Aesthetic Alteration of Marble Surfaces Caused by Biofilm Formation: Effects of Chemical Cleaning
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Sites and Chemical and Mechanical Cleaning Treatment
2.2. Stone Characterization and Evaluation of the State of Conservation
2.3. Visualization of Biofilm Structure
2.4. DNA Extraction and Sequencing
3. Results
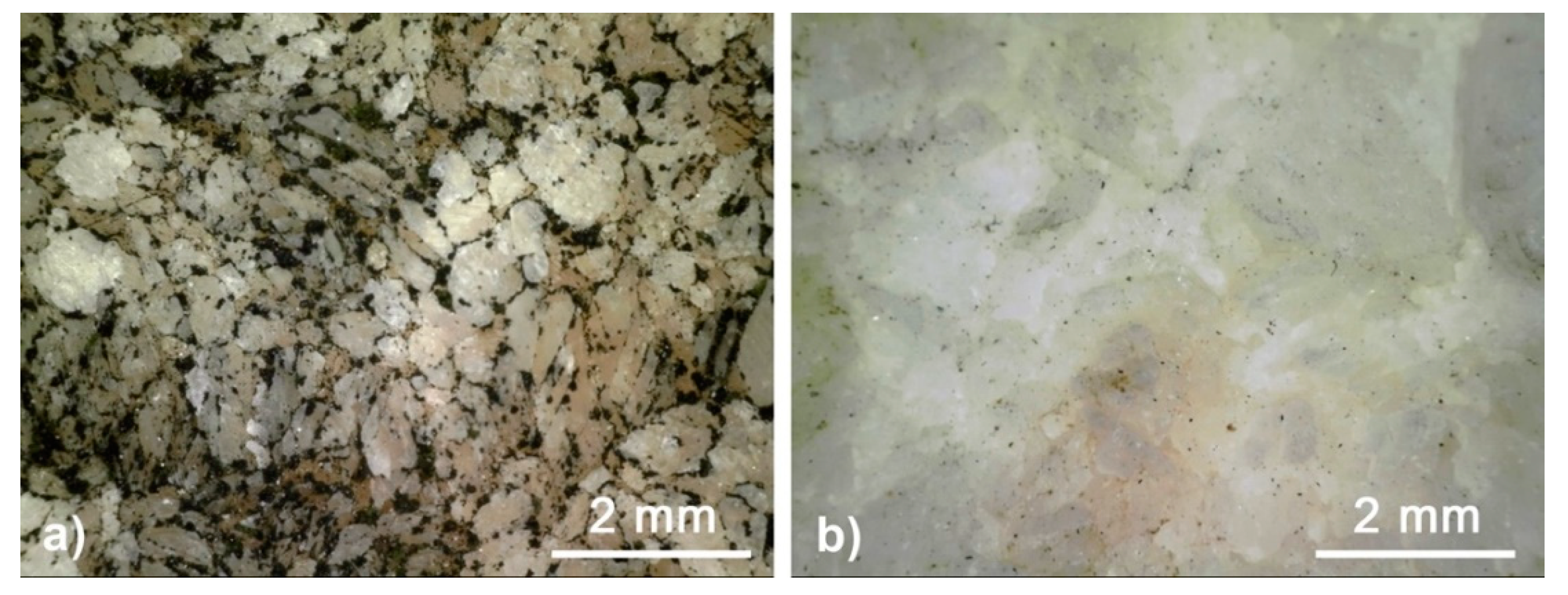
3.1. Evaluation of the State of Conservation of the Stone Substrate before and after the Cleaning Treatment
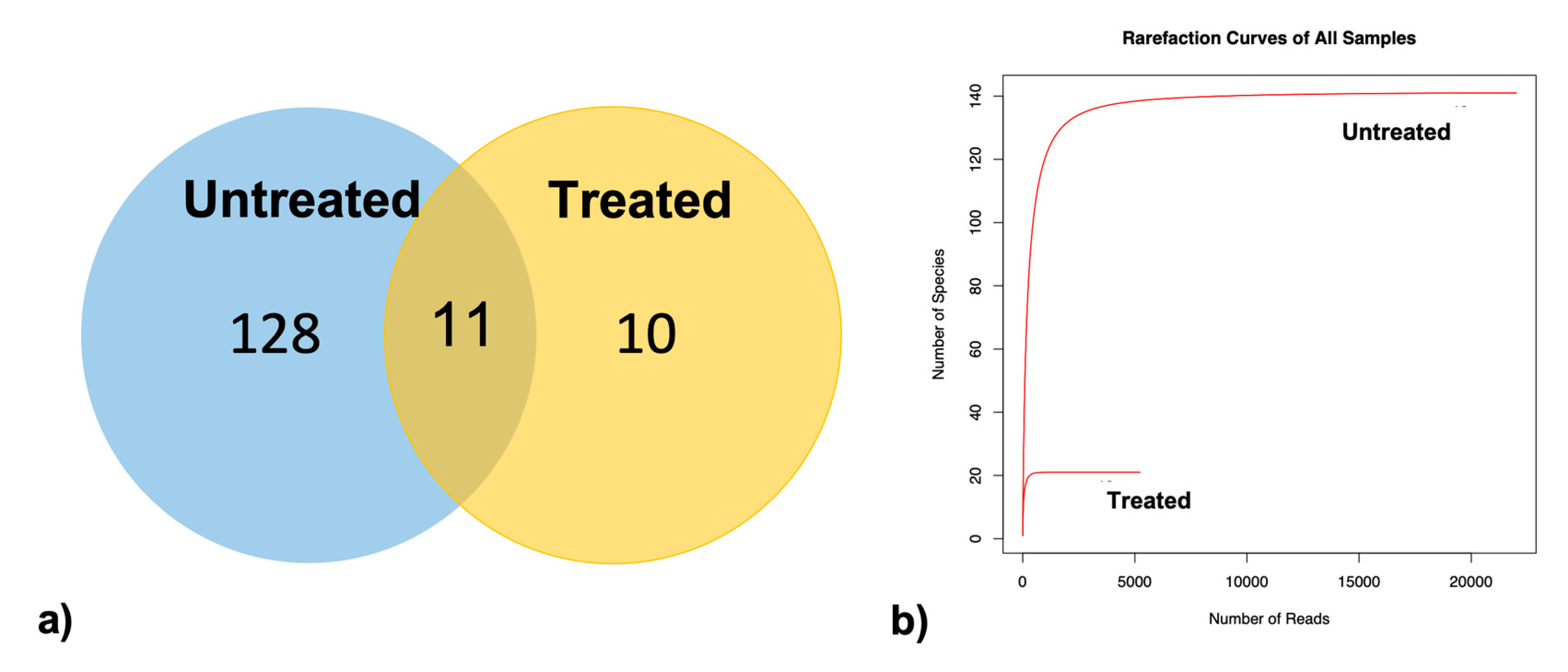
3.2. Biofilm Characterization
3.3. Effects of the Cleaning Treatment on the Biofilm Composition
3.4. Effects of the Cleaning Treatment on Biofilm Functional Profiles
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gulotta, D.; Villa, F.; Cappitelli, F.; Toniolo, L. Biofilm colonization of metamorphic lithotypes of a renaissance cathedral exposed to urban atmosphere. Sci. Total Environ. 2018, 639, 1480–1490. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.D.; Anjos, M.V.; Charola, A.E. Recolonization of marble sculptures in a garden environment. In Biocolonization of Stone: Control and Preventative Methods; Charola, A.E., McNamara, C., Koestler, R.J., Eds.; Smithsonian Institution Scholarly Press: Washington, WA, USA, 2011; pp. 71–84. [Google Scholar]
- Matteucci, E.; Scarcella, A.V.; Croveri, P.; Marengo, A.; Borghi, A.; Benelli, C.; Hamdan, O.; Favero-Longo, S.E. Lichens and other lithobionts on the carbonate rock surfaces of the heritage site of the tomb of Lazarus (Palestinian territories): Diversity, biodeterioration, and control issues in a semi-arid environment. Ann. Microbiol. 2019, 69, 1033–1046. [Google Scholar] [CrossRef]
- Nowicka-Krawczyk, P.; Żelazna-Wieczorek, J.; Koźlecki, T. Silver nanoparticles as a control agent against facades coated by aerial algae—A model study of Apatococcus lobatus (green algae). PLoS ONE 2017, 12, e0183276. [Google Scholar] [CrossRef] [PubMed]
- Sanmartín, P.; Villa, F.; Polo, A.; Silva, B.; Prieto, B.; Cappitelli, F. Rapid evaluation of three biocide treatments against the cyanobacterium Nostoc sp. PCC 9104 by color changes. Ann. Microbiol. 2015, 65, 1153–1158. [Google Scholar]
- Savvides, A.L.; Nikolakopoulou, T.L.; Kyratsous, N.; Katsifas, E.A.; Kanini, G.; Karagouni, A.D. Bacterial deterioration of marble monuments: A case study of the Conservation Project of Acropolis Monuments. Geomicrobiol. J. 2014, 31, 726–736. [Google Scholar] [CrossRef]
- Shirakawa, M.A.; Gaylarde, C.C.; Gaylarde, P.M.; John, V.; Gambale, W. Fungal colonization and succession on newly painted buildings and the effect of biocide. FEMS Microbiol. Ecol. 2002, 39, 165–173. [Google Scholar] [CrossRef]
- Sterflinger, K.; Piñar, G. Microbial deterioration of cultural heritage and works of art — tilting at windmills? Appl. Microbiol. Biotechnol. 2013, 97, 9637–9646. [Google Scholar] [CrossRef]
- Mulder, I.; Siemens, J.; Sentek, V.; Amelung, W.; Smalla, K.; Jechalke, S. Quaternary ammonium compounds in soil: Implications for antibiotic resistance development. Rev. Environ. Sci. Biotechnol. 2018, 17, 159–185. [Google Scholar] [CrossRef]
- Faimon, J.; Štelcl, J.; Kubešová, S.; Zimák, J. Environmentally acceptable effect of hydrogen peroxide on cave ‘‘lamp-flora’’, calcite speleothems and limestones. Environ. Pollut. 2003, 122, 417–422. [Google Scholar] [CrossRef]
- Mulec, J.; Kosi, G. Lampenflora algae and methods of growth control. J. Cave Karst Stud. 2009, 71, 109–115. [Google Scholar]
- Jurado, V.; Porca, E.; Cuezva, S.; Fernandez-Cortes, A.; Sanchez-Moral, S.; Saiz-Jimenez, C. Fungal outbreak in a show cave. Sci. Total Environ. 2010, 408, 3632–3638. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Marine, M.; Gonzalez-del Valle, M.A.; Ortiz-Martinez, A.; Laiz, L.; Saiz-Jimenez, C. Effect of Algophase on the cyanobacterium Gloeothece membranacea CCAP 1430/3. In Molecular Biology and Cultural Heritage; Saiz-Jimenez, C., Ed.; Swets & Zeitlinger: Lisse, The Netherlands, 2003; pp. 195–200. [Google Scholar]
- Tretiach, M.; Crisafulli, P.; Imai, N.; Kashiwadani, H.; Moon, K.H.; Wada, H.; Salvadori, O. Efficacy of a biocide tested on selected lichens and its effects on their substrata. Int. Biodeter. Biodegr. 2007, 59, 44–54. [Google Scholar] [CrossRef]
- Maxwell, I. Cleaning Sandstone: Risks and Consequences. Inform: Information for Historic Building Owners. Historic Scotland, Technical Conservation, Research and Education Group: Edinburgh, UK. Available online: https://www.historicenvironment.scot/archives-and-research/publications/publication/?publicationid=cfcd9855-eff4-40d7-9ccf-a59500b10077 (accessed on 16 December 2019).
- Leplat, J.; Francois, A.; Bousta, F. White fungal covering on the wall paintings of the Saint-Savin-sur-Gartempe Abbey church crypt: A case study. Int. Biodeter. Biodegr. 2017, 122, 29–37. [Google Scholar] [CrossRef]
- Mitova, M.M.; Iliev, M.; Nováková, A.; Gorbushina, A.A.; Groudeva, V.I.; Martin-Sanchez, P.M. Diversity and biocide susceptibility of fungal assemblages dwelling in the Art Gallery of Magura Cave, Bulgaria. Int. J. Speleol. 2017, 46, 67–80. [Google Scholar] [CrossRef]
- Favero-Longo, S.E.; Brigadeci, F.; Segimiro, A.; Voyrona, S.; Cardinali, M.; Girlanda, M.; Piervittori, R. Biocide efficacy and consolidant effect on the mycoflora of historical stuccos in indoor environment. J. Cult. Herit. 2018, 34, 33–42. [Google Scholar] [CrossRef]
- Warscheid, T.; Leisen, H. Microbiological studies on stone deterioration and development of conservation measures at Angkor Wat. In Biocolonization of Stone: Control and Preventative Methods; Charola, A.E., McNamara, C., Koestler, R.J., Eds.; Smithsonian Institution Scholarly Press: Washington, WA, USA, 2011; pp. 1–18. [Google Scholar]
- Barresi, G.; Cammarata, M.; Palla, F. Biocide. In Biotechnology and Conservation of Cultural Heritage; Palla, F., Barresi, G., Eds.; Springer: Cham, Switzerland, 2017; pp. 49–65. [Google Scholar]
- Ascaso, C.; Wierzchos, J.; Souza-Egipsy, V.; de los Rios, A.; Delgado Rodrigues, J. In situ evaluation of the biodeteriorating action of microorganisms and the effects of biocides on carbonate rock of the Jeronimos Monastery (Lisbon). Int. Biodeter. Biodegr. 2002, 49, 1–12. [Google Scholar] [CrossRef]
- Urzì, C.; De Leo, F.; Krakova, L.; Pangallo, D.; Bruno, L. Effects of biocide treatments on the biofilm community in Domitilla’s catacombs in Rome. Sci. Total Environ. 2016, 572, 252–262. [Google Scholar] [CrossRef]
- Martin-Sanchez, P.M.; Nováková, A.; Bastian, F.; Alabouvette, C.; Saiz-Jimenez, C. Use of biocides for the control of fungal outbreaks in subterranean environments: The case of the Lascaux cave in France. Environ. Sci. Technol. 2012, 46, 3762–3770. [Google Scholar] [CrossRef]
- Gulotta, D.; Toniolo, L. Conservation of the built heritage: Pilot site approach to design a sustainable process. Heritage 2019, 2, 797–812. [Google Scholar] [CrossRef]
- Villa, F.; Pitts, B.; Lauchnor, E.; Cappitelli, F.; Stewart, P. Development of a laboratory model of a phototroph-heterotroph mixed-species biofilm at the stone/air interface. Front. Microbiol. 2015, 6, 1251. [Google Scholar] [CrossRef]
- Roldán, M.; Ascaso, C.; Wierzchos, J. Fluorescent fingerprints of endolithic phototrophic cyanobacteria living within halite rocks in the Atacama Desert. Appl. Environ. Microbiol. 2014, 80, 2998–3006. [Google Scholar] [CrossRef] [PubMed]
- Polo, A.; Gulotta, D.; Santo, N.; Di Benedetto, C.; Fascio, U.; Toniolo, L.; Villa, F.; Cappitelli, F. Importance of subaerial biofilms and airborne microflora in the deterioration of stonework: A molecular study. Biofouling 2012, 28, 1093–1106. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rapin, A.; Pattaroni, C.; Marsland, B.J.; Harris, N.L. Microbiota analysis using an illumina MiSeq platform to sequence 16S rRNA genes. Curr. Protoc. Mouse Biol. 2017, 7, 100–129. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I. PICRUSt2: An improved and extensible approach for metagenome inference. BioRxiv 2019, 672295. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing: Vienna, Austria. Available online: http://www.r-project.org/ (accessed on 16 December 2019).
- De Leo, F.; Iero, A.; Zammit, G.; Urzì, C. Chemoorganotrophic bacteria isolated from biodeteriorated surfaces in cave and catacombs. Int. J. Speleol. 2012, 41, 125–136. [Google Scholar] [CrossRef]
- Rosado, T.; Gil, M.; Caldeira, A.T.; Martins, M.D.R.; Dias, C.B.; Carvalho, L.; Candeias, A.E. Material characterization and biodegradation assessment of mural paintings: Renaissance frescoes from Santo Aleixo Church, Southern Portugal. Int. J. Archit. Herit. 2014, 8, 835–852. [Google Scholar] [CrossRef]
- Di Martino, P. What about biofilms on the surface of stone monuments? Open Conf. Proc. J. 2016, 7, 14–28. [Google Scholar] [CrossRef]
- Berdoulay, M.; Salvado, J.C. Genetic characterization of microbial communities living at the surface of building stones. Lett. Appl. Microbiol. 2009, 49, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Portillo, M.C.; Saiz-Jimenez, C.; Gonzalez, J.M. Molecular characterization of total and metabolically active bacterial communities of “white colonizations” in the Altamira cave, Spain. Res. Microbiol. 2009, 160, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Hiraishi, A.; Shimada, K. Aerobic anoxygenic photosynthetic bacteria with zinc-bacteriochlorophyll. J. Gen. Appl. Microbiol. 2001, 47, 161–180. [Google Scholar] [CrossRef] [PubMed]
- Carugno, M.; Consonni, D.; Randia, G.; Catelan, D.; Grisotto, L.; Bertazzi, P.A.; Biggeri, A.; Baccini, M. Air pollution exposure, cause-specific deaths and hospitalizations in a highly polluted Italian region. Environ. Res. 2016, 147, 415–424. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, B.; He, Z.; Yang, X. Distribution and diversity of bacteria and fungi colonization in stone monuments analyzed by high-throughput sequencing. PLoS ONE 2016, 11, e0163287. [Google Scholar] [CrossRef]
- Miller, A.Z.; Laiz, L.; Dionísio, A.; Macedo, M.F.; Saiz-Jimenez, C. Growth of phototrophic biofilms from limestone monuments under laboratory conditions. Int. Biodeter. Biodegr. 2009, 63, 860–867. [Google Scholar] [CrossRef]
- Favet, J.; Lapanje, A.; Giongo, A.; Kennedy, S.; Aung, Y.-Y.; Cattaneo, A.; Davis-Richardson, A.G.; Brown, C.T.; Kort, R.; Brumsack, H.-J.; et al. Microbial hitchhikers on intercontinental dust: Catching a lift in Chad. ISME J. 2013, 7, 850–867. [Google Scholar] [CrossRef]
- Brewer, T.E.; Fierer, N. Tales from the tomb: The microbial ecology of exposed rock surfaces. Environ. Microbiol. 2018, 20, 958–970. [Google Scholar] [CrossRef]
- Maszenan, A.M.; Tay, J.-H.; Schumann, P.; Jiang, H.-L.; Tay, S.T.-L. Quadrisphaera granulorum gen. nov.; sp. nov.; a Gram-positive polyphosphate-accumulating coccus in tetrads or aggregates isolated from aerobic granules. Int. J. Syst. Evol. Microbiol. 2005, 55, 1771–1777. [Google Scholar] [CrossRef]
- Pulschen, A.A.; Bendia, A.G.; Fricker, A.D.; Pellizari, V.H.; Galante, D.; Rodrigues, F. Isolation of uncultured bacteria from Antarctica using long incubation periods and low nutritional media. Front. Microbiol. 2017, 8, 1346. [Google Scholar] [CrossRef]
- Ivanova, N.; Rohde, C.; Munk, C.; Nolan, M.; Lucas, S.; Glavina Del Rio, T.; Tice, H.; Deshpande, S.; Cheng, J.; Tapia, R. Complete genome sequence of Truepera radiovictrix type strain (RQ-24T). Stand. Genomic Sci. 2011, 4, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Gaylarde, C.C.; Gaylarde, P.M.; Copp, J.; Neilan, B. Polyphasic detection of cyanobacteria in terrestrial biofilms. Biofouling 2004, 20, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Hauer, T.; Mühlsteinová, R.; Bohunická, M.; Kaštovský, J.; Mareš, J. Diversity of cyanobacteria on rock surfaces. Biodivers. Conserv. 2015, 24, 759–779. [Google Scholar] [CrossRef]
- Lee, L.-F.; Huang, Y.-J.; Chen, C.W. Repressed multidrug resistance genes in Streptomyces lividans. Arch. Microbiol. 2003, 180, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Herraiz, M.; Jurado, V.; Cuezva, S.; Laiz, L.; Pallecchi, P.; Tiano, P.; Sanchez-Moral, S.; Saiz-Jimenez, C. The actinobacterial colonization of Etruscan paintings. Sci. Rep. 2013, 3, 1440. [Google Scholar] [CrossRef] [PubMed]
- Odić, D.; Prah, J.; Avguštin, G. Identification of bacterial contaminants from calcium carbonate filler production lines and an evaluation of biocide based decontamination procedures. Biofouling 2017, 33, 327–335. [Google Scholar] [CrossRef]
- Piccardi, P.; Vessman, B.; Mitri, S. Toxicity drives facilitation between 4 bacterial species. PNAS 2019, 116, 15979–15984. [Google Scholar] [CrossRef]
- Wang, T.-J.; Su, N.-N.; Lei, P.; Qiu, M.-Y.; Chen, Z.-J.; Yao, L.-G.; Han, H.E. Community structure of heavy metal immobilized bacteria in the lettuce (Lactuca sativa L.) rhizosphere in soil polluted by heavy metals and its effects on reducing heavy metal accumulation in lettuce. Huanjing Kexue/Environ. Sci. 2019, 40, 5133–5141. [Google Scholar]
- Wang, B.; Wang, Y.; Cui, X.; Zhang, Y.; Yu, Z. Bioconversion of coal to methane by microbial communities from soil and from an opencast mine in the Xilingol grassland of northeast China. Biotechnol. Biofuels 2019, 12, 236. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Y.; Feng, H.; Wang, Y.; Yang, X.; Wang, Z. Genome-guided identification and characterization of bacteria for simultaneous degradation of polycyclic aromatic hydrocarbons and resistance to hexavalent chromium. Int. Biodeter. Biodegr. 2019, 138, 78–86. [Google Scholar] [CrossRef]
- Stackebrandt, E.; Brambilla, E.; Richert, K. Gene sequence phylogenies of the family Microbacteriaceae. Curr. Microbiol. 2007, 55, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Dhanjal, S.; Kaur, I.; Korpole, S.; Schumann, P.; Singh Cameotra, S.; Pukall, R.; Klenk, H.-P.; Mayilraj, S. Agrococcus carbonis sp. nov., isolated from soil of a coal mine. Int. J. Syst. Evol. Microbiol. 2011, 61, 1253–1258. [Google Scholar] [CrossRef] [PubMed]
- Wieser, M. Agrococcus citreus sp. nov.; isolated from a medieval wall painting of the chapel of Castle Herberstein (Austria). Int. J. Syst. Bacteriol. 1999, 49, 1165–1170. [Google Scholar] [CrossRef] [PubMed]
- Rauch, M.E.; Graef, H.W.; Rozenzhak, S.M.; Jones, S.E.; Bleckmann, C.A.; Kruger, R.L.; Naik, R.R.; Stone, M.O. Characterization of microbial contamination in United States Air Force aviation fuel tanks. J. Ind. Microbiol. Biotechnol. 2006, 33, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Tekaya, S.B.; Tipayno, S.; Kim, K.; Subramanian, P.; Sa, T. Rhizobacteria: Restoration of Heavy Metal-Contaminated Soils. In Physiological Mechanisms and Adaptation Strategies in Plants Under Changing Environment; Ahmad, P., Wani, M., Eds.; Springer: New York, NY, USA, 2014; pp. 297–323. [Google Scholar]
- Li, H.; Chi, Z.; Li, J.; Wu, H.; Yan, B. Bacterial community structure and function in soils from tidal freshwater wetlands in a Chinese delta: Potential impacts of salinity and nutrient. Sci Total Environ. 2019, 696, 134029. [Google Scholar] [CrossRef]
- Li, J.; Cai, M.H.; Miao, Y.; Luo, G.; Li, W.T.; Li, Y.; Li, A.M. Bacterial community structure and predicted function in an acidogenic sulfate-reducing reactor: Effect of organic carbon to sulfate ratios. Bioresour. Technol. 2019, 293, 122020. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, B.; Yang, X.; Ge, Q. Deterioration-associated microbiome of stone monuments: Structure, variation, and assembly. Appl. Environ. Microbiol. 2018, 84, e02680-17. [Google Scholar] [CrossRef]
- De Mandal, S.; Chatterjee, R.; Kumar, N.S. Dominant bacterial phyla in caves and their predicted functional roles in C and N cycle. BMC Microbiol. 2017, 17, 90. [Google Scholar] [CrossRef]
- Ren, Z.; Gao, H.; Elser, J.J.; Zhao, Q. Microbial functional genes elucidate environmental drivers of biofilm metabolism in glacier-fed streams. Sci Rep. 2017, 7, 12668. [Google Scholar] [CrossRef]
- Bomberg, M.; Lamminmäki, T.; Itävaara, M. Microbial communities and their predicted metabolic characteristics in deep fracture groundwaters of the crystalline bedrock at Olkiluoto, Finland. Biogeosciences 2016, 13, 6031–6047. [Google Scholar] [CrossRef]
- Vikram, S.; Guerrero, L.D.; Makhalanyane, T.P.; Le, P.T.; Seely, M.; Cowan, D.A. Metagenomic analysis provides insights into functional capacity in a hyperarid desert soil niche community. Environ. Microbiol. 2016, 18, 1875–1888. [Google Scholar] [CrossRef] [PubMed]
- Shirai, M.; Okuda, M.; Motohashi, K.; Imoto, M.; Furihata, K.; Matsuo, Y.; Katsuta, A.; Shizuri, Y.; Seto, H. Terpenoids produced by actinomycetes: Isolation, structural elucidation and biosynthesis of new diterpenes, gifhornenolones A and B from Verrucosispora gifhornensis YM28-088. J. Antibiot. (Tokyo) 2010, 63, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Moody, S.C.; Zhao, B.; Lei, L.; Nelson, D.R.; Mullins, J.G.; Waterman, M.R.; Kelly, S.L.; Lamb, D.C. Investigating conservation of the albaflavenone biosynthetic pathway and CYP170 bifunctionality in streptomycetes. FEBS J. 2012, 279, 1640–1649. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Kwon, H.C. New naphthoquinone terpenoids from marine actinobacterium, Streptomyces sp. CNQ-509. Mar. Drugs 2018, 16, 90. [Google Scholar] [CrossRef] [PubMed]
- Bastian, F.; Alabouvette, C. Lights and shadows on the conservation of a rock art cave: The case of Lascaux Cave. Int. J. Speleol. 2009, 38, 55–60. [Google Scholar] [CrossRef]
- Jones, M.; Herd, T.; Christie, H. Resistance of Pseudomonas aeruginosa to amphoteric and quaternary ammonium biocides. Microbios 1989, 58, 49–61. [Google Scholar]
- Levy, S.B. Active efflux, a common mechanism for biocide and antibiotic resistance. J. Appl. Microbiol. 2002, 92, 65S–71S. [Google Scholar] [CrossRef]
- Nagai, K.; Murata, T.; Ohta, S.; Zenda, H.; Ohnishi, M.; Hayashi, T. Two different mechanisms are involved in the extremely high-level benzalkonium chloride resistance of a Pseudomonas fluorescens strain. Microbiol. Immunol. 2003, 47, 709–715. [Google Scholar] [CrossRef]
- Kim, M.; Weigand, M.R.; Oh, S.; Hatt, J.K.; Krishnan, R.; Tezel, U.; Pavlostathis, S.G.; Konstantinidis, K.T. Widely used benzalkonium chloride disinfectants can promote antibiotic resistance. Appl. Environ. Microbiol. 2018, 84, e01201-18. [Google Scholar] [CrossRef]
- Ceragioli, M.; Mols, M.; Moezelaar, R.; Ghelardi, E.; Senesi, S.; Abee, T. Comparative transcriptomic and phenotypic analysis of the responses of Bacillus cereus to various disinfectant treatments. Appl. Environ. Microbiol. 2010, 76, 3352–3360. [Google Scholar] [CrossRef]
- Ahmad, A.; Majaz, S.; Nouroz, F. Two-component systems regulate ABC transporters in antimicrobial peptide production, immunity and resistance. Microbiology 2019. [Google Scholar] [CrossRef] [PubMed]
- Greene, N.P.; Kaplan, E.; Crow, A.; Koronakis, V. Antibiotic resistance mediated by the macB ABC transporter family: A structural and functional perspective. Front. Microbiol. 2018, 9, 950. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Geng, Y.; Ren, S.; Yu, T.; Li, Y.; Liu, G.; Wang, H.; Meng, H.; Shi, L. The VirAB-VirSR-AnrAB multicomponent system is involved in resistance of Listeria monocytogenes EGD-e to cephalosporins, bacitracin, nisin, benzalkonium chloride, and ethidium bromide. Appl. Environ. Microbiol. 2019, 85, e01470-19. [Google Scholar] [CrossRef] [PubMed]
- Forbes, S.; Morgan, N.; Humphreys, G.J.; Amézquita, A.; Mistry, H.; McBain, A.J. Loss of function in Escherichia coli exposed to environmentally relevant concentrations of benzalkonium chloride. Appl. Environ. Microbiol. 2019, 85, e02417-18. [Google Scholar] [CrossRef] [PubMed]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villa, F.; Gulotta, D.; Toniolo, L.; Borruso, L.; Cattò, C.; Cappitelli, F. Aesthetic Alteration of Marble Surfaces Caused by Biofilm Formation: Effects of Chemical Cleaning. Coatings 2020, 10, 122. https://doi.org/10.3390/coatings10020122
Villa F, Gulotta D, Toniolo L, Borruso L, Cattò C, Cappitelli F. Aesthetic Alteration of Marble Surfaces Caused by Biofilm Formation: Effects of Chemical Cleaning. Coatings. 2020; 10(2):122. https://doi.org/10.3390/coatings10020122
Chicago/Turabian StyleVilla, Federica, Davide Gulotta, Lucia Toniolo, Luigimaria Borruso, Cristina Cattò, and Francesca Cappitelli. 2020. "Aesthetic Alteration of Marble Surfaces Caused by Biofilm Formation: Effects of Chemical Cleaning" Coatings 10, no. 2: 122. https://doi.org/10.3390/coatings10020122
APA StyleVilla, F., Gulotta, D., Toniolo, L., Borruso, L., Cattò, C., & Cappitelli, F. (2020). Aesthetic Alteration of Marble Surfaces Caused by Biofilm Formation: Effects of Chemical Cleaning. Coatings, 10(2), 122. https://doi.org/10.3390/coatings10020122








