Polyethylene Glycol Pulsed Electrodeposition for the Development of Antifouling Coatings on Titanium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Electrodeposition Process
2.3. Surface Characterization
2.4. In Vitro Biological Characterization
2.5. Statistical Analysis
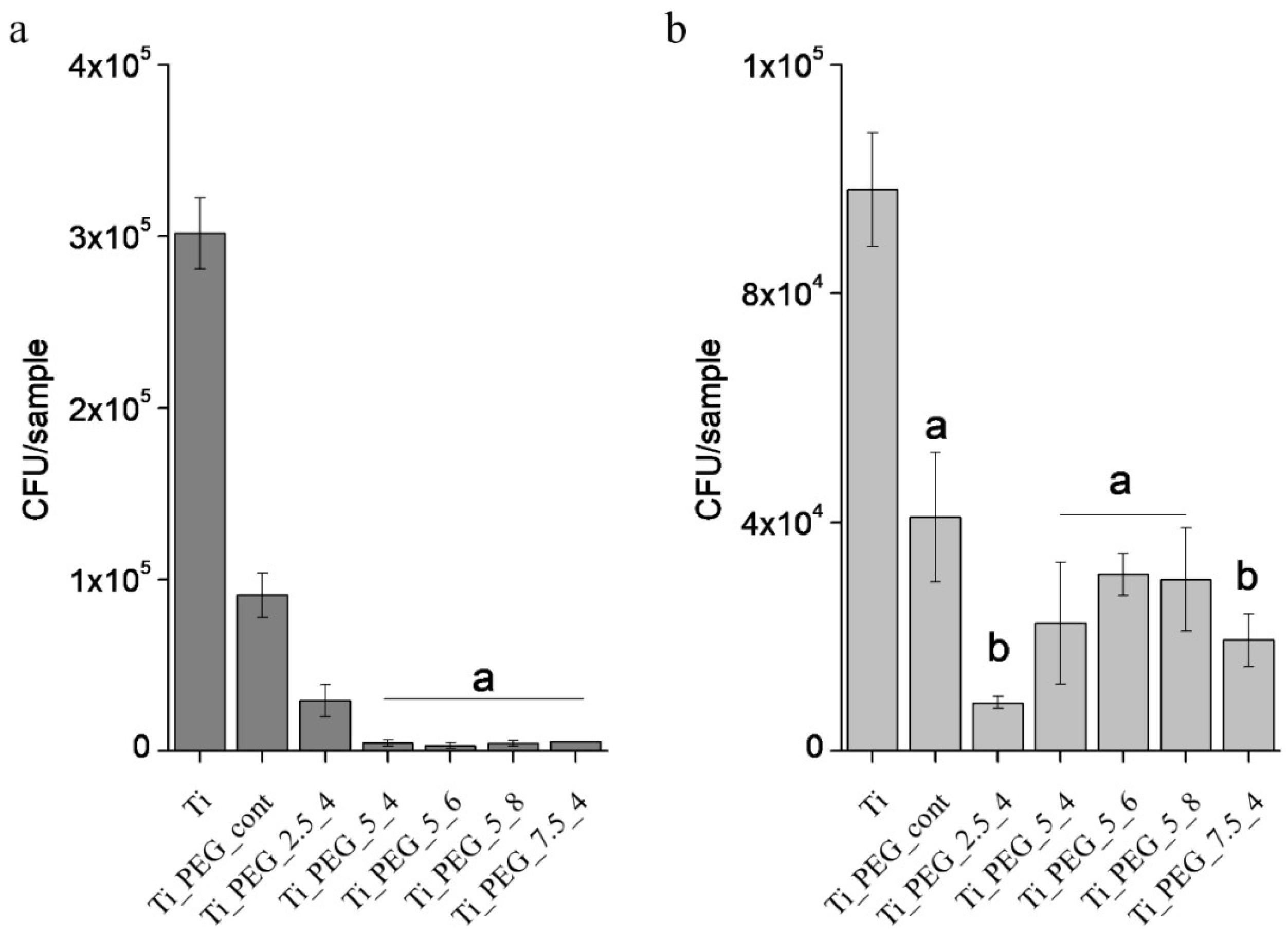
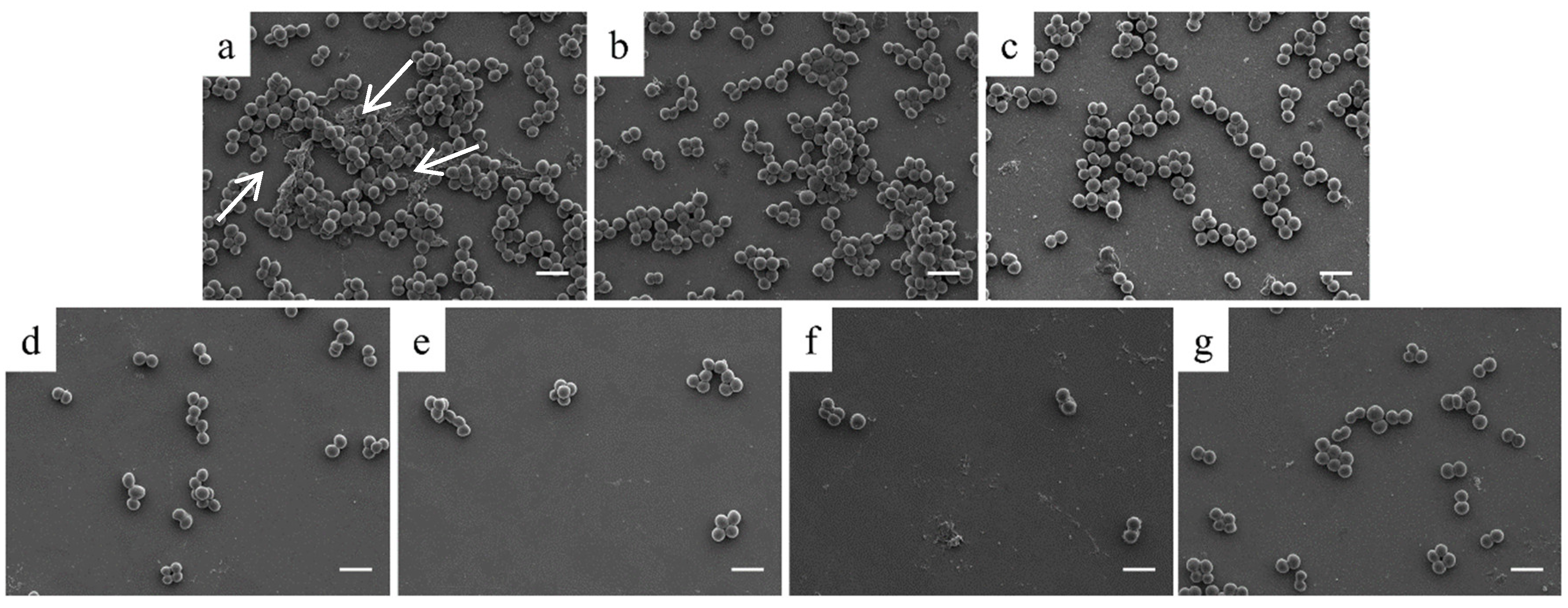
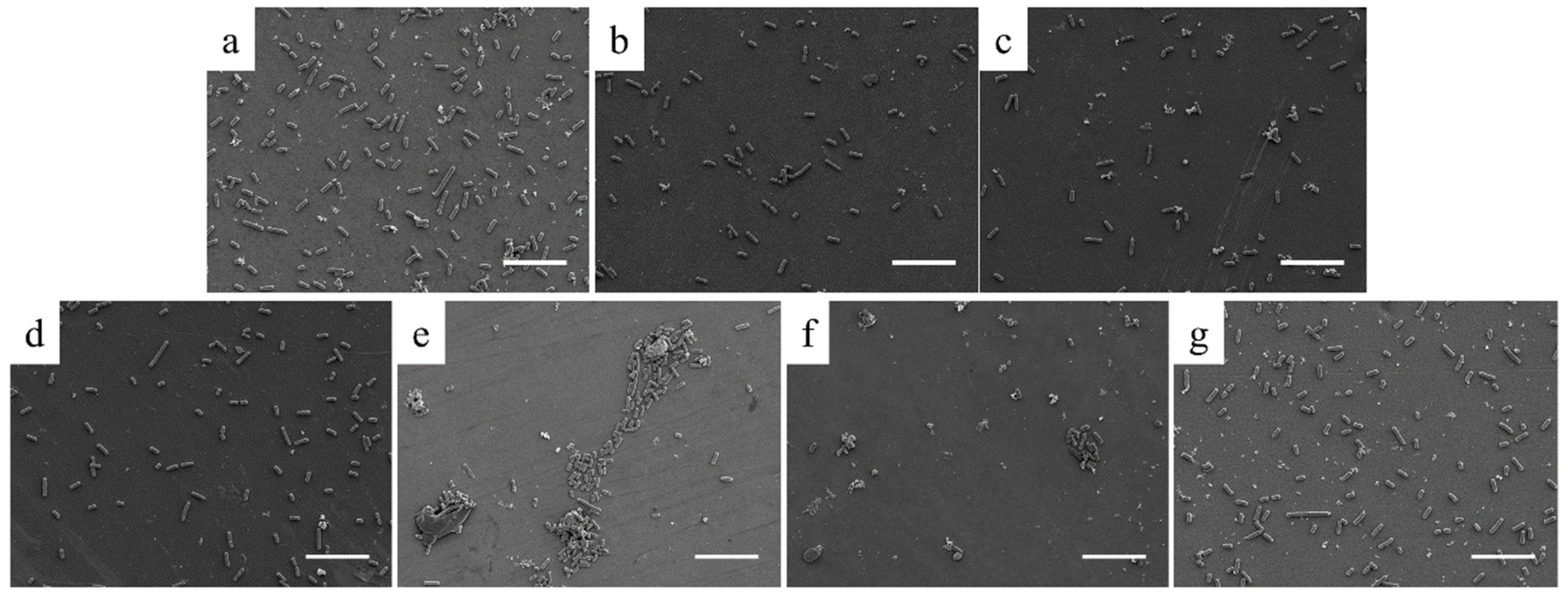
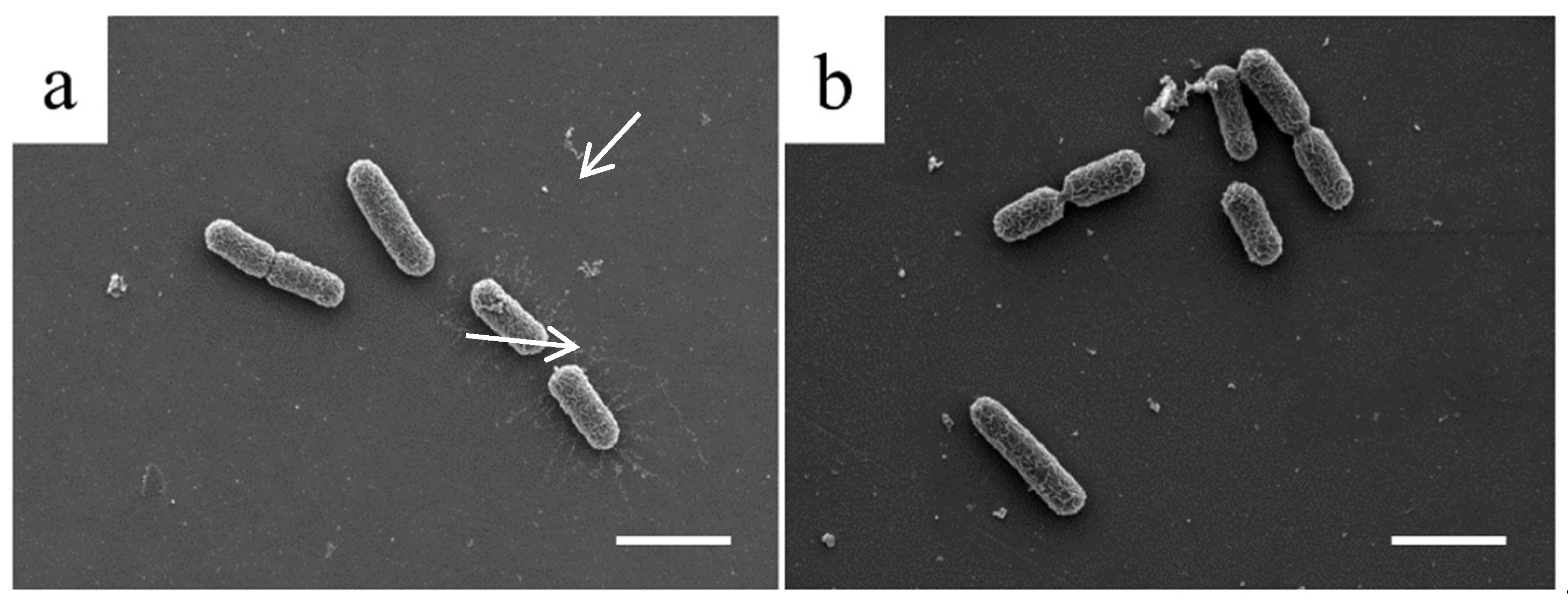
3. Results
3.1. Physicochemical Characterization
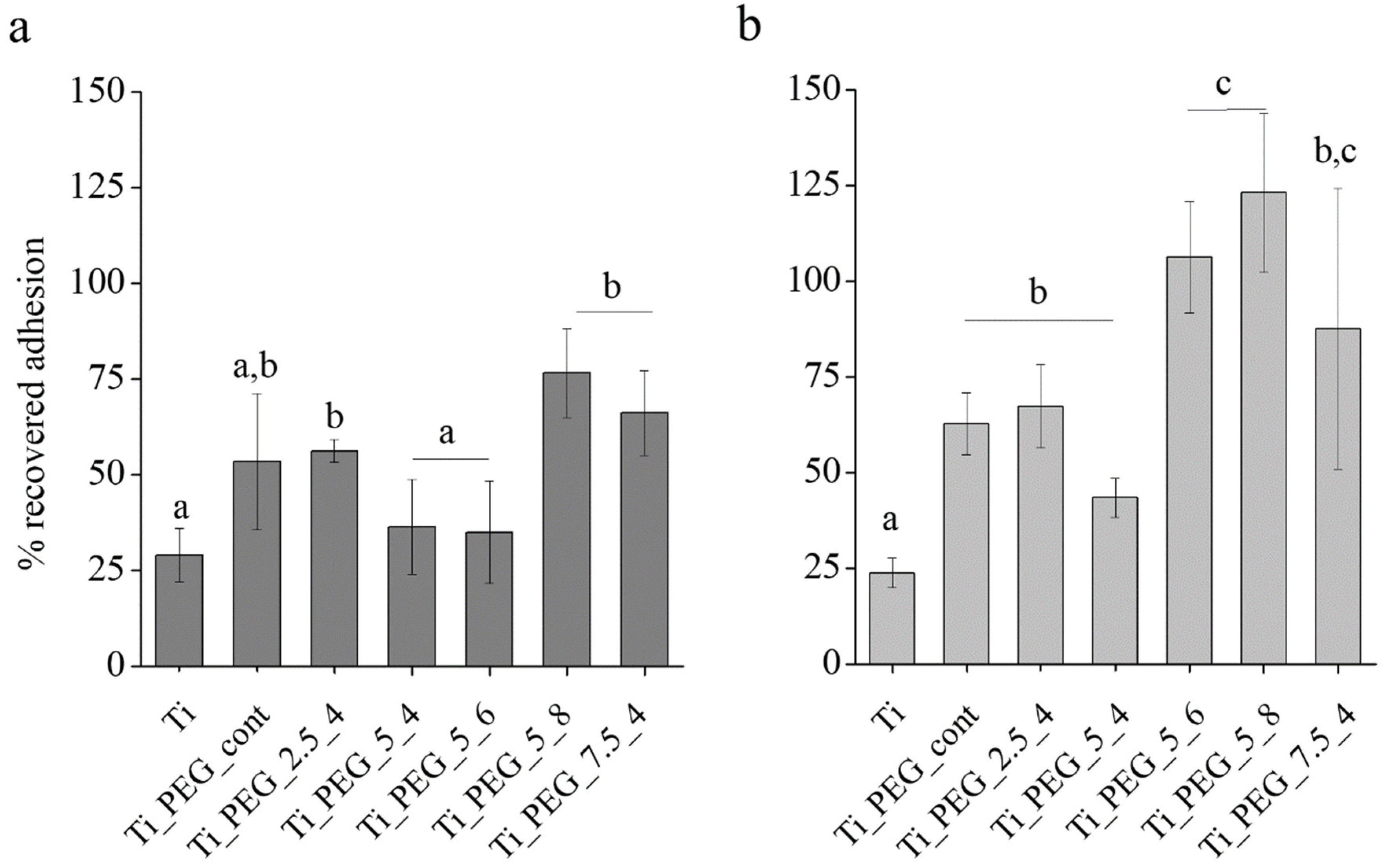
3.2. In Vitro Characterization
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- French, D.; Grandin, H.M.; Ofec, R. Retrospective cohort study of 4,591 dental implants: Analysis of risk indicators for bone loss and prevalence of peri-implant mucositis and peri-implantitis. J. Periodontol. 2019, 90, 691–700. [Google Scholar] [CrossRef] [Green Version]
- Oshida, Y. Bioscience and Bioengineering of Titanium Materials; Elsevier: Amsterdam, The Netherlands, 2007; ISBN 9780080451428. [Google Scholar]
- Watnick, P.; Kolter, R. Biofilm, City of Microbes. J. Bacteriol. 2000, 182, 2675–2679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hannig, C.; Hannig, M. The oral cavity—A key system to understand substratum-dependent bioadhesion on solid surfaces in man. Clin. Oral Investig. 2009, 13, 123–139. [Google Scholar] [CrossRef] [PubMed]
- Atsuta, I.; Ayukawa, Y.; Ogino, Y.; Moriyama, Y.; Jinno, Y.; Koyano, K. Evaluations of epithelial sealing and peri-implant epithelial down-growth around “step-type” implants. Clin. Oral Implants Res. 2012, 23, 459–466. [Google Scholar] [CrossRef]
- Chai, W.L.; Brook, I.M.; Palmquist, A.; van Noort, R.; Moharamzadeh, K. The biological seal of the implant-soft tissue interface evaluated in a tissue-engineered oral mucosal model. J. R. Soc. Interface 2012, 9, 3528–3538. [Google Scholar] [CrossRef] [PubMed]
- Parsek, M.R.; Singh, P.K. Bacterial biofilms: An emerging link to disease pathogenesis. Annu. Rev. Microbiol. 2003, 57, 677–701. [Google Scholar] [CrossRef]
- Costerton, J.W. Biofilm theory can guide the treatment of device-related orthopaedic infections. Clin. Orthop. Relat. Res. 2005, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Bhadra, C.M.; Khanh Truong, V.; Pham, V.T.H.; Al Kobaisi, M.; Seniutinas, G.; Wang, J.Y.; Juodkazis, S.; Crawford, R.J.; Ivanova, E.P. Antibacterial titanium nano-patterned arrays inspired by dragonfly wings. Sci. Rep. 2015, 5, 16817. [Google Scholar] [CrossRef] [Green Version]
- Lorenzetti, M.; Dogša, I.; Stošicki, T.; Stopar, D.; Kalin, M.; Kobe, S.; Novak, S. The Influence of Surface Modification on Bacterial Adhesion to Titanium-Based Substrates. ACS Appl. Mater. Interfaces 2015, 7, 1644–1651. [Google Scholar] [CrossRef]
- Fraioli, R.; Tsimbouri, P.M.; Fisher, L.E.; Nobbs, A.H.; Su, B.; Neubauer, S.; Rechenmacher, F.; Kessler, H.; Ginebra, M.-P.; Dalby, M.J.; et al. Towards the cell-instructive bactericidal substrate: Exploring the combination of nanotopographical features and integrin selective synthetic ligands. Sci. Rep. 2017, 7, 16363. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Schlaich, C.; Willem Kulka, M.; Donskyi, I.S.; Schwerdtle, T.; Unger, W.E.S.; Haag, R. Mussel-inspired coatings with tunable wettability, for enhanced antibacterial efficiency and reduced bacterial adhesion. J. Mater. Chem. B 2019, 7, 3438–3445. [Google Scholar] [CrossRef]
- Ferraris, S.; Spriano, S. Antibacterial titanium surfaces for medical implants. Mater. Sci. Eng. C 2016, 61, 965–978. [Google Scholar] [CrossRef] [PubMed]
- Godoy-Gallardo, M.; Rodríguez-Hernández, A.G.; Delgado, L.M.; Manero, J.M.; Javier Gil, F.; Rodríguez, D. Silver deposition on titanium surface by electrochemical anodizing process reduces bacterial adhesion of Streptococcus sanguinis and Lactobacillus salivarius. Clin. Oral Implants Res. 2014, 26, 1170–1179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuchiya, H.; Shirai, T.; Nishida, H.; Murakami, H.; Kabata, T.; Yamamoto, N.; Watanabe, K.; Nakase, J. Innovative antimicrobial coating of titanium implants with iodine. J. Orthop. Sci. 2012, 17, 595–604. [Google Scholar] [CrossRef]
- Buxadera-Palomero, J.; Canal, C.; Torrent-Camarero, S.; Garrido, B.; Javier Gil, F.; Rodríguez, D. Antifouling coatings for dental implants: Polyethylene glycol-like coatings on titanium by plasma polymerization. Biointerphases 2015, 10, 029505. [Google Scholar] [CrossRef]
- Wei, Q.; Haag, R. Universal polymer coatings and their representative biomedical applications. Mater. Horiz. 2015, 2, 567–577. [Google Scholar] [CrossRef] [Green Version]
- Wei, Q.; Becherer, T.; Angioletti-Uberti, S.; Dzubiella, J.; Wischke, C.; Neffe, A.T.; Lendlein, A.; Ballauff, M.; Haag, R. Protein Interactions with Polymer Coatings and Biomaterials. Angew. Chem. Int. Ed. 2014, 53, 8004–8031. [Google Scholar] [CrossRef]
- Ungureanu, C.; Pirvu, C.; Mindroiu, M.; Demetrescu, I. Antibacterial polymeric coating based on polypyrrole and polyethylene glycol on a new alloy TiAlZr. Prog. Org. Coat. 2012, 75, 349–355. [Google Scholar] [CrossRef]
- De Giglio, E.; Cometa, S.; Ricci, M.A.; Cafagna, D.; Savino, A.M.; Sabbatini, L.; Orciani, M.; Ceci, E.; Novello, L.; Tantillo, G.M.; et al. Ciprofloxacin-modified electrosynthesized hydrogel coatings to prevent titanium-implant-associated infections. Acta Biomater. 2011, 7, 882–891. [Google Scholar] [CrossRef]
- Stigter, M.; Bezemer, J.; de Groot, K.; Layrolle, P. Incorporation of different antibiotics into carbonated hydroxyapatite coatings on titanium implants, release and antibiotic efficacy. J. Control. Release 2004, 99, 127–137. [Google Scholar] [CrossRef]
- Rocas, P.; Hoyos-Nogués, M.; Rocas, J.; Manero, J.M.; Gil, J.; Albericio, F.; Mas-Moruno, C. Installing Multifunctionality on Titanium with RGD-Decorated Polyurethane-Polyurea Roxithromycin Loaded Nanoparticles: Toward New Osseointegrative Therapies. Adv. Healthc. Mater. 2015, 4, 1956–1960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoyos-Nogués, M.; Buxadera-Palomero, J.; Ginebra, M.-P.; Manero, J.M.; Gil, F.J.; Mas-Moruno, C. All-in-one trifunctional strategy: A cell adhesive, bacteriostatic and bactericidal coating for titanium implants. Colloids Surfaces B Biointerfaces 2018, 169, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Hoyos-Nogués, M.; Velasco, F.; Ginebra, M.P.; Manero, J.M.; Gil, F.J.; Mas-Moruno, C. Regenerating Bone via Multifunctional Coatings: The Blending of Cell Integration and Bacterial Inhibition Properties on the Surface of Biomaterials. ACS Appl. Mater. Interfaces 2017, 9, 21618–21630. [Google Scholar] [CrossRef] [PubMed]
- Mas-Moruno, C.; Su, B.; Dalby, M.J. Multifunctional Coatings and Nanotopographies: Toward Cell Instructive and Antibacterial Implants. Adv. Healthc. Mater. 2019, 8, 1801103. [Google Scholar] [CrossRef]
- Ostuni, E.; Chapman, R.G.; Holmlin, R.E.; Takayama, S.; Whitesides, G.M. A Survey of Structure−Property Relationships of Surfaces that Resist the Adsorption of Protein. Langmuir 2001, 17, 5605–5620. [Google Scholar] [CrossRef]
- Buxadera-Palomero, J.; Calvo, C.; Torrent-Camarero, S.; Gil, F.J.; Mas-Moruno, C.; Canal, C.; Rodríguez, D. Biofunctional polyethylene glycol coatings on titanium: An in vitro-based comparison of functionalization methods. Colloids Surfaces B Biointerfaces 2017. [Google Scholar] [CrossRef]
- Huang, Y.-W.W.; Gupta, V.K. Influence of Polymer Flux and Chain Length on Adsorption of Poly(Ethylene Oxide) on Physically Heterogeneous Surfaces. Langmuir 2002, 18, 2280–2287. [Google Scholar] [CrossRef]
- Godoy-Gallardo, M.; Mas-Moruno, C.; Yu, K.; Manero, J.M.; Gil, J.; Kizhakkedathu, J.N.; Rodriguez, D. Antibacterial properties of hLf1-11 peptide onto titanium surfaces: A comparison study between silanization and surface initiated polymerization. Biomacromolecules 2015, 16, 483–496. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, Y.; Doi, H.; Iwasaki, Y.; Hiromoto, S.; Yoneyama, T.; Asami, K.; Imai, H.; Hanawa, T. Electrodeposition of amine-terminated poly(ethylene glycol) to titanium surface. Mater. Sci. Eng. C 2007, 27, 206–212. [Google Scholar] [CrossRef]
- Hansson, K.M.; Tosatti, S.; Isaksson, J.; Wetterö, J.; Textor, M.; Lindahl, T.L.; Tengvall, P. Whole blood coagulation on protein adsorption-resistant PEG and peptide functionalised PEG-coated titanium surfaces. Biomaterials 2005, 26, 861–872. [Google Scholar] [CrossRef]
- Huang, N.-P.; Csucs, G.; Emoto, K.; Nagasaki, Y.; Kataoka, K.; Textor, M.; Spencer, N.D. Covalent Attachment of Novel Poly(ethylene glycol)−Poly(dl -lactic acid) Copolymeric Micelles to TiO2 Surfaces. Langmuir 2002, 18, 252–258. [Google Scholar] [CrossRef]
- Saxer, S.; Portmann, C.; Tosatti, S.; Gademann, K.; Zürcher, S.; Textor, M. Surface Assembly of Catechol-Functionalized Poly( l -lysine)- graft -poly(ethylene glycol) Copolymer on Titanium Exploiting Combined Electrostatically Driven Self-Organization and Biomimetic Strong Adhesion. Macromolecules 2010, 43, 1050–1060. [Google Scholar] [CrossRef]
- Cometa, S.; Bonifacio, M.A.; Mattioli-Belmonte, M.; Sabbatini, L.; De Giglio, E. Electrochemical Strategies for Titanium Implant Polymeric Coatings: The Why and How. Coatings 2019, 9, 268. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Zhang, X.; Gu, J.; Yang, H.; Nie, J.; Ma, G. Electrodeposition of alginate/chitosan layer-by-layer composite coatings on titanium substrates. Carbohydr. Polym. 2014, 103, 38–45. [Google Scholar] [CrossRef]
- Wang, J.; Li, B.; Li, Z.; Ren, K.; Jin, L.; Zhang, S.; Chang, H.; Sun, Y.; Ji, J. Electropolymerization of dopamine for surface modification of complex-shaped cardiovascular stents. Biomaterials 2014, 35, 7679–7689. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekar, M.S.; Pushpavanam, M. Pulse and pulse reverse plating—Conceptual, advantages and applications. Electrochim. Acta 2008, 53, 3313–3322. [Google Scholar] [CrossRef]
- Natter, H.; Hempelmann, R. Nanocrystalline Copper by Pulsed Electrodeposition: The Effects of Organic Additives, Bath Temperature, and pH. J. Phys. Chem. 1996, 100, 19525–19532. [Google Scholar] [CrossRef]
- Zhou, H.; Wen, J.; Ning, X.; Fu, C.; Chen, J.; Kuang, Y. Electrosynthesis of polyaniline films on titanium by pulse potentiostatic method. Synth. Met. 2007, 157, 98–103. [Google Scholar] [CrossRef]
- International Organization for Standardization. ISO 10993-5: Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity; ISO: Geneva, Switzerland, 2009. [Google Scholar]
- Zhao, B.; van der Mei, H.C.; Subbiahdoss, G.; de Vries, J.; Rustema-Abbing, M.; Kuijer, R.; Busscher, H.J.; Ren, Y. Soft tissue integration versus early biofilm formation on different dental implant materials. Dent. Mater. 2014, 30, 716–727. [Google Scholar] [CrossRef]
- Godoy-Gallardo, M.; Guillem-Marti, J.; Sevilla, P.; Manero, J.M.; Gil, F.J.; Rodriguez, D. Anhydride-functional silane immobilized onto titanium surfaces induces osteoblast cell differentiation and reduces bacterial adhesion and biofilm formation. Mater. Sci. Eng. C. Mater. Biol. Appl. 2016, 59, 524–532. [Google Scholar] [CrossRef] [Green Version]
- Lo Porto, C.; Palumbo, F.; Buxadera-Palomero, J.; Canal, C.; Jelinek, P.; Zajickova, L.; Favia, P. On the plasma deposition of vancomycin-containing nano-capsules for drug-delivery applications. Plasma Process. Polym. 2018, 15, 1700232. [Google Scholar] [CrossRef]
- Vidal, E.; Buxadera-Palomero, J.; Pierre, C.; Manero, J.M.; Ginebra, M.P.; Cazalbou, S.; Combes, C.; Rupérez, E.; Rodríguez, D. Single-step pulsed electrodeposition of calcium phosphate coatings on titanium for drug delivery. Surf. Coat. Technol. 2019, 358, 266–275. [Google Scholar] [CrossRef] [Green Version]
- Quinton, D.; Galtayries, A.; Prima, F.; Griveau, S. Functionalization of titanium surfaces with a simple electrochemical strategy. Surf. Coat. Technol. 2012, 206, 2302–2307. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [Green Version]
- De Giglio, E.; Guascito, M.; Sabbatini, L.; Zambonin, G. Electropolymerization of pyrrole on titanium substrates for the future development of new biocompatible surfaces. Biomaterials 2001, 22, 2609–2616. [Google Scholar] [CrossRef]
- Drevet, R.; Velard, F.; Potiron, S.; Laurent-Maquin, D.; Benhayoune, H. In vitro dissolution and corrosion study of calcium phosphate coatings elaborated by pulsed electrodeposition current on Ti6Al4V substrate. J. Mater. Sci. Mater. Med. 2011, 22, 753–761. [Google Scholar] [CrossRef]
- Allahyarzadeh, M.; Alofkhazraei, M.; Sabour Rouhaghdam, A.; Torabinejad, V. Structure and wettability of pulsed electrodeposited Ni-W-Cu-(α-alumina) nanocomposite. Surf. Coat. Technol. Part A 2016, 15, 525–533. [Google Scholar] [CrossRef]
- Fukuhara, Y.; Kyuzo, M.; Tsutsumi, Y.; Nagai, A.; Chen, P.; Hanawa, T. Phospholipid polymer electrodeposited on titanium inhibits platelet adhesion. J. Biomed. Mater. Res. B. Appl. Biomater. 2015. [Google Scholar] [CrossRef]
- Thalla, P.K.; Contreras-García, A.; Fadlallah, H.; Barrette, J.; De Crescenzo, G.; Merhi, Y.; Lerouge, S. A versatile star PEG grafting method for the generation of nonfouling and nonthrombogenic surfaces. BioMed Res. Int. 2013, 2013, 962376. [Google Scholar] [CrossRef]
- Papra, A.; Gadegaard, N.; Larsen, N.B. Characterization of Ultrathin Poly(ethylene glycol) Monolayers on Silicon Substrates. Langmuir 2001, 17, 1457–1460. [Google Scholar] [CrossRef]
- Teughels, W.; Van Assche, N.; Sliepen, I.; Quirynen, M. Effect of material characteristics and/or surface topography on biofilm development. Clin. Oral Implants Res. 2006, 17, 68–81. [Google Scholar] [CrossRef] [PubMed]
- Wassmann, T.; Kreis, S.; Behr, M.; Buergers, R. The influence of surface texture and wettability on initial bacterial adhesion on titanium and zirconium oxide dental implants. Int. J. Implant Dent. 2017, 3, 32. [Google Scholar] [CrossRef] [PubMed]
- Boru, Z.; Thomas, E.; Rico, G.; Deborah, L. Chain-length dependence of the protein and cell resistance of oligo(ethylene glycol)-terminated self-assembled monolayers on gold. J. Biomed. Mater. Res. 2001, 56, 406–416. [Google Scholar]
- Sharma, S.; Johnson, R.W.; Desai, T.A. XPS and AFM analysis of antifouling PEG interfaces for microfabricated silicon biosensors. Biosens. Bioelectron. 2004, 20, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Sofia, S.; Premnath, V.; Merrill, E. Poly(ethylene oxide) Grafted to Silicon Surfaces: Grafting Density and Protein Adsorption. Macromolecules 1998, 31, 5059–5070. [Google Scholar] [CrossRef]
- Altankov, G.; Thom, V.; Groth, T.; Jankova, K.; Jonsson, G.; Ulbricht, M. Modulating the biocompatibility of polymer surfaces with poly(ethylene glycol): Effect of fibronectin. J. Biomed. Mater. Res. 2000, 52, 219–230. [Google Scholar] [CrossRef]
- Röttgermann, P.J.F.; Hertrich, S.; Berts, I.; Albert, M.; Segerer, F.J.; Moulin, J.-F.; Nickel, B.; Rädler, J.O. Cell Motility on Polyethylene Glycol Block Copolymers Correlates to Fibronectin Surface Adsorption. Macromol. Biosci. 2014, 14, 1755–1763. [Google Scholar] [CrossRef]
- Tanaka, Y.; Matin, K.; Gyo, M.; Okada, A.; Tsutsumi, Y.; Doi, H.; Nomura, N.; Tagami, J.; Hanawa, T. Effects of electrodeposited poly(ethylene glycol) on biofilm adherence to titanium. J. Biomed. Mater. Res. A 2010, 95, 1105–1113. [Google Scholar] [CrossRef]
- Beloin, C.; Roux, A.; Ghigo, J.M. Escherichia coli biofilms. Curr. Top. Microbiol. Immunol. 2008, 322, 249–289. [Google Scholar]
- Blumer, C.; Kleefeld, A.; Lehnen, D.; Heintz, M.; Dobrindt, U.; Nagy, G.; Michaelis, K.; Emödy, L.; Polen, T.; Rachel, R.; et al. Regulation of type 1 fimbriae synthesis and biofilm formation by the transcriptional regulator LrhA of Escherichia coli. Microbiology 2005, 151, 3287–3298. [Google Scholar] [CrossRef] [Green Version]
- Gristina, A.G. Biomaterial-centered infection: Microbial adhesion versus tissue integration. Science 1987, 237, 1588–1595. [Google Scholar] [CrossRef] [PubMed]
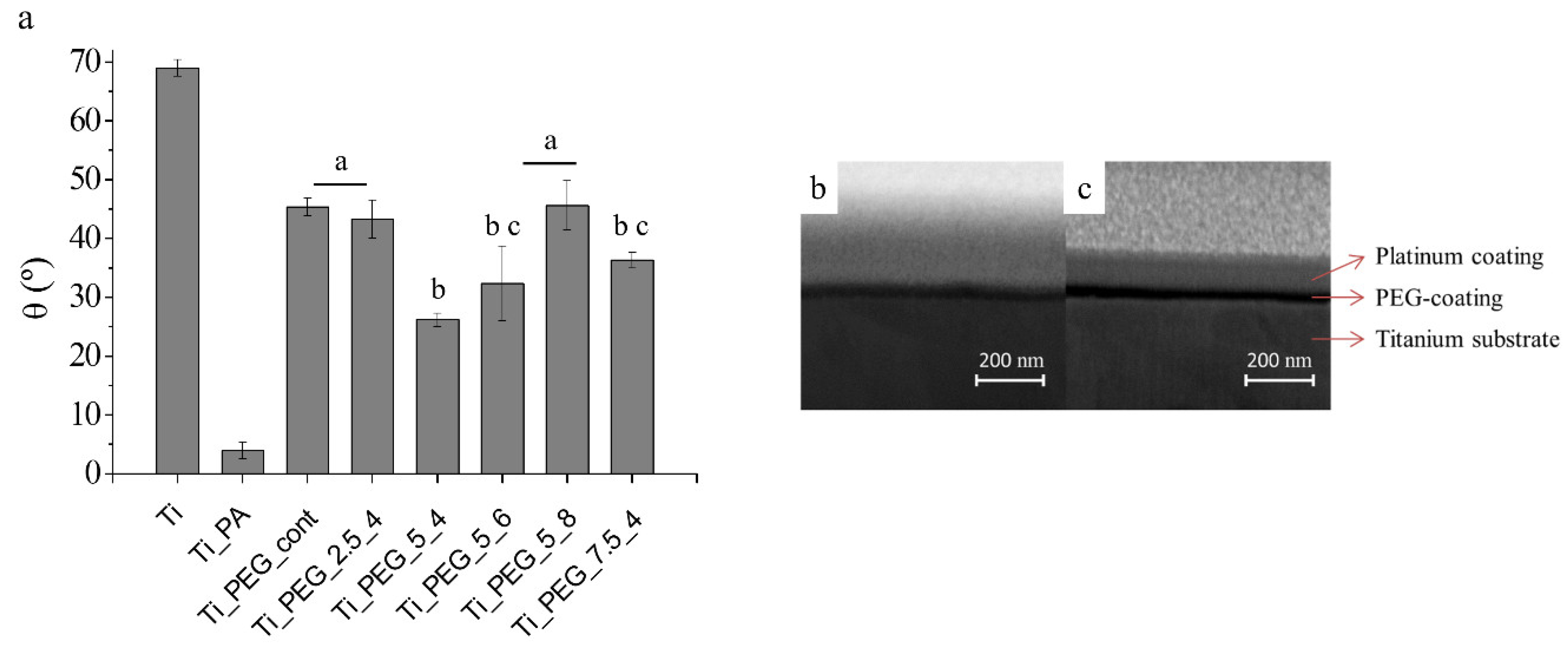
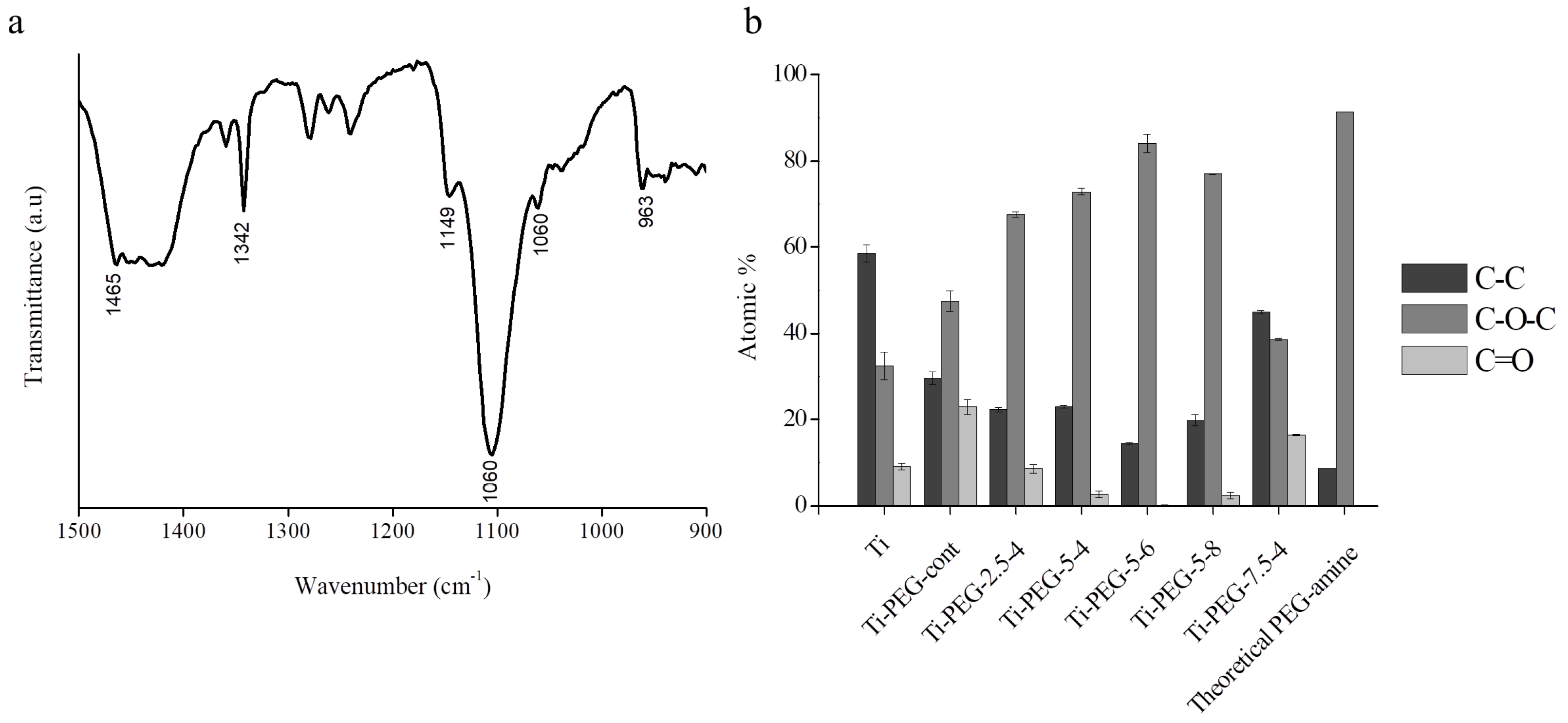
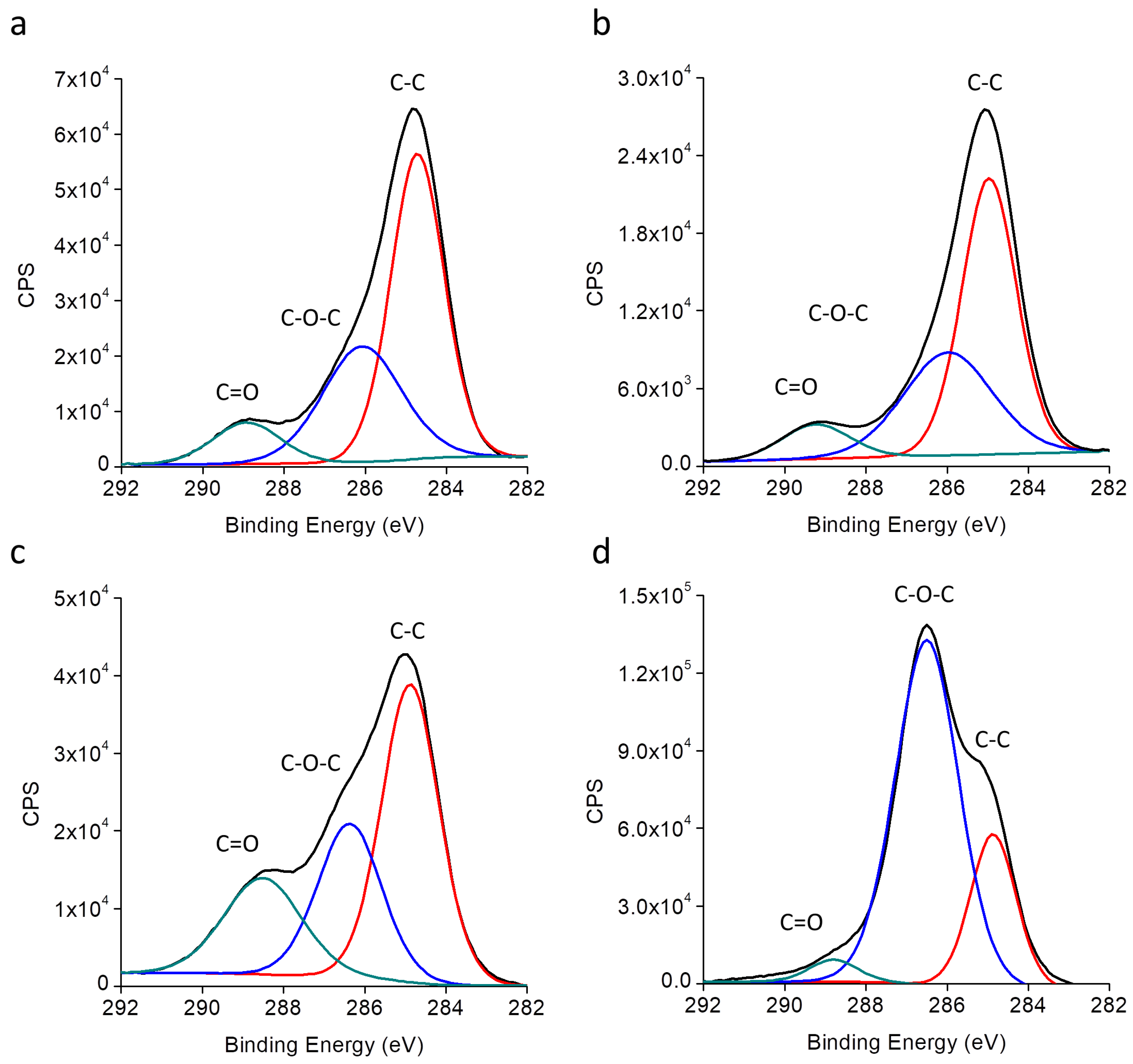
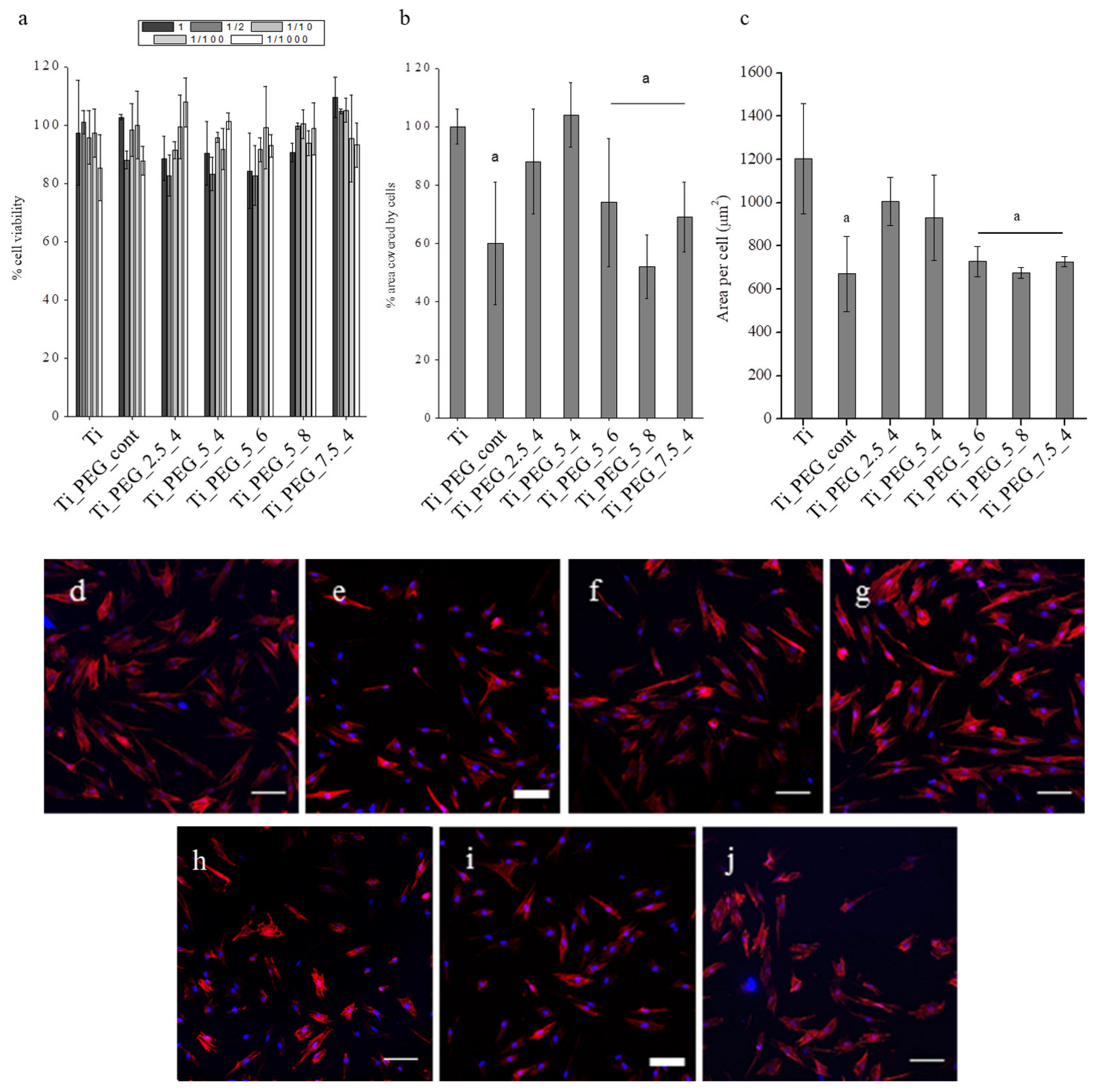
| Sample Code | Vmin (V) | Vmax (V) | t1 = t2 (ms) | f (Hz) |
|---|---|---|---|---|
| Ti_PEG_cont | 0 | −5 | - | - |
| Ti_PEG_2.5_4 | −2.5 | −5 | 4 | 125 |
| Ti_PEG_5_4 | 0 | −5 | 4 | 125 |
| Ti_PEG_5_6 | 0 | −5 | 6 | 83.3 |
| Ti_PEG_5_8 | 0 | −5 | 8 | 62.5 |
| Ti_PEG_7.5_4 | 2.5 | −5 | 4 | 125 |
| Sample Code | C 1s | O 1s | N 1s | Ti 2p |
|---|---|---|---|---|
| Ti | 23.5 ± 0.8 | 59.6 ± 0.8 | 0.6 ± 0.1 | 16.3 ± 0.1 |
| Ti-PA | 15.0 ± 0.7 | 62.1 ± 0.7 | 0.3 ± 0.1 | 22.6 ± 0.1 |
| Ti_PEG_cont | 38.1 ± 0.9 | 47.7 ± 1.0 | 1.2 ± 0.1 | 13.9 ± 0.1 |
| Ti_PEG_2.5_4 | 73.6 ± 1.0 | 24.8 ± 0.9 | 1.1 ± 0.1 | 0.1 ± 0.2 |
| Ti_PEG_5_4 | 70.8 ± 0.5 | 26.3 ± 0.5 | 1.0 ± 0.1 | 1.2 ± 0.4 |
| Ti_PEG_5_6 | 68.3 ± 0.2 | 29.2 ± 0.2 | 1.5 ± 0.4 | 0.5 ± 0.2 |
| Ti_PEG_5_8 | 71.9 ± 0.6 | 26.2 ± 0.9 | 1.1 ± 0.1 | 0.7 ± 0.7 |
| Ti_PEG_7.5_4 | 63.7 ± 0.3 | 30.1 ± 0.2 | 1.4 ± 0.7 | 4.7 ± 0.3 |
| Theoretical_PEG | 67.3 | 30.8 | 1.9 | - |
| Sample Code | N 1s |
|---|---|
| Ti | 7.9 ± 0.2 |
| Ti_PEG_cont | 3.1 ± 0.7 |
| Ti_PEG_2.5_4 | 2.7 ± 0.9 |
| Ti_PEG_5_4 | 5.0 ± 0.6 |
| Ti_PEG_5_6 | 3.27 ± 0.02 |
| Ti_PEG_5_8 | 2.2 ± 0.4 |
| Ti_PEG_7.5_4 | 5.0 ± 0.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buxadera-Palomero, J.; Albó, K.; Gil, F.J.; Mas-Moruno, C.; Rodríguez, D. Polyethylene Glycol Pulsed Electrodeposition for the Development of Antifouling Coatings on Titanium. Coatings 2020, 10, 456. https://doi.org/10.3390/coatings10050456
Buxadera-Palomero J, Albó K, Gil FJ, Mas-Moruno C, Rodríguez D. Polyethylene Glycol Pulsed Electrodeposition for the Development of Antifouling Coatings on Titanium. Coatings. 2020; 10(5):456. https://doi.org/10.3390/coatings10050456
Chicago/Turabian StyleBuxadera-Palomero, Judit, Kim Albó, Francisco Javier Gil, Carlos Mas-Moruno, and Daniel Rodríguez. 2020. "Polyethylene Glycol Pulsed Electrodeposition for the Development of Antifouling Coatings on Titanium" Coatings 10, no. 5: 456. https://doi.org/10.3390/coatings10050456
APA StyleBuxadera-Palomero, J., Albó, K., Gil, F. J., Mas-Moruno, C., & Rodríguez, D. (2020). Polyethylene Glycol Pulsed Electrodeposition for the Development of Antifouling Coatings on Titanium. Coatings, 10(5), 456. https://doi.org/10.3390/coatings10050456








