3.1. Chemistry and Morphology of the Samples
Changes in the chemistry of the samples were observed from an empirical point of view during the entire process, since both the visual appearance and hydrophilicity of the surfaces were noticeably changed after certain stages. In particular, the water affinity of the surface after the sensitization bath was greatly increased, likely due to the attachment of the first metal species to the surface. Furthermore, it was already mentioned that the samples underwent a change in their color after activation, more specifically from dark black to a more blueish tone.
Figure 2 presents an understandable schematic illustration of the evolution of the sample surface during all processes. As shown in the figure, different species were expected to attach sequentially to the surface until the last stage, when copper was deposited in large amounts. The attachment of catalytic metal during activation by the substitution of tin was already described by Kuzmik [
13]. When exposing the sensitized sample to the activation bath, part of the tin ions that were adsorbed at the surface were oxidized in Sn
4+, whereas silver in the solution was reduced to the metal state in a redox process. This reaction led to the formation of the catalytic nuclei on the part, which promoted the electron exchange during the electroless copper plating.
The EDS test results agreed with the expected change in chemical composition of the surface of the part. For the three spectra shown in
Figure 2, peaks at low energy (below 0.67 keV) corresponded to that of carbon and oxygen of the resin at the substrate. However, part of the oxygen that was found in the test could have been due to low amounts of atmospheric gas that were still trapped in the vacuum chamber of the SEM. In addition, a significant sulfur peak could be found in all three spectra left from the acid etch. Sulfate ions probably got attached to the surface, and, even though a thorough rinsing was carried out in between all pre-treatment stages, a relevant amount of this species was present during the entire process.
Spectrum B shows a high presence of tin atoms on the sample. The sensitization bath led to the adsorption of the tin cations at the surface of the CFRP. In addition, chlorine traces were detected, probably either left by the hydrochloric acid that was employed to stabilize the stannous ion in the solution or from the metal salt that was employed.
When analyzing the activated part, spectrum C shows the presence of silver, which means that the process left metal seeds from the catalyst. However, peaks related to tin were slightly lower as compared to the previous spectrum. These differences could be explained by taking into account that the sampling locations of the study were different, and, thus, local variations in the chemical composition may have occurred. In addition, the different amount of counts between spectra changed the vertical scale in the graphs, which in turn affected the peak heights. Either way, a lower presence of tin was expected after the activation due to the oxidation of Sn
2+. According to the description of the activation process by several authors [
13,
16], the catalyst ions are reduced to metal state with the electrons delivered by the tin oxidation from Sn
2+ to Sn
4+. Additionally, detached tin aggregates could have been removed during rinsing cycles. Furthermore, the masking effect of the outer silver that was deposited during activation could have led to a lower detection of tin.
Physical and chemical changes in a surface can be evaluated simply by contact angle measurements. Surface wettability is a useful sign to know how the surface will interact with incoming species [
17]. A clear evolution in this sense was seen during the pretreatment, and the results of sessile drop tests with water are presented in
Figure 3. The neat CFRP surface initially showed a certain grade of affinity to water. The contact angle slightly increased after chemical etch from 61° to 70°. Contrary to what was thought, the change in the surface morphology and chemistry during the acid attack led to a more hydrophobic behavior. It seems likely that the specific surface increase that was obtained by material removal had a greater effect on the contact angle than the chemical attachment of hydroxyl groups.
By contrast, sensitization with tin salt bath significantly improved the wettability to a strong hydrophilic surface showing less than 12° of contact angle. The metal species that were attached to the sample greatly improved the water affinity and thus the surface energy. Just before electroless plating, the activated samples showed 20° of contact angle due to the remaining wetting capability of the surface.
The SEM inspection of the sample surfaces during pre-treatment revealed strong changes in the microstructure, as expected from wettability results. In its initial state, the CFRP presented a flat polymeric surface, only showing small irregularities and heterogeneities, probably due to defects during the fabrication process and external pollution (see
Figure 4A). Once exposed to chemical etching and a sensitization bath, a continuous layer of 30–50 nm nuclei was formed at the surface and it exhibited big micro-aggregates (
Figure 4B). In addition, the activation seems to have had an effect on the previous structure by growing the micro-aggregates into bigger particles of 60–90 nm in size (
Figure 4C). These observable physical changes were combined with changes in the chemical composition, as seen in the EDS spectra.
The samples that were coated with copper were also inspected under SEM to obtain more information about the morphology of the deposit. As observed at the macroscale, an uninterrupted and homogeneous layer of copper along the parts was revealed at the microscale (see
Figure 5). The reduction of copper occurred extensively at the surfaces that were exposed to the bath. It could be easily observed that the structure presented a continuous layer, on top of which small copper promontories of approximately 0.5–3 μm in diameter were visible, and these created a dotted-like effect.
According to previous studies related to the formation of electroless copper layers, the deposition process follows the nucleation, growth and coalescence mechanisms. Furthermore, Paunovic [
18] stated that the layer formation happens in two different stages, namely the thin film stage and the bulk stage. It was also stated in his work that during the inception of the deposition at the first stage, the predominant phenomenon is nucleation. The creation of three-dimensional crystallites at this point increases the average density in terms of number of copper nuclei. Once this number reaches a maximum, individual crystals begin the coalescence process, in which the copper grains grow laterally to form a continuous film. The quality and strength of the final coating strongly depends on how the crystallites are merged during coalescence, since a favorable orientation of the nuclei results in a lower number of defects and pores and, thus, better overall properties. Furthermore, during the bulk stage, the thickness of the film is increased by the growth of favorably oriented grains and later by the nucleation of new grains on top of the previous layer [
18].
The evolution during growth of coatings was paid especial attention. Copper layer growth was carefully observed to check whether the behavior reported in literature was also reproduced in our process. A set of samples was dedicated to this study, and while their surface preparation was realized in the same conditions as the main samples, they were intentionally coated for insufficient times (under 5 min). This allowed for the obtainment of information about the early stages of the deposition process by employing SEM inspection.
Figure 6A shows the sample that was exposed to an electroless bath for barely 60–90 s. It can be easily observed that a structure of isolated cubic copper crystals began to form on the substrate. Most of these cubes were 150–200 nm in width, but smaller grains below 100 nm could be found, which means that some of the crystals were not fully developed. At this stage, nucleation was the predominant ongoing process because a significant part of the surface still lacked copper crystals. In addition, fine rounded particles were found on the dark background layer when observed in detail, which might have been be attributed to catalyst seeds that were inherited from activation or incipient copper crystals.
By contrast, it can be seen in
Figure 6B that the entire surface was already covered by copper cubes only 180 s after immersion. The formation of new particles on the substrate seemed not possible anymore, and, at this point, the cubic nuclei already grew laterally such that they were now forming a continuous layer. It was observed that during their growth, the crystals eventually reached the neighbors and created some sort of interference between differently oriented grains. The coalescence of the cubic structures led to a thick layer with presence of small gaps, and, according to the process description in literature [
18], the thin film stage ended. Moreover,
Figure 6C shows a fully developed layer after 300 s (sample S1) in which the initial cubic structures were harder to distinguish. The base grains grew vertically but also laterally, so the gaps between them were closed or completely filled with copper. In addition, a new generation was created on top, and some of them grew selectively, forming globular structures at the surface.
The transversal cuts of sample S3 in
Figure 6D show the copper layer in two different regions of the coating. The grain structure, in which domains of different orientations merged to form the layer, can be observed in the pictures. Additionally, some type of defects can be noticed in the bulk of the copper layer in form of lack of material. The presence of these voids in the deposited material could have been due to a local interruption of metal reduction. Also, trapped H
2 bubbles could have promoted the void formation. Both mechanisms were observed by Nakahara et al. [
19] in their study. For a better understanding of the process, one can write the reaction proposed in literature for electroless copper deposition with formaldehyde as the reducing agent [
16]:
As previously detailed, bubbling was observed at the sample surface during copper plating. Basically, the electron exchange between the reducing agent and the copper ions produced hydrogen gas, among other side species. The generation of H
2 gas was proportional to the amount of copper reduced, and when the reaction intensified, the hydrogen production did too. It has been reported in literature that the electroless copper process exhibits two different rates of reaction from the electrochemical point of view: the induction period and the steady state. The induction period refers to the time until the anodic and cathodic reactions (HCHO oxidation and Cu
2+ reduction, respectively) reach their mixed potential and begin to produce a stable reaction called the steady state [
16]. Thus, hydrogen gas production increases over time, until the steady state is reached and the process remains stable.
As also reported in literature, the duration of the periods depends on several factors, such as ion concentration, temperature and pH. Indeed, when looking carefully at Equation (2), one can realize the strong influence of the pH in the chemical reaction due to the presence of the term OH− in the chemical balance. In fact, the consumption of this species is the cause of the rapid decrease of pH, and it makes it necessary to frequently replenish the pH regulator during reaction.
However,
Figure 6D shows a highly heterogeneous cross section with a very rough coating surface at some sites. The presence of regrowth globules on top of the copper base layer strongly affected the observed thickness of the coating. Additionally, another important source of inhomogeneity may have been lying on the high roughness of the sensitization layer, which presented undesired micrometric and sub-micrometric grains on top (see
Figure 4B2). The formation of these structures could be probably avoided by a finer tune of the bath conditions such as reagent concentration, exposure time, and temperature. Additionally, either a more vigorous stirring of the fluid during sensitization or subsequent short ultrasonication cycles might cause nocuous particle detaching.
A new specific experimental method was designed in order to have reliable data of the thickness by employing the profilometry of relatively large areas.
Figure 7 shows the sequence for obtaining the layer thickness from the surface data. The process began by physically creating a sharp edge in the copper coating (
Figure 7A). The samples were partially immersed in a solution of ammonium persulfate, which is a common oxidizing agent that is employed for copper etching. Copper and any other metallic species on the samples were totally removed at the immersed area. The sample was then thoroughly rinsed with water to prevent any migration of the etching solution to the boundaries of the remaining layer. After that, the samples were inspected with a profilometer (
Figure 7B). Four-to-six 3 mm × 3 mm edge areas were analyzed in each sample in 15 traces with a spatial resolution of 200 µm in the traverse direction. Near 10
5 points in total were extracted from each area (
Figure 7C). The collected data were then processed to remove the sample tilt by attending to the average level that was presented in the uncoated region (
Figure 7D). After levelling, the coated region was isolated, and the height of the points was averaged by taking the average level of the uncoated composite as zero (
Figure 7E).
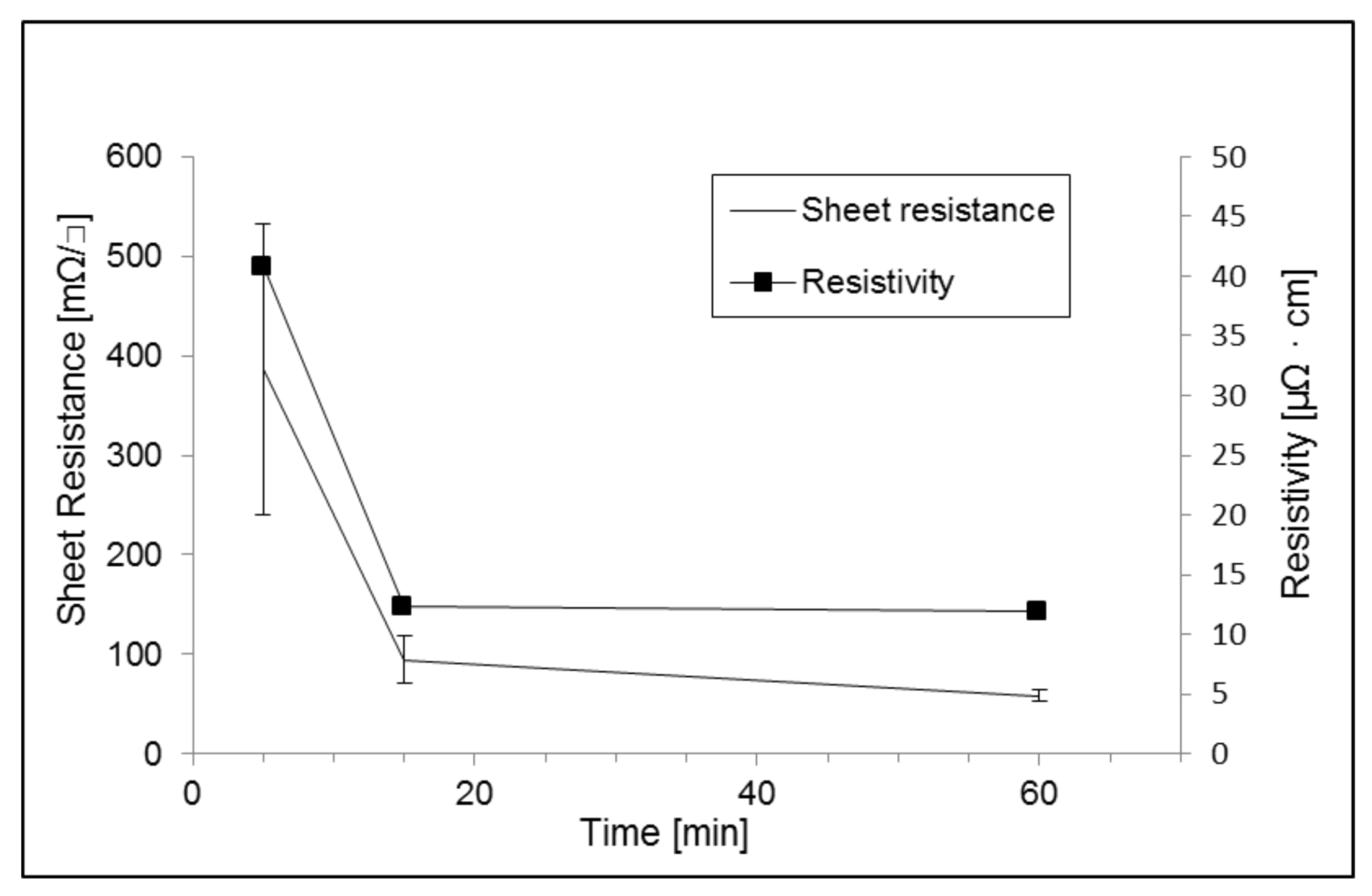
The processed thickness data are plotted in
Figure 8A. Obviously, there was a strong dependence of the thickness of coatings with the exposure to electroless bath. The layer of the 5 min samples was near 1 µm thick, and there was an uptrend showing values of 1.3 and 2 µm for 15 and 60 min, respectively. Nevertheless, the growth rate was lower for higher times, which was in accordance with the literature. Paunovic stated that at some point, the growth rate of crystals is reduced due to the higher degree of inhibition and overpotential in them [
18]. The cessation of perpendicular growth is always dependent on the plating solution variables and kinetic parameters of the particular reaction. The depletion of reactants, for example, can play an important role in the growth rate and, thus, in the thickness of the final layer.
The roughness of the samples was also investigated by using the profilometer, and the data are plotted in
Figure 8B. The average roughness of the coatings also increased with the time of deposition. The initial value of the as-manufactured CFRP was 0.81 µm of surface roughness (
Ra), and this was the minimum roughness observed. After only 5 min of electroless deposition, the
Ra already reached about 1.33 µm, whereas for 15 min, it became 1.55 µm. The highest
Ra that was observed was 2.17 µm for the 60 min plated samples. Again, a non-linear rate in the increasing trend of this parameter could be seen, as with the copper thickness. As previously noticed in SEM micrographs, the formation of regrowth grains on top of the coalesced crystals layer can have an important role in the average roughness. These particles have been observed to be bigger at higher times of deposition, and the surface texture is accordingly affected.