhanced Osteogenic Differentiation Ability with Electrical Stimulation" Coatings 10, no. 12: 1189. https://doi.org/10.3390/coatings10121189
APA StyleHe, Y., Dai, L., Zhang, X., Sun, Y., Shi, W., & Ge, D. (2020). The Bioactive Polypyrrole/Polydopamine Nanowire Coating with Enhanced Osteogenic Differentiation Ability with Electrical Stimulation. Coatings, 10(12), 1189. https://doi.org/10.3390/coatings10121189



