Fabrication of Zinc Oxide-Xanthan Gum Nanocomposite via Green Route: Attenuation of Quorum Sensing Regulated Virulence Functions and Mitigation of Biofilm in Gram-Negative Bacterial Pathogens
Abstract
1. Introduction
2. Materials and Experimental Methods
2.1. Chemicals
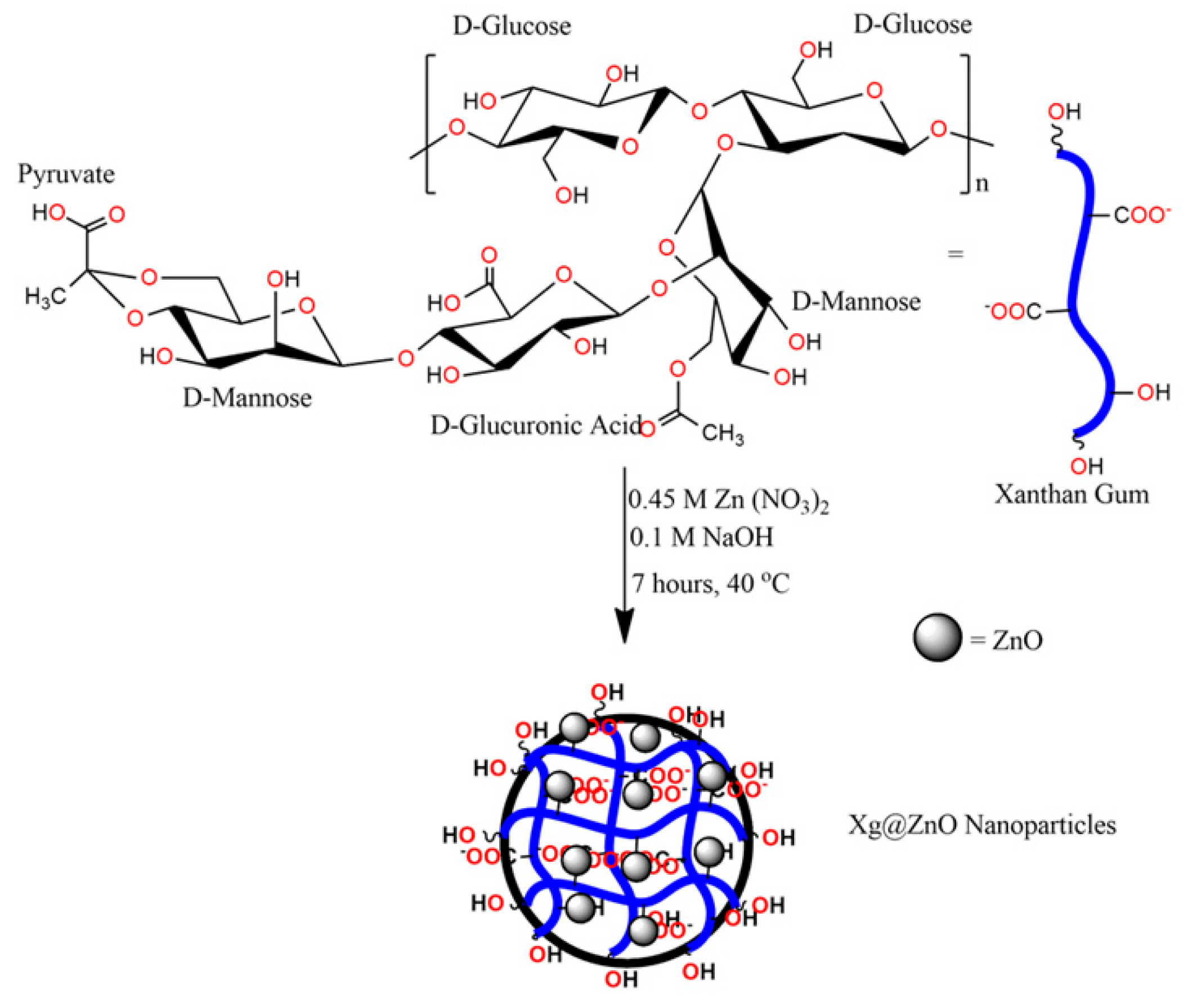
2.2. One-Pot Green Synthesis of ZnO@XG Nanoparticles
2.3. Analytical Techniques Used for Characterization
2.4. Bacterial Strains
2.5. Determination of Minimum Inhibitory Concentration (MIC)
2.6. Violacein Inhibition Assay
2.7. Chitinolytic Activity
2.8. Prodigiosin Assay
2.9. Protease Assay
2.10. Biofilm Inhibition Studies
2.10.1. Microtiter Plate Assay
2.10.2. Confocal Laser Scanning Microscopic (CLSM) Visualization of Biofilm Structure
2.10.3. Quantification of EPS
2.10.4. Swarming Motility
2.10.5. Microbial Adhesion to Hydrocarbon (MATH) Assay
2.11. Disruption of Preformed Biofilms
3. Results and Discussion
3.1. FTIR
3.2. XRD
3.3. Morphological Analysis: SEM and TEM
3.4. BET
3.5. MIC
3.6. QS Interference in C. violaceum
3.7. QS Interference in S. marcescens
3.8. Effect on Biofilm and Biofilm-Related Virulence Functions
3.8.1. Inhibition of Biofilm Formation
3.8.2. EPS Production
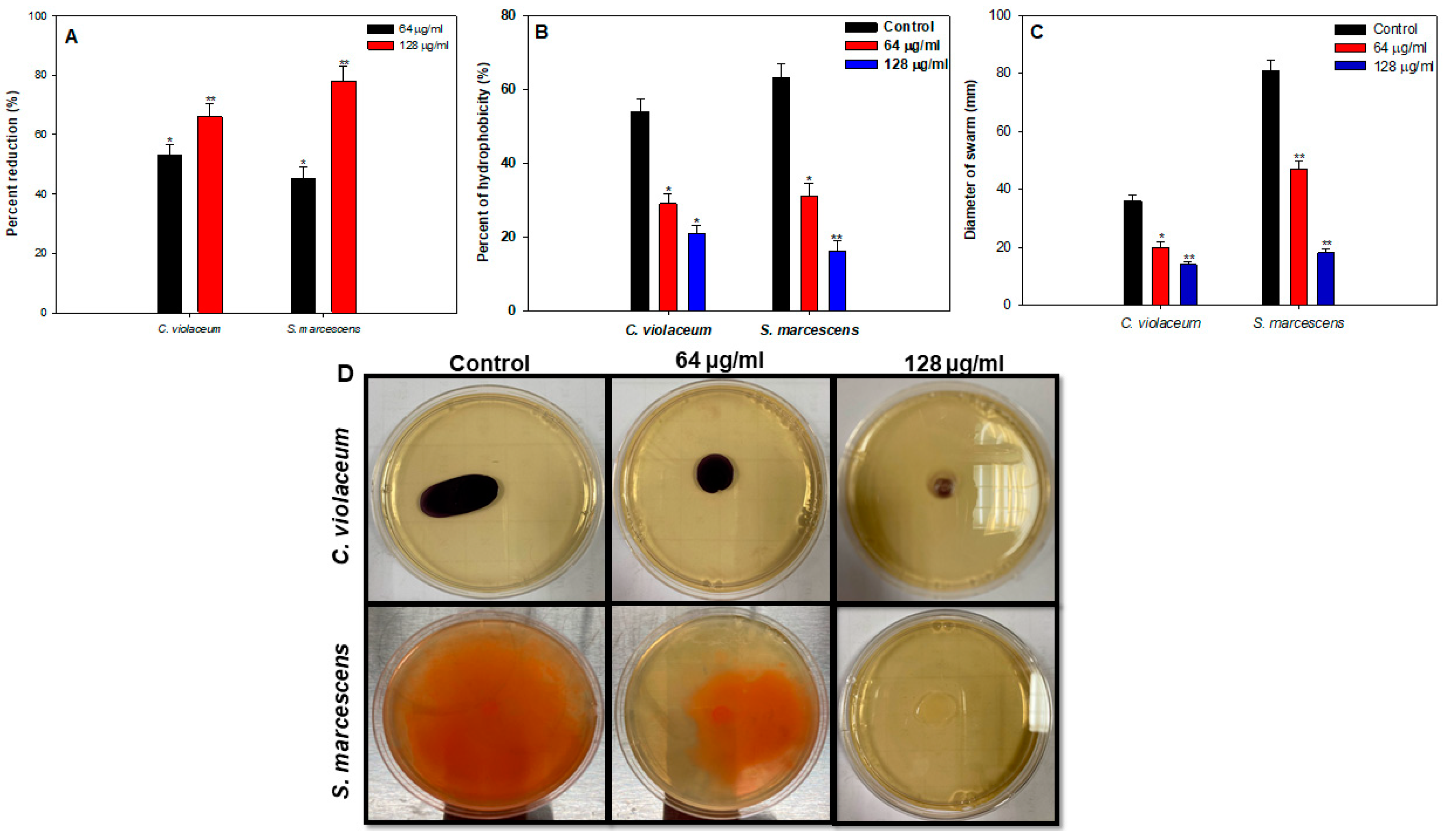
3.8.3. Cell-Surface Hydrophobicity (CSH)
3.8.4. Swarming Motility
3.9. Disruption of Preformed Biofilm
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alizadeh-Sani, M.; Ehsani, A.; Moghaddas Kia, E.; Khezerlou, A. Microbial gums: Introducing a novel functional component of edible coatings and packaging. Appl. Microbiol. Biotechnol. 2019, 103, 6853–6866. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Jin, W.; Huang, K.; Huang, L.; Lou, Y.; Li, J.; Liu, X.; Li, B. Interfacial and emulsion stabilized behavior of lysozyme/xanthan gum nanoparticles. Int. J. Biol. Macromol. 2018, 117, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Ghorai, S.; Sarkar, A.; Panda, A.B.; Pal, S. Evaluation of the flocculation characteristics of polyacrylamide grafted xanthan gum/silica hybrid nanocomposite. Ind. Eng. Chem. Res. 2013, 52, 9731–9740. [Google Scholar] [CrossRef]
- Joshy, K.S.; Jose, J.; Li, T.; Thomas, M.; Shankregowda, A.M.; Sreekumaran, S.; Kalarikkal, N.; Thomas, S. Application of novel zinc oxide reinforced xanthan gum hybrid system for edible coatings. Int. J. Biol. Macromol. 2020, 151, 806–813. [Google Scholar] [CrossRef]
- Al-Shabib, N.A.; Husain, F.M.; Hassan, I.; Khan, M.S.; Ahmed, F.; Qais, F.A.; Oves, M.; Rahman, M.; Khan, R.A.; Khan, A.; et al. Biofabrication of zinc oxide nanoparticle from ochradenus baccatus leaves: Broad-spectrum antibiofilm activity, protein binding studies, and in vivo toxicity and Stress studies. J. Nanomater. 2018, 2018. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on zinc oxide nanoparticles: Antibacterial activity and toxicity mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef]
- Vikesland, P.; Garner, E.; Gupta, S.; Kang, S.; Maile-Moskowitz, A.; Zhu, N. Differential drivers of antimicrobial resistance across the world. Acc. Chem. Res. 2019, 52, 916–924. [Google Scholar] [CrossRef]
- WHO. The Top 10 Causes of Death; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- Dadgostar, P. Antimicrobial resistance: Implications and costs. Infect. Drug Resist. 2019, 12, 3903–3910. [Google Scholar] [CrossRef]
- WHO. No Time to Wait: Securing the Future from Drug-Resistant Infections; WHO: Geneva, Switzerland, 2019. [Google Scholar]
- Shakoor, S.; Platts-Mills, J.A.; Hasan, R. Antibiotic-resistant enteric infections. Infect. Dis. Clin. N. Am. 2019, 33, 1105–1123. [Google Scholar] [CrossRef]
- Andleeb, S.; Majid, M.; Sardar, S. Environmental and public health effects of antibiotics and AMR/ARGs. In Antibiotics and Antimicrobial Resistance Genes in the Environment; Elsevier: Amsterdam, The Netherlands, 2020; pp. 269–291. [Google Scholar]
- Ahmad, I.; Qais, F.A.; Abulreesh, H.H.; Ahmad, S.; Rumbaugh, K.P. Antibacterial drug discovery: Perspective insights. In Antibacterial Drug Discovery to Combat MDR; Springer: Singapore, 2019; pp. 1–21. [Google Scholar]
- Albrich, W.C.; Monnet, D.L.; Harbarth, S. Antibiotic selection pressure and resistance in streptococcus pneumoniae and streptococcus pyogenes. Emerg. Infect. Dis. 2004, 10, 514–517. [Google Scholar] [CrossRef]
- Miller, K. Response of Escherichia coli hypermutators to selection pressure with antimicrobial agents from different classes. J. Antimicrob. Chemother. 2002, 49, 925–934. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, S.T.; Bassler, B.L. Bacterial quorum sensing: Its role in virulence and possibilities for its control. Cold Spring Harb. Perspect. Med. 2012, 2, a012427. [Google Scholar] [CrossRef] [PubMed]
- Qais, F.A.; Khan, M.S.; Ahmad, I. Nanoparticles as quorum sensing inhibitor: Prospects and limitations. In Biotechnological Applications of Quorum Sensing Inhibitors; Kalia, V.C., Ed.; Springer: Singapore, 2018; pp. 227–244. [Google Scholar]
- Davies, D.G.; Parsek, M.R.; Pearson, J.P.; Iglewski, B.H.; Costerton, J.W.; Greenberg, E.P. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science 1998, 280, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Percival, S.L.; Hill, K.E.; Williams, D.W.; Hooper, S.J.; Thomas, D.W.; Costerton, J.W. A review of the scientific evidence for biofilms in wounds. Wound Repair Regen. 2012, 20, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Martins, N.; Rodrigues, C.F. Biomaterial-related infections. J. Clin. Med. 2020, 9, 722. [Google Scholar] [CrossRef] [PubMed]
- Lasa, I.; del Pozo, J.L.; Penadés, J.R.; Leiva, J. Biofilms bacterianos e infección. An. Sist. Sanit. Navar. 2005, 28, 163–175. [Google Scholar] [CrossRef]
- Qais, F.A.; Ahmad, I. Green synthesis of metal nanoparticles: Characterization and their antibacterial efficacy. In Antibacterial Drug Discovery to Combat MDR; Springer: Singapore, 2019; pp. 635–680. [Google Scholar]
- Al-Shabib, N.A.; Husain, F.M.; Ahmad, N.; Qais, F.A.; Khan, A.; Khan, A.; Khan, M.S.; Khan, J.M.; Shahzad, S.A.; Ahmad, I. Facile synthesis of tin oxide hollow nanoflowers interfering with quorum sensing-regulated functions and bacterial biofilms. J. Nanomater. 2018, 2018. [Google Scholar] [CrossRef]
- Husain, F.M.; Ansari, A.A.; Khan, A.; Ahmad, N.; Albadri, A.; Albalawi, T.H. Mitigation of acyl-homoserine lactone (AHL) based bacterial quorum sensing, virulence functions, and biofilm formation by yttrium oxide core/shell nanospheres: Novel approach to combat drug resistance. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Basha, S.K.; Lakshmi, K.V.; Kumari, V.S. Ammonia sensor and antibacterial activities of green zinc oxide nanoparticles. Sens. Bio-Sens. Res. 2016, 10, 34–40. [Google Scholar] [CrossRef]
- Andrews, J.M. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 2001, 48, 5–16. [Google Scholar] [CrossRef]
- Husain, F.M.; Ahmad, I. Doxycycline interferes with quorum sensing-mediated virulence factors and biofilm formation in Gram-negative bacteria. World J. Microbiol. Biotechnol. 2013, 29. [Google Scholar] [CrossRef] [PubMed]
- Matz, C.; Deines, P.; Boenigk, J.; Arndt, H.; Eberl, L.; Kjelleberg, S.; Jurgens, K. Impact of violacein-producing bacteria on survival and feeding of bacterivorous nanoflagellates. Appl. Environ. Microbiol. 2004, 70, 1593–1599. [Google Scholar] [CrossRef] [PubMed]
- Chernin, L.S.; Winson, M.K.; Thompson, J.M.; Haran, S.; Bycroft, B.W.; Chet, I.; Williams, P.; Stewart, G.S.A.B. Chitinolytic activity in chromobacterium violaceum: Substrate analysis and regulation by quorum sensing. J. Bacteriol. 1998, 180, 4435–4441. [Google Scholar] [CrossRef]
- Champalal, L.; Kumar, U.S.; Krishnan, N.; Vaseeharan, B.; Mariappanadar, V.; Raman, P. Modulation of quorum sensing-controlled virulence factors in chromobacterium violaceum by selective amino acids. FEMS Microbiol. Lett. 2018, 365, 1–8. [Google Scholar] [CrossRef]
- Morohoshi, T.; Shiono, T.; Takidouchi, K.; Kato, M.; Kato, N.; Kato, J.; Ikeda, T. Inhibition of quorum sensing in serratia marcescens AS-1 by synthetic analogs of N-acylhomoserine lactone. Appl. Environ. Microbiol. 2007, 73, 6339–6344. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, D.; Ramanathan, S.; Arunachalam, K.; Jeyaraj, G.P.; Shunmugiah, K.P.; Arumugam, V.R. Phytosynthesized silver nanoparticles as antiquorum sensing and antibiofilm agent against the nosocomial pathogen Serratia marcescens: An in vitro study. J. Appl. Microbiol. 2018, 124, 1425–1440. [Google Scholar] [CrossRef]
- Al-Shabib, N.A.; Husain, F.M.; Nadeem, M.; Khan, M.S.; Al-Qurainy, F.; Alyousef, A.A.; Arshad, M.; Khan, A.; Khan, J.M.; Alam, P.; et al. Bio-inspired facile fabrication of silver nanoparticles from: In vitro grown shoots of Tamarix nilotica: Explication of its potential in impeding growth and biofilms of Listeria monocytogenes and assessment of wound healing ability. RSC Adv. 2020, 10. [Google Scholar] [CrossRef]
- Al-Shabib, N.A.; Husain, F.M.; Qais, F.A.; Ahmad, N.; Khan, A.; Alyousef, A.A.; Arshad, M.; Noor, S.; Khan, J.M.; Alam, P.; et al. Phyto-mediated synthesis of porous titanium dioxide nanoparticles from withania somnifera root extract: Broad-spectrum attenuation of biofilm and cytotoxic properties against HepG2 cell lines. Front. Microbiol. 2020, 11, 1680. [Google Scholar] [CrossRef]
- Husain, F.M.; Ahmad, I.; Baig, M.H.; Khan, M.S.; Khan, M.S.; Hassan, I.; Al-Shabib, N.A. Broad-spectrum inhibition of AHL-regulated virulence factors and biofilms by sub-inhibitory concentrations of ceftazidime. RSC Adv. 2016, 6. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Qais, F.A.; Shafiq, A.; Ahmad, I.; Husain, F.M.; Khan, R.A.; Hassan, I. Green synthesis of silver nanoparticles using Carum copticum: Assessment of its quorum sensing and biofilm inhibitory potential against gram negative bacterial pathogens. Microb. Pathog. 2020, 144, 104172. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, M.; Gutnick, D.; Rosenberg, E. Adherence of bacteria to hydrocarbons: A simple method for measuring cell-surface hydrophobicity. FEMS Microbiol. Lett. 1980, 9, 29–33. [Google Scholar] [CrossRef]
- Al-Shabib, N.A.; Husain, F.M.; Rehman, M.T.; Alyousef, A.A.; Arshad, M.; Khan, A.; Masood Khan, J.; Alam, P.; Albalawi, T.A.; Shahzad, S.A.; et al. Food color ‘Azorubine’ interferes with quorum sensing regulated functions and obliterates biofilm formed by food associated bacteria: An in vitro and in silico approach. Saudi J. Biol. Sci. 2020, 27, 1080–1090. [Google Scholar] [CrossRef] [PubMed]
- Mohsin, A.; Zhang, K.; Hu, J.; Tariq, M.; Zaman, W.Q.; Khan, I.M.; Zhuang, Y.; Guo, M. Optimized biosynthesis of xanthan via effective valorization of orange peels using response surface methodology: A kinetic model approach. Carbohydr. Polym. 2018, 181, 793–800. [Google Scholar] [CrossRef]
- Chikkanna, M.M.; Neelagund, S.E.; Rajashekarappa, K.K. Green synthesis of Zinc oxide nanoparticles (ZnO NPs) and their biological activity. SN Appl. Sci. 2019, 1, 117. [Google Scholar] [CrossRef]
- Thirumavalavan, M.; Huang, K.-L.; Lee, J.-F. Preparation and morphology studies of nano Zinc oxide obtained using native and modified chitosans. Materials 2013, 6, 4198–4212. [Google Scholar] [CrossRef]
- Muhammad, W.; Ullah, N.; Haroon, M.; Abbasi, B.H. Optical, morphological and biological analysis of zinc oxide nanoparticles (ZnO NPs) using Papaver somniferum L. RSC Adv. 2019, 9, 29541–29548. [Google Scholar] [CrossRef]
- Scherrer, P. Estimation of the size and internal structure of colloidal particles by means of X rays. Nachs. Gissel. Wiss. Gott. 1918, 26, 98–100. [Google Scholar]
- Meng, A.; Xing, J.; Li, Z.; Li, Q. Cr-doped ZnO nanoparticles: Synthesis, characterization, adsorption property, and recyclability. ACS Appl. Mater. Interfaces 2015, 7, 27449–27457. [Google Scholar] [CrossRef]
- Wang, J.; Xia, Y.; Dong, Y.; Chen, R.; Xiang, L.; Komarneni, S. Defect-rich ZnO nanosheets of high surface area as an efficient visible-light photocatalyst. Appl. Catal. B Environ. 2016, 192, 8–16. [Google Scholar] [CrossRef]
- Le, T.K.; Nguyen, T.M.T.; Nguyen, H.T.P.; Nguyen, T.K.L.; Lund, T.; Nguyen, H.K.H.; Huynh, T.K.X. Enhanced photocatalytic activity of ZnO nanoparticles by surface modification with KF using thermal shock method. Arab. J. Chem. 2020, 13, 1032–1039. [Google Scholar] [CrossRef]
- Nagaraju, P.; Puttaiah, S.H.; Wantala, K.; Shahmoradi, B. Preparation of modified ZnO nanoparticles for photocatalytic degradation of chlorobenzene. Appl. Water Sci. 2020, 10, 137. [Google Scholar] [CrossRef]
- McLean, R.J.C.; Pierson, L.S.; Fuqua, C. A simple screening protocol for the identification of quorum signal antagonists. J. Microbiol. Methods 2004, 58, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Al-Shabib, N.A.; Husain, F.M.; Ahmed, F.; Khan, R.A.; Ahmad, I.; Alsharaeh, E.; Khan, M.S.; Hussain, A.; Rehman, M.T.; Yusuf, M.; et al. Biogenic synthesis of Zinc oxide nanostructures from Nigella sativa seed: Prospective role as food packaging material inhibiting broad-spectrum quorum sensing and biofilm. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- Liu, G.Y.; Nizet, V. Color me bad: Microbial pigments as virulence factors. Trends Microbiol. 2009, 17, 406–413. [Google Scholar] [CrossRef]
- Sethupathy, S.; Ananthi, S.; Selvaraj, A.; Shanmuganathan, B.; Vigneshwari, L.; Balamurugan, K.; Mahalingam, S.; Pandian, S.K. Vanillic acid from Actinidia deliciosa impedes virulence in Serratia marcescens by affecting S-layer, flagellin and fatty acid biosynthesis proteins. Sci. Rep. 2017, 7, 1–17. [Google Scholar] [CrossRef]
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial biofilms: From the natural environment to infectious diseases. Nat. Rev. Microbiol. 2004, 2, 95–108. [Google Scholar] [CrossRef]
- Rice, S.A.; Koh, K.S.; Queck, S.Y.; Labbate, M.; Lam, K.W.; Kjelleberg, S. Biofilm formation and sloughing in serratia marcescens are controlled by quorum sensing and nutrient cues. J. Bacteriol. 2005, 187, 3477–3485. [Google Scholar] [CrossRef]
- Zhou, J.-W.; Ruan, L.-Y.; Chen, H.-J.; Luo, H.-Z.; Jiang, H.; Wang, J.-S.; Jia, A.-Q. Inhibition of quorum sensing and virulence in serratia marcescens by hordenine. J. Agric. Food Chem. 2019, 67, 784–795. [Google Scholar] [CrossRef]
- Hasan, I.; Qais, F.A.; Husain, F.M.; Khan, R.A.; Alsalme, A.; Alenazi, B.; Usman, M.; Jaafar, M.H.; Ahmad, I. Eco-friendly green synthesis of dextrin based poly (methyl methacrylate) grafted silver nanocomposites and their antibacterial and antibiofilm efficacy against multi-drug resistance pathogens. J. Clean. Prod. 2019, 230, 1148–1155. [Google Scholar] [CrossRef]
- LewisOscar, F.; MubarakAli, D.; Nithya, C.; Priyanka, R.; Gopinath, V.; Alharbi, N.S.; Thajuddin, N. One pot synthesis and anti-biofilm potential of copper nanoparticles (CuNPs) against clinical strains of Pseudomonas aeruginosa. Biofouling 2015, 31, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Van Houdt, R.; Givskov, M.; Michiels, C.W. Quorum sensing in Serratia. FEMS Microbiol. Rev. 2007, 31, 407–424. [Google Scholar] [CrossRef] [PubMed]
- Fux, C.A.; Costerton, J.W.; Stewart, P.S.; Stoodley, P. Survival strategies of infectious biofilms. Trends Microbiol. 2005, 13, 34–40. [Google Scholar] [CrossRef] [PubMed]









| Component | 2θ | FWHM (βhkl) | Interlayer Spacing (A°) at 2θ | Crystallite Size (nm) at 2θ | Dislocation Density (δ) × 1015 Lines (m−2) | % Crystallinity (%) |
|---|---|---|---|---|---|---|
| ZnO NPs | 36.16 | 0.51 | 0.24 | 16.31 | 3.75 | 73 |
| ZnO@XG | 35.89 | 0.45 | 0.18 | 14.89 | 3.12 | 46 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Husain, F.M.; Hasan, I.; Qais, F.A.; Khan, R.A.; Alam, P.; Alsalme, A. Fabrication of Zinc Oxide-Xanthan Gum Nanocomposite via Green Route: Attenuation of Quorum Sensing Regulated Virulence Functions and Mitigation of Biofilm in Gram-Negative Bacterial Pathogens. Coatings 2020, 10, 1190. https://doi.org/10.3390/coatings10121190
Husain FM, Hasan I, Qais FA, Khan RA, Alam P, Alsalme A. Fabrication of Zinc Oxide-Xanthan Gum Nanocomposite via Green Route: Attenuation of Quorum Sensing Regulated Virulence Functions and Mitigation of Biofilm in Gram-Negative Bacterial Pathogens. Coatings. 2020; 10(12):1190. https://doi.org/10.3390/coatings10121190
Chicago/Turabian StyleHusain, Fohad Mabood, Imran Hasan, Faizan Abul Qais, Rais Ahmad Khan, Pravej Alam, and Ali Alsalme. 2020. "Fabrication of Zinc Oxide-Xanthan Gum Nanocomposite via Green Route: Attenuation of Quorum Sensing Regulated Virulence Functions and Mitigation of Biofilm in Gram-Negative Bacterial Pathogens" Coatings 10, no. 12: 1190. https://doi.org/10.3390/coatings10121190
APA StyleHusain, F. M., Hasan, I., Qais, F. A., Khan, R. A., Alam, P., & Alsalme, A. (2020). Fabrication of Zinc Oxide-Xanthan Gum Nanocomposite via Green Route: Attenuation of Quorum Sensing Regulated Virulence Functions and Mitigation of Biofilm in Gram-Negative Bacterial Pathogens. Coatings, 10(12), 1190. https://doi.org/10.3390/coatings10121190







