Graphene Oxide Decorated with Titanium Nanoparticles to Reinforce the Anti-Corrosion Performance of Epoxy Coating
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of GO
2.3. Preparation of f-GO and f-Ti
2.4. Preparation of GO-Ti
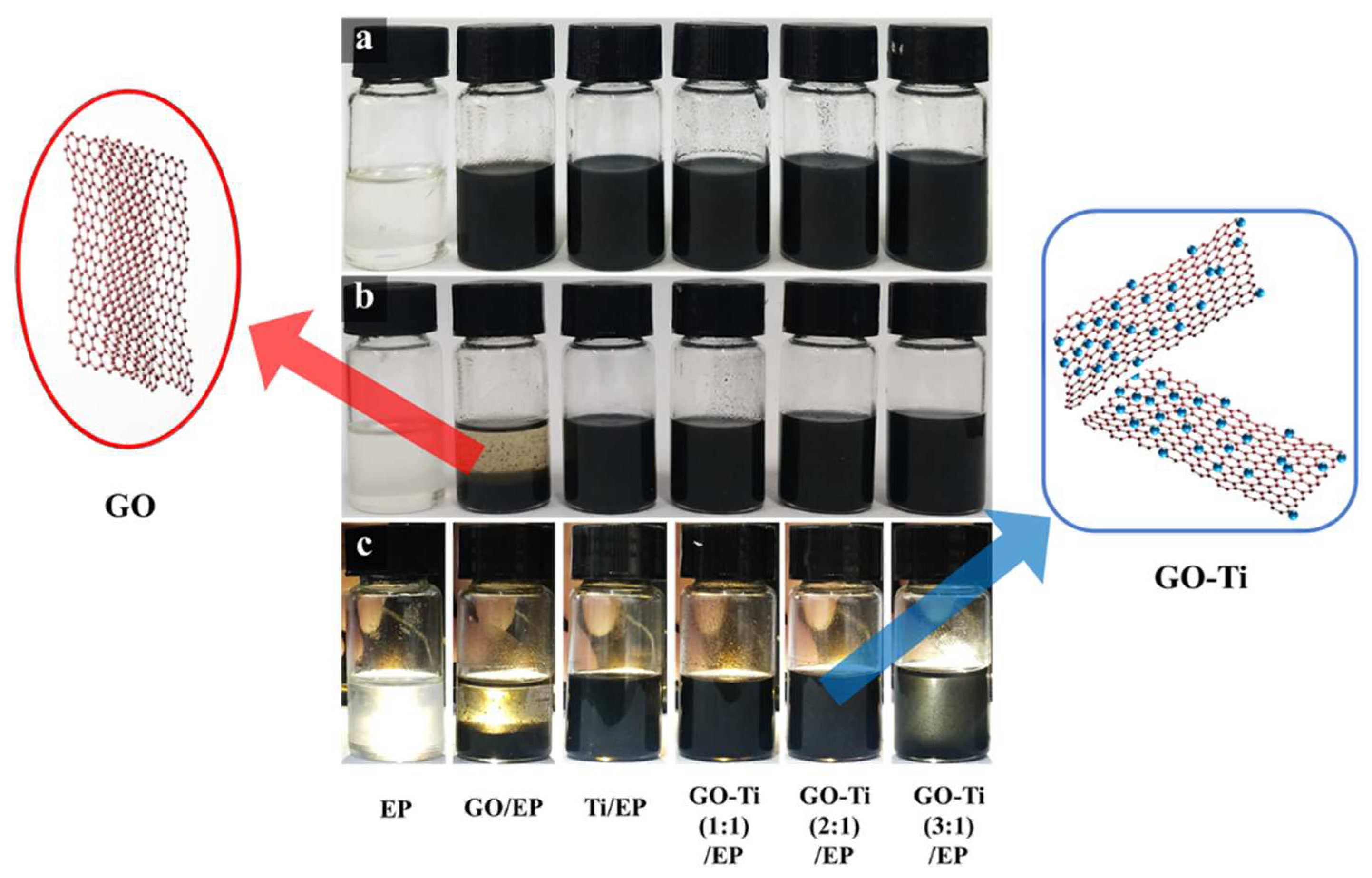
2.5. Preparation of GO/EP, Ti/EP, and GO-Ti /EP Composite Coatings
2.6. Measurements
3. Results and Discussions
3.1. Structure and Morphology of GO-Ti Composites
3.2. The Properties of GO-Ti/EP Composite Coatings
4. Conclusions
- Nano-Ti particles are successfully uniformly loaded on the GO sheets, and the best composite ratio of GO and Nano-Ti is 2:1. The GO-Ti sheet spacing is increased to 1.02 nm at most, and the composites have good compatibility with epoxy resin.
- The |Z|0.01Hz of GO-Ti/EP is higher than that of GO/EP, showing an excellent anti-corrosion effect. This is because the composites exhibit the sheet structure that blocks the micropores and hinders the invasion of corrosive medium.
- The addition of GO-Ti effectively improves the wear resistance of the coatings, mainly because the composites can play a lubricating and cross-linking buffering role in the coating.
Author Contributions
Funding
Conflicts of Interest
References
- Bian, J.; Wang, W.; Cuan, C. Metal Corrosion and Progress in Anti-Corrosion Organic Coatings. J. Mater. Sci. Eng. 2003, 5, 769–772. (In Chinese) [Google Scholar]
- Ma, H.; Cui, C.; Chen, T. Brief Introduction to Metal Corrosion and Protection. Electrochemistry 2011, 3, 55–58. [Google Scholar]
- Nazeer, A.A.; Madkour, M. Potential use of smart coatings for corrosion protection of metals and alloys: A review. J. Mol. Liq. 2018, 253, 11–22. [Google Scholar] [CrossRef]
- Sugiman, S.; Putra, I.K.P.; Setyawan, P.D. Effects of the media and ageing condition on the tensile properties and fracture toughness of epoxy resin. Polym. Degrad. Stab. 2016, 134, 311–321. [Google Scholar] [CrossRef]
- Park, S.-J.; Heo, G.-Y.; Jin, F.-L. Cure behaviors and thermal stabilities of tetrafunctional epoxy resin toughened by polyamideimide. Macromol. Res. 2015, 23, 320–324. [Google Scholar] [CrossRef]
- Paluvai, N.R.; Mohanty, S.; Nayak, S.K. Fabrication and evaluation of acrylated epoxidized castor oil-toughened diglycidyl ether of bisphenol A nanocomposites. Can. J. Chem. Eng. 2015, 93, 2107–2116. [Google Scholar] [CrossRef]
- Yahyaie, H.; Ebrahimi, M.; Tahami, H.V.; Mafi, E.R. Toughening mechanisms of rubber modified thin film epoxy resins. Prog. Org. Coat. 2013, 76, 286–292. [Google Scholar] [CrossRef]
- Smith, A.T.; Lachance, A.M.; Zeng, S.; Liu, B.; Sun, L. Synthesis, properties, and applications of graphene oxide/reduced graphene oxide and their nanocomposites. Nano Mater. Sci. 2019, 1, 31–47. [Google Scholar] [CrossRef]
- Wang, J.; Jin, X.; Li, C.; Wang, W.; Wu, H.; Guo, S. Graphene and graphene derivatives toughening polymers: Toward high toughness and strength. Chem. Eng. J. 2019, 370, 831–854. [Google Scholar] [CrossRef]
- Ding, R.; Chen, S.; Lv, J.; Zhang, W.; Zhao, X.-D.; Liu, J.; Wang, X.; Gui, T.-J.; Li, B.-J.; Tang, Y.-Z.; et al. Study on graphene modified organic anti-corrosion coatings: A comprehensive review. J. Alloys Compd. 2019, 806, 611–635. [Google Scholar] [CrossRef]
- Wu, Y.; Zhu, X.; Zhao, W.; Wang, Y.; Wang, C.; Xue, Q. Corrosion mechanism of graphene coating with different defect levels. J. Alloys Compd. 2019, 777, 135–144. [Google Scholar] [CrossRef]
- Sun, W.; Wang, L.; Wu, T.; Wang, M.; Yang, Z.; Pan, Y.; Liu, G. Inhibiting the Corrosion-Promotion Activity of Graphene. Chem. Mater. 2015, 27, 2367–2373. [Google Scholar] [CrossRef]
- Schriver, M.; Regan, W.; Gannett, W.J.; Zaniewski, A.M.; Crommie, M.F.; Zettl, A. Graphene as a Long-Term Metal Oxidation Barrier: Worse Than Nothing. ACS Nano 2013, 7, 5763–5768. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.; Coleman, J.N.; Zhang, H.; Shin, H.; Chhowalla, M.; Zheng, Z. Production of Two-Dimensional Nanomaterials via Liquid-Based Direct Exfoliation. Small 2016, 12, 272–293. [Google Scholar] [CrossRef] [PubMed]
- Fankhänel, J.; Arash, B.; Rolfes, R. Elastic interphase properties of nanoparticle/epoxy nanocomposites: A molecular dynamics study. Compos. Part B Eng. 2019, 176, 107211. [Google Scholar] [CrossRef]
- Jamkhande, P.G.; Ghule, N.W.; Bamer, A.H.; Kalaskar, M.G. Metal nanoparticles synthesis: An overview on methods of preparation, advantages and disadvantages, and applications. J. Drug Deliv. Sci. Technol. 2019, 53, 101174. [Google Scholar] [CrossRef]
- Di, H.; Yu, Z.; Ma, Y.; Li, F.; Lv, L.; Pan, Y.; Lin, Y.; Liu, Y.; He, Y. Graphene oxide decorated with Fe3O4 nanoparticles with advanced anticorrosive properties of epoxy coatings. J. Taiwan Inst. Chem. Eng. 2016, 64, 244–251. [Google Scholar] [CrossRef]
- Yu, Z.; Di, H.; Ma, Y.; Lv, L.; Pan, Y.; Zhang, C.; He, Y. Fabrication of graphene oxide–alumina hybrids to reinforce the anti-corrosion performance of composite epoxy coatings. Appl. Surf. Sci. 2015, 351, 986–996. [Google Scholar] [CrossRef]
- Das, M.R.; Sarma, R.K.; Borah, S.C.; Kumari, R.; Saikia, R.; Deshmukh, A.B.; Shelke, M.V.; Sengupta, P.; Szunerits, S.; Boukherroub, R. The synthesis of citrate-modified silver nanoparticles in an aqueous suspension of graphene oxide nanosheets and their antibacterial activity. Colloids Surf. B Biointerfaces 2013, 105, 128–136. [Google Scholar] [CrossRef]
- Liu, J.; Yu, Q.; Yu, M.; Li, S.; Zhao, K.; Xue, B.; Zu, H. Silane modification of titanium dioxide-decorated graphene oxide nanocomposite for enhancing anticorrosion performance of epoxy coatings on AA-2024. J. Alloys Compd. 2018, 744, 728–739. [Google Scholar] [CrossRef]
- Yan, S.; Song, G.-L.; Li, Z.; Wang, H.; Zheng, D.; Cao, F.; Horynova, M.; Dargusch, M.S.; Zhou, L. A state-of-the-art review on passivation and biofouling of Ti and its alloys in marine environments. J. Mater. Sci. Technol. 2018, 34, 421–435. [Google Scholar] [CrossRef]
- Zhang, X. Effects of Nano-sized Titanium Powder on the Anti-corrosion Property of Epoxy Coatings on Steel. Kem. u Ind. 2014, 63, 317–322. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, F.; Du, Y. Effect of nano-sized titanium powder addition on corrosion performance of epoxy coatings. Surf. Coat. Technol. 2007, 201, 7241–7245. [Google Scholar] [CrossRef]
- William, S.H.; Richard, E.O. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar]
- Ahmadi-Moghadam, B.; Sharafimasooleh, M.; Shadlou, S.; Taheri, F. Effect of functionalization of graphene nanoplatelets on the mechanical response of graphene/epoxy composites. Mater. Des. 2015, 66, 142–149. [Google Scholar] [CrossRef]
- Xu, L.; Feng, J.; Li, J.; Liu, X.; Jiang, S. Graphene oxide bonded fused-silica fiber for solid-phase microextraction-gas chromatography of polycyclic aromatic. J. Sep. Sci. 2012, 35, 93–100. [Google Scholar] [CrossRef]
- Bruun, S.W.; Kohler, A.; Adt, I.; Sockalingum, G.D.; Manfait, M.; Martens, H. Correcting Attenuated Total Reflection—Fourier Transform Infrared Spectra for Water Vapor and Carbon Dioxide. Appl. Spectrosc. 2006, 60, 1029–1039. [Google Scholar] [CrossRef]
- Muniyalakshmi, M.; Sethuraman, K.; Silambarasan, D. Synthesis and characterization of graphene oxide nanosheets. Mater. Today Proc. 2019, in press. [Google Scholar] [CrossRef]
- Alrashed, M.M.; Soucek, M.D.; Jana, S.C. Role of graphene oxide and functionalized graphene oxide in protective hybrid coatings. Prog. Org. Coat. 2019, 134, 197–208. [Google Scholar] [CrossRef]
- Di, H.; Yu, Z.; Ma, Y.; Pan, Y.; Shi, H.; Lv, L.; Li, F.; Wang, C.; Long, T.; He, Y. Anchoring calcium carbonate on graphene oxide reinforced with anticorrosive properties of composite epoxy coatings. Polym. Adv. Technol. 2016, 27, 915–921. [Google Scholar] [CrossRef]
- Sbirrazzuoli, N.; Mititelu-Mija, A.; Vincent, L.; Alzina, C. Isoconversional kinetic analysis of stoichiometric and off-stoichiometric epoxy-amine cures. Thermochim. Acta 2006, 447, 167–177. [Google Scholar] [CrossRef]
- Amrollahi, S.; Ramezanzadeh, B.; Yari, H.; Ramezanzadeh, M.; Mahdavian, M. In-situ growth of ceria nanoparticles on graphene oxide nanoplatelets to be used as a multifunctional (UV shield/radical scavenger/anticorrosive) hybrid compound for exterior coatings. Prog. Org. Coat. 2019, 136, 105241. [Google Scholar] [CrossRef]
- Kalsoom, U.-I.; Bashir, S.; Ali, N. SEM, AFM, EDX and XRD analysis of laser ablated Ti in nonreactive and reactive ambient environments. Surf. Coat. Technol. 2013, 235, 297–302. [Google Scholar] [CrossRef]
- Liu, X.; Shao, Y.; Zhang, Y.; Meng, G.; Zhang, T.; Wang, F. Using high-temperature mechanochemistry treatment to modify iron oxide and improve the corrosion performance of epoxy coating–I. High-temperature ball milling treatment. Corros. Sci. 2015, 90, 451–462. [Google Scholar] [CrossRef]
- Liu, X.; Xiong, J.; Lv, Y.; Zuo, Y. Study on corrosion electrochemical behavior of several different coating systems by EIS. Prog. Org. Coat. 2009, 64, 497–503. [Google Scholar] [CrossRef]
- Zhu, C.; Xie, R.; Xue, J.; Song, L. Studies of the impedance models and water transport behaviors of cathodically polarized coating. Electrochim. Acta 2011, 56, 5828–5835. [Google Scholar] [CrossRef]
- Shi, G.; Zhang, M.Q.; Rong, M.Z.; Wetzel, B.; Friedrich, K. Friction and wear of low nanometer Si3N4 filled epoxy composites. Wear 2003, 254, 784–796. [Google Scholar] [CrossRef]







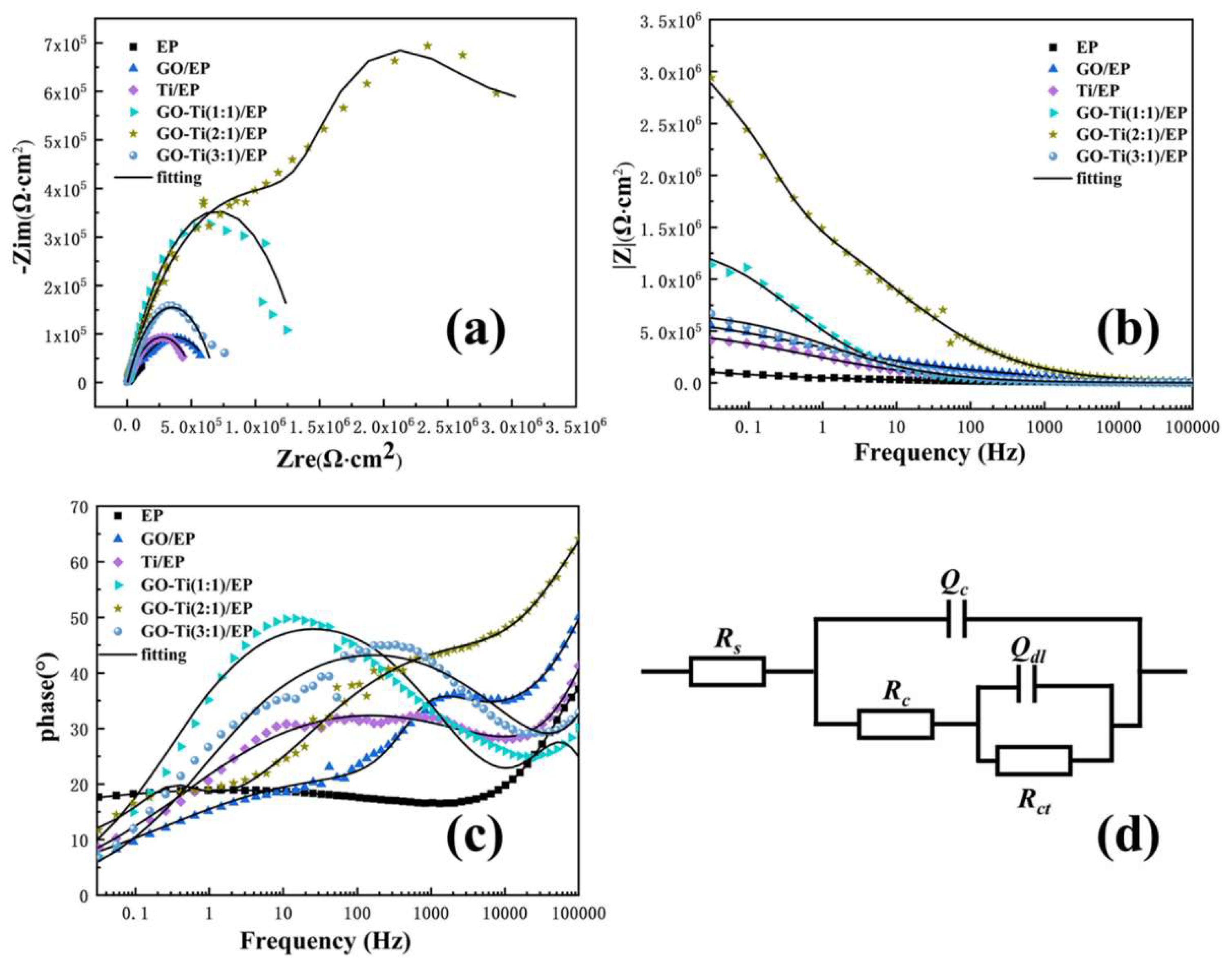



| Sample | Time/h | Qc/(Ω−1 cm−2 sn) | Rc/(Ω cm2) | Qdl/(Ω−1 cm−2 sn) | Rct/(Ω cm2) |
|---|---|---|---|---|---|
| EP | 72 | 1.72 × 10−8 | 7.34 × 103 | 1.086 × 10−5 | 2.486 × 10−5 |
| GO/EP | 96 | 1.859 × 10−7 | 1.066 × 104 | 8.181 × 10−7 | 6.01 × 105 |
| Ti/EP | 72 | 1.284 × 10−10 | 9.56 × 103 | 1.24 × 10−6 | 5.208 × 105 |
| GO-Ti(1:1)/EP | 120 | 1.159 × 10−7 | 1.437 × 105 | 3.452 × 10−7 | 1.327 × 106 |
| GO-Ti(2:1)/EP | 192 | 9.014 × 10−11 | 3.591 × 105 | 3.104 × 10−7 | 4.404 × 106 |
| GO-Ti(3:1)/EP | 120 | 1.058 × 10−6 | 2.455 × 104 | 2.538 × 10−8 | 5.556 × 105 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, H.; Qi, F.; Zhao, N.; Wan, P.; Zhang, B.; Xiong, H.; Liao, B.; Ouyang, X. Graphene Oxide Decorated with Titanium Nanoparticles to Reinforce the Anti-Corrosion Performance of Epoxy Coating. Coatings 2020, 10, 129. https://doi.org/10.3390/coatings10020129
Yuan H, Qi F, Zhao N, Wan P, Zhang B, Xiong H, Liao B, Ouyang X. Graphene Oxide Decorated with Titanium Nanoparticles to Reinforce the Anti-Corrosion Performance of Epoxy Coating. Coatings. 2020; 10(2):129. https://doi.org/10.3390/coatings10020129
Chicago/Turabian StyleYuan, Heng, Fugang Qi, Nie Zhao, Pengying Wan, Biao Zhang, Hailong Xiong, Bin Liao, and Xiaoping Ouyang. 2020. "Graphene Oxide Decorated with Titanium Nanoparticles to Reinforce the Anti-Corrosion Performance of Epoxy Coating" Coatings 10, no. 2: 129. https://doi.org/10.3390/coatings10020129






