Synthesis and Physical Properties of Antiperovskite CuNFe3 Thin Films via Solution Processing for Room Temperature Soft-Magnets
Abstract
1. Introduction
2. Materials and Methods
3. Results
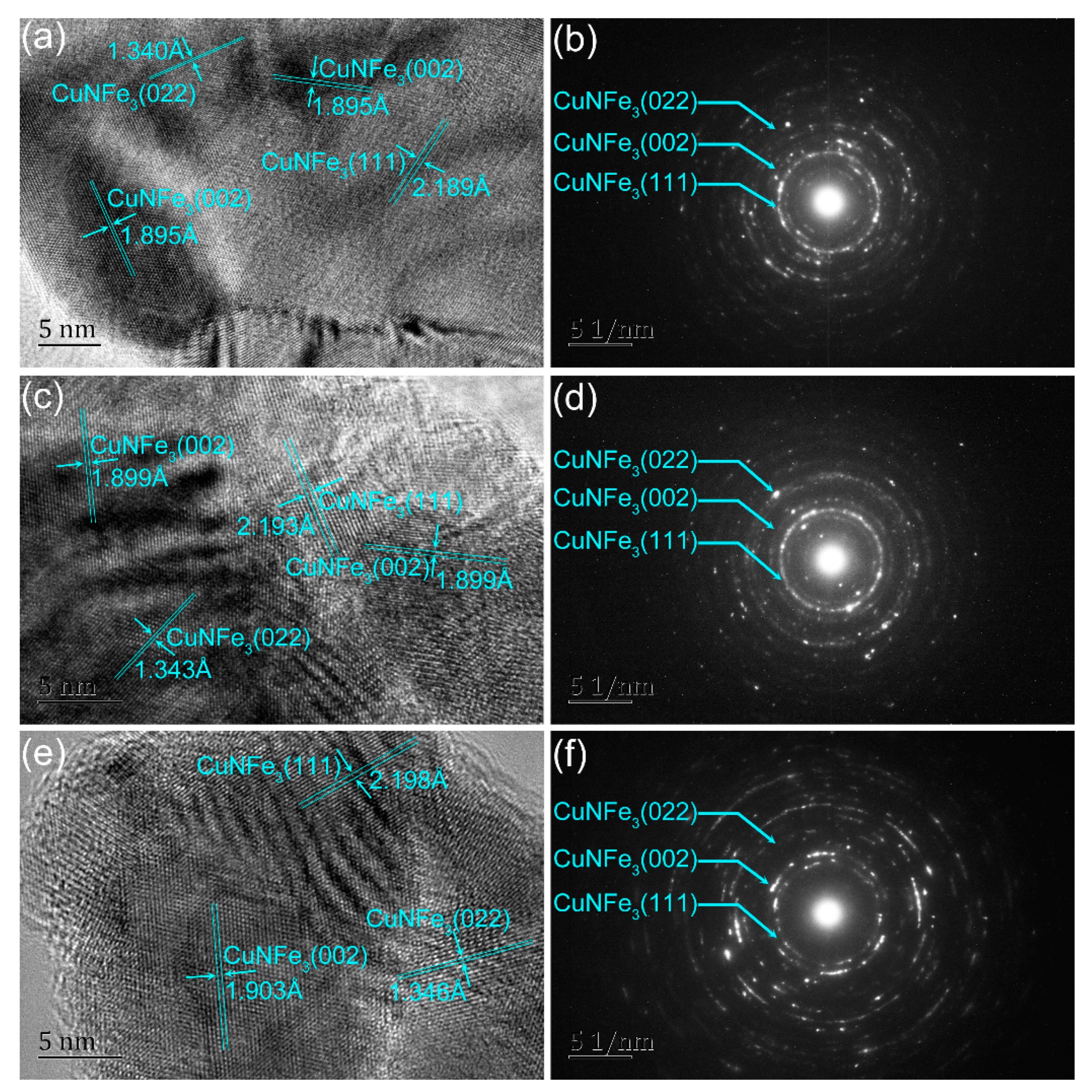
3.1. Structural and Surface Morphology Studies
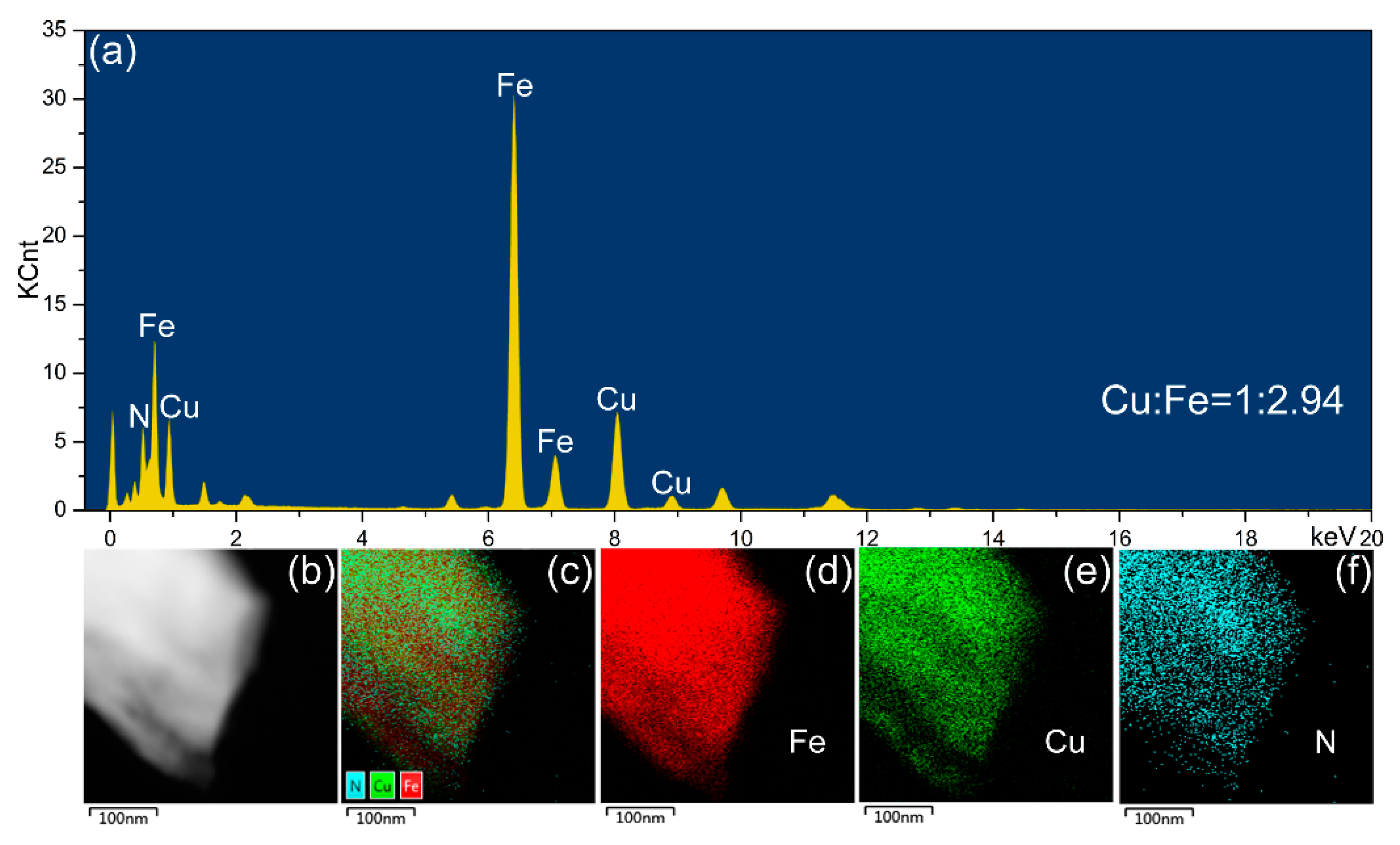
3.2. Nitrogen Content Variations
3.3. Magnetic Properties
3.4. Electrical Transport Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, H.L.; Hui, Z.Z.; Tang, X.W.; Wei, R.H.; Yang, J.; Dai, J.M.; Song, W.H.; Luo, H.M.; Zhu, X.B.; Sun, Y.P. Self-assembled c-axis oriented δ-MoN thin films on Si substrates by chemical solution deposition: Growth, transport and superconducting properties. J. Alloy. Comps. 2017, 704, 453–458. [Google Scholar] [CrossRef]
- Hui, Z.Z.; Tang, X.W.; Shao, D.F.; Lei, H.C.; Yang, J.; Song, W.H.; Luo, H.M.; Zhu, X.B.; Sun, Y.P. Epitaxial antiperovskite superconducting CuNNi3 thin films synthesized by chemical solution deposition. Chem. Commun. 2014, 50, 12734–12737. [Google Scholar] [CrossRef]
- Peng, X.L.; Yu, S.Y.; Chang, J.S.; Ge, M.H.; Li, J.; Ellis, T.; Yang, Y.T.; Xu, J.C.; Hong, B.; Jin, D.F.; et al. Preparation and magnetic properties of Fe4N/Fe soft magnetic composites fabricated by gas nitridation. J. Magn. Magn. Mater. 2020, 166407. [Google Scholar] [CrossRef]
- Gao, B.; Li, X.X.; Ding, K.; Huang, C.; Li, Q.W.; Chu, P.K.; Huo, K.F. Recent progress in nanostructured transition metal nitrides for advanced electrochemical energy storage. J. Mater. Chem. A 2019, 7, 14–37. [Google Scholar] [CrossRef]
- Kolaklieva, L.; Chitanov, V.; Szekeres, A.; Antonova, K.; Terziyska, P.; Fogarassy, Z.; Petrik, P.; Mihaiescu, I.N.; Duta, L. Pulsed Laser Deposition of Aluminum Nitride Films: Correlation between Mechanical, Optical, and Structural Properties. Coatings 2019, 9, 195. [Google Scholar] [CrossRef]
- Benmalem, Y.; Abbad, A.; Benstaali, W.; Bentounes, H.A.; Seddik, T.; Lantri, T. Thermoelectric, electronic and structural properties of CuNMn3 cubic antiperovskite. J. Comput. Electron. 2018, 17, 881–887. [Google Scholar] [CrossRef]
- Guo, X.G.; Lin, J.C.; Tong, P.; Lin, S.; Yang, C.; Lu, W.J.; Song, W.H.; Luo, H.M.; Zhu, X.B.; Sun, Y.P. Anomalous low-temperature heat capacity in antiperovskite compounds. Chin. Phys. B 2017, 26, 026501. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Song, Y.; Cai, J.Y.; Zheng, X.S.; Han, D.D.; Wu, Y.S.; Zang, Y.P.; Niu, S.W.; Liu, Y.; Zhu, J.F.; et al. Tailoring the d-Band centers enables Co4N nanosheets to be highly active for hydrogen evolution catalysis. Angew. Chem. Int. Edit. 2018, 57, 5076–5080. [Google Scholar] [CrossRef] [PubMed]
- Hui, Z.Z.; Tang, X.W.; Shao, D.F.; Wei, R.H.; Yang, J.; Tong, P.; Song, W.H.; Zhu, X.B.; Sun, Y.P. Self-assembled c-axis oriented antiperovskite soft-magnetic CuNCo3 thin films by chemical solution deposition. J. Mater. Chem. C 2015, 3, 4438. [Google Scholar] [CrossRef]
- Meinert, M. Exchange interactions and Curie temperatures of the tetrametal nitrides Cr4N, Mn4N, Fe4N, Co4N, and Ni4N. J. Phys. Condens. Mat. 2016, 28, 056006. [Google Scholar] [CrossRef]
- Luo, X.; Liu, S.X. Preparation and chemical stability of iron-nitride-coated iron microparticles. J. Magn. Magn. Mater. 2007, 308, L1–L4. [Google Scholar] [CrossRef]
- Monachesi, P.; Björkman, T.; Gasche, T.; Eriksson, O. Electronic structure and magnetic properties of Mn, Co, and Ni substitution of Fe in Fe4N. Phys. Rev. B 2013, 88, 054420. [Google Scholar] [CrossRef]
- Li, F.S.; Shen, B.L.; Makino, A.; Inoue, A. Excellent soft-magnetic properties of (Fe, Co)-Mo-(P, C, B, Si) bulk glassy alloys with ductile deformation behavior. Appl. Phys. Lett. 2007, 91, 4096. [Google Scholar] [CrossRef]
- Skulmowska, K.; Pelka, R.; Arabczyk, W. Oscillatory kinetics in the process of reduction of nanocrystalline iron nitride. J. Phys. Chem. C 2017, 121, 141. [Google Scholar] [CrossRef]
- Mi, W.B.; Guo, Z.B.; Feng, X.P.; Bai, H.L. Reactively sputtered epitaxial γ′−Fe4N films: Surface morphology, microstructure, magnetic and electrical transport properties. Acta Mater. 2013, 61, 6387–6395. [Google Scholar] [CrossRef]
- Wang, W.; Kan, X.C.; Liu, X.S.; Feng, S.J.; Liu, C.C.; Rehman, K.M.U.; Shezad, M.; Wu, Q.Y.; Wang, Y.Y. Effect of Cu on microstructure, magnetic properties of antiperovskite nitrides CuxNFe4−x. J. Mate. Sci. Mater. Electron. 2019, 30, 10383–10390. [Google Scholar] [CrossRef]
- Costa-Krämer, J.L.; Borsa, D.M.; García-Martín, J.M.; Martín-González, M.S.; Boerma, D.O.; Briones, F. Structure and magnetism of single-phase epitaxial γ′−Fe4N. Phys. Rev. B 2004, 69, 144402. [Google Scholar] [CrossRef]
- Subramanyam, G.; Cole, M.W.; Sun, N.X.; Kalkur, T.S.; Sbrockey, N.M.; Tompa, G.S.; Guo, X.M.; Chen, C.L.; Alpay, S.P.; Rossetti, G.A.; et al. Challenges and opportunities for multi-functional oxide thin films for voltage tunable radio frequency/microwave components. J. Appl. Phys. 2013, 114, 191301. [Google Scholar] [CrossRef]
- Haertling, G.H. Dielectric and ferroelectric properties of acetatederived PBZT and PSZT thin films. Integr. Ferroelectr. 1995, 7, 279–289. [Google Scholar] [CrossRef]
- Lange, F.F. Chemical solution routes to single-crystal thin films. Science 1996, 273, 903–909. [Google Scholar] [CrossRef]
- Schwartz, R.W.; Schneller, T.; Waser, R. Chemical solution deposition of electronic oxide films. C. R. Chim. 2004, 7, 433–461. [Google Scholar] [CrossRef]
- Mexmain, J. Contribution a l’etude du ferrite cuivreux et de ses solutions solides avec le ferrite cuivrique. Ann. Chim. 1971, 1971, 297–308. [Google Scholar]
- Schwartz, R.W. Chemical solution deposition of perovskite thin films. Chem. Mater. 1997, 9, 2325–2329. [Google Scholar] [CrossRef]
- Thompson, C.V. Structure evolution during processing of polycrystalline films. Ann. Rev. Mater. 2000, 30, 159. [Google Scholar] [CrossRef]
- Zhang, L.; Deng, Y.; Yu, J.Y.; Zhang, M.; Huang, K.; Li, Y.B.; Li, P.; Zhu, X.B. Synthesis and properties of Co5.47N thin films by chemical solution deposition. Mater. Lett. 2018, 225, 145–148. [Google Scholar] [CrossRef]
- Matar, S.F.; Houari, A.; Belkhir, M.A. Ab initio studies of magnetic properties of cobalt and tetracobalt nitride Co4N. Phys. Rev. B 2007, 75, 245109. [Google Scholar] [CrossRef]
- Ma, X.G.; Jiang, J.J.; Liang, P.; Wang, J.; Ma, Q.; Zhang, Q.K. Structural stability and magnetism of γ′-Fe4N and CoFe3N compounds. J. Alloy. Compd. 2009, 480, 475–480. [Google Scholar] [CrossRef]
- Heime, K. Der zusammenhang zwischen metall-halbleiter-barrieren-höhe und elektronegativität sowie kernladungszahl des metalles. Solid State Electron. 1970, 13, 1505–1507. [Google Scholar] [CrossRef]
- Thompson, C.V. Grain growth in thin films. Annu. Rev. Mater. Sci. 1990, 20, 245–268. [Google Scholar] [CrossRef]
- Yu, F.; Ren, L.; Meng, M.; Wang, Y.; Yang, M.; Wu, S.; Li, S. Growth and magnetic property of antiperovskite manganese nitride films doped with Cu by molecular beam epitaxy. J. Appl. Phys. 2014, 115, 133911. [Google Scholar] [CrossRef]
- Moulder, J.F. Handbook of X-ray photoelectron spectroscopy. Phys. Electron. 1995, 230–232. [Google Scholar]
- Merey, R.A.; Karajou, J.; Issa, H. X-ray fluorescence analysis of geological samples: Exploring the effect of sample thickness on the accuracy of results. Appl. Radiat. Isot. 2005, 62, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jia, H.; Zheng, W.T.; Chen, Y.; Feng, S. Structural and magnetic properties of Co-N thin films synthesized by direct current magnetron sputtering. Thin Solid Films 2009, 517, 4419–4424. [Google Scholar] [CrossRef]
- Silva, C.; Vovk, A.; Da Silva, R.C.; Strichovanec, P.; Algarabel, P.A.; Gonçalves, A.P.; Borges, R.P.; Godinho, M.; Cruz, M.M. Magnetic properties of Co-N thin films deposited by reactive sputtering. Thin Solid Films 2014, 556, 125–127. [Google Scholar] [CrossRef]
- Anzai, A.; Takata, F.; Gushi, T.; Toko, K.; Suemasu, T. Epitaxial growth and magnetic properties of Fe4−xMnxN thin films grown on MgO (001) substrates by molecular beam epitaxy. J. Cryst. Growth 2018, 489, 20–23. [Google Scholar] [CrossRef]
- Pandey, N.; Pütter, S.; Amir, S.M.; Reddy, V.R.; Phase, D.M.; Stahn, J.; Gupta, A.; Gupta, M. Effect of interfacial interdiffusion on magnetism in epitaxial Fe4N films on LaAlO3 substrates. Phys. Rev. Mater. 2019, 3, 114414. [Google Scholar] [CrossRef]
- Hui, Z.Z.; Meng, Q.M.; Wei, R.H.; Tang, X.W.; Zhu, X.D.; Ouyang, Z.R.; Dai, J.M.; Song, W.H.; Luo, H.M.; Zhu, X.B.; et al. CrN thin films with ultra-low magnetoresistance prepared via solution processing for large-area applications. J. Alloys Compd. 2017, 696, 844–849. [Google Scholar] [CrossRef]
- Chattopadhyay, S.K.; Meikap, A.K.; Lal, K.; Biswas, D.; Chatterjee, S.K.; Ghosh, M.; Baba, K.; Hatada, R. Transport properties of iron nitride films prepared by ion beam assisted deposition. Solid State Commun. 1998, 108, 977–982. [Google Scholar] [CrossRef]
- Sajitha, E.P.; Prasad, V.; Subramanyam, S.V. Low temperature electrical transport properties of carbon matrix containing iron nanoparticles. J. Appl. Phys. 2009, 105, 073708. [Google Scholar] [CrossRef]
- Altshuler, B.L.; Aronov, A.G. Zero bias anomaly in tunnel resistance and electron-electron interaction. Solid State Commun. 1979, 30, 115–117. [Google Scholar] [CrossRef]
- Lee, P.A.; Ramakrishnan, T.V. Disordered electronic systems. Rev. Mod. Phys. 1985, 57, 287–337. [Google Scholar] [CrossRef]
- Lee, P.A.; Ramakrishnan, T.V. Magnetoresistance of weakly disordered electrons. Phys. Rev. B 1982, 26, 4009. [Google Scholar] [CrossRef]
- Lin, S.; Wang, B.S.; Lin, J.C.; Huang, Y.N.; Lu, W.J.; Zhao, B.C.; Tong, P.; Song, W.H.; Sun, Y.P. Tunable room-temperature zero temperature coefficient of resistivity in antiperovskite compounds Ga1−xCFe3 and Ga1−yAlyCFe3. Appl. Phys. Lett. 2012, 101, 011908. [Google Scholar] [CrossRef]
- Chi, E.O.; Kim, W.S.; Hur, N.H. Nearly zero temperature coefficient of resistivity in antiperovskite compound CuNMn3. Solid State Commun. 2001, 120, 307–310. [Google Scholar] [CrossRef]
- Mobius, A. Comment on metal-insulator-transition in amorphous Si1-xCrx. Solid State Commun. 1990, 73, 215–219. [Google Scholar] [CrossRef]






| CNF | af (Å) | Average Grain Size (nm) | Binding Energy of Cu 2p3/2 (eV) | Binding Energy of Fe 2p3/2 (eV) | Binding Energy of N 1s (eV) |
|---|---|---|---|---|---|
| CNF/700 | 3.792 | 75 | 932.7 | 710.5 | 397.5 |
| CNF/800 | 3.799 | 100 | 933.0 | 710.7 | 397.7 |
| CNF/900 | 3.807 | 114 | 933.2 | 710.8 | 398.1 |
| CNF | Ms (emu/cm3) | Hc (Oe) | ρ300K (μΩ·cm) | σ0 (μΩ·cm)−1 | p | dρ/dT | TCR (ppm/K) | Temperature Range (K) |
|---|---|---|---|---|---|---|---|---|
| CNF/700 | 579 | 19.8 | 338.6 | 0.015 | 0.133 | 0.029 | 86 | 220–360 |
| CNF/800 | 692 | 16.7 | 246.7 | 0.026 | 0.128 | 0.084 | 347 | 314–360 |
| CNF/900 | 950 | 3.7 | 139.4 | 0.061 | 0.111 | 0.064 | 469 | 314–360 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hui, Z.; Zhu, Q.; Liu, C.; Wei, J.; Tang, J.; Ye, L.; Ye, X.; Wang, X.; Zuo, X.; Zhu, X. Synthesis and Physical Properties of Antiperovskite CuNFe3 Thin Films via Solution Processing for Room Temperature Soft-Magnets. Coatings 2020, 10, 270. https://doi.org/10.3390/coatings10030270
Hui Z, Zhu Q, Liu C, Wei J, Tang J, Ye L, Ye X, Wang X, Zuo X, Zhu X. Synthesis and Physical Properties of Antiperovskite CuNFe3 Thin Films via Solution Processing for Room Temperature Soft-Magnets. Coatings. 2020; 10(3):270. https://doi.org/10.3390/coatings10030270
Chicago/Turabian StyleHui, Zhenzhen, Qi Zhu, Chuan Liu, Jumeng Wei, Jing Tang, Longqiang Ye, Xiangju Ye, Xuchun Wang, Xuzhong Zuo, and Xuebin Zhu. 2020. "Synthesis and Physical Properties of Antiperovskite CuNFe3 Thin Films via Solution Processing for Room Temperature Soft-Magnets" Coatings 10, no. 3: 270. https://doi.org/10.3390/coatings10030270
APA StyleHui, Z., Zhu, Q., Liu, C., Wei, J., Tang, J., Ye, L., Ye, X., Wang, X., Zuo, X., & Zhu, X. (2020). Synthesis and Physical Properties of Antiperovskite CuNFe3 Thin Films via Solution Processing for Room Temperature Soft-Magnets. Coatings, 10(3), 270. https://doi.org/10.3390/coatings10030270







