Reinforced Superhydrophobic Anti-Corrosion Epoxy Resin Coating by Fluorine–Silicon–Carbide Composites
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of Superhydrophobic Nano-SiC
2.3. Preparation of Epoxy Composite Coating
2.4. Characterization
3. Results
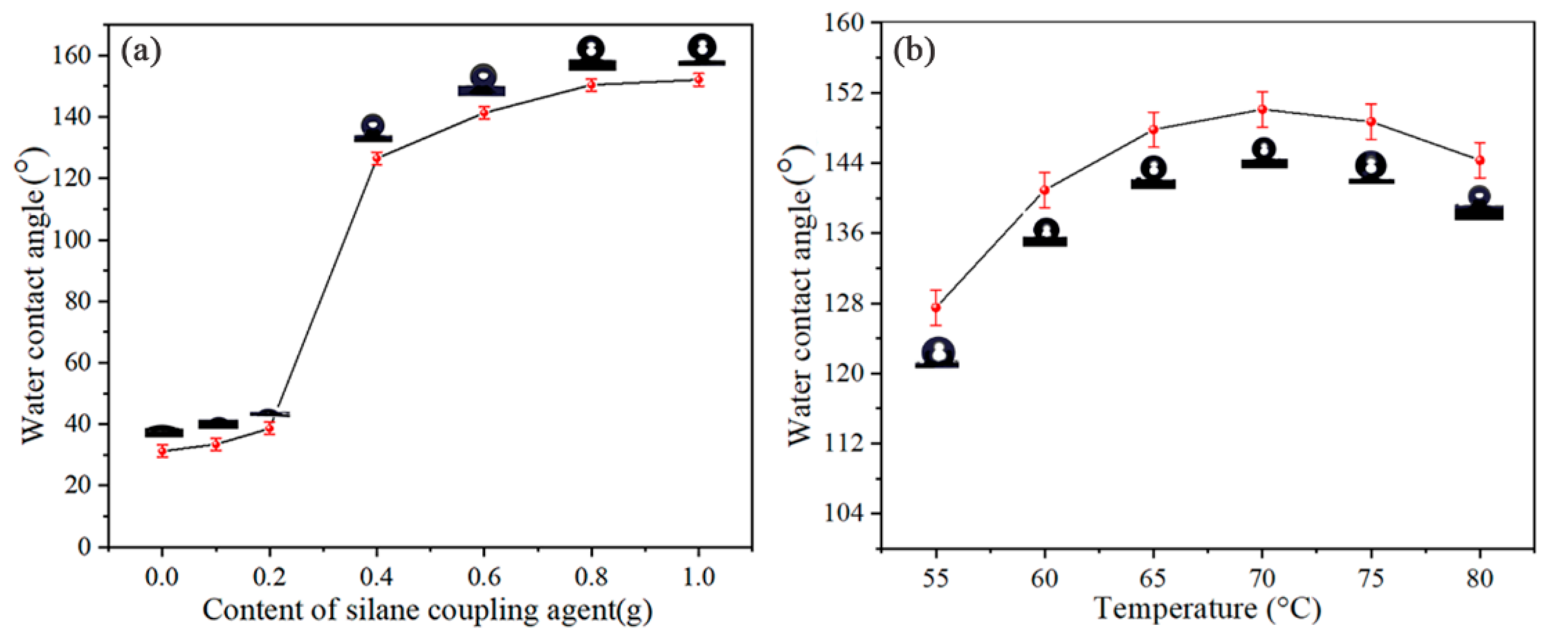
3.1. The Reaction Conditions for the Modification of SiC Powder by FAS
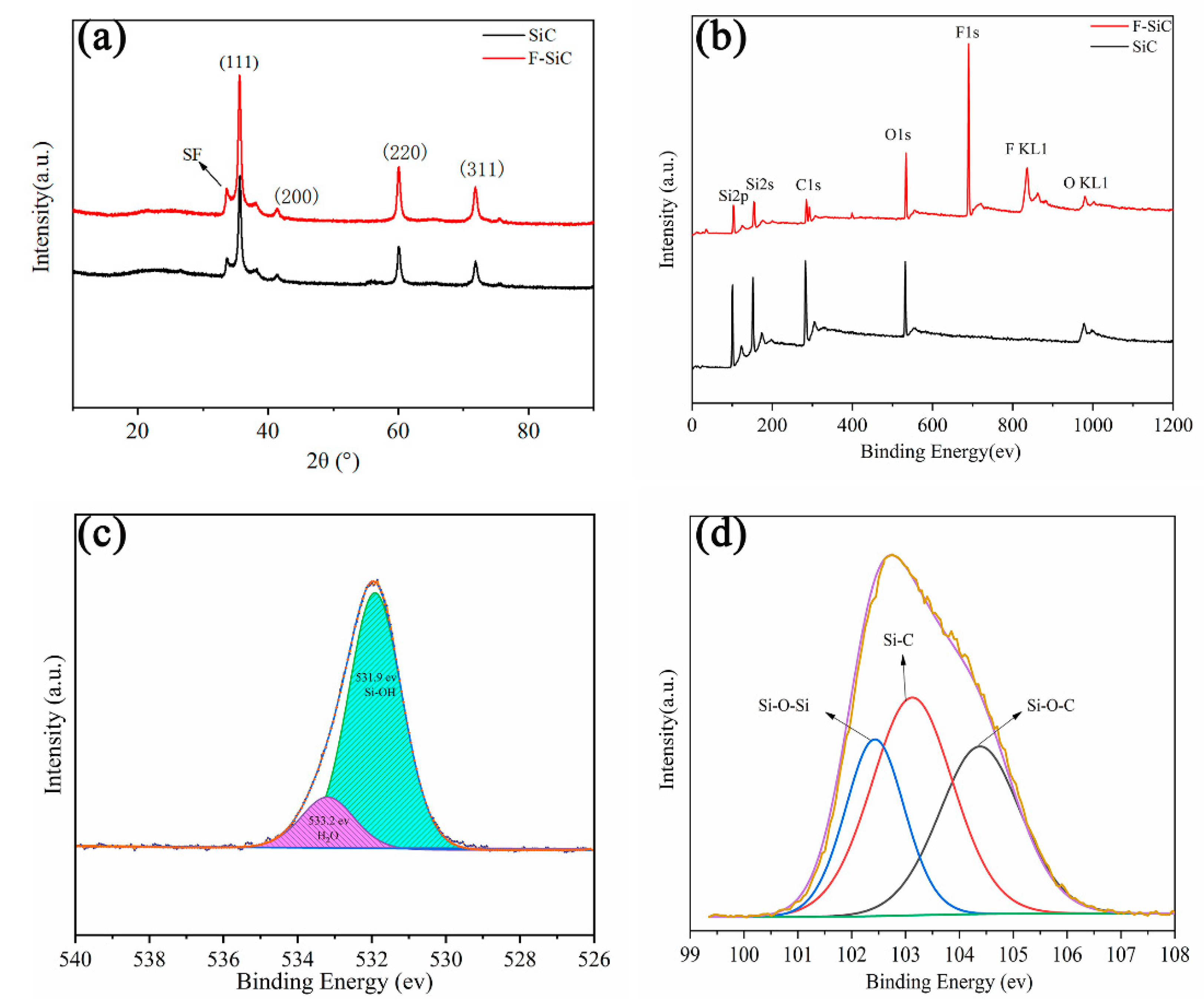
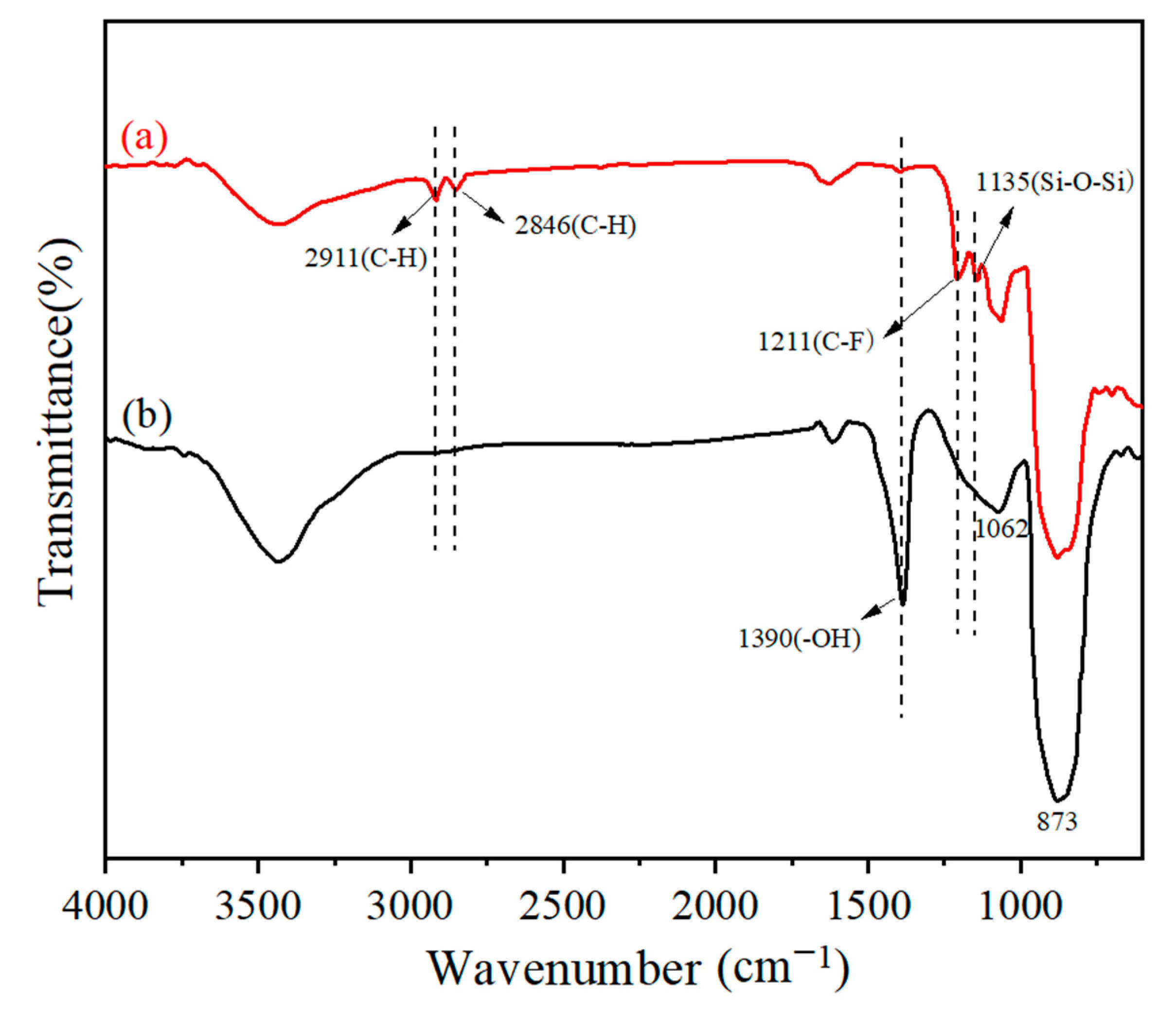
3.2. The Structural Characterization of F–SiC Powder
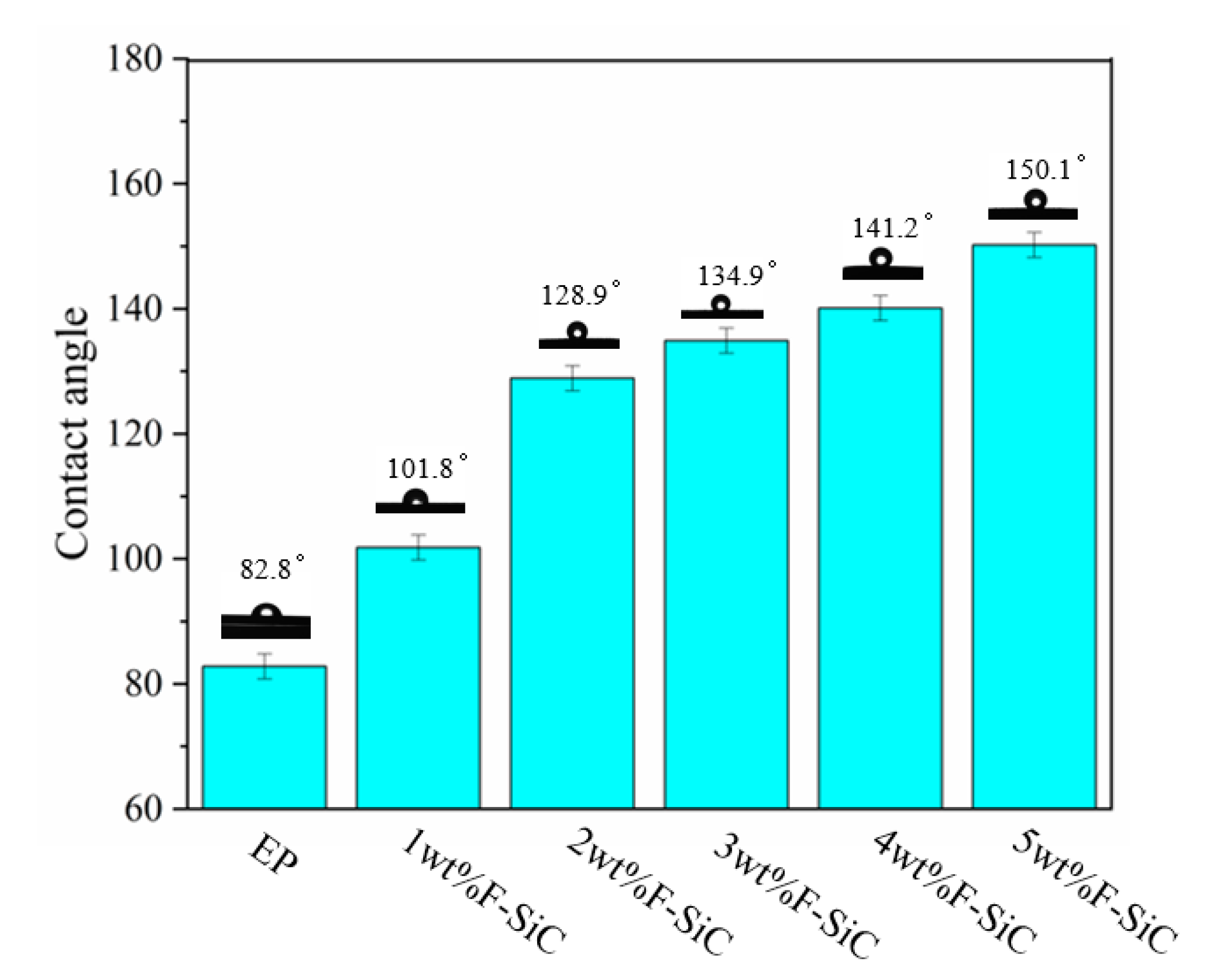
3.3. The Structural Characterization of the Coating System
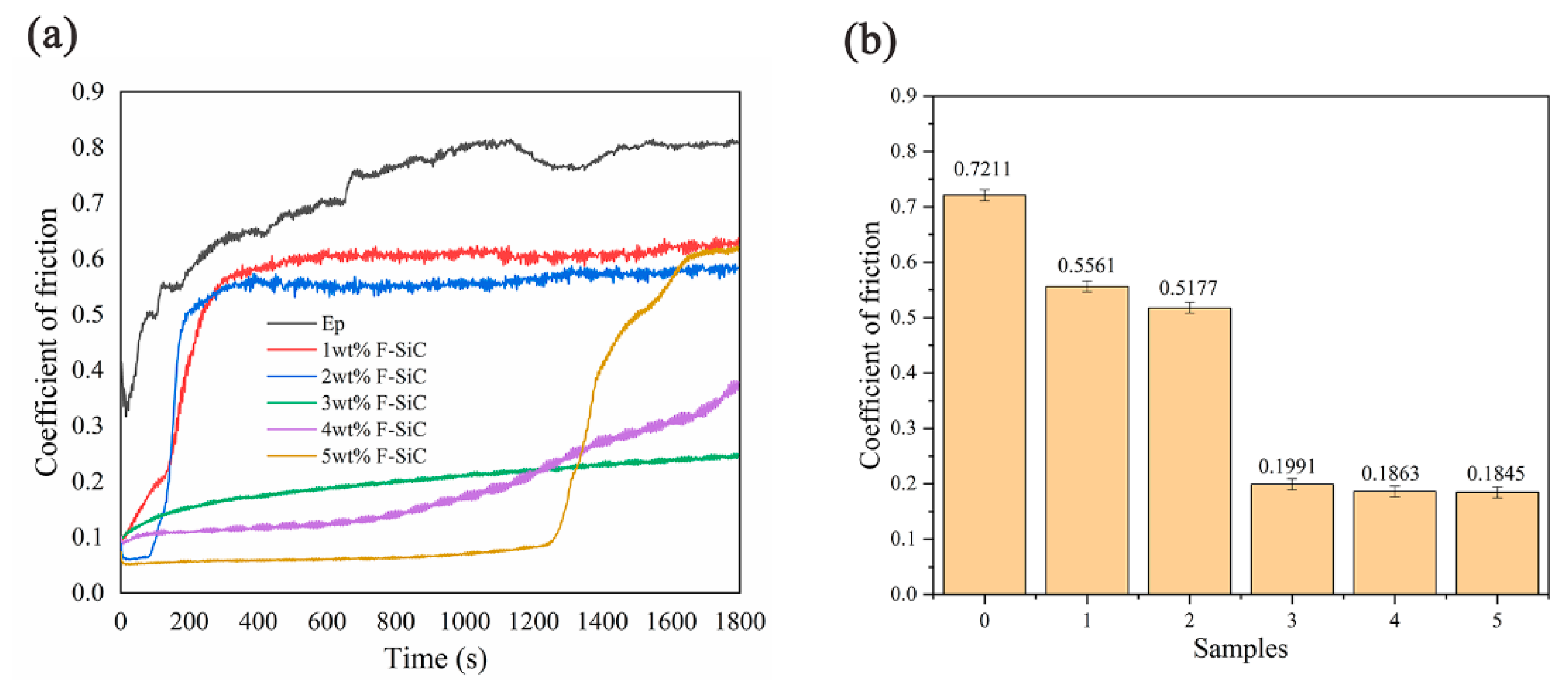
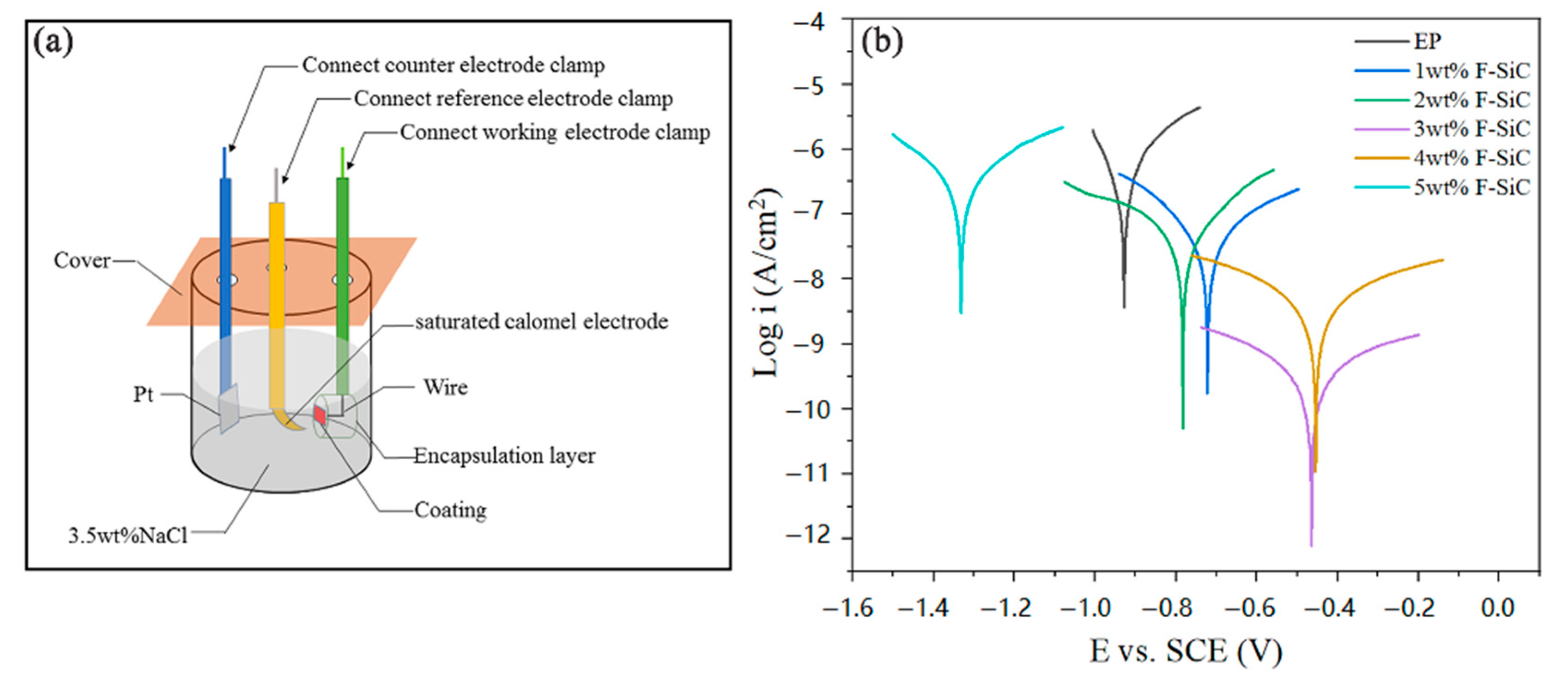
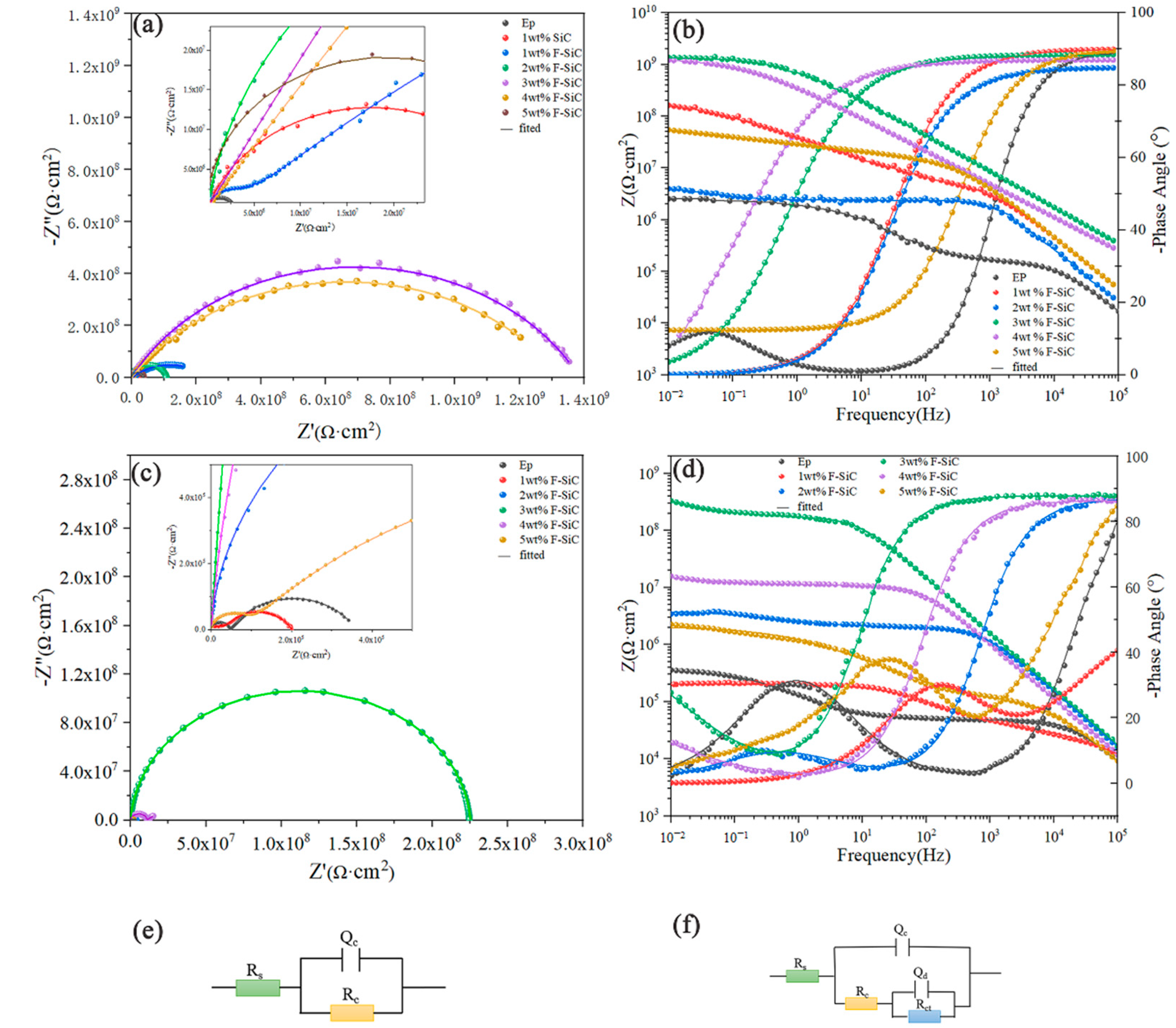
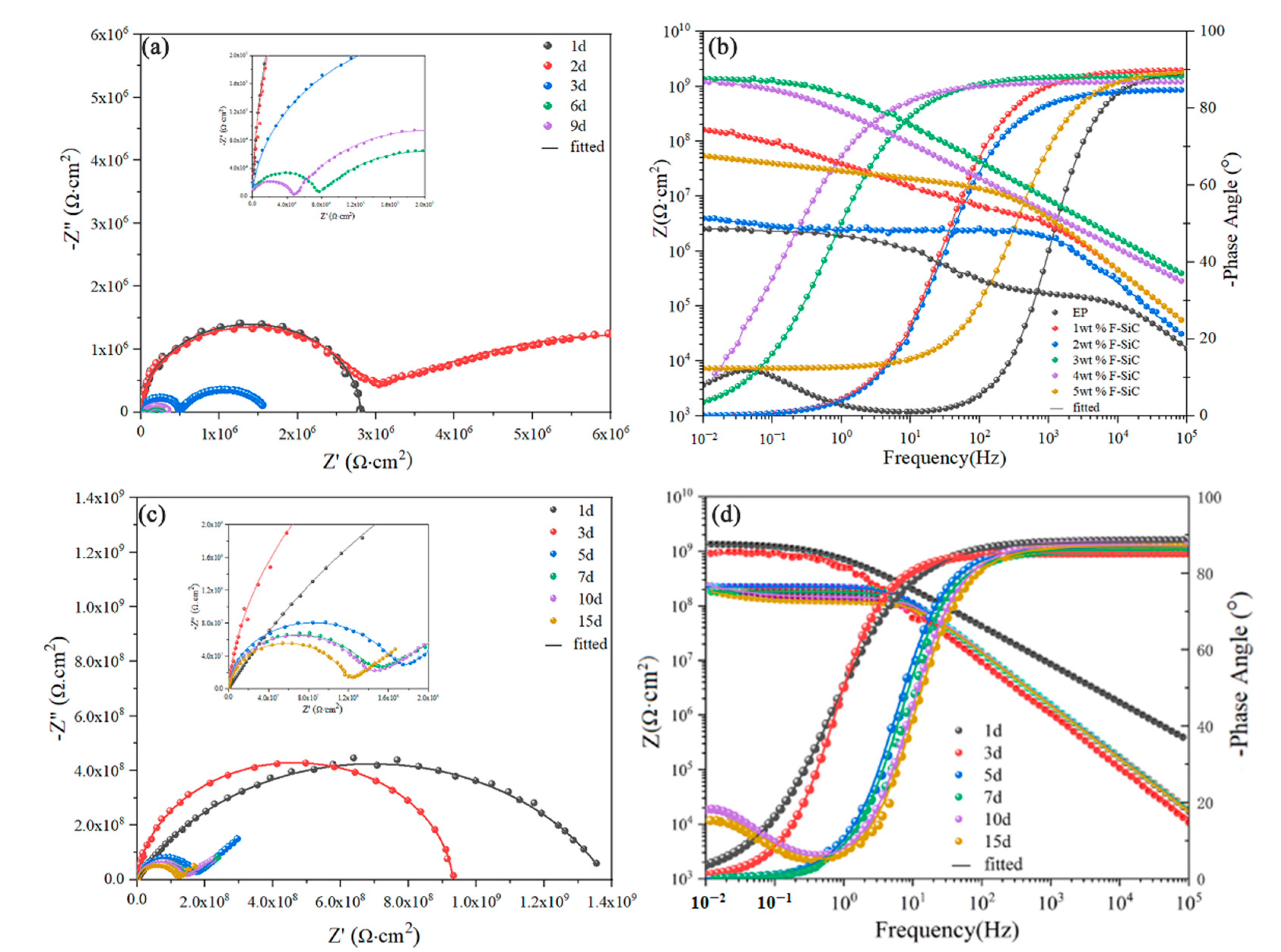
3.4. Corrosion Inhibitive Performance of Composite Coatings
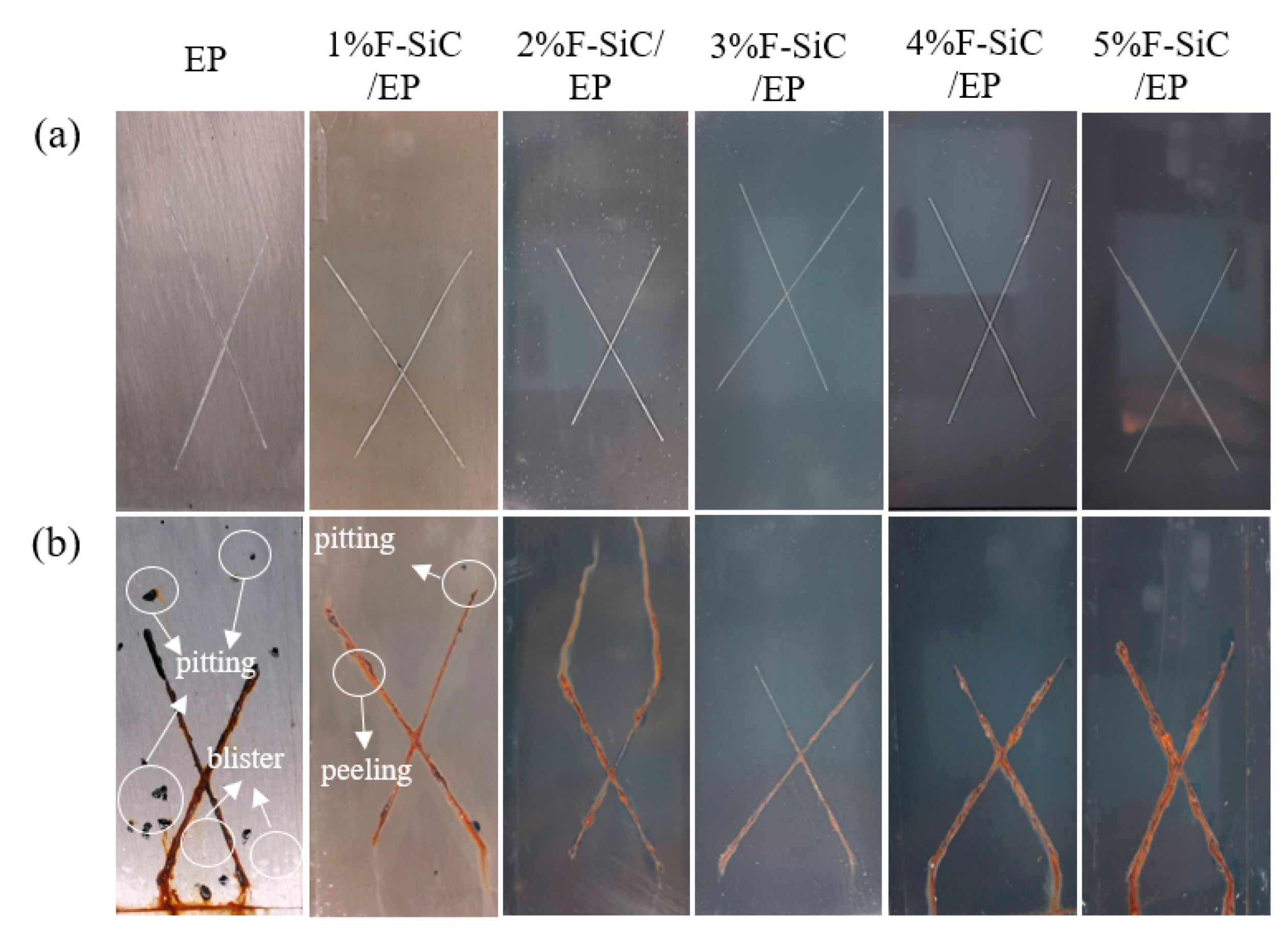
3.5. Salt Spray Corrosion Resistance
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chauhan, D.S.; Quraishi, M.A.; Ansari, K.R.; Saleh, T.A. Graphene and graphene oxide as new class of materials for corrosion control and protection: Present status and future scenario. Prog. Org. Coat. 2020, 147, 105741. [Google Scholar] [CrossRef]
- Sun, P.; Wang, Z.; Lu, Y.; Shen, S.; Yang, R.; Xue, A.; Parker, T.; Wang, J.; Wang, Q. Analysis of the corrosion failure of a semiconductor polycrystalline distillation column. Process Saf. Environ. Prot. 2020, 135, 244–256. [Google Scholar] [CrossRef]
- Bradl, H.B. Adsorption of heavy metal ions on soils and soils constituents. J. Colloid Interface Sci. 2004, 277, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Chajduk, E.; Bojanowska-Czajka, A. Corrosion mitigation in coolant systems in nuclear power plants. Prog. Nucl. Energy 2016, 88, 1–9. [Google Scholar] [CrossRef]
- Tang, Z. A review of corrosion inhibitors for rust preventative fluids. Curr. Opin. Solid State Mater. Sci. 2019, 23, 100759. [Google Scholar] [CrossRef]
- Grundmeier, G.; Schmidt, W.; Stratmann, M. Corrosion protection by organic coatings: Electrochemical mechanism and novel methods of investigation. Electrochim. Acta 2000, 45, 2515–2533. [Google Scholar] [CrossRef]
- Li, M.; Hu, P.; Zhang, Y.; Chang, Y. Enhancing performance of the CuCrZrTiV alloys through increasing recrystallization resistance and two-step thermomechanical treatments. J. Nucl. Mater. 2021, 543, 152482. [Google Scholar] [CrossRef]
- Zhang, J.J.; Liu, J.C.; Liao, H.M.; Zeng, M.; Ma, S. A review on relationship between morphology of boride of Fe-B alloys and the wear/corrosion resistant properties and mechanisms. Mater. Today Proc. 2020, 8, 6308–6320. [Google Scholar] [CrossRef]
- Fallah, P.; Rajagopalan, S.; McDonald, A.; Yue, S. Development of hybrid metallic coatings on carbon fiber-reinforced polymers (CFRPs) by cold spray deposition of copper-assisted copper electroplating process. Surf. Coat. Technol. 2020, 400, 126231. [Google Scholar] [CrossRef]
- Lim, J.; Lee, C. The rf-power dependences of the deposition rate, the hardness and the corrosion-resistance of the chromium nitride film deposited by using a dual ion beam sputtering system. Mater. Chem. Phys. 2006, 95, 164–168. [Google Scholar] [CrossRef]
- Olajire, A.A. Recent advances on organic coating system technologies for corrosion protection of offshore metallic structures. J. Mol. Liq. 2018, 269, 572–606. [Google Scholar] [CrossRef]
- Han, Y.H.; Taylor, A.; Knowles, K.M. Scratch resistance and adherence of novel organic-inorganic hybrid coatings on metallic and non-metallic substrates. Surf. Coat. Technol. 2009, 203, 2871–2877. [Google Scholar] [CrossRef]
- Deering, J.; Clifford, A.; D’Elia, A.; Zhitomirsky, I.; Grandfield, K. Composite dip coating improves biocompatibility of porous metallic scaffolds. Mater. Lett. 2020, 274, 128057. [Google Scholar] [CrossRef]
- Jin, F.L.; Li, X.; Park, S.J. Synthesis and application of epoxy resins: A review. J. Ind. Eng. Chem. 2015, 29, 1–11. [Google Scholar] [CrossRef]
- Xia, Y.; He, Y.; Chen, C.; Wu, Y.; Chen, J. MoS2 nanosheets modified SiO2 to enhance the anticorrosive and mechanical performance of epoxy coating. Prog. Org. Coat. 2019, 132, 316–327. [Google Scholar] [CrossRef]
- Li, B.; Mao, B.; Wang, X.; He, T. Fabrication and frictional wear property of bamboo-like SiC nanowires reinforced SiC coating. Surf. Coat. Technol. 2020, 389, 125647. [Google Scholar] [CrossRef]
- Sekhavat Pour, Z.; Ghaemy, M.; Bordbar, S.; Karimi-Maleh, H. Effects of surface treatment of TiO2 nanoparticles on the adhesion and anticorrosion properties of the epoxy coating on mild steel using electrochemical technique. Prog. Org. Coat. 2018, 119, 99–108. [Google Scholar] [CrossRef]
- Oliveira, J.D.; Rocha, R.C.; Galdino, A.G.D.S. Effect of Al2O3 particles on the adhesion, wear, and corrosion performance of epoxy coatings for protection of umbilical cables accessories for subsea oil and gas production systems. J. Mater. Res. Technol. 2019, 8, 1729–1736. [Google Scholar] [CrossRef]
- Hu, C.; Li, Y.; Kong, Y.; Ding, Y. Preparation of poly(o-toluidine)/nano ZnO/epoxy composite coating and evaluation of its corrosion resistance properties. Synth. Met. 2016, 214, 62–70. [Google Scholar] [CrossRef]
- Wu, R.; Zhou, K.; Yue, C.Y.; Wei, J.; Pan, Y. Recent progress in synthesis, properties and potential applications of SiC nanomaterials. Prog. Mater. Sci. 2015, 72, 1–60. [Google Scholar] [CrossRef]
- Fan, J.Y.; Wu, X.L.; Chu, P.K. Low-dimensional SiC nanostructures: Fabrication, luminescence, and electrical properties. Prog. Mater. Sci. 2006, 51, 983–1031. [Google Scholar] [CrossRef]
- Yang, Y.; Lai, L.Y.; Ding, G.F.; Chen, T. SiC nanowire-based SU-8 with enhanced mechanical properties for MEMS structural layer design. Nanotechnol. Precis. Eng. 2020, 2, 169–176. [Google Scholar] [CrossRef]
- Zhang, W.; Yamashita, S.; Kita, H. Progress in tribological research of SiC ceramics in unlubricated sliding-A review. Mater. Des. 2020, 190, 108528. [Google Scholar] [CrossRef]
- Rahaman, M.N.; Boiteux, Y.; Dejonghe, L.C. Surface characterization of silicon nitride and silicon carbide powders. Am. Ceram. Soc. Bull. 1985, 65, 171–176. [Google Scholar]
- Naeimirad, M.; Zadhoush, A.; Neisiany, R.E. Fabrication and characterization of silicon carbide/epoxy nanocomposite using silicon carbide nanowhisker and nanoparticle reinforcements. J. Compos. Mater. 2016, 50, 435–446. [Google Scholar] [CrossRef]
- Kychkin, A.A.; Anan’Eva, E.S.; Tankova, K.I.; Kychkin, A.K.; Tuisov, A.G. Influence of ultrafine silicon carbide powders on the properties of epoxy resin. Struct. Integr. 2019, 20, 185–189. [Google Scholar] [CrossRef]
- Nigrawal, A.; Buddi, T.; Rana, R.S.; Purohit, R. Development of epoxy/nano SiC composites and their mechanical studies. Mater. Today Proc. 2019, 18, 4384–4391. [Google Scholar] [CrossRef]
- Iijima, M.; Kamiya, H. Surface modification of silicon carbide nanoparticles by azo radical initiators. J. Phys. Chem. C 2008, 112, 397–398. [Google Scholar] [CrossRef]
- Huang, L.Y.; Lu, C.X.; Wang, F.; Dong, X.Z. Piezoelectric property of PVDF/graphene composite films using 1H,1H,2H,2H-Perfluorooctyltriethoxysilane as a modifying agent. J. Alloys Compd. 2016, 688, 885–892. [Google Scholar] [CrossRef]
- Shang, X.; Zhu, Y.; Li, Z. Surface modification of silicon carbide with silane coupling agent and hexadecyl iodiele. Appl. Surf. Sci. 2017, 394, 169–177. [Google Scholar] [CrossRef]
- Esmeryan, K.D.; Mchale, G.; Trabi, C.L.; Geraldi, N.R.; Newton, M.I. Manipulated wettability of a superhydrophobic quartz crystal microbalance through electrowetting. J. Phys. D Appl. Phys. 2013, 46, 345307. [Google Scholar] [CrossRef]
- Liu, H.D.; Zhang, H.; Pang, J.; Ning, Y.J.; Jia, F.; Yuan, W.F.; Gu, B.; Zhang, Q.P. Superhydrophobic property of epoxy resin coating modified with octadecylamine and SiO2 nanoparticles. Mater. Lett. 2019, 247, 204–207. [Google Scholar] [CrossRef]
- Usman, B.J.; Scenini, F.; Curioni, M. The effect of exposure conditions on performance evaluation of post-treated anodic oxides on an aerospace aluminium alloy: Comparison between salt spray and immersion testing. Surf. Coat. Technol. 2020, 399, 126157. [Google Scholar] [CrossRef]
- Kulkarni, S.A.; Ogale, S.B.; Vijayamohanan, K.P. Tuning the hydrophobic properties of silica particles by surface silanization using mixed self-assembled monolayers. J. Colloid Interface Sci. 2008, 318, 372–379. [Google Scholar] [CrossRef]
- Palimi, M.J.; Rostami, M.; Mahdavian, M.; Ramezanzadeh, B. Surface modification of Cr2O3 nanoparticles with 3-amino propyl trimethoxy silane (APTMS). Part 1: Studying the mechanical properties of polyurethane/Cr2O3 nanocomposites. Prog. Org. Coat. 2014, 77, 1663–1673. [Google Scholar] [CrossRef]
- Palimi, M.J.; Rostami, M.; Mahdavian, M.; Ramezanzadeh, B. Surface modification of Fe2O3 nanoparticles with 3-aminopropyltrimethoxysilane (APTMS): An attempt to investigate surface treatment on surface chemistry and mechanical properties of polyurethane/Fe2O3 nanocomposites. Appl. Surf. Sci. 2014, 320, 60–72. [Google Scholar] [CrossRef]
- Sabzi, M.; Mirabedini, S.M.; Zohuriaan-Mehr, J.; Atai, M. Surface modification of TiO2 nano-particles with silane coupling agent and investigation of its effect on the properties of polyurethane composite coating. Prog. Org. Coat. 2009, 65, 222–228. [Google Scholar] [CrossRef]
- Lin, J.; Siddiqui, J.A.; Ottenbrite, R.M. Surface modification of inorganic oxide particles with Silane coupling agent and organic dyes. Polym. Adv. Technol. 2001, 12, 285–292. [Google Scholar] [CrossRef]
- Nechanicky, M.A.; Chew, K.W.; Sellinger, A.; Laine, R.M. α-Silicon carbide/β-silicon carbide particulate composites via polymer infiltration and pyrolysis (PIP) processing using polymethylsilane. J. Am. Ceram. Soc. 2000, 20, 441–451. [Google Scholar] [CrossRef]
- Chew, K.W.; Sellinger, A.; Laine, R.M. Processing of aluminum, nitride/silicon carbide composites via polymer infiltration and pyrolysis of polymethylsilane, a precursor to stoichiometric SiC. J. Am. Ceram. Soc. 1999, 82, 857–866. [Google Scholar] [CrossRef] [Green Version]
- Iwata, H.P.; Lindefelt, U.; Öberg, S.; Briddon, P.R. Stacking faults in silicon carbide. Physica B 2003, 340, 165–170. [Google Scholar] [CrossRef]
- Choi, H.-J.; Lee, J.-G. Stacking faults in silicon carbide whiskers. Ceram. Int. 2000, 26, 7–12. [Google Scholar] [CrossRef]
- Yuan, H.; Qi, F.; Zhao, N.; Wan, P.; Zhang, B.; Xiong, H.; Liao, B.; Ouyang, X. Graphene oxide decorated with titanium nanoparticles to reinforce the anti-corrosion performance of epoxy coating. Coatings 2020, 10, 129. [Google Scholar] [CrossRef] [Green Version]
- Georgakilas, V.; Bourlinos, A.B.; Zboril, R.; Trapalis, C. Synthesis, Characterization and aspects of superhydrophobic functionalized carbon nanotubes. Chem. Mater. 2008, 20, 2884–2886. [Google Scholar] [CrossRef]
- Shi, G.; Zhang, M.Q.; Rong, M.Z.; Wetzel, B.; Friedrich, K. Friction and wear of low nanometer Si3N4 filled epoxy composites. Wear 2003, 254, 784–796. [Google Scholar] [CrossRef]
- Mishchenko, L.; Hatton, B.; Bahadur, V.; Taylor, J.A.; Krupenkin, T.; Aizenberg, J. Design of ice-free nanostructured surfaces based on repulsion of impacting water droplets. ACS Nano 2010, 4, 7699–7707. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Li, S.; Yu, J.; Yuan, Z.; Qi, B. Epoxy nanocomposites filled with thermotropic liquid crystalline epoxy grafted graphene oxide. RSC Adv. 2013, 3, 8915–8923. [Google Scholar] [CrossRef]
- Bortz, D.R.; Heras, E.G.; Martin-Gullon, I. Impressive fatigue life and fracture toughness improvements in graphene oxide/epoxy composites. Macromolecules 2012, 45, 238–245. [Google Scholar] [CrossRef]
- Pourhashem, S.; Ghasemy, E.; Rashidi, A.; Vaezi, M.R. Corrosion protection properties of novel epoxy nanocomposite coatings containing silane functionalized graphene quantum dots. J. Alloys Compd. 2018, 731, 1112–1118. [Google Scholar] [CrossRef]
- Xia, W.; Xue, H.; Wang, J.; Wang, T.; Song, L.; Guo, H.; Fan, X.; Gong, H.; He, J. Functionlized graphene serving as free radical scavenger and corrosion protection in gamma-irradiated epoxy composites. Carbon 2016, 101, 315–323. [Google Scholar] [CrossRef]
- Cano, E.; Lafuente, D.; Bastidas, D.M. Use of EIS for the evaluation of the protective properties of coatings for metallic cultural heritage: A review. J. Solid State Electrochem. 2010, 14, 381–391. [Google Scholar] [CrossRef] [Green Version]
- Tian, W.; Meng, F.; Liu, L.; Li, Y.; Wang, F. The failure behaviour of a commercial highly pigmented epoxy coating under marine alternating hydrostatic pressure. Prog. Org. Coat. 2015, 82, 101–112. [Google Scholar] [CrossRef]
- Zhi, D.; Wang, H.; Jiang, D.; Parki, I.P.; Zhang, X. Reactive silica nanoparticles turn epoxy coating from hydrophilic to super-robust superhydrophobic. RSC Adv. 2019, 9, 12547–12554. [Google Scholar] [CrossRef] [Green Version]
- Xiong, H.; Qi, F.; Zhao, N.; Yuan, H.; Wan, P.Y.; Liao, B.; Ouyang, X.P. Effect of organically modified sepiolite as inorganic nanofiller on the anti-corrosion resistance of epoxy coating. Mater. Lett. 2019, 260, 126941. [Google Scholar] [CrossRef]
- Xu, Y.S.H.; Gao, D.; Dong, Q.; Li, M.H.; Liu, A.; Wang, X.C.; Wang, S.F.; Liu, Q. Anticorrosive behavior of epoxy coating modified with hydrophobic nano-silica on phosphatized carbon steel. Prog. Org. Coat. 2021, 151, 106051. [Google Scholar] [CrossRef]
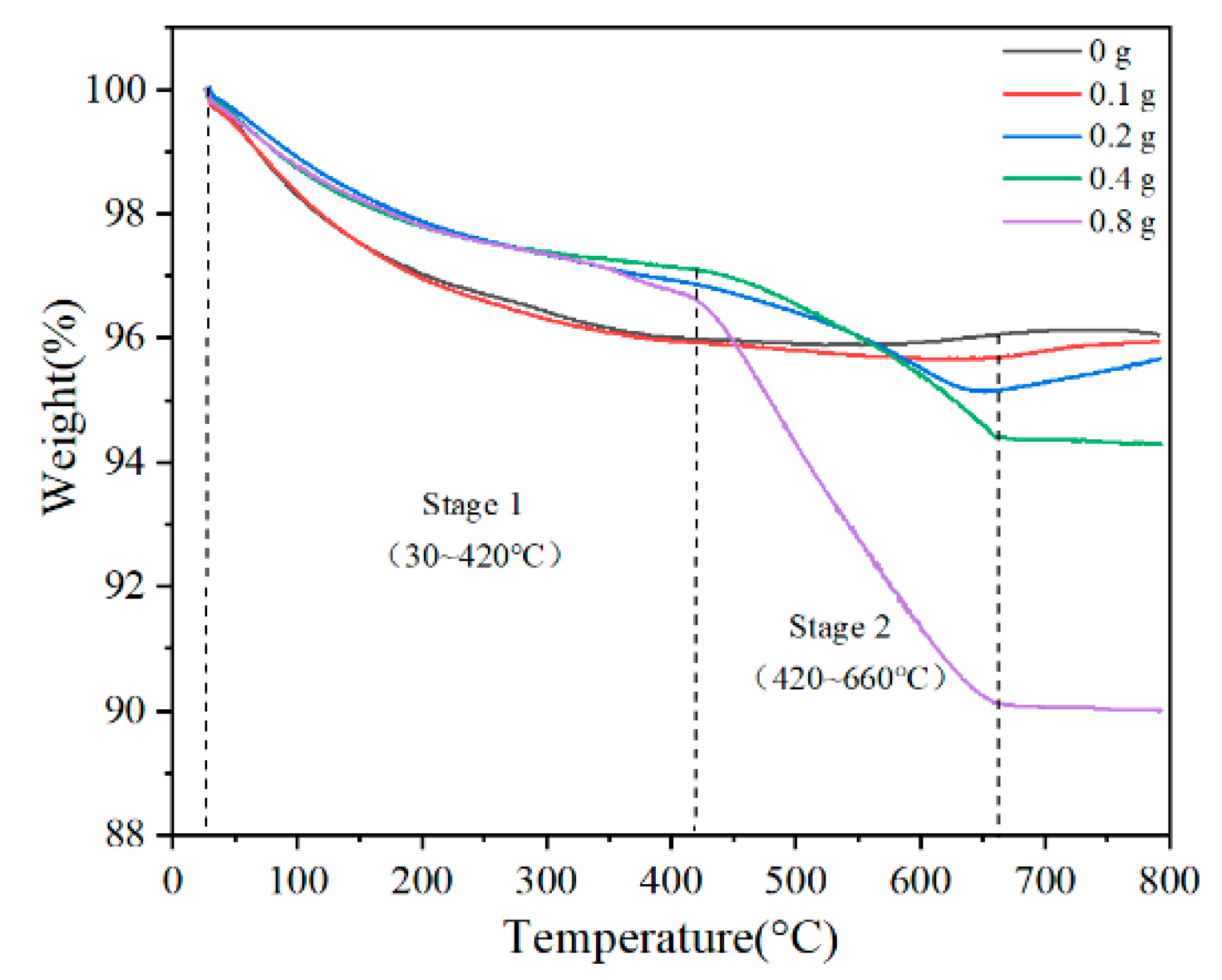


| Samples | Stage 1 Weight Loss (%) | Stage 2 Weight Loss (%) | Total Weight Loss at 800 °C (%) |
|---|---|---|---|
| 0 g | 4.02 | –0.09 | 3.93 |
| 0.1 g | 4.02 | 0.29 | 4.31 |
| 0.2 g | 3.14 | 1.71 | 4.35 |
| 0.4 g | 2.89 | 2.71 | 5.71 |
| 0.8 g | 3.39 | 6.49 | 10.01 |
| Samples | Ecorr (V vs. SCE) | icorr (A/cm2) | βa (V/dec) | −βc (V/dec) |
|---|---|---|---|---|
| Blank solution | −0.928 | 1.524 × 10−7 | 0.066 | 0.071 |
| 1 wt % F–SiC/EP | −0.722 | 1.754 × 10−8 | 0.118 | 0.111 |
| 2 wt % F–SiC/EP | −0.786 | 2.667 × 10−8 | 0.155 | 0.155 |
| 3 wt % F–SiC/EP | −0.468 | 1.837 × 10−10 | 0.375 | 0.417 |
| 4 wt % F–SiC/EP | −0.455 | 1.751× 10−9 | 0.171 | 0.162 |
| 5 wt % F–SiC/EP | −1.332 | 1.770 × 10−7 | 0.154 | 0.139 |
| Samples | Time | Qc | Rc (Ω∙cm2) | Qd | Rct (Ω∙cm2) | ||
|---|---|---|---|---|---|---|---|
| Y0 (Ω−1 cm−2 sn) | n | Y0 (Ω−1 cm−2 sn) | n | ||||
| EP | 1 d | 6.243 × 10−11 | 0.998 | 2.799 × 106 | – | – | – |
| 2 d | 1.194 × 10−10 | 0.9931 | 2.593 × 106 | 2.090 × 10−7 | 0.4094 | 7.35 × 106 | |
| 3 d | 1.757 × 10−10 | 0.9655 | 4.900 × 105 | 6.899 × 10−7 | 0.7169 | 1.137 × 106 | |
| 4 d | 4.486 × 10−10 | 0.929 | 7.531 × 104 | 2.418 × 10−7 | 0.6027 | 2.493 × 105 | |
| 5 d | 3.159 × 10−10 | 0.953 | 4.788 × 104 | 2.592 × 10−7 | 0.689 | 3.126 × 105 | |
| 3 wt % F–SiC | 1 d | 2.558 × 10−10 | 0.963 | 1.386 × 109 | – | – | – |
| 3 d | 2.494 × 10−10 | 0.9494 | 9.307 × 108 | – | – | – | |
| 5 d | 1.378 × 10−10 | 0.9654 | 2.247 × 108 | – | – | – | |
| 7 d | 1.522 × 10−10 | 0.9584 | 1.821 × 108 | – | – | – | |
| 10 d | 1.252 × 10−10 | 0.9794 | 1.431 × 108 | 4.826 × 10−8 | 0.7496 | 2.36 0× 108 | |
| 15 d | 1.378 × 10−10 | 0.9714 | 1.202 × 108 | 7.424 × 10−8 | 0.7802 | 1.386 × 108 | |
| Samples | Addition Amount | Water Contact Angle | Abrasion Tests | Electrochemical Impedance Spectroscopy | Salt Spray Test |
|---|---|---|---|---|---|
| F–SiC/EP | 3 wt % | 134.9° | The friction coefficient was reduced by 73.5% | 1.386 × 109 Ω∙cm2 | 240 h |
| SiO2/EP-ODA [32] | >35 wt % | 153.6° | The coatings have considerably stable abrasion resistance. | – | – |
| SiO2/EP [53] | 40 wt % | 156° | The retention ratio was about 78% | – | – |
| OMSEP/EP [54] | 3 wt % | 101° | – | about 109 Ω∙cm2 | 240 h |
| MEC [55] | 9.09 wt % | 95.9° | – | 5.25 × 105 Ω∙cm2 | 336 h |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Zhao, N.; Qi, F.; Zhang, B.; Liao, B.; Ouyang, X. Reinforced Superhydrophobic Anti-Corrosion Epoxy Resin Coating by Fluorine–Silicon–Carbide Composites. Coatings 2020, 10, 1244. https://doi.org/10.3390/coatings10121244
Zhang Z, Zhao N, Qi F, Zhang B, Liao B, Ouyang X. Reinforced Superhydrophobic Anti-Corrosion Epoxy Resin Coating by Fluorine–Silicon–Carbide Composites. Coatings. 2020; 10(12):1244. https://doi.org/10.3390/coatings10121244
Chicago/Turabian StyleZhang, Zhicai, Nie Zhao, Fugang Qi, Biao Zhang, Bin Liao, and Xiaoping Ouyang. 2020. "Reinforced Superhydrophobic Anti-Corrosion Epoxy Resin Coating by Fluorine–Silicon–Carbide Composites" Coatings 10, no. 12: 1244. https://doi.org/10.3390/coatings10121244
APA StyleZhang, Z., Zhao, N., Qi, F., Zhang, B., Liao, B., & Ouyang, X. (2020). Reinforced Superhydrophobic Anti-Corrosion Epoxy Resin Coating by Fluorine–Silicon–Carbide Composites. Coatings, 10(12), 1244. https://doi.org/10.3390/coatings10121244






