4-(Trifluoromethoxy)phenyl-Containing Polymers as Promising Anodic Materials for Electrochromic Devices
Abstract
:1. Introduction
2. Experimental
2.1. Materials
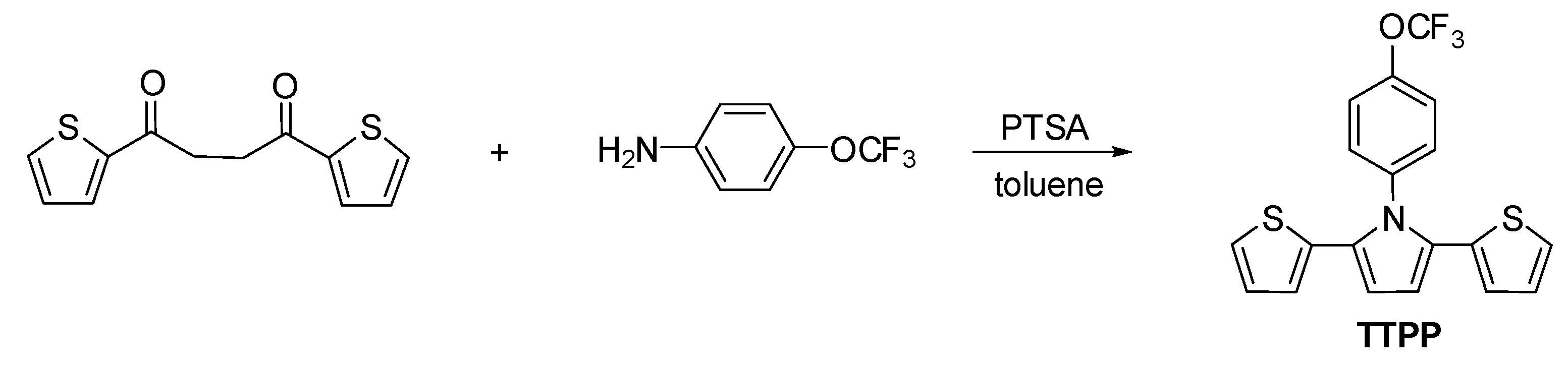
2.2. Preparation of 2,5-Di(Thiophen-2-yl)-1-(4-(Trifluoromethoxy)Phenyl)Pyrrole (TTPP)
2.3. Electrochemical and Electrochromic Characterizations
2.4. Fabrication of ECDs
3. Results and Discussion
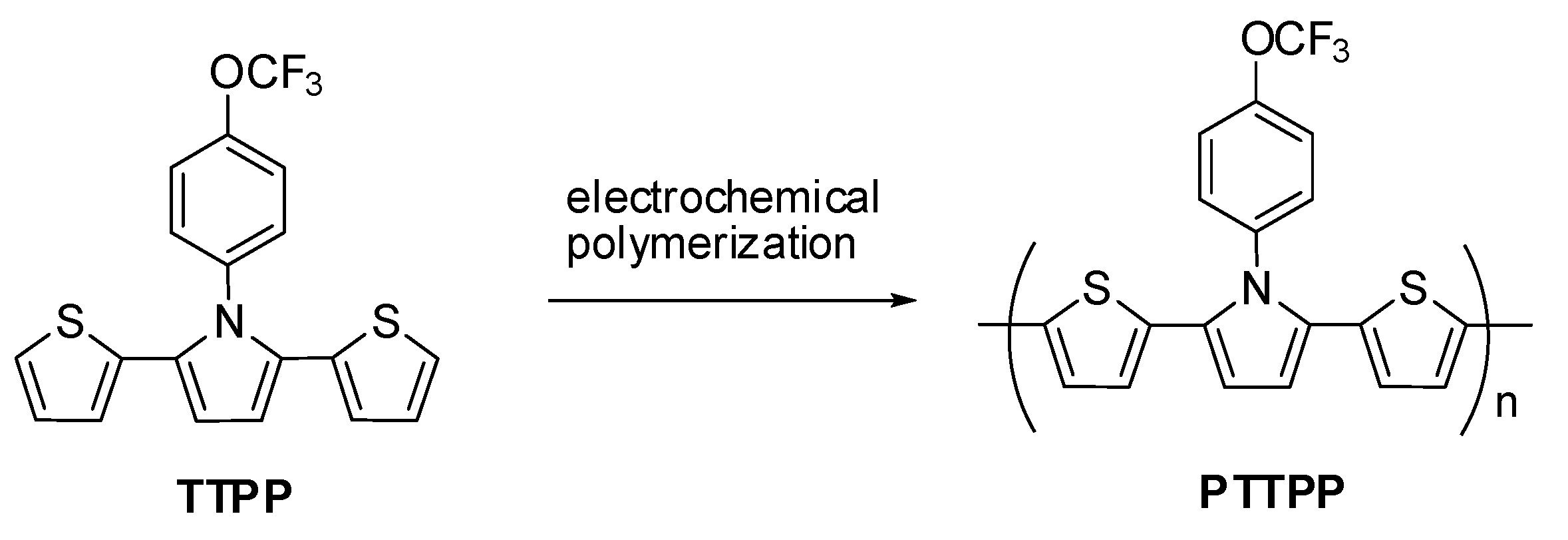
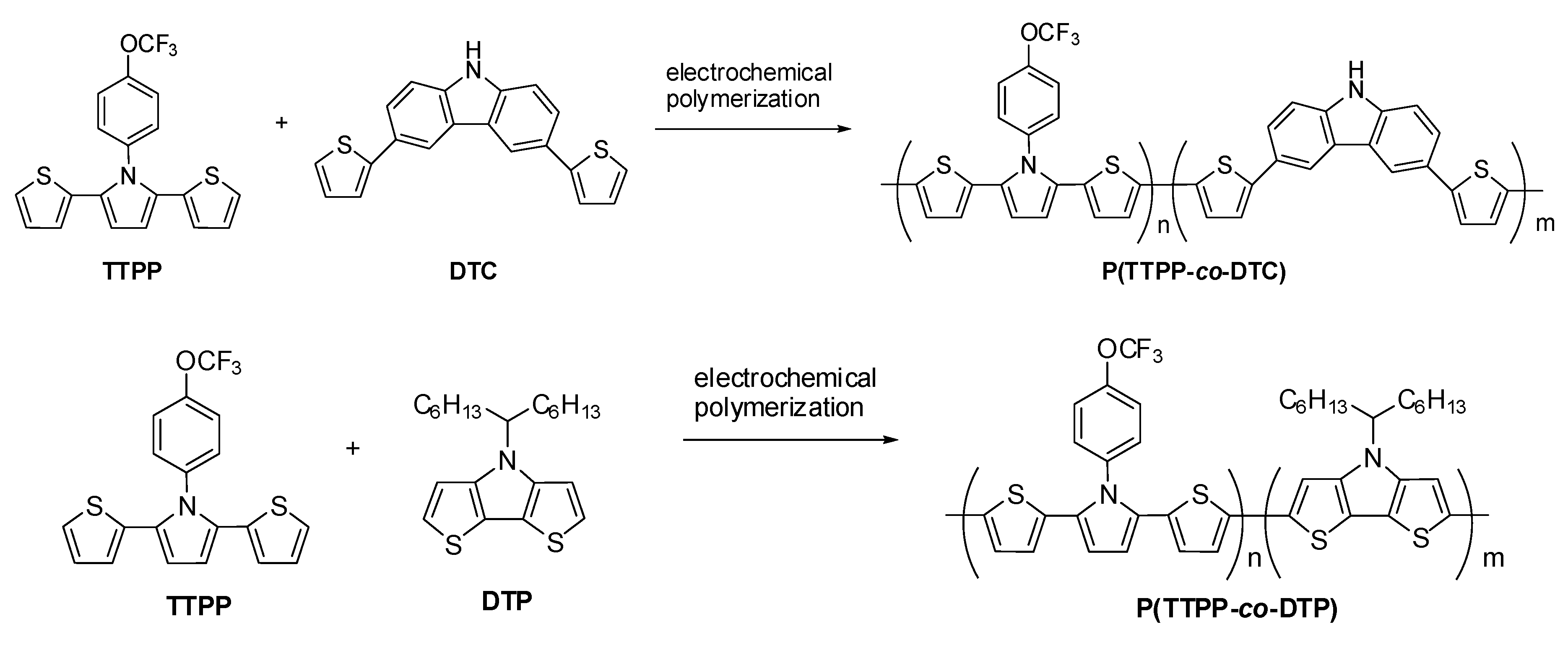
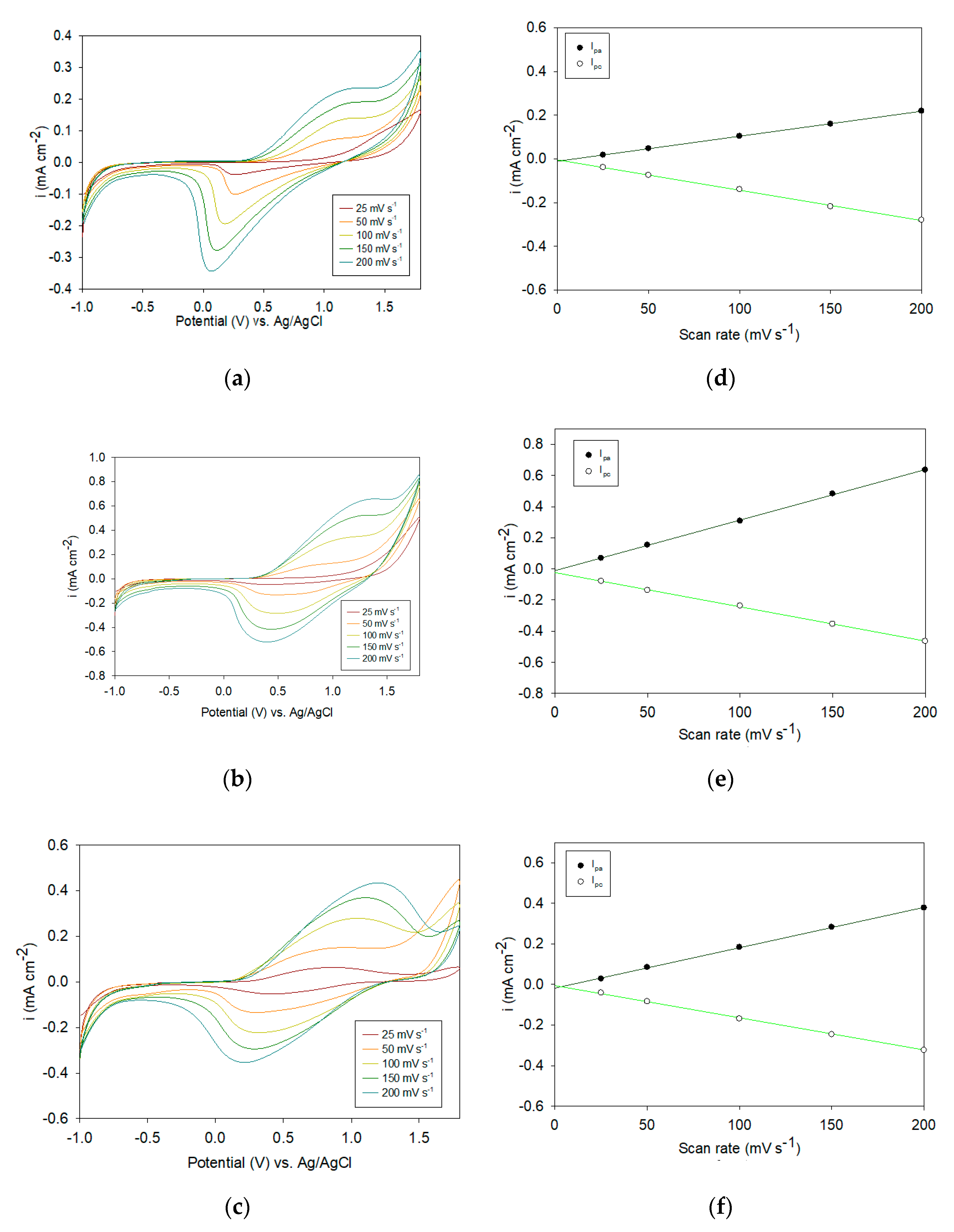
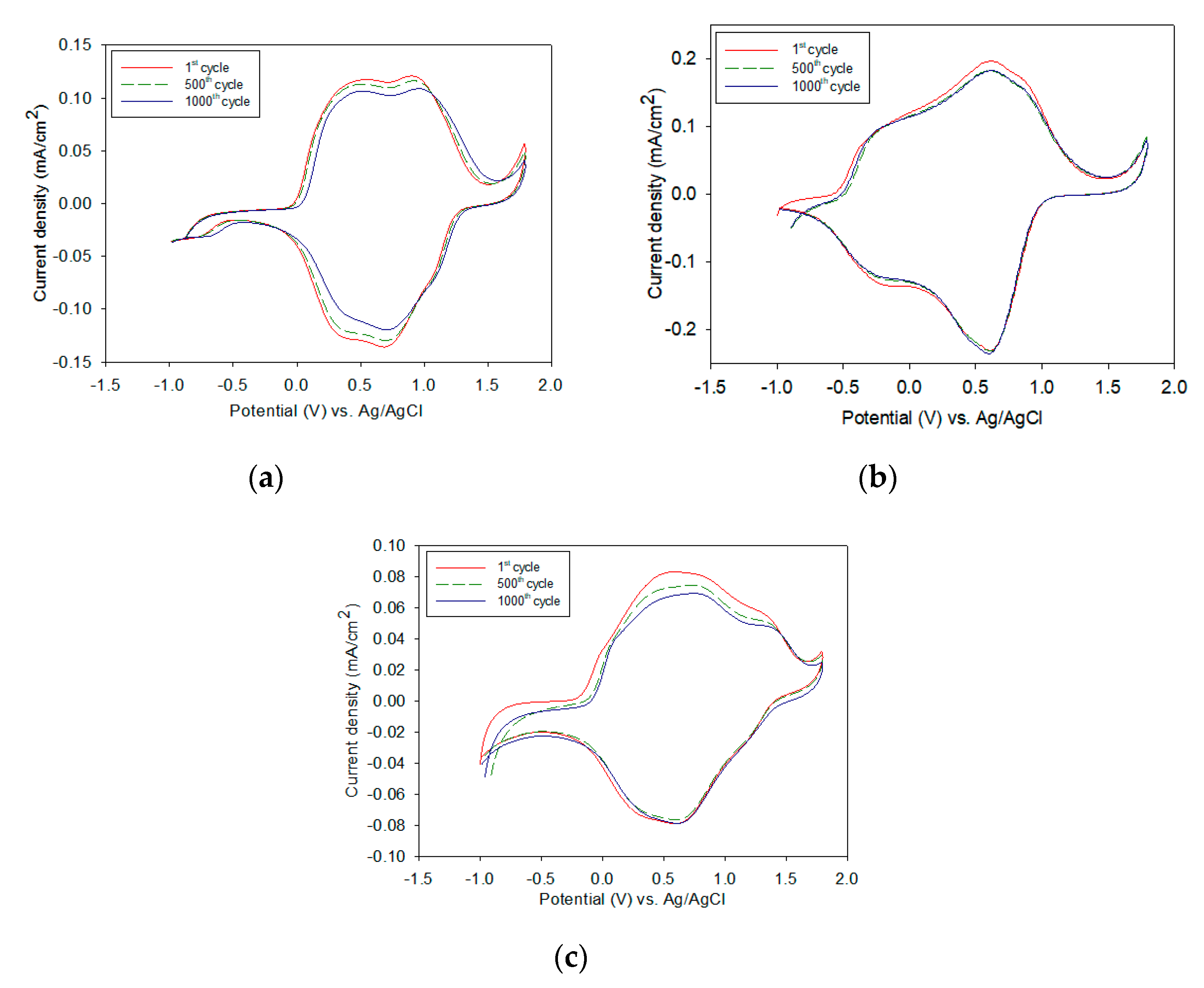
3.1. Electrochemical Preparation of Electrodes
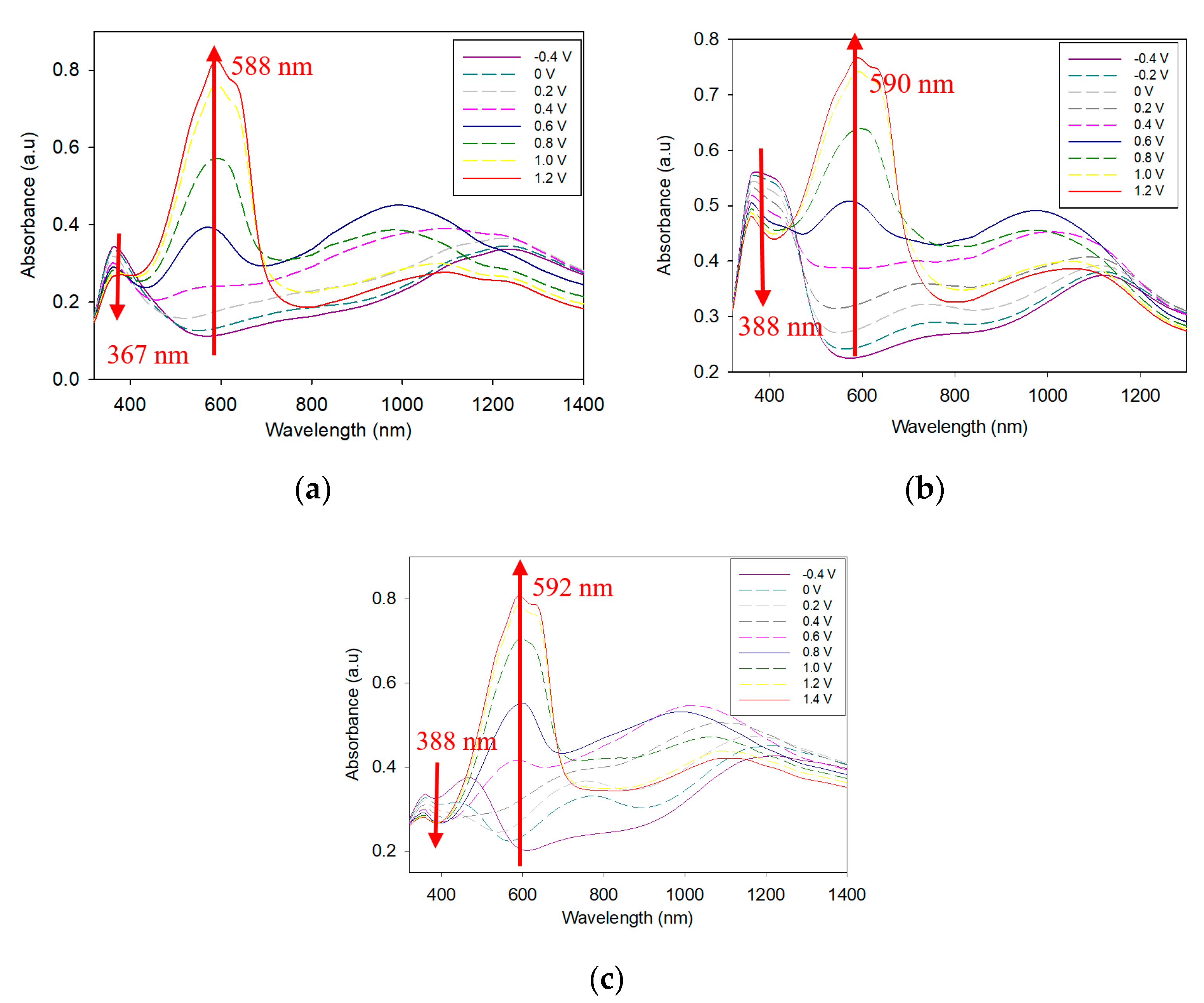
3.2. Electrochromic Characterizations of PTTPP, P(TTPP-co-DTC), and P(TTPP-co-DTP)
3.3. Spectroelectrochemical Characterization of Electrochromic Devices
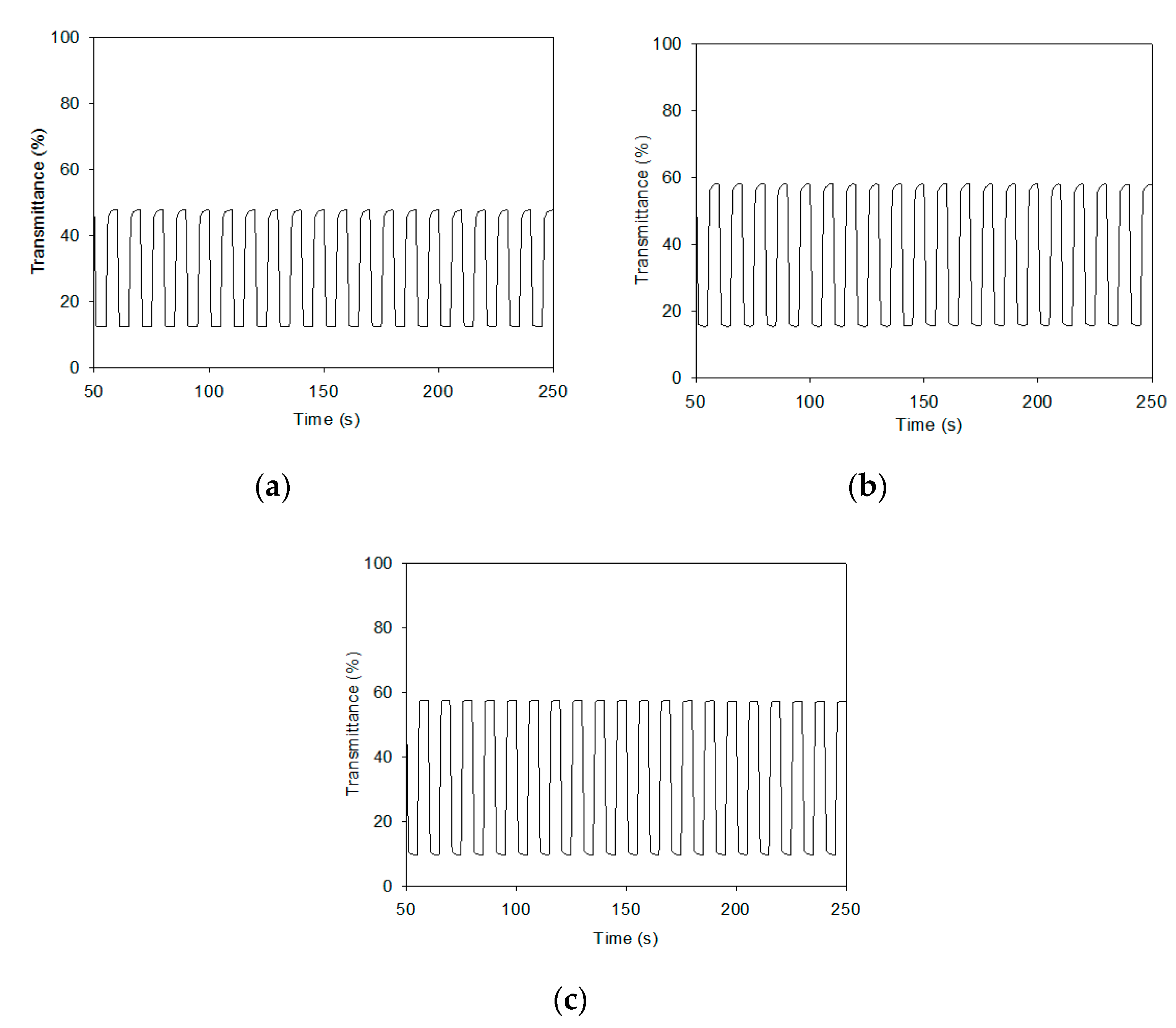
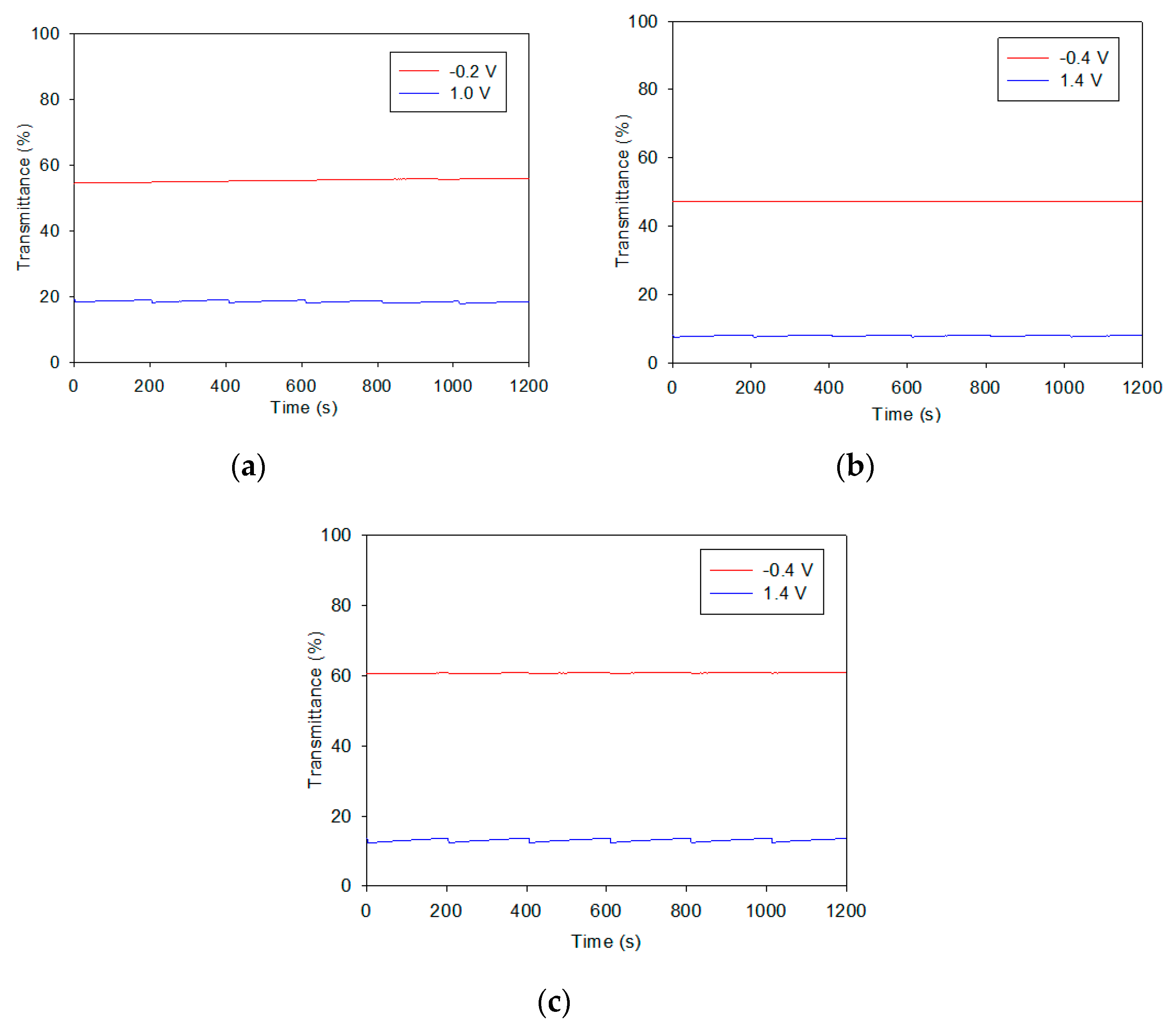
3.4. Cycling Stability and Optical Memory of Electrochromic Devices
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cai, G.F.; Wang, J.X.; Lee, P.S. Next-generation multifunctional electrochromic devices. Acc. Chem. Res. 2016, 49, 1469–1476. [Google Scholar] [CrossRef]
- Beaujuge, P.M.; Reynolds, J.R. Color control in π-conjugated organic polymers for use in electrochromic devices. Chem. Rev. 2010, 110, 268–320. [Google Scholar] [CrossRef]
- Rosseinsky, D.R.; Mortimer, R.J. Electrochromic systems and the prospects for devices. Adv. Mater. 2001, 13, 783–793. [Google Scholar] [CrossRef]
- Mortimer, R.J. Electrochromic materials. Annu. Rev. Mater. Res. 2011, 41, 241–268. [Google Scholar] [CrossRef]
- Kuo, C.-W.; Wu, B.-W.; Chang, J.-K.; Chang, J.-C.; Lee, L.-T.; Wu, T.-Y.; Ho, T.-H. Electrochromic devices based on poly(2,6-di(9H-carbazol-9-yl)pyridine)-type polymer films and PEDOT-PSS. Polymers 2018, 10, 604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuo, C.W.; Wu, T.Y.; Fan, S.C. Applications of poly(indole-6-carboxylic acid-co-2,2′-bithiophene) films in high-contrast electrochromic devices. Coatings 2018, 8, 102. [Google Scholar] [CrossRef] [Green Version]
- Hsiao, S.H.; Lin, S.W. Electrochemical synthesis of electrochromic polycarbazole films from N-phenyl-3,6-bis(N-carbazolyl)carbazoles. Polym. Chem. 2016, 7, 198–211. [Google Scholar] [CrossRef]
- Kuo, C.W.; Hsieh, T.H.; Hsieh, C.K.; Liao, J.W.; Wu, T.Y. Electrosynthesis and characterization of four electrochromic polymers based on carbazole and indole-6-carboxylic acid and their applications in high-contrast electrochromic devices. J. Electrochem. Soc. 2014, 161, D782–D790. [Google Scholar] [CrossRef]
- Camurlu, P. Polypyrrole derivatives for electrochromic applications. RSC Adv. 2014, 4, 55832–55845. [Google Scholar] [CrossRef]
- Hacioglu, S.O.; Yiğit, D.; Ermis, E.; Soylemez, S.; Güllü, M.; Toppare, L. Syntheses and electrochemical characterization of low oxidation potential nitrogen analogs of pedot as electrochromic materials. J. Electrochem. Soc. 2016, 163, E293–E299. [Google Scholar] [CrossRef]
- Kuo, C.W.; Chen, B.K.; Li, W.B.; Tseng, L.Y.; Wu, T.Y.; Tseng, C.G.; Chen, H.R.; Huang, Y.C. Effects of supporting electrolytes on spectroelectrochemical and electrochromic properties of polyaniline-poly(styrene sulfonic acid) and poly(ethylenedioxythiophene)-poly(styrene sulfonic acid)-based electrochromic device. J. Chin. Chem. Soc. 2014, 61, 563–570. [Google Scholar] [CrossRef]
- Otley, M.T.; Alamer, F.A.; Zhu, Y.; Singhaviranon, A.; Zhang, X.; Li, M.; Kumar, A.; Sotzing, G.A. Acrylated poly(3,4-propylenedioxythiophene) for enhancement of lifetime and optical properties for single-layer electrochromic devices. ACS Appl. Mater. Interfaces 2014, 6, 1734–1739. [Google Scholar] [CrossRef]
- Ouyang, M.; Wang, G.H.; Zhang, Y.J.; Hua, C.; Zhang, C. Multicolored electrochromic copolymer based on 1,4-di(thiophen-3-yl)benzene and 3,4-ethylenedioxythiophene. J. Electroanal. Chem. 2011, 653, 21–26. [Google Scholar] [CrossRef]
- Atılgan, N.; Cihaner, A.; Önal, A.M. Electrochromic performance and ion sensitivity of a terthienyl based fluorescent polymer. React. Funct. Polym. 2010, 70, 244–250. [Google Scholar] [CrossRef]
- Chang, K.H.; Wang, H.P.; Wu, T.Y.; Sun, I.W. Optical and electrochromic characterizations of four 2,5-dithienylpyrrole-based conducting polymer films. Electrochim. Acta 2014, 119, 225–235. [Google Scholar] [CrossRef]
- Kuo, C.W.; Chang, J.C.; Huang, Y.T.; Chang, J.K.; Lee, L.T.; Wu, T.Y. Applications of copolymers consisting of 2,6-di(9H-carbazol-9-yl)pyridine and 3,6-di(2-thienyl)carbazole units as electrodes in electrochromic devices. Materials 2019, 12, 1251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Li, J.; Zhang, B.; Qin, J. Highly fluorescent conjugated copolymers containing dithieno [3,2-b:2′,3′-d] pyrrole. Macromol. Rapid Commun. 2008, 29, 1603–1608. [Google Scholar] [CrossRef]
- Su, Y.S.; Chang, J.C.; Wu, T.Y. Applications of three dithienylpyrroles-based electrochromic polymers in high-contrast electrochromic devices. Polymers 2017, 9, 114. [Google Scholar] [CrossRef] [Green Version]
- Guzela, M.; Karatasbz, E.; Ak, M. Synthesis and fluorescence properties of carbazole based asymmetric functionalized star shaped polymer. J. Electrochem. Soc. 2017, 164, H49–H55. [Google Scholar] [CrossRef]
- Kuo, C.W.; Lee, P.Y. Electrosynthesis of copolymers based on 1,3,5-tris(N-carbazolyl)benzene and 2,2′-bithiophene and their applications in electrochromic devices. Polymers 2017, 9, 518. [Google Scholar] [CrossRef] [Green Version]
- Kuo, C.W.; Wu, T.Y.; Huang, M.W. Electrochromic characterizations of copolymers base on 4,4′-bis(N-carbazole)-1,1′-biphenyl and indole-6-carboxylic acid and their application in electrochromic devices. J. Taiwan Inst. Chem. Eng. 2016, 68, 481–488. [Google Scholar] [CrossRef]
- Su, Y.S.; Wu, T.Y. Three carbazole-based polymers as potential anodically coloring materials for high-contrast electrochromic devices. Polymers 2017, 9, 284. [Google Scholar] [CrossRef] [Green Version]
- Welsh, D.M.; Kumar, A.; Meijer, E.W.; Reynolds, J.R. Enhanced contrast ratios and rapid switching in electrochromics based on poly(3,4-propylenedioxythiophene) derivatives. Adv. Mater. 1999, 11, 1379–1382. [Google Scholar] [CrossRef]
- Wu, T.Y.; Chen, B.K.; Hao, L.; Lin, K.F.; Sun, I.W. Thermophysical properties of a room temperature ionic liquid (1-methyl-3-pentyl-imidazolium hexafluorophosphate) with poly(ethylene glycol). J. Taiwan Inst. Chem. Eng. 2011, 42, 914–921. [Google Scholar] [CrossRef]
- Wu, T.Y.; Liao, J.W.; Chen, C.Y. Electrochemical synthesis, characterization and electrochromic properties of indan and 1,3-benzodioxole-based poly(2,5-dithienylpyrrole) derivatives. Electrochim. Acta 2014, 150, 245–262. [Google Scholar] [CrossRef]
- Wu, T.Y.; Su, Y.S.; Chang, J.C. Dithienylpyrrole- and tris[4-(2-thienyl)phenyl]amine-containing copolymers as promising anodic layers in high-contrast electrochromic devices. Coatings 2018, 8, 164. [Google Scholar] [CrossRef] [Green Version]
- Oğuztűrk, H.E.; Tirkeș, S.; Őnal, A.M. Electrochemical synthesis of new conjugated polymers based on carbazole and furan units. J. Electroanal. Chem. 2015, 750, 1–8. [Google Scholar] [CrossRef]
- Wu, T.Y.; Tsao, M.H.; Chen, F.L.; Su, S.G.; Chang, C.W.; Wang, H.P.; Lin, Y.C.; Ou-Yang, W.C.; Sun, I.W. Synthesis and characterization of organic dyes containing various donors and acceptors. Int. J. Mol. Sci. 2010, 11, 329–353. [Google Scholar] [CrossRef] [Green Version]
- Tsao, M.H.; Wu, T.Y.; Wang, H.P.; Sun, I.W.; Su, S.G.; Lin, Y.C.; Chang, C.W. An efficient metal free sensitizer for dye-sensitized solar cells. Mater. Lett. 2011, 65, 583–586. [Google Scholar] [CrossRef]
- Kuo, C.W.; Chang, J.K.; Lin, Y.C.; Wu, T.Y.; Lee, P.Y.; Ho, T.H. Poly(tris(4-carbazoyl-9-ylphenyl)amine)/three poly(3,4-ethylenedioxythiophene) derivatives in complementary high-contrast electrochromic devices. Polymers 2017, 9, 543. [Google Scholar] [CrossRef] [Green Version]
- Hsiao, S.H.; Liao, Y.C. Facile synthesis of electroactive and electrochromic triptycene poly(ether-imide)s containing triarylamine units via oxidative electro-coupling. Polymers 2017, 9, 497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Algi, M.P.; Öztaş, Z.; Tirkes, S.; Cihaner, A.; Algi, F. A new electrochromic copolymer based on dithienylpyrrole and EDOT. Org. Electron. 2013, 14, 1094–1102. [Google Scholar] [CrossRef]
- Hu, B.; Luo, W.; Jin, L.; Liu, Z.; Wang, M.; Zhou, L.; Li, C. Electrochemical and spectroelectrochemical properties of poly(carbazole-EDOT)s derivatives functionalized with benzonitrile and phthalonitrile units. ECS J. Solid State Sci. Technol. 2016, 5, P21–P26. [Google Scholar] [CrossRef] [Green Version]
- Cheng, X.; Zhao, J.; Fu, Y.; Cui, C.; Zhang, X. Electrosynthesis and characterization of a multielectrochromic copolymer of tris[4 -(2-thienyl)phenyl]amine with 3,4-ethylenedioxythiophene. J. Electrochem. Soc. 2013, 160, G6–G13. [Google Scholar] [CrossRef]
- Turkarslan, O.; Ak, M.; Tanyeli, C.; Akhmedov, I.M.; Toppare, L. Enhancing electrochromic properties of conducting polymers via copolymerization: Copolymer of 1-(4-fluorophenyl)-2,5-di(thiophen-2-yl)-1H-pyrrole with 3,4-ethylene dioxythiophene. J. Polym. Sci. Pol. Chem. 2007, 45, 4496–4503. [Google Scholar] [CrossRef]
- Feng, F.; Kong, L.; Du, H.; Zhao, J.; Zhang, J. Donor-acceptor-type copolymers based on 3,4-propylenedioxy-thiophene and 5,6-difluorobenzotriazole: Synthesis and electrochromic properties. Polymers 2018, 10, 427. [Google Scholar] [CrossRef] [Green Version]




| Polymers | E/V | Photographs | L* | a* | b* |
|---|---|---|---|---|---|
| PTTPP | 0 |  | 92.11 | −2.13 | 19.48 |
| 1.0 |  | 91.09 | −2.13 | 17.43 | |
| 1.4 |  | 75.4 | 6.8 | 4.77 | |
| P(TTPP-co-DTC) | 0 |  | 86.23 | −3.16 | 43.75 |
| 1.0 |  | 71.81 | −3.3 | 9.7 | |
| 1.2 |  | 68.18 | −0.87 | 7.17 | |
| P(TTPP-co-DTP) | 0 |  | 82.49 | 14.04 | 14.73 |
| 0.6 |  | 87.79 | −4.73 | 3.79 | |
| 1.2 |  | 84.85 | −3.03 | −1.81 |
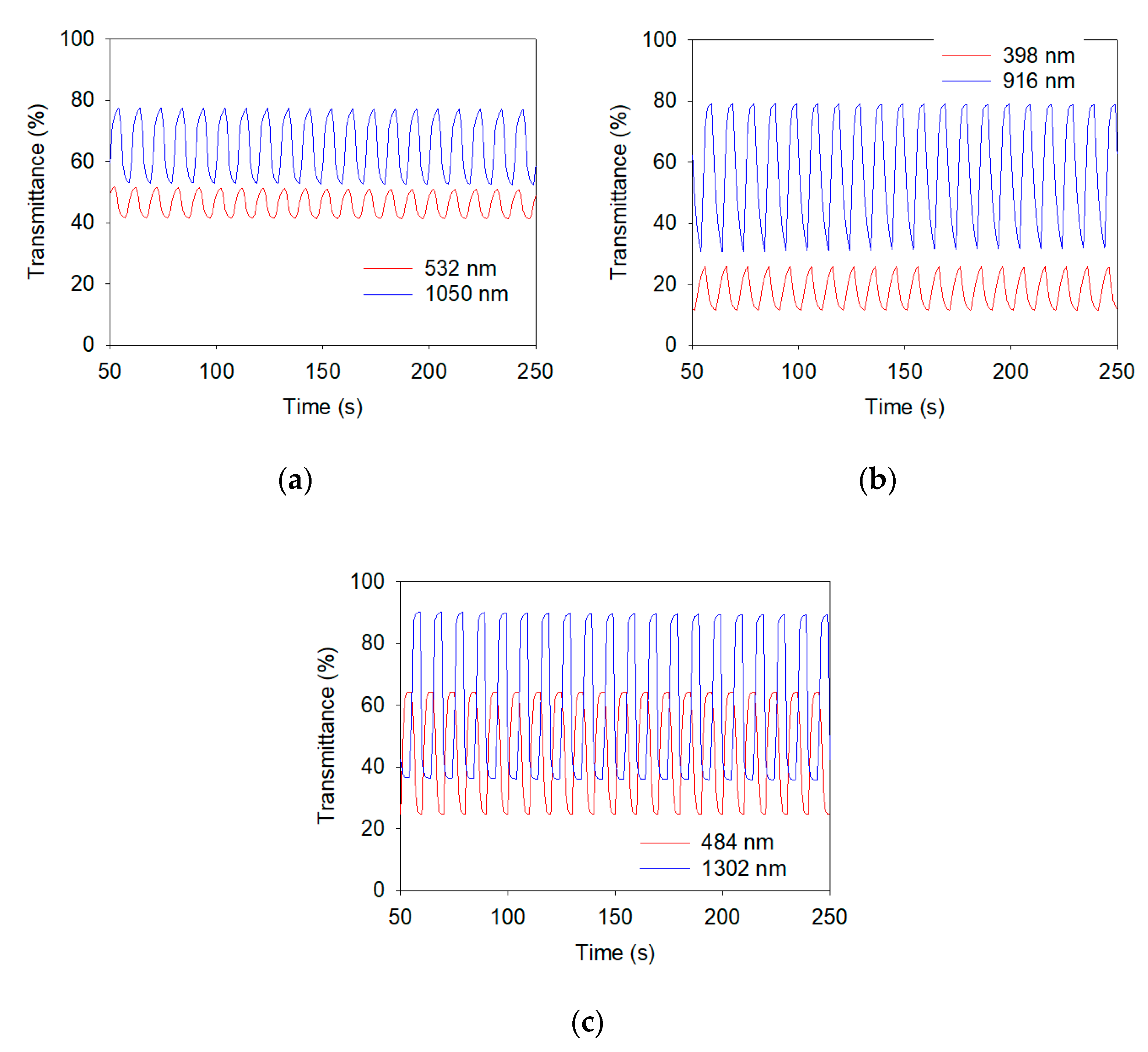
| Polymers and ECDs | λ/nm | Eg/eV | ΔTmax/% | ΔODmax/% | ηmax/cm2 C−1 |
|---|---|---|---|---|---|
| PTTPP | 1050 | 2.34 | 24.5 | 16.5 | 379.64 |
| P(TTPP-co-DTC) | 916 | - | 49.0 | 41.9 | 171.29 |
| P(TTPP-co-DTP) | 1302 | - | 53.8 | 39.5 | 394.82 |
| poly(1-co-EDOT) [32] | 500 | - | 32.9 | - | 173 |
| PBCB [33] | 1000 | 1.77 | 44 | - | 268.43 |
| PBCP [33] | 1000 | 1.74 | 39 | - | 236.18 |
| PTTPP/PProDOT-Et2 ECD | 588 | - | 35.7 | 59.4 | 890.96 |
| P(TTPP-co-DTC)/PProDOT-Et2 ECD | 590 | - | 42.6 | 56.9 | 512.79 |
| P(TTPP-co-DTP)/PProDOT-Et2 ECD | 592 | - | 48.1 | 77.8 | 519.27 |
| P(TTPA-co-EDOT)/PEDOT ECD [34] | 650 | - | 24 | - | 545 |
| P(FPTP-co-EDOT)/PEDOT ECD [35] | 555 | - | 23 | - | - |
| Polymers and ECDs | λmax/nm | Cycle No. | T | τ (T90%) | Stability (100 cycles) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Tb/% | Tc/% | ΔT/% | τc/s | τb/s | |||||
| PTTPP in [EPI+][TFSI–] | 532 | 1 | 51.9 | 41.7 | 10.2 | 2.32 | 1.99 | - | |
| - | - | 50 | 49.6 | 40.7 | 8.9 | 2.33 | 1.86 | - | |
| - | - | 100 | 48.7 | 40.4 | 8.3 | 2.29 | 2.07 | 81.4% | |
| - | 1050 | 1 | 77.7 | 53.2 | 24.5 | 2.41 | 1.87 | - | |
| - | - | 50 | 76.4 | 51.9 | 24.5 | 2.41 | 1.89 | - | |
| - | - | 100 | 75.3 | 52.4 | 22.9 | 2.39 | 1.95 | 93.5% | |
| P(TTPP-co-DTC) in | 398 | 1 | 26.0 | 11.5 | 14.5 | 2.34 | 2.18 | - | |
| [EPI+][TFSI–] | - | 50 | 25.6 | 11.4 | 14.2 | 2.41 | 2.28 | - | |
| - | - | 100 | 25.4 | 11.4 | 14.0 | 2.30 | 2.33 | 96.6% | |
| - | 916 | 1 | 79.2 | 30.2 | 49.0 | 2.03 | 2.42 | - | |
| - | - | 50 | 78.7 | 33.1 | 45.6 | 1.94 | 2.46 | - | |
| - | - | 100 | 78.6 | 35.4 | 43.2 | 1.96 | 2.49 | 88.2% | |
| P(TTPP-co-DTP) in | 484 | 1 | 64.3 | 24.7 | 39.6 | 1.85 | 1.88 | - | |
| [EPI+][TFSI–] | - | 50 | 64.0 | 24.5 | 39.5 | 1.87 | 1.86 | - | |
| - | - | 100 | 63.9 | 24.4 | 39.5 | 1.97 | 1.91 | 99.7% | |
| - | 1302 | 1 | 90.1 | 36.3 | 53.8 | 1.96 | 1.92 | - | |
| - | - | 50 | 88.9 | 35.5 | 53.4 | 1.94 | 2.02 | - | |
| - | - | 100 | 88.7 | 35.4 | 53.3 | 1.92 | 1.89 | 99.1% | |
| PTTPP/ PProDOT-Et2 ECD | 588 | 1 | 47.9 | 12.2 | 35.7 | 0.96 | 0.97 | - | |
| - | 50 | 47.8 | 12.8 | 35.0 | 0.94 | 0.94 | - | ||
| - | 100 | 48.0 | 13.9 | 34.1 | 0.99 | 0.92 | 95.5% | ||
| P(TTPP-co-DTC)/ | 590 | 1 | 58.3 | 15.7 | 42.6 | 0.99 | 0.94 | - | |
| PProDOT-Et2 ECD | - | 50 | 58.0 | 15.6 | 42.4 | 0.93 | 0.91 | - | |
| - | - | 100 | 56.8 | 15.7 | 41.1 | 0.97 | 0.90 | 96.5% | |
| P(TTPP-co-DTP)/ | 592 | 1 | 57.7 | 9.6 | 48.1 | 0.94 | 0.88 | - | |
| PProDOT-Et2 ECD | - | 50 | 56.9 | 9.7 | 47.2 | 0.92 | 0.90 | - | |
| - | - | 100 | 56.7 | 10.1 | 46.6 | 0.87 | 0.94 | 96.9% | |
| ECDs | E/V | Photoimages | L* | a* | b* |
|---|---|---|---|---|---|
| PTTPP/PProDOT-Et2 | −0.4 |  | 87.38 | −1.64 | 8.91 |
| 0 |  | 85.98 | −2.28 | 5.47 | |
| 0.6 |  | 72.62 | 4.5 | −10.44 | |
| 0.8 |  | 65.31 | 4.11 | −19.6 | |
| 1.2 |  | 56.03 | 5.65 | −30.98 | |
| P(TTPP-co-DTC)/PProDOT-Et2 | 0 |  | 81.05 | −7.69 | 19.29 |
| 0.4 |  | 73.43 | −3.39 | 6.91 | |
| 0.6 |  | 67.22 | −1.06 | −2.54 | |
| 0.8 |  | 61.72 | −0.31 | −10.81 | |
| 1.2 |  | 59 | 1.65 | −15.34 | |
| P(TTPP-co-DTP)/PProDOT-Et2 | −0.4 |  | 81.78 | 8.71 | 5.4 |
| 0 |  | 85.15 | −1.07 | 4.43 | |
| 0.6 |  | 76.9 | 1.94 | −10.44 | |
| 0.8 |  | 70.37 | 3.26 | −19.12 | |
| 1.4 |  | 57.72 | 5.9 | −35.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.-H.; Chang, J.-C.; Lee, P.-Y.; Lin, Y.-C.; Wu, T.-Y. 4-(Trifluoromethoxy)phenyl-Containing Polymers as Promising Anodic Materials for Electrochromic Devices. Coatings 2020, 10, 1251. https://doi.org/10.3390/coatings10121251
Wang W-H, Chang J-C, Lee P-Y, Lin Y-C, Wu T-Y. 4-(Trifluoromethoxy)phenyl-Containing Polymers as Promising Anodic Materials for Electrochromic Devices. Coatings. 2020; 10(12):1251. https://doi.org/10.3390/coatings10121251
Chicago/Turabian StyleWang, Wen-Hsin, Jui-Cheng Chang, Pei-Ying Lee, Yuan-Chung Lin, and Tzi-Yi Wu. 2020. "4-(Trifluoromethoxy)phenyl-Containing Polymers as Promising Anodic Materials for Electrochromic Devices" Coatings 10, no. 12: 1251. https://doi.org/10.3390/coatings10121251





