Abstract
The effective utilization of many conventional pesticide formulations is less than 30%, which can increase the environmental impact of these substances. This degree of waste could be reduced by improving the adhesion of pesticides to foliage. In the present work, a complex comprising tannic acid (TA) and Fe3+ ions was used to encapsulate azoxystrobin and avermectin water dispersible granule (WDG) formulations (termed Az-WDG-TA and Av-WDG-TA) to improve adhesion. The treated pesticides exhibited improved photostability as well as sustained continuous release behavior. The retention proportions of the Az-WDG-TA and Av-WDG-TA on cucumber and lettuce foliage were improved by more than 50%. The ability of solutions of these materials to wet foliage was also enhanced after coating, such that the toxicity of Av-WDG-TA to aphids and the antifungal activity of Az-WDG-TA to Fusarium oxysporum were increased by nearly 50%. Given the low cost of TA and Fe3+ compounds and the simple synthesis process, this method represents a promising means of producing foliage-adhesive pesticide formulations with increased retention and bioavailability.
1. Introduction
Constant increases in the worldwide population are leading to a growing demand for food and other resources; the population in the world will exceed 9.2 billion by 2050, which means nearly 0.92 million tons of food was supplied by the United Nation predicted [1,2]. Pesticides, also known as agrochemicals or agricultural growth regulators, have played a vital role in meeting the attendant requirements for agricultural production [3,4]. However, due to the poor dispersion, sedimentation, and low biological activity of currently available pesticides at typical dosages, much of these materials are not only wasted, but also pose a threat to local ecosystems; the misuse of pesticides leads to more than 70.6% rivers being polluted in China [5,6,7,8,9]. Silent Spring, published in 1962, was one of the first warnings of the potential for pesticides to damage the environment [10]. According to many studies, off-target application, run off, and leaching during the traditional spray-based application of pesticides cause nearly 70% of the compounds to be unused, meaning that they enter air, soil, and aquatic ecosystems where they may undergo bioconcentration and ultimately threaten human health [11,12]. The weak adhesion of pesticides to crop leaves is the main reason for the waste associated with the spraying of pesticides [13,14,15]. Therefore, enhancing this adhesion along with reduction of loss, such as rolling down, leaching and bounce off, could increase the effective utilization efficiency of pesticides while positively contributing to the maintenance of various ecosystems.
Natural adhesive behaviors occur in many living systems, like gecko, mussel, beetles, and octopus [16,17,18,19,20,21]. Inspired by the mussel, polydopamines (containing catechol groups that play a major role in adhesion) have been widely used as adhesive materials [22,23,24,25]. However, given the high cost of dopamine, it is not practical to use polydopamines to promote contact between pesticides and foliage [26]. Tannic acid (TA), a naturally occurring polyphenol, is widely distributed in various organs of plants, and exhibits good biocompatibility, high chemical reactivity, and suitable thermal stability [27,28]. TA is also readily available and shows unique biological activities and physicochemical properties, such that this compound has numerous applications in surface modified, drug delivery, and prepared other materials [29,30,31]. TA can also interact with various functional chemical groups or molecules via processes such as coordination or hydrogen bonding [32,33]. As an example, the chelation of metal ions by TA has been widely used and coordination complexes with ions such as Fe3+, Cu2+, and Al3+ are commonly applied for preparing AgNPs and bionic function interfaces [34,35,36]. The U.S. Food and Drug Administration has recognized the combination of TA and Fe3+ as safe [37].
Thus, the aim of the present study was to improve the adhesion of two pesticides to foliage and enhance the retention of these substances, by using the eco-friendly materials. The objective of this study is to characterize Az-WDG, Av-WDG treated with TA and Fe3+, to compare their photodegradation, wettability, and their retention rates with the standard to test their kinetics release. We hope our studies provide an advanced way to solve problems during the pesticide using this process.
2. Materials and Methods
2.1. Materials
The commercially-available pesticides Az-WDG and Av-WDG used in this work were donated by the Kewin Company (Yancheng, China). TA and iron chloride hexahydrate (FeCl3∙6H2O) were obtained from J&K Scientific, Ltd. (Beijing, China). The methanol, acetonitrile, and dichloromethane HPLC grade were purchased from Thermo Fisher Scientific (Tustin, CA, USA). The azoxystrobin and avermectin standards were obtained from AccuStandard, lnc. (New Haven, CT, USA). The support films for electron microscopy were purchased from the Beijing Keyi Films Technology Co., Ltd. (Beijing, China) Deionized water (18.2 MΩ cm, total organic compounds ≤ 4 ppb) was used in all experiments and was generated using a Milli-Q system. The cucumber and lettuce seedlings were cultivated in a sterile incubator. Fusarium oxysporum was obtained by the Beijing Academy of Agriculture and Forestry Sciences (Beijing, China).
2.2. Methods
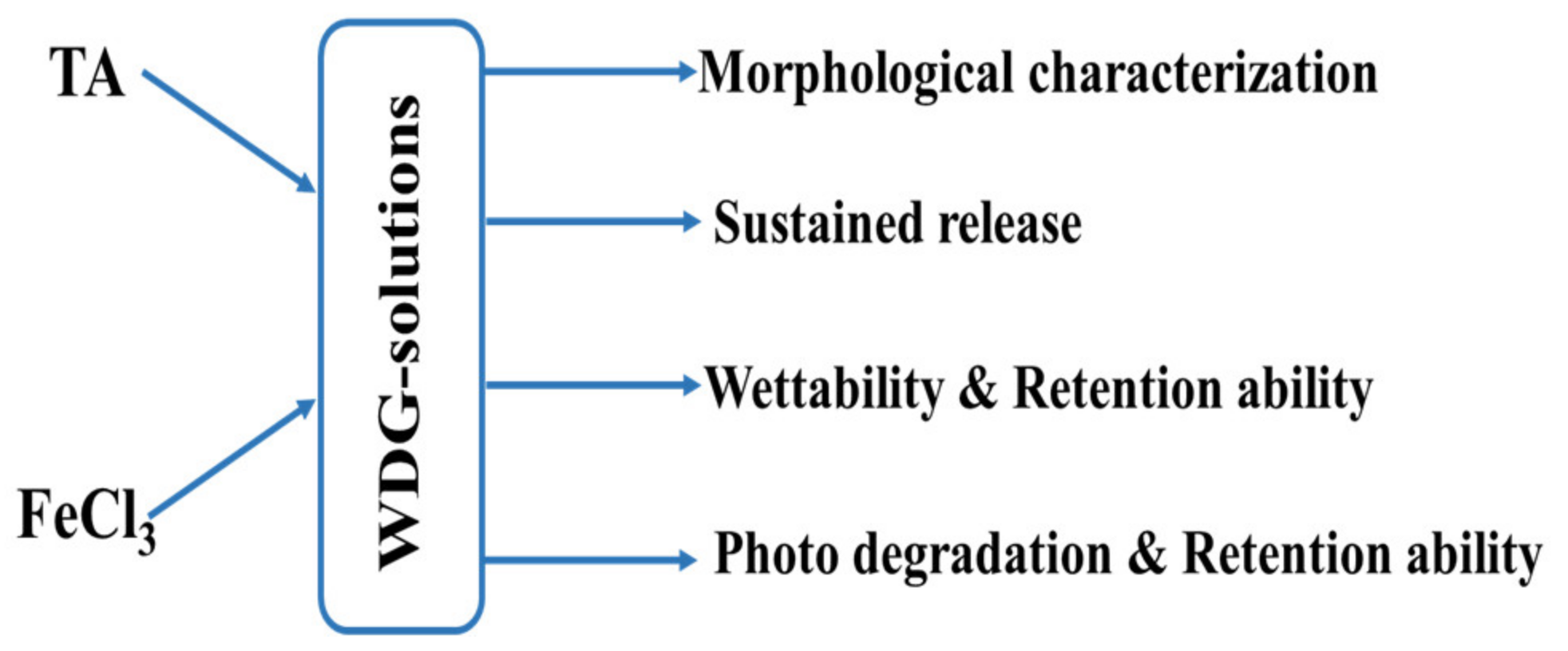
The method we conducted in this study was summarized in the following flowchart (Scheme 1).
Scheme 1.
The diagram of research routes.
2.2.1. Preparation of Az-WDG-TA and Av-WDG-TA with TA/Fe3+ Coatings
The single step process presented in Scheme 2 was used to form microcapsules over the pesticide particles, employing a mixture containing Av-WDG or Az-WDG, 50 μg/mL TA, and 40 μg/mL FeCl3. In this process, the required 0.1 g of Av-WDG or Az-WDG was transferred into a beaker and the stirring process started. Thus, the TA solution was added and stirring was maintained for an additional 5 min, followed by slow dropwise addition of the FeCl3. The resulting precipitate was collected by centrifugation for 5 min at 6000 rpm. This material was washed three times with deionized water to remove excess TA and Fe3+, and then formed into a fine powder by lyophilization.
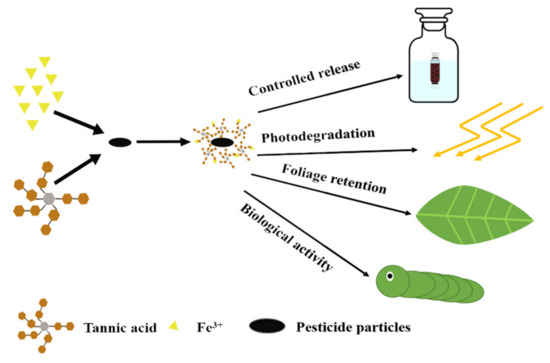
Scheme 2.
Synthesis of the tannic acid/Fe3+ microencapsulated pesticide particles and its features.
2.2.2. The Morphological Characteristics of Particles
The morphological characteristics of Az-WDG, Az-WDG-TA, Av-WDG, and Av-WDG-TA particles were observed using transmission electron microscopy (TEM; JEOL. LTD, JEM-2100F, Tokyo, Japan). In addition, energy-dispersive X-Ray spectroscopy (EDS; Oxford Instruments, Abingdon, UK) was employed to further assess the extent of chelation of the Fe3+ by the TA on the particle surfaces, based on a method previously described by Yang [38]. In preparation for these analyses, a 4 μL aliquot of a suspension of the coated pesticide was dropped onto a support film and moved it into the exsiccator until dehydrated.
2.2.3. Sustained Release Kinetics of Avermectin and Azoxystrobin Formulations
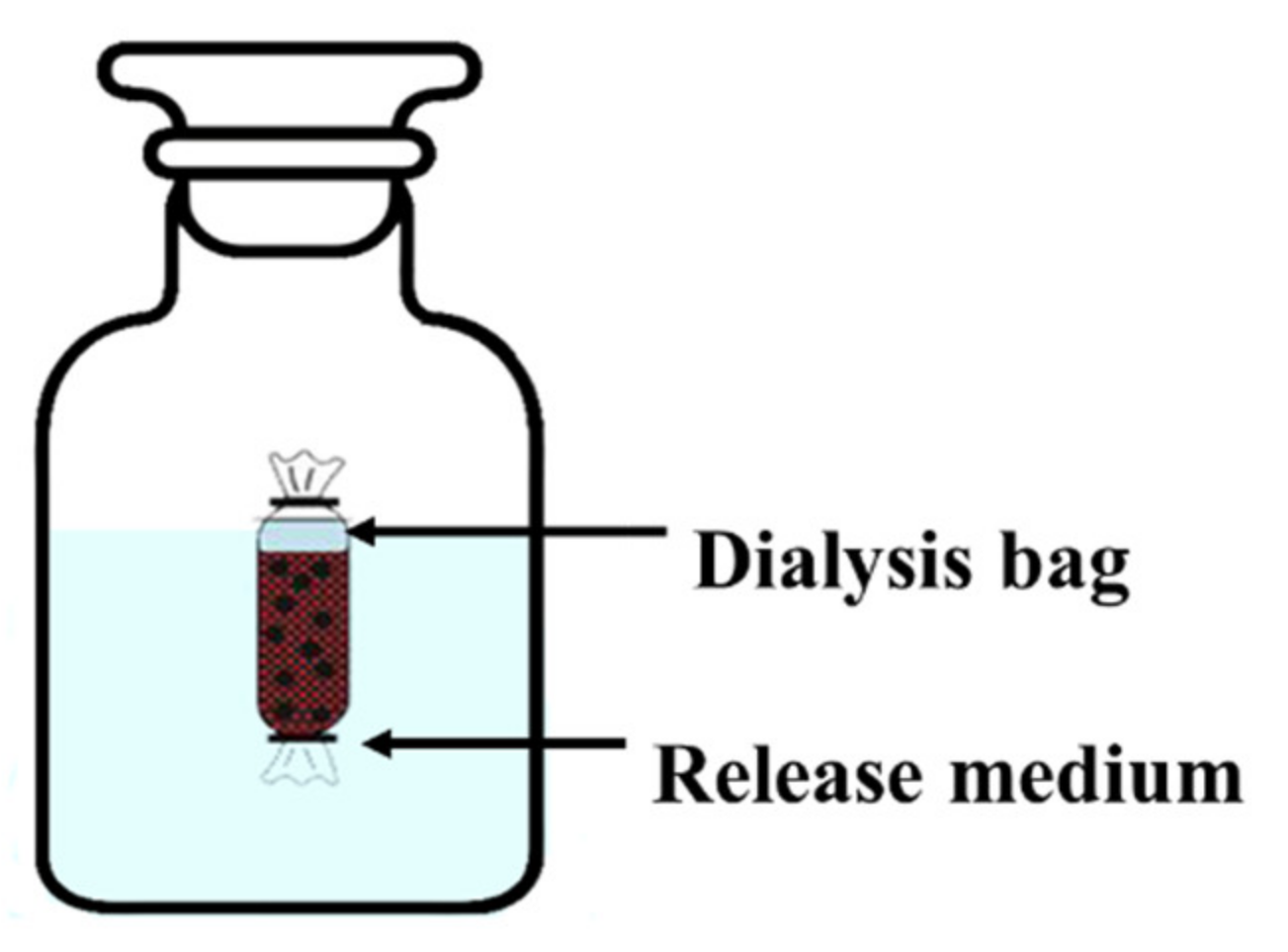
Methanol/deionized water (90:10, v/v) was used as the release medium for kinetics assessments. In each trial, an 8 cm opening was cut in a dialysis bag, and 5 mg of the coated Av-WDG was transferred into the bag along with 5 mL of the release medium. The two sides of the bag were subsequently wrapped with a polyaramid cord, and the bag was placed in a bottle holding 95 mL of a medium solution (Scheme 3). Each sample was then agitated on a shaking table (THZ-98C, Shanghai, China) at 100 rpm and 25 °C for 144 h. At every time interval (0.5, 1, 2, 3, 4, 6, 8 ….. 144 h), a 2 mL quantity of the release solution was extracted from the bottle and quickly replaced by a fresh 2 mL quantity of the same medium. The pesticide concentration in each aliquot was determined using high performance liquid chromatography (HPLC) with a 30 °C column temperature, 245 nm UV detector at the flow rate 1 mL/min. The release kinetics of the coated Az-WDG were also assessed using the same process but employing methanol/deionized water (70:30, v/v) as the release medium with the method as Yao described [39].
Scheme 3.
The diagram of release systems.
2.2.4. Biological Activity Tests of Avermectin and Azoxystrobin Formulations
To test the antifungal activity of the Az-WDG formulations, Fusarium oxysporum was incubated in a PDA medium. The toxicity regression equation and median lethal concentration (LC50, μg/mL) were subsequently determined using the SPSS 20.0 software package (IBM, Armonk, NY, USA). In these trials, the cultures were exposed to 5, 10, 20, 25, and 50 μg/mL solutions of Az-WDG or Az-WDG-TA in 5 mm diameter hyphae plates in an incubator at 25 °C for 2 days, using the crossing method to determine the diameter of the hyphae. Each trial was repeated three times to assess reproducibility.
Av is a famous insecticide around the world that has better control efficiency compared to aphids. In the case of the Av-WDG, we employed Myzus persicae (Homoptera, Aphid family) as the target species to test the bioactivity and employed Av-WDG or Av-WDG-TA solutions with concentrations of 1.5625, 3.125, 6.25, 12.5, 25 and 50 μg/mL by the leaf dipping method. In detail, firstly, the lettuce foliage was cut into 8 mm diameter circles, secondly, the circles were put into different concentration solutions for 10 s; after drying the circles under the room temperature, finally, the target species were put onto the leaf. After 3 days, the activity of the creature was observed [15].
2.2.5. The Wettability of Foliage by the Avermectin and Azoxystrobin Formulations
The poor deposition of pesticides on crop leaves is the main reason for the low effectiveness of these compounds, and the development of microcapsules or microspheres has been proposed as one means of counteracting this problem [40]. Portions of the finely powdered Av-WDG, Av-WDG-TA, Az-WDG, and Az-WDG-TA were diluted into the concentration of 490 μg/mL recommended by the manufacturer in deionized water and then applied to cucumber leaves, which served as model foliage. The resulting contact angles (CAs) were measured using a CA contact angle meter (JC2000D2 M, Zhongchen Digital Technology Apparatus, Shanghai, China). In each experiment, an aliquot of approximately 8 μL of the pesticide solution was dropped onto the leaf using an automated micro-drop injector, and photographic images were acquired after the drop stabilized by the imaging system. The angle data were collected via ellipsometry based on analyses of these images [41,42]. Each solution was used for 10 replicate measurements.
2.2.6. Retention Rates of Avermectin and Azoxystrobin Formulations
The retention of these pesticides on cucumber and lettuce leaves was determined based on HPLC analyses using a previously described method [15]. In each case, 2 mL of one of the four sample solutions was uniformly sprayed on the leaves, after which the leaves were transferred to a dark room. After drying, the leaves were divided into two parts. One half was washed with 20 L/m2 deionized water to simulate rain water, while the other half served as a control. The leaves were subsequently cut into very small pieces and transferred into a Soxhlet extraction apparatus where they were extracted in dichloromethane for 24 h. The solvent was subsequently removed under reduced pressure at room temperature, after which the solid residue was dissolved in 5 mL of a mixture of acetonitrile, methanol, and deionized water (80:15:5, v/v/v). Each sample was then stirred for 1 h and ultrasonicated for 10 min, after which an HPLC analysis was employed to ascertain the concentration of the pesticide.
2.2.7. Assessing Photodegradation
The ability of the TA/Fe3+ encapsulation to slow photodegradation of the Av-WDG was also assessed. Samples of the coated Av-WDG-TA and the original Av-WDG were separately dissolved in a methanol/deionized water mixture (90:10, v/v) at concentrations of 250 μg/mL, after which a 1 mL aliquot of each solution was transferred to a silica culture vessel. Each sample was allowed to dry in a dark room to produce a film-like specimen and these films were then exposed to UV light generated by a xenon lamp. At various intervals, pesticide film specimens were dissolved in methanol/deionized water (90:10, v/v) and then analyzed using HPLC. The tests at each time interval were repeated three times.
2.2.8. Statistical and Analysis
The SPSS 20.0 (IBM, Armonk, NY, USA) software carried out all the experiments data. The results all showed as “Mean ± Standard deviation”. The different letters indicated the significant difference between experimental settings with the S-N-K test of One-Way analysis of variance (p < 0.05).
3. Results and Discussion
3.1. Morphological Characterization of Avermectin and Azoxystrobin WDG Formulations
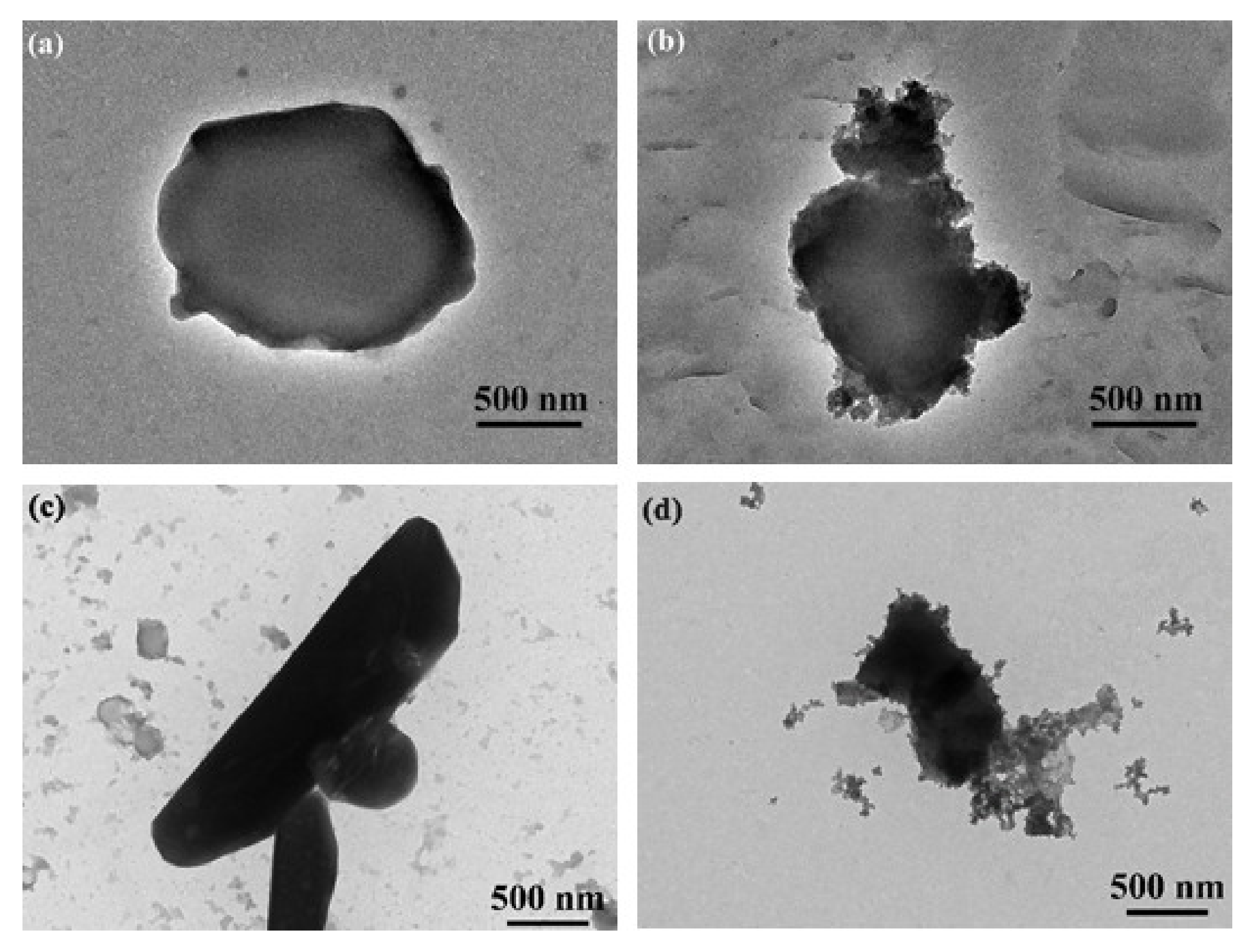
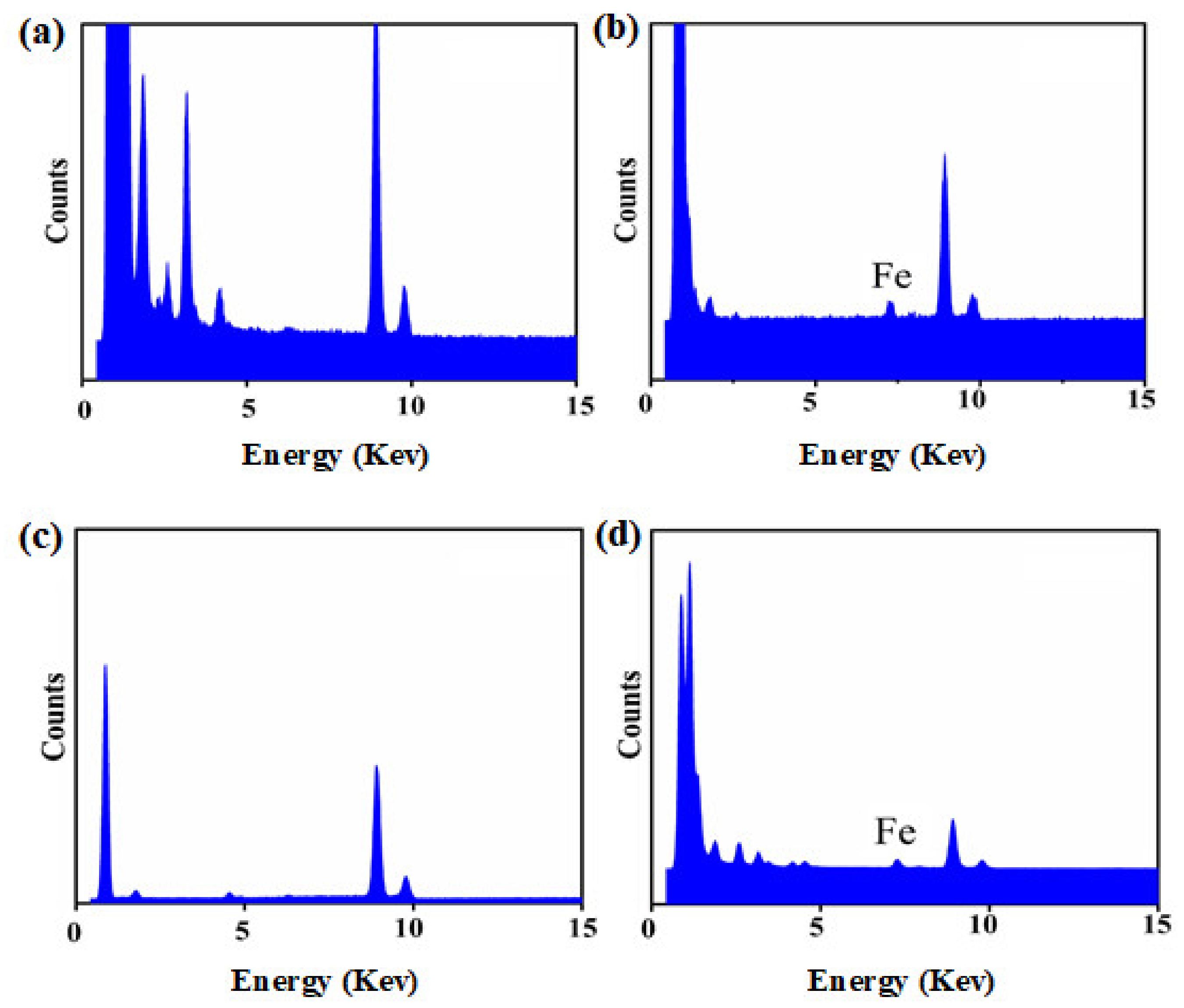
The results demonstrated that the pesticide particles were readily coated using a combination of TA and Fe3+ ions, with the Fe3+ coordinated to the TA. TEM images of uncoated Av-WDG and Az-WDG particles are shown in Figure 1a,c, respectively, and demonstrate that the particle surfaces were smooth. Following the application of the TA and Fe3+, the surfaces became rough (Figure 1b,d), indicating that they had been coated with films of these materials. This result is in good agreement with Ejima’s work, in which a complex formed from TA and Fe3+ ions was used to coat Au nanoparticles that subsequently exhibited rough surfaces [43]. EDS was used to provide further evidence for the successful coating of the pesticide particles by confirming the chelation interaction between TA and Fe3+ on the particle surfaces according to previous reports [44,45]. Figure 2 demonstrates the chelation of Fe3+ ions by the TA to form irregular films on the particle surfaces, with Fe3+ concentrations in the TA films of 2% and 1.9% in Az-WDG-TA and Av-WDG-TA, respectively.
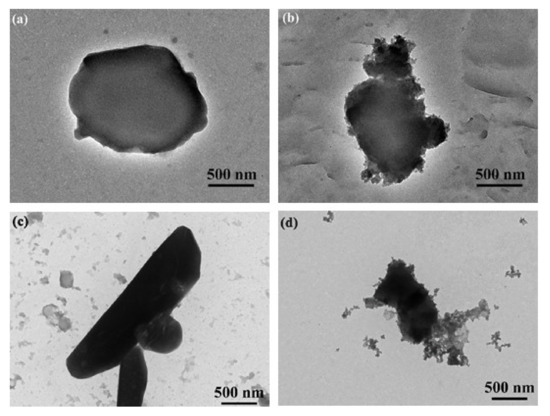
Figure 1.
TEM images of (a) Az-WDG, (b) Az-WDG-TA, (c) Av-WDG, and (d) Av-WDG-TA. The scale bar is 500 nm.
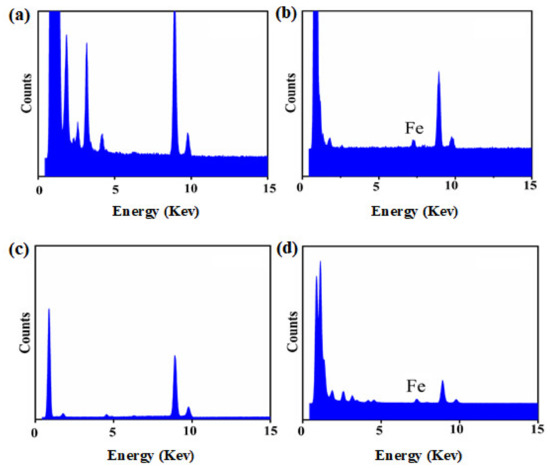
Figure 2.
The EDS spectrums of (a) Az-WDG, (b) Az-WDG-TA, (c) Av-WDG, and (d) Av-WDG-TA.
3.2. Sustained Release Kinetics of Avermectin and Azoxystrobin WDG Formulations
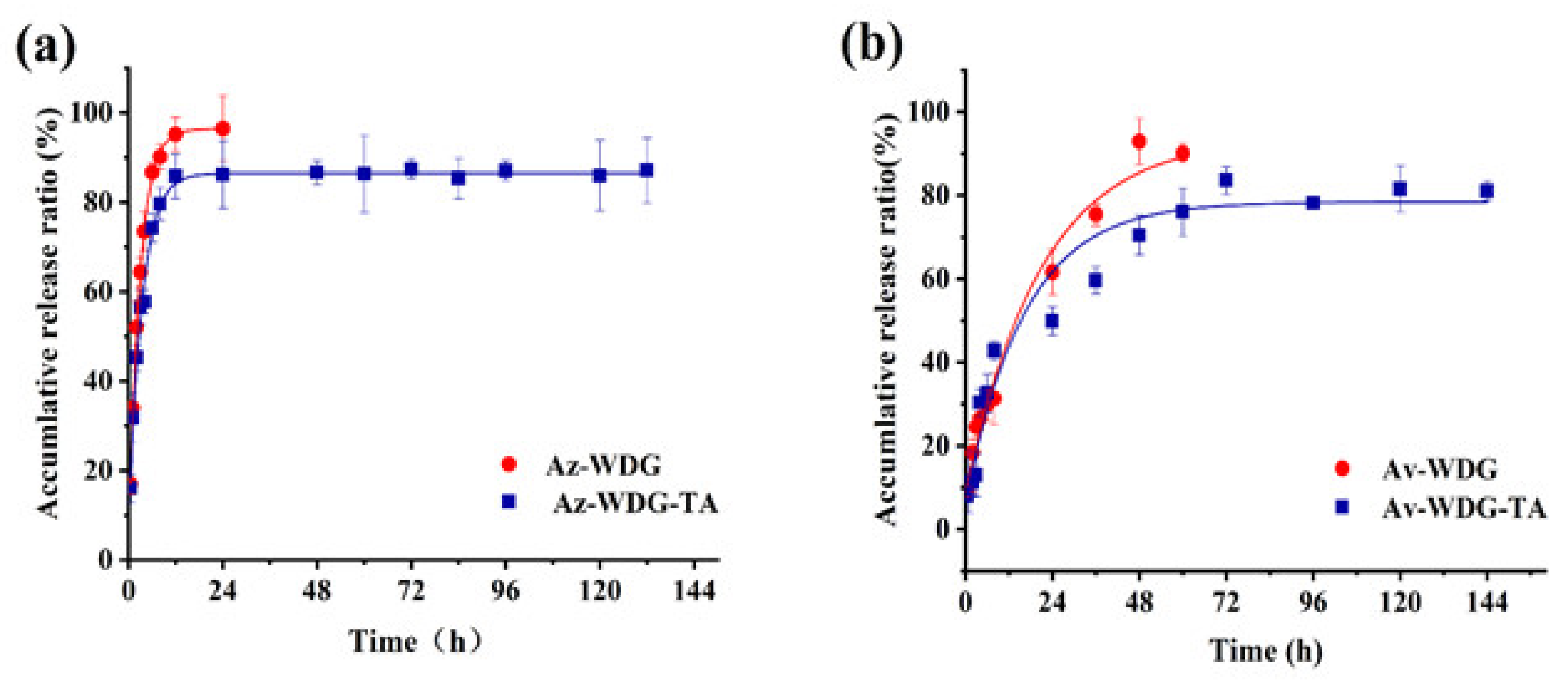
The microencapsulation of pesticides can potentially provide increased deposition, suitable sustained release effects, reduced toxicity, and less waste [28,29]. In the present work, the eco-friendly materials TA and Fe3+ were used for the purpose of encapsulation via a one step process. Figure 3 summarizes the release kinetics of the coated and uncoated Av-WDG and Az-WDG. In each case, the data correspond closely to first-order kinetics as indicated, with R2 values greater than 0.99. It is also evident that the Av-WDG-TA and Az-WDG-TA had slower release rates than the uncoated samples, implying desirable sustained release behavior. In these trials, 95% of the uncoated Az-WDG and Av-WDG was released after 10 and 48 h, respectively, while much more gradual release rates were obtained from the coated Av-WDG-TA and Az-WDG-TA. The complex of TA/Fe3+ has great application on drug delivery or control release; Shen found it had the good ability to delay the urea release [35].
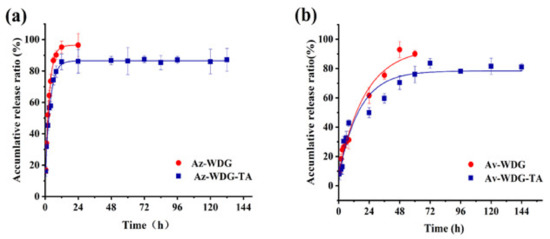
Figure 3.
Sustained release profiles of (a) Az-WDG and Az-WDG-TA and (b) Av-WDG and Av-WDG-TA.
3.3. The Photodegradation of Avermectin WDG Formulations
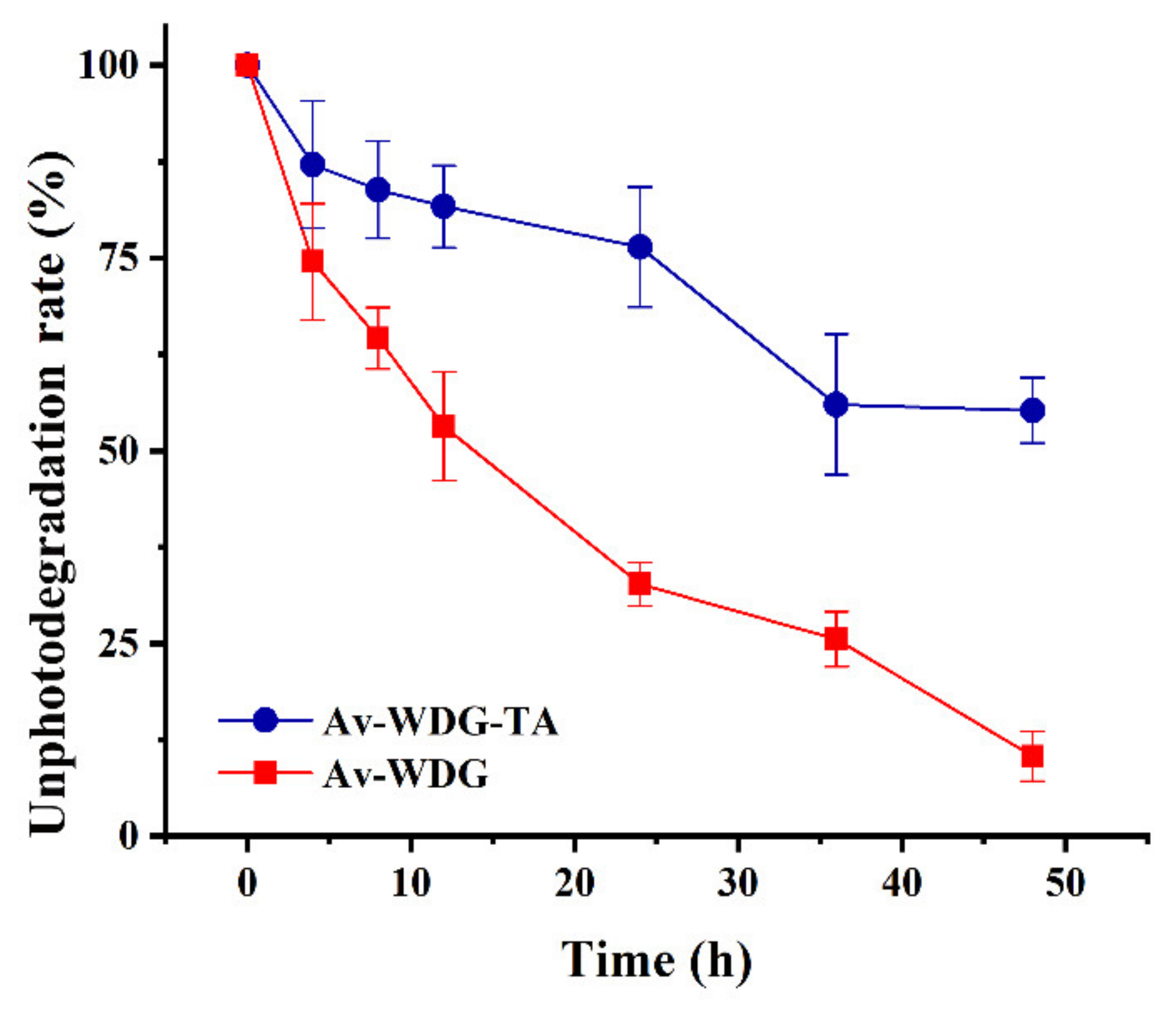
Av is one of the most widely used pesticides worldwide but is also highly sensitive to UV light, leading to low utilization efficiencies. Thus, it would be beneficial to increase the photostability of this pesticide. Polyphenols have exhibited good resistance to UV radiation and to photothermal effects [46], and, for these reasons, are typically used as sunscreens in various cosmetics. Prior work has also demonstrated that the encapsulation of Av is an effective way to improve photostability [15,47]. In the present work, the formation of TA/Fe3+ films on the Av particles was predicted to partly shield these granules from UV light, and this is confirmed by the photodegradation results presented in Figure 4. It is evident that the Av-WDG-TA exhibited better stability in response to UV radiation, while the photodegradation rate of the uncoated Av-WDG was relatively fast. The photodegradation proportion of the original Av-WDG was determined to be nearly 90%, whereas that of the coated Av-WDG-TA was 45% after 48 h of continuous UV irradiation. These results establish that the photostability of the Av-WDG-TA was substantially improved. The better anti-photodegradation could enhance the control efficiency and obtain the holding time.
Figure 4.
Photodegradation data for the Av-WDG and Av-WDG-TA.
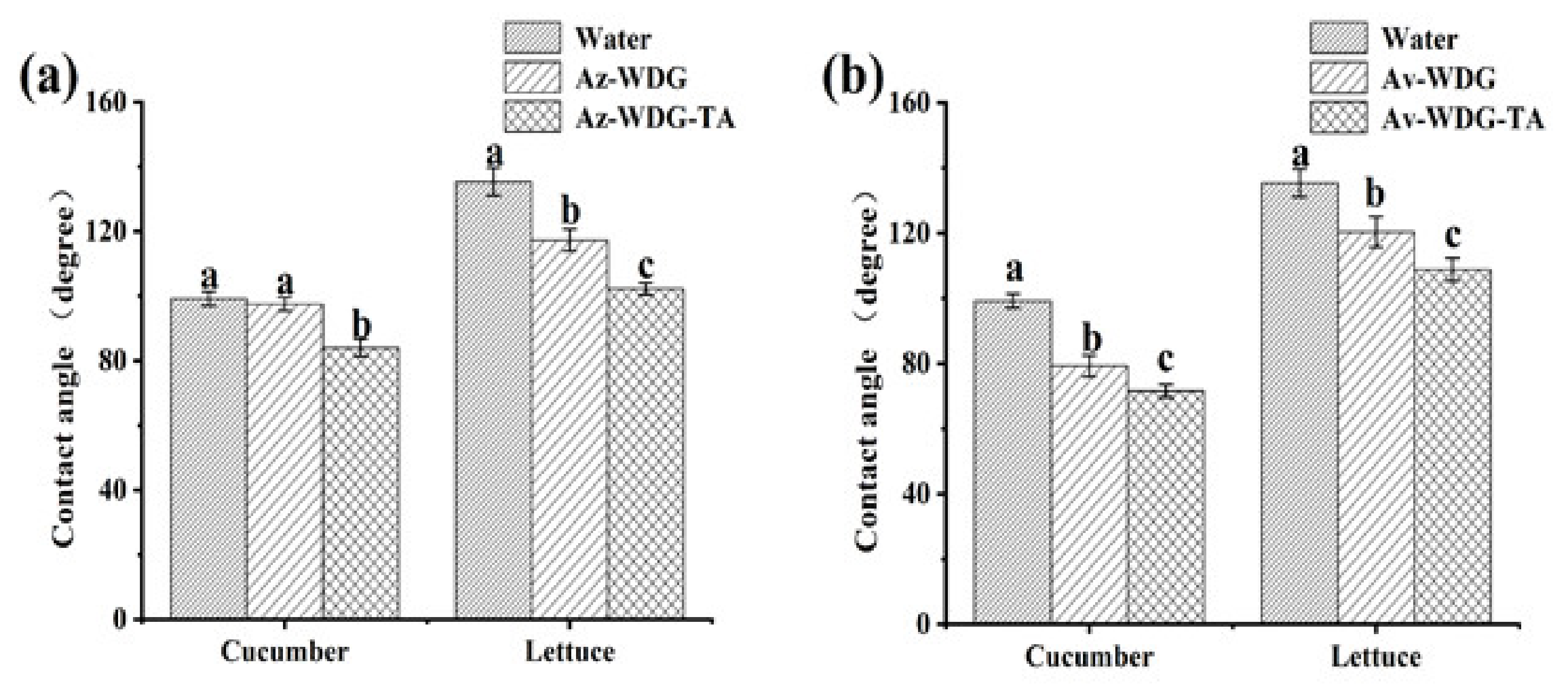
3.4. The Effects of Encapsulation on Contact Angle and Retention
The contact angle (CA) is an indirect way of assessing the wetting ability of a pesticide due to the waxy hydrophobic surface of crop foliage, which typically inhibits contact between pesticide droplets and the foliage surface. Pesticide solutions with low contact angles should spread over the foliage more easily, which would be expected to assist in retention [31]. The CA values determined for solutions of the Az-WDG, Az-WDG-TA, Av-WDG, and Av-WDG-TA are presented in Figure 5. Following the application of the TA/Fe3+ to the Az-WDG and Av-WDG, the CA values of Az-WDG-TA and Av-WDG-TA were decreased significantly on cucumber and lettuce surfaces. In prior work, complexes made of TA and metal ions together with diethylenetriamine were found to decrease the CA and increase the wettability on many materials, such as quartz, glass, and high polymers [48,49]. These prior results suggest that TA and its complexes when applied to the surfaces of pesticide particles could enhance wettability and reduce losses on crop foliage, which not only lead to cutting down on the usage of pesticide but also decrease the cost for farming.
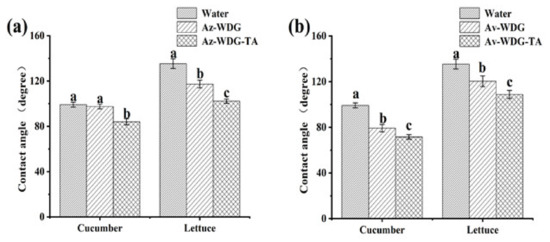
Figure 5.
Contact angles of (a) Az-WDG, Az-WDG-TA and (b) Av-WDG, Av-WDG-TA on different foliage. Based on a one-way ANOVA using the S-N-K test, values with different letters are significantly different (p < 0.05).
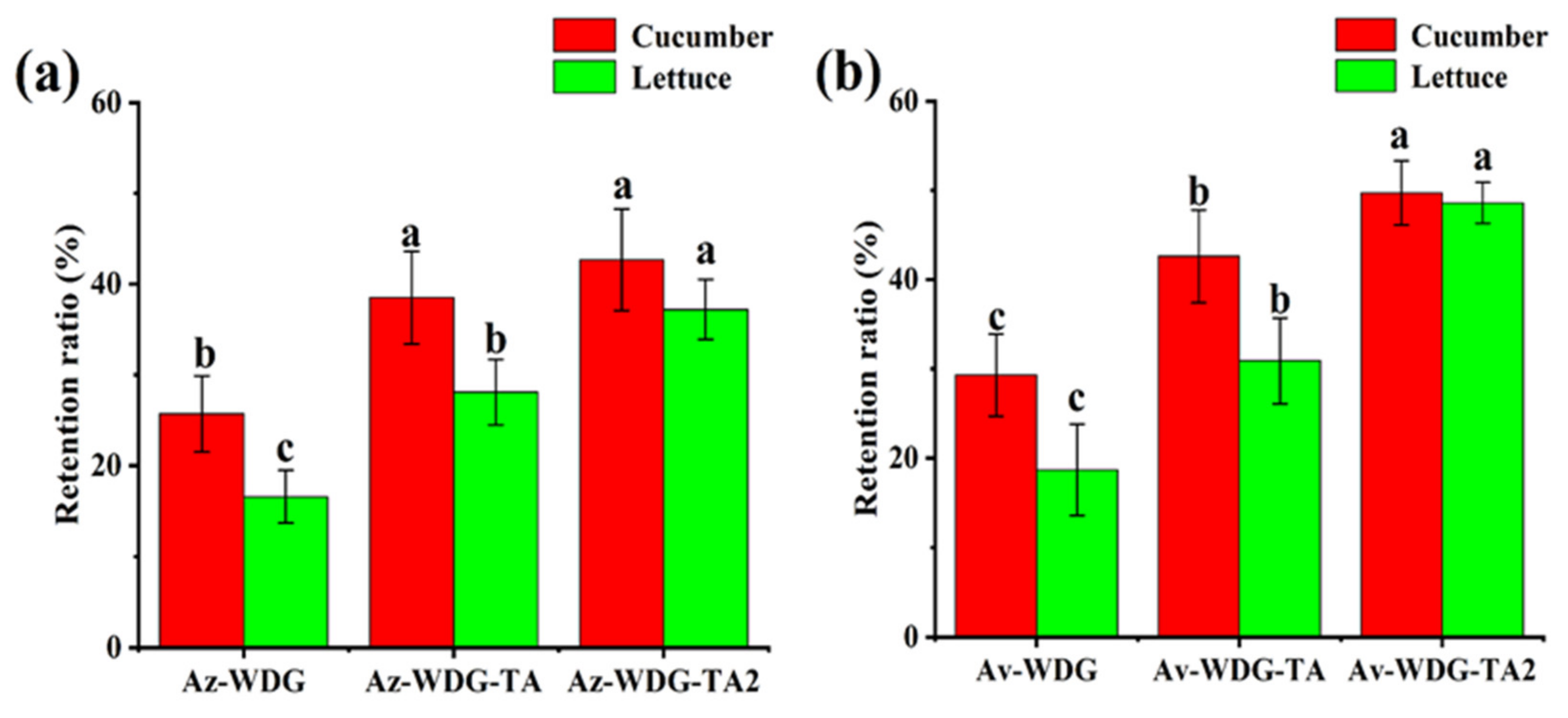
Pesticides are typically applied to foliage by spraying with the intent of killing targeted organisms. The retention time on foliage is highly correlated with the adhesion of the pesticide, such that improving adhesion could increase utilization efficiency. To further demonstrate the effects of applying the TA/Fe3+ complex on the retention of the pesticides, retention data were acquired based on HPLC analyses. As shown in Figure 6, compared with Av-WDG, the retention proportions of the Av-WDG-TA on the cucumber and lettuce foliage were enhanced by 1.51 and 1.55 fold, respectively, after coating with the TA/Fe3+ complex. The retention proportions of Az-WDG-TA on the cucumber and lettuce foliage were enhanced by 1.45 and 1.67 fold, respectively, relative to Az-WDG. Interestingly, the retention was found to be highly correlated with the amount of the TA/Fe3+ complex on the surfaces of the pesticide particles. The TA molecule contains numerous phenol groups and thus would be expected to form strong hydrogen or coordinate bonds with the foliage, based on previous reports of polyphenol adhesive chemistry [50].
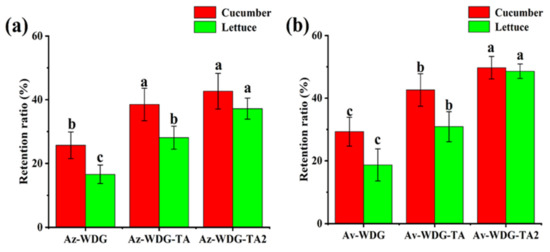
Figure 6.
Retention rates of (a) Az-WDG and (b) Av-WDG on cucumber and lettuce foliage surfaces, where Az-WDG-TA2 and Av-WDG-TA2 mean twice the amount of TA/Fe3+ complex on the surfaces of the pesticide particles.
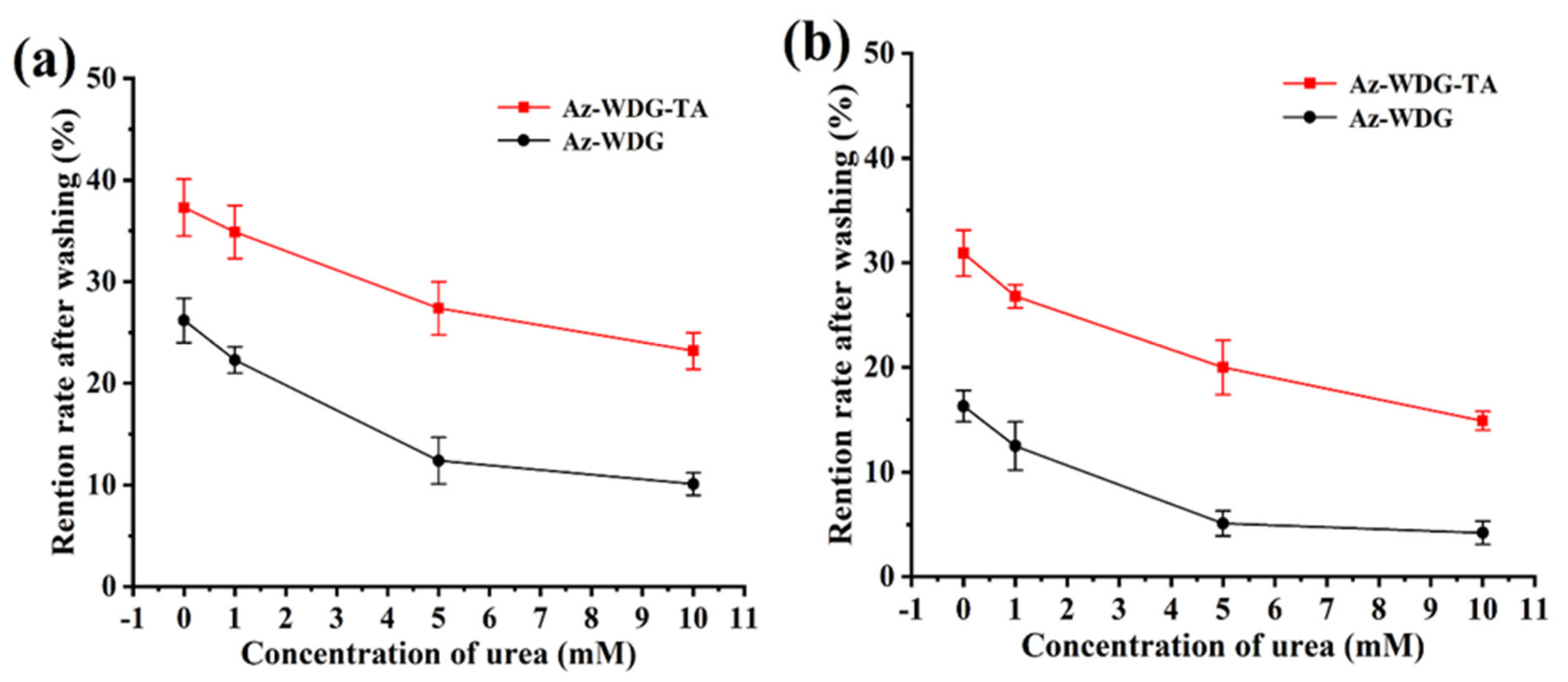
To better understand the adhesion mechanism, urea, as a hydrogen bond interfering agent, was added to the various solutions [51]. The retentions of both the Az-WDG and Az-WDG-TA were observed to significantly decrease on both the cucumber and lettuce foliage following the addition of urea, and the retention was inversely proportional to the urea concentration (Figure 7). These results suggest that the interactions between the coated pesticide particles and the foliage were primarily due to hydrogen bonding due to the groups of the TA molecule similar to previous reports [52,53]
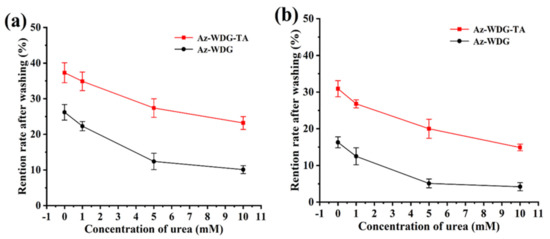
Figure 7.
The retention rates variations of Az-WDG and Az-WDG-TA with different urea concentrations washing on the cucumber (a) and lettuce foliage surface (b).
3.5. The Biological Activities of Avermectin and Azoxystrobin WDG Formulations
In this work, the biological activities of the Av-WDG and Av-WDG-TA were evaluated, and the results obtained from toxicity regression equations (that is, LC50 and toxicity index values) are summarized in Table 1 and Table 2. The LC50 value typically reflects the toxicity of the Av-WDG-TA to aphids was 1.5 times higher that of Av-WDG. These data suggest that coating the pesticide with the TA/Fe3+ led to improved retention on the foliage, resulting in higher toxicity. These values are also in good agreement with the foliage retention results. The antifungal activities of the Az-WDG and Az-WDG-TA as measured using Fusarium oxysporum were investigated by disk diffusion assays, and the antifungal activity of the Az-WDG-TA was 1.6 times higher than that of Az-WDG. (Table 2). Thus, the Az-WDG-TA exhibited higher antifungal activity, presumably owing to increased interaction with the Fusarium oxysporum. These biological activity results indicate that the Av-WDG-TA and Az-WDG-TA, both of which showed enhanced adhesion, also demonstrated increased efficacy against the target organisms.

Table 1.
Biological activities of Av-WDG and Av-WDG-TA against aphids (Myzus persicae L.).

Table 2.
Antifungal activities of Az-WDG and Az-WDG-TA against Fusarium oxysporum.
The facile approach to fabricate Av/Az-WDG-TA formulations with increasing the foliage retention of pesticides based on coating with a tannic acid/Fe3+ complex makes them industrialization possible, due to their high utilization efficiency, low cost, eco-friendly materials, and easily scalable production. The field trails are in progress. It is believed that the development of folia-adhesive pesticide formulations with lower losses and effective utilization efficiency, leading to decreased spraying dosage, residue, and pollution in food and the environment, will be prioritized.
4. Conclusions
The poor retention ability may lead to the abuse of pesticide. To achieve the goal, the farmer also sprayed a lot during the activity, which is a huge threat to humans and environmental systems. Considering the further application of Av/Az-WDG, the fast way to solve the problem during the spraying process should be mentioned. Inspired by the mussel’s strong adhesive ability, the eco-friendly materials TA and Fe3+ were applied to coat above the pesticide particles to improve the retention ability on foliage to increase the utilization efficiency and reduce the damage to the environmental systems.
Av-WDG-TA and Az-WDG-TA were synthesized and found to exhibit good adhesion to crop foliage. This synthesis was based on a simple one-step chemical complexation coating of the surface of conventional WDG particles using TA and Fe3+. These materials showed improved continuous sustained release properties, and the Av-WDG-TA was determined to be more resistant to UV light. The affinity of both pesticides for foliage was highly enhanced, leading to improved adhesion and longer retention times on foliage surfaces. This improved adhesive force is primarily ascribed to hydrogen bonding between phenols in the TA and various functional groups on the foliage. These results demonstrate a promising means of obtaining pesticide formulations with improved adhesion as a means of reducing pollution and increasing efficacy.
Author Contributions
H.Z. wrote the paper. M.Y. and J.Y. provided assistance in experimental operations and data analysis. Z.Z. and H.C. provided the theoretical guidance. C.S., B.C., X.Z. and Y.W. contributed with the references. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the National Key Project of Research and Development Plan Program of China (2017YFD0200900) and China Postdoctoral Science Foundation (2018M630234).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gerber, L.C.; Koehler, F.M.; Grass, R.N.; Stark, W.J. Incorporating microorganisms into polymer layers provides bioinspired functional living materials. Proc. Natl. Acad. Sci. USA 2012, 109, 90–94. [Google Scholar] [CrossRef] [PubMed]
- McGuire, S.; FAO; IFAD; WFP. The State of Food Insecurity in the World 2015: Meeting the 2015 International Hunger Targets: Taking Stock of Uneven Progress; FAO: Rome, Italy; Oxford University Press: Oxford, UK, 2015. [Google Scholar]
- Balaure, P.C.; Gudovan, D.; Gudovan, I. Nanopesticides: A new paradigm in crop protection. In New Pesticides and Soil Sensors; Elsevier: Amsterdam, The Netherlands, 2017; pp. 129–192. [Google Scholar]
- Carvalho, F.P. Agriculture, pesticides, food security and food safety. Environ. Sci. Policy 2006, 9, 685–692. [Google Scholar] [CrossRef]
- Bradberry, S.M.; Proudfoot, A.T.; Vale, J.A. Poisoning due to chlorophenoxy herbicides. Toxicol. Rev. 2004, 23, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Ghormade, V.; Deshpande, M.V.; Paknikar, K.M. Perspectives for nano-biotechnology enabled protection and nutrition of plants. Biotechnol. Adv. 2011, 29, 792–803. [Google Scholar] [CrossRef] [PubMed]
- Malaj, E.; Peter, C.; Grote, M.; Kühne, R.; Mondy, C.P.; Usseglio-Polatera, P.; Brack, W.; Schäfer, R.B. Organic chemicals jeopardize the health of freshwater ecosystems on the continental scale. Proc. Natl. Acad. Sci. USA 2014, 111, 9549–9554. [Google Scholar] [CrossRef]
- Schreiner, V.C.; Szöcs, E.; Bhowmik, A.K.; Vijver, M.G.; Schäfer, R.B. Pesticide mixtures in streams of several European countries and the USA. Sci. Total Environ. 2016, 573, 680–689. [Google Scholar] [CrossRef]
- Yang, X.; Chen, L.; Li, Y.; Xi, W.; Chen, L. Rule-based land use/land cover classification in coastal areas using seasonal remote sensing imagery: A case study from Lianyungang City, China. Environ. Monit. Assess. 2015, 187, 449. [Google Scholar] [CrossRef]
- Stein, K.F. Silent Spring (1962); Houghton Mifflin: New York, NY, USA, 2012. [Google Scholar]
- Nuruzzaman, M.; Rahman, M.M.; Liu, Y.; Naidu, R. Nanoencapsulation, nano-guard for pesticides: A new window for safe application. J. Agric. Food Chem. 2016, 64, 1447–1483. [Google Scholar] [CrossRef]
- Wang, B.; Song, J.L.; Zeng, A.J.; Liu, Y.J.; Zhang, J.; He, X.K. Effects of formulations and surfactants on the behavior of pesticide liquid spreading in the plant leaves. Chin. J. Pestic. Sci. 2012, 14, 334–340. [Google Scholar]
- Allagui, A.; Bahrouni, H.; M’Sadak, Y. Deposition of Pesticide to the Soil and Plant Retention during Crop Spraying: The Art State. J. Agric. Sci. 2018, 10, 12. [Google Scholar] [CrossRef]
- Zhang, M.; Zhu, Z.; Yuan, S.; Wang, S.; Yang, C.; Dwivedi, P.; Si, T.; Xu, R.X. One-step microencapsulation and spraying of pesticide formulations for improved adhesion and sustained release. J. Microencapsul. 2019, 36, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Yao, J.; Liang, J.; Zeng, Z.; Cui, B.; Zhao, X.; Sun, C.; Wang, Y.; Liu, G.; Cui, H. Development of functionalized abamectin poly (lactic acid) nanoparticles with regulatable adhesion to enhance foliar retention. RSC Adv. 2017, 7, 11271–11280. [Google Scholar] [CrossRef]
- Autumn, K.; Liang, Y.A.; Hsieh, S.T.; Zesch, W.; Chan, W.P.; Kenny, T.W.; Fearing, R.; Full, R.J. Adhesive force of a single gecko foot-hair. Nature 2000, 405, 681–685. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.; Glass, P.; Pothen, J.M.; Sitti, M.; Washburn, N.R. Enhanced Adhesion of Dopamine Methacrylamide Elastomers via Viscoelasticity Tuning. Biomacromolecules 2011, 12, 342–347. [Google Scholar] [CrossRef]
- Darwin, C. The Movements and Habits of Climbing Plants; John Murray: London, UK, 1875. [Google Scholar]
- Federle, W.; Riehle, M.; Curtis, S.A.; Full, J.R. An Integrative Study of Insect Adhesion: Mechanics and Wet Adhesion of Pretarsal Pads in Ants. Integr. Comp. Biol. 2002, 42, 1100–1106. [Google Scholar] [CrossRef]
- Yang, F.L.; Li, X.G.; Zhu, F.; Lei, C.L. Structural Characterization of Nanoparticles Loaded with Garlic Essential Oil and Their Insecticidal Activity against Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae). J. Agric. Food Chem. 2009, 57, 10156–10162. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef]
- Lee, H.; Rho, J.; Messersmith, P.B. Facile conjugation of biomolecules onto surfaces via mussel adhesive protein inspired coatings. Adv. Mater. 2009, 21, 431–434. [Google Scholar] [CrossRef]
- Postma, A.; Yan, Y.; Wang, Y.; Zelikin, A.N.; Tjipto, E.; Caruso, F. Self-polymerization of dopamine as a versatile and robust technique to prepare polymer capsules. Chem. Mater. 2009, 21, 3042–3044. [Google Scholar] [CrossRef]
- Cheng, C.; Li, S.; Nie, S.; Zhao, W.; Yang, H.; Sun, S.; Zhao, C. General and biomimetic approach to biopolymer-functionalized graphene oxide nanosheet through adhesive dopamine. Biomacromolecules 2012, 13, 4236–4246. [Google Scholar] [CrossRef]
- Xiong, X.; Liu, Y.; Shi, F.; Zhang, G.; Weng, J.; Qu, S. Enhanced Adhesion of Mussel-inspired Adhesive through Manipulating Contents of Dopamine Methacrylamide and Molecular Weight of Polymer. J. Bionic Eng. 2018, 15, 461–470. [Google Scholar] [CrossRef]
- Hollon, N.G.; Arnold, M.M.; Gan, J.O.; Walton, M.E.; Phillips, P.E. Dopamine-associated cached values are not sufficient as the basis for action selection. Proc. Natl. Acad. Sci. USA 2014, 111, 18357–18362. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Han, L.; Jia, L. A novel platelet-repellent polyphenolic surface and its micropattern for platelet adhesion detection. ACS Appl. Mater. Interfaces 2016, 8, 26570–26577. [Google Scholar] [CrossRef] [PubMed]
- Riedl, K.M.; Hagerman, A.E. Tannin—Protein complexes as radical scavengers and radical sinks. J. Agric. Food Chem. 2001, 49, 4917–4923. [Google Scholar] [CrossRef]
- Serrano, J.; Puupponen-Pimiä, R.; Dauer, A.; Aura, A.M.; Saura-Calixto, F. Tannins: Current knowledge of food sources, intake, bioavailability and biological effects. Mol. Nutr. Food Res. 2009, 53, S310–S329. [Google Scholar] [CrossRef]
- Li, B.X.; Wang, W.C.; Zhang, X.P.; Zhang, D.X.; Ren, Y.P.; Gao, Y.; Mu, W.; Liu, F. Using coordination assembly as the microencapsulation strategy to promote the efficacy and environmental safety of pyraclostrobin. Adv. Funct. Mater. 2017, 27, 1701841. [Google Scholar] [CrossRef]
- Kim, B.-S.; Lee, H.-i.; Min, Y.; Poon, Z.; Hammond, P.T. Hydrogen-bonded multilayer of pH-responsive polymeric micelles with tannic acid for surface drug delivery. Chem. Commun. 2009, 4194–4196. [Google Scholar] [CrossRef]
- Proust, A.; Matt, B.; Villanneau, R.; Guillemot, G.; Gouzerh, P.; Izzet, G. Functionalization and post-functionalization: A step towards polyoxometalate-based materials. Chem. Soc. Rev. 2012, 41, 7605–7622. [Google Scholar] [CrossRef]
- Decher, G.; Schlenoff, J.B. Multilayer Thin Films: Sequential Assembly of Nanocomposite Materials; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Guo, J.; Ping, Y.; Ejima, H.; Alt, K.; Meissner, M.; Richardson, J.J.; Yan, Y.; Peter, K.; Von Elverfeldt, D.; Hagemeyer, C.E. Engineering multifunctional capsules through the assembly of metal-phenolic networks. Angew. Chem. Int. Ed. 2014, 53, 5546–5551. [Google Scholar] [CrossRef]
- Shen, Y.; Du, C.; Zhou, J.; Ma, F. Application of nano FeIII-tannic acid complexes in modifying aqueous acrylic latex for controlled-release coated urea. J. Agric. Food Chem. 2017, 65, 1030–1036. [Google Scholar] [CrossRef]
- Shen, H.; Duan, C.; Guo, J.; Zhao, N.; Xu, J. Facile in situ synthesis of silver nanoparticles on boron nitride nanosheets with enhanced catalytic performance. J. Mater. Chem. A 2015, 3, 16663–16669. [Google Scholar] [CrossRef]
- Zou, Y.; Guo, J.; Yin, S.-W.; Wang, J.-M.; Yang, X.-Q. Pickering emulsion gels prepared by hydrogen-bonded zein/tannic acid complex colloidal particles. J. Agric. Food Chem. 2015, 63, 7405–7414. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Han, L.; Ren, J.; Wei, H.; Jia, L. Coating process and stability of metal-polyphenol film. Coll. Surf. A Physicochem. Eng. ASP 2015, 484, 197–205. [Google Scholar] [CrossRef]
- Yao, J.; Cui, B.; Zhao, X.; Wang, Y.; Zeng, Z.; Sun, C.; Cui, H. Preparation, characterization, and evaluation of azoxystrobin nanosuspension produced by wet media milling. Appl. Nanosci. 2018, 8, 297–307. [Google Scholar] [CrossRef]
- Chen, M.; Jensen, S.P.; Hill, M.R.; Moore, G.; He, Z.; Sumerlin, B.S. Synthesis of amphiphilic polysuccinimide star copolymers for responsive delivery in plants. Chem. Commun. 2015, 51, 9694–9697. [Google Scholar] [CrossRef]
- Wang, C.; Guo, L.; Yao, J.; Wang, A.; Gao, F.; Zhao, X.; Cui, B. Preparation, characterization and antifungal activity of pyraclostrobin solid nanodispersion by self-emulsifying technique. Pest Manag. Sci. 2019, 75, 2785–2793. [Google Scholar] [CrossRef]
- Bo, C.; Chunxin, W.; Xiang, Z.; Junwei, Y.; Zhanghua, Z.; Yan, W.; Changjiao, S.; Guoqiang, L.; Haixin, C.; Amitava, M. Characterization and evaluation of avermectin solid nanodispersion prepared by microprecipitation and lyophilisation techniques. PLoS ONE 2018, 13, e0191742. [Google Scholar]
- Ejima, H.; Richardson, J.J.; Liang, K.; Best, J.P.; van Koeverden, M.P.; Such, G.K.; Cui, J.; Caruso, F. One-step assembly of coordination complexes for versatile film and particle engineering. Science 2013, 341, 154–157. [Google Scholar] [CrossRef]
- Liu, P.-Y.; Miao, Z.-H.; Li, K.; Yang, H.; Zhen, L.; Xu, C.-Y. Biocompatible Fe3+-TA coordination complex with high photothermal conversion efficiency for ablation of cancer cells. Coll. Surf. B Biointerfaces 2018, 167, 183–190. [Google Scholar] [CrossRef]
- Yin, X.; Zhang, F.; Zhang, W. Fabrication of hybrid magnetic Sr5xBa3x (PO4) 3 (OH)/Fe3O4 nanorod and its highly efficient adsorption performance for acid fuchsin dye. Appl. Surf. Sci. 2015, 359, 714–722. [Google Scholar] [CrossRef]
- Li, Z.-Z.; Xu, S.-A.; Wen, L.-X.; Liu, F.; Liu, A.-Q.; Wang, Q.; Sun, H.-Y.; Yu, W.; Chen, J.-F. Controlled release of avermectin from porous hollow silica nanoparticles: Influence of shell thickness on loading efficiency, UV-shielding property and release. J. Control Release 2006, 111, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Qie, R.; Li, W.; Hong, N.; Li, Y.; Li, C.; Wang, R.; Shi, Y.; Guo, X.; Jia, X. Preparation of avermectin microcapsules with anti-photodegradation and slow-release by the assembly of lignin derivatives. New J. Chem. 2017, 41, 3190–3195. [Google Scholar] [CrossRef]
- Li, L.; Zhang, G.; Su, Z. One-step assembly of phytic acid metal complexes for superhydrophilic coatings. Angew. Chem. Int. Ed. 2016, 55, 9093–9096. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ren, P.-F.; Yang, H.-C.; Wan, L.-S.; Xu, Z.-K. Co-deposition of tannic acid and diethlyenetriamine for surface hydrophilization of hydrophobic polymer membranes. Appl. Surf. Sci. 2016, 360, 291–297. [Google Scholar] [CrossRef]
- Simon, S.; Disalvo, E.; Gawrisch, K.; Borovyagin, V.; Toone, E.; Schiffman, S.; Needham, D.; McIntosh, T. Increased adhesion between neutral lipid bilayers: Interbilayer bridges formed by tannic acid. Biophys. J. 1994, 66, 1943–1958. [Google Scholar] [CrossRef]
- McGrane, S.J.; Mainwaring, D.E.; Cornell, H.J.; Rix, C.J. The role of hydrogen bonding in amylose gelation. Starch-Stärke 2004, 56, 122–131. [Google Scholar] [CrossRef]
- Kozlovskaya, V.; Kharlampieva, E.; Drachuk, I.; Cheng, D.; Tsukruk, V.V. Responsive microcapsule reactors based on hydrogen-bonded tannic acid layer-by-layer assemblies. Soft Matter 2010, 6, 3596–3608. [Google Scholar] [CrossRef]
- Liu, F.; Kozlovskaya, V.; Zavgorodnya, O.; Martinez-Lopez, C.; Catledge, S.; Kharlampieva, E. Encapsulation of anticancer drug by hydrogen-bonded multilayers of tannic acid. Soft Matter 2014, 10, 9237–9247. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).