The Utilization of Carbon Dioxide to Prepare TiCxOy Films with Low Friction and High Anti-Corrosion Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Films Preparation
2.2. Characterization Methods
2.3. Friction Test
3. Results
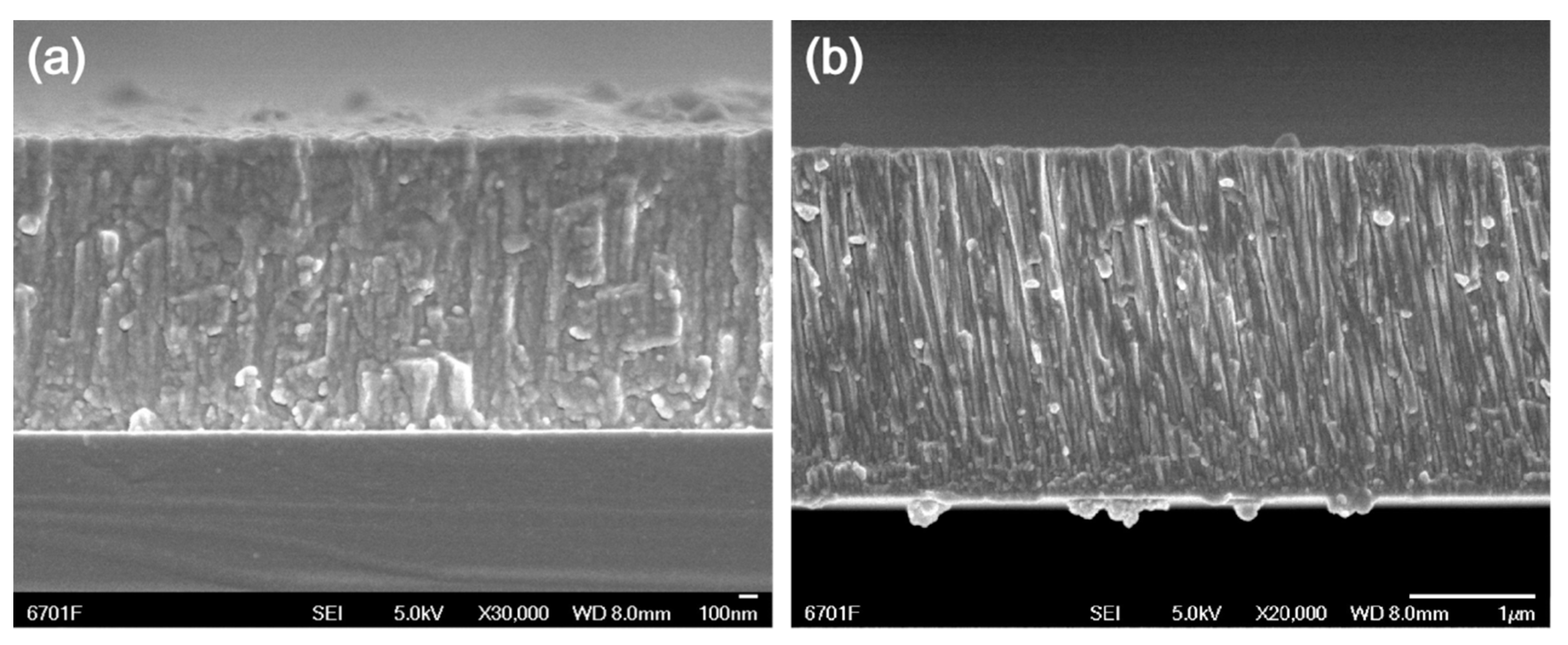
3.1. The Mechanical and Frictional Performance of the Films
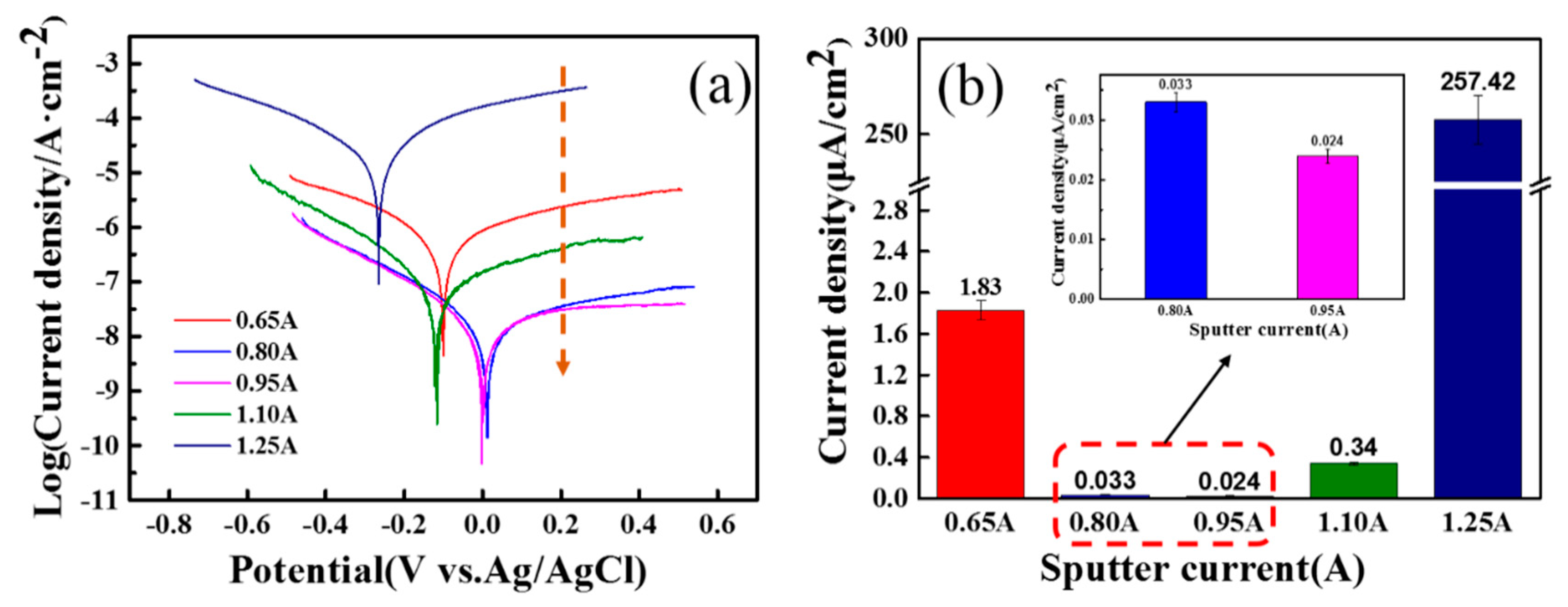
3.2. The Electrochemical Corrosion Performances of Films
3.3. The XRD Results of Films
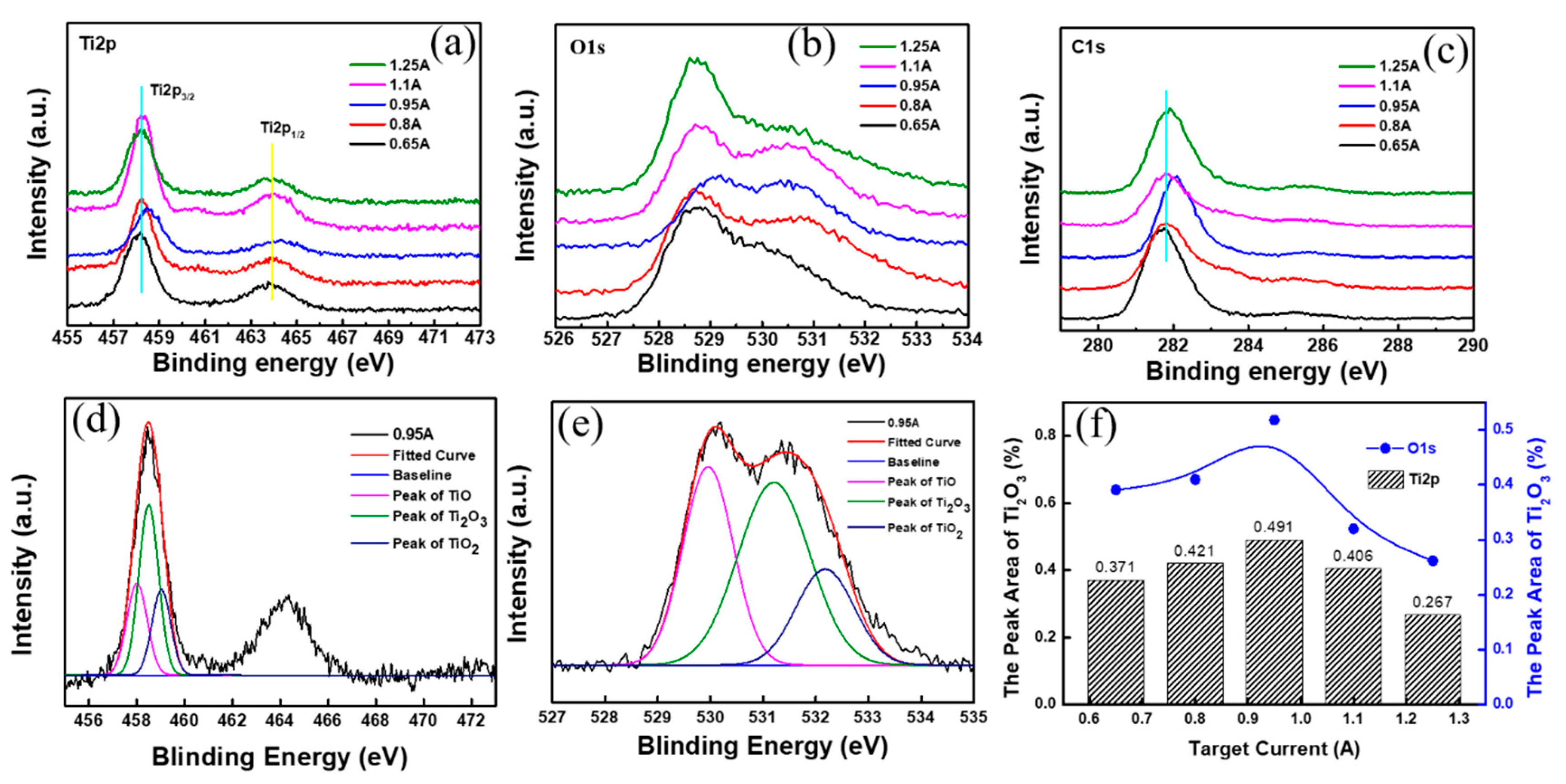
3.4. The Results of XPS
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sanglimsuwan, K. Carbon dioxide emissions and economic growth: An econometric analysis. Int. Res. J. Financ. Econ. 2011, 67, 97–102. [Google Scholar]
- Raupach, M.R.; Marland, G.; Ciais, P.; Le Quéré, C.; Canadell, J.G.; Klepper, G.; Field, C.B. Global and regional drivers of accelerating CO2 emissions. Proc. Natl. Acad. Sci. USA 2007, 104, 10288–10293. [Google Scholar] [CrossRef] [Green Version]
- Mardani, A.; Streimikiene, D.; Cavallaro, F.; Loganathan, N.; Khoshnoudi, M. Carbon dioxide (CO2) emissions and economic growth: A systematic review of two decades of research from 1995 to 2017. Sci. Total. Environ. 2019, 649, 31–49. [Google Scholar] [CrossRef] [PubMed]
- Graciani, J.; Mudiyanselage, K.; Xu, F.; Baber, A.E.; Evans, J.; Senanayake, S.D.; Stacchiola, D.J.; Liu, P.; Hrbek, J.; Sanz, J.F. Highly active copper-ceria and copper-ceria-titania catalysts for methanol synthesis from CO2. Science 2014, 345, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.; Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 2012, 488, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Dresselhaus, M.S.; Thomas, I.L. Alternative energy technologies. Nature 2001, 414, 332–337. [Google Scholar] [CrossRef]
- Steinlechner, C.; Junge, H. Renewable methane generation from carbon dioxide and sunlight. Angew. Chem. Int. Ed. 2018, 57, 44–45. [Google Scholar] [CrossRef]
- Kar, S.; Sen, R.; Kothandaraman, J.; Goeppert, A.; Chowdhury, R.; Munoz, S.B.; Haiges, R.; Prakash, G.K.S. Mechanistic insights into ruthenium-pincer-catalyzed amine-assisted homogeneous hydrogenation of CO2 to methanol. J. Am. Chem. Soc. 2019, 141, 3160–3170. [Google Scholar] [CrossRef]
- Lee, H.; Shinn, Y.J.; Ong, S.H.; Woo, S.W.; Park, K.G.; Lee, T.J.; Moon, S.W. Fault reactivation potential of an offshore CO2 storage site, Pohang Basin, South Korea. J. Pet. Sci. Eng. 2017, 152, 427–442. [Google Scholar] [CrossRef]
- Kamali, A.R. Nanocatalytic conversion of CO2 into nanodiamonds. Carbon 2017, 123, 205–215. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, T.; Wang, H.; Wang, X.; Dai, X.; Shi, F. Active catalyst construction for CO2 recycling via catalytic synthesis of N-doped carbon on supported Cu. Nat. Commun. 2019, 10, 2599. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, Z.; Cao, S.W.; Cao, S.W.; Xue, C. Au/Pt nanoparticle-decorated TiO2 nanofibers with plasmon-enhanced photocatalytic activities for Solar-to-Fuel conversion. J. Phys. Chem. C 2013, 117, 25939–25947. [Google Scholar] [CrossRef]
- Esrafilzadeh, D.; Zavabeti, A.; Jalili, R.; Atkin, P.; Choi, J.; Carey, B.J.; Brkljača, R.; O’Mullane, A.P.; Dickey, M.D.; Officer, D.L.; et al. Room temperature CO2 reduction to solid carbon species on liquid metals featuring atomically thin ceria interfaces. Nature 2019, 10, 865. [Google Scholar]
- Kang, U.; Choi, S.K.; Ham, D.J.; Ji, S.M.; Choi, W.; Han, D.S.; Abdel-Wahab, A.; Park, H. Photosynthesis of formate from CO2 and water at 1% energy efficiency via copper iron oxide catalysis. Energy Environ. Sci. 2015, 8, 2638–2643. [Google Scholar] [CrossRef]
- Chen, G.; Silv, T.; Georgieva, V.; Godfroid, T.; Britun, N.R.; Delplancke-ogletree, P.M. Simultaneous dissociation of CO2 and H2O to syngas in a surface-wave microwave discharge. Int. J. Hydrog. Energy 2015, 40, 3789–3796. [Google Scholar] [CrossRef]
- Hyoung, S.K.; Han, S.U.; Yong, C.H.; Eun, H.C. Disintegration of carbon dioxide molecules in a microwave plasma torch. Sci. Rep. 2015, 5, 18436. [Google Scholar]
- Wang, W.; Snoeckx, R.; Zhang, X.; Cha, M.S.; Bogaerts, A. Modeling plasma-based CO2 and CH4 conversion in mixtures with N2, O2 and H2O: the bigger plasma chemistry. J. Phys. Chem. C 2018, 122, 8704–8723. [Google Scholar] [CrossRef] [Green Version]
- Bernhardsen, I.M.; Knuutila, H.K. A review of potential amine solvents for CO2 absorption process: Absorption capacity, cyclic capacity and pKa. Int. J. Greenh. Gas Control 2017, 61, 27–48. [Google Scholar] [CrossRef]
- Takashi, K.; Ryo, Y.; Toshihiko, M.; Kingo, A.; Setsuo, N. Preparation of TiN films by reactive high-power pulsed sputtering Penning discharges. Jpn. J. Appl. Phys. 2018, 57, 06JE02. [Google Scholar]
- Cao, X.; Xu, W.; He, W. A method for evaluating the impact wear behavior of multilayer TiN/Ti coating. Coatings 2020, 10, 132. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, D.N.; Mahesh, V.; Nguyen, T.H. Corrosion behavior of TiN, TiAlN, TiAlSiN-coated 316L stainless steel in simulated proton exchange membrane fuel cell environment. J. Power Sources 2014, 268, 240–245. [Google Scholar]
- Krell, A.K.; Czyżniewski, A.; Gilewicz, A.; Gajowiec, G. Experimental study of the influence of deposition of multilayer CrN/CrCN PVD coating on austenitic steel on resistance to cavitation erosion. Coatings 2020, 10, 487. [Google Scholar] [CrossRef]
- Lian, G.; Zhao, C.; Zhang, Y.; Huang, X.; Chen, C.; Jiang, J. Investigation of micro-hardness, wear resistance and defects of 316L stainless steel and TiC composite coating fabricated by laser engineered net Shaping. Coatings 2019, 9, 498. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.A.; Yang, S.W.; Liu, Y.W.; Lai, P.L. Effect of Cu and Ni undercoatings on the electrochemical corrosion behaviour of Cr–C-coated steel samples in 0.1 M H2SO4 solution with 1 g/L NaCl. Coatings 2019, 9, 531. [Google Scholar] [CrossRef] [Green Version]
- Aijaz, A.; Ferreira, F.; Oliveira, J.; Kubart, T. Mechanical properties of hydrogen free diamond-like carbon thin films deposited by high power impulse magnetron sputtering with Ne. Coatings 2018, 8, 385. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Gao, K.; Zhang, B.; Gong, Z.; Wei, X.; Zhang, J. Verification study of nanostructure evolution with heating treatment between thin and thick fullerene-like hydrogen carbon films. Coatings 2019, 9, 82. [Google Scholar] [CrossRef] [Green Version]
- Qiang, L.; Gao, K.; Zhang, L.; Wang, J.; Zhang, B.; Zhang, J. Further improving the mechanical and tribological properties of low content Ti-doped DLC film by W incorporating. Appl. Surf. Sci. 2015, 353, 522–529. [Google Scholar] [CrossRef]
- Gao, K.; Wang, Y.; Wei, X.; Qiang, L.; Zhang, B.; Zhang, J. Hydrogenated amorphous carbon films with different nanostructure: a comparative study. Chem. Phys. Lett. 2019, 715, 330–334. [Google Scholar] [CrossRef]
- Zhao, Q.; Mou, Z.; Zhang, B.; Zhang, X.; Wang, Z.; Wang, K.; Gao, K.; Jia, Q. Revealing the corrosion resistance of amorphous carbon films under heat shock via annealing. Diam. Relat. Mater. 2020, 102, 107692. [Google Scholar] [CrossRef]
- Wang, Z.; Gong, Z.; Zhang, B.; Wang, Y.; Gao, K.; Zhang, J.; Liu, G. Heating induced nanostructure and superlubricity evolution of fullerene-like hydrogenated carbon films. Solid State Sci. 2019, 90, 29–33. [Google Scholar] [CrossRef]
- Qiang, L.; Zhang, B.; Zhou, Y.; Zhang, J. Improving the internal stress and wear resistance of DLC film by low content Ti doping. Solid State Sci. 2013, 20, 17–22. [Google Scholar] [CrossRef]
- Fernandes, A.C.; Carvalho, P.; Vaz, F.; Lanceros-Méndez, S.; Machado, A.V.; Parreir, N.M.G.; Pierson, J.F.; Martin, N. Property change in multifunctional TiCxOy thin films: Effect of the O/Ti ratio. Thin Solid Films 2006, 515, 866–871. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, A.C.; Vaz, F.; Rebouta, L.; Pinto, A.; Alves, E.; Parreir, N.M.G.; Goudeau, Ph.; Bourhis, E.L.; Rivière, J.P. Influence of the O/C ratio in the behaviour of TiCxOy thin films. Surf. Coat. Technol. 2007, 201, 5587–5591. [Google Scholar] [CrossRef]
- Carvalho, P.; Vaz, F.; Rebouta, L.; Cunha, L.; Tavares, C.J.; Moura, C.; Alves, E.; Cavaleiro, A.; Goudeau, P.; Bourhis, E.L.; et al. Structural, electrical, optical and mechanical characterization of decorative ZrOxNy thin films. J. Appl. Phys. 2005, 98, 023715. [Google Scholar] [CrossRef] [Green Version]
- Mathew, M.T.; Ariza, E.; Rocha, L.A.; Fernandes, A.C.; Vaz, F. TiCxOy thin films for decorative applications: Tribocorrosion mechanisms and synergism. Tribol. Int. 2008, 41, 603–615. [Google Scholar] [CrossRef] [Green Version]
- Mathew, M.T.; Ariza, E.; Rocha, L.A.; Fernandes, A.C.; Vaz, F. Tribocorrosion behaviour of TiCxOy thin films in bio-fluids. Electrochim. Acta 2010, 56, 929–937. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Cosio, G.; Calixto-Rodriguez, M.; Martinez, H. Low-pressure plasma discharge of Ar/N2/CO2 ternary mixture. Topic number B6200954. In Proceedings of the 29th International Conference on Phenomena in Ionized Gases (ICPIG 2009), Cancun, Mexico, 12–17 July 2009. [Google Scholar]
- Leyland, A.; Matthews, A. On the significance of the H/E ratio in wear control: A nanocomposite coating approach to optimised tribological behaviour. Wear 2000, 246, 1–11. [Google Scholar] [CrossRef]
- Galvan, D.; Pei, Y.T.; Hosson, J.T.M.D. Deformation and failure mechanism of nano-composite coatings under nano-indentation. Surf. Coat. Technol. 2006, 200, 6718–6726. [Google Scholar] [CrossRef] [Green Version]
- Devia, D.M.; Restrepo, J.; Ruden, A.; González, J.M.; Sequeda, F.; Arango, P.J. The tribological characteristics of TiN, TiC, TiC/TiN films prepared by reactive pulsed arc evaporation technique. In Proceedings of the 52nd Annual Technical Conference, Santa Clara, CA, USA, 9–14 May 2009. [Google Scholar]
- Chen, Y.; Mao, J. Sol–gel preparation and characterization of black titanium oxides Ti2O3 and Ti3O5. J. Mater. Sci.: Mater. Electron 2014, 25, 1284–1288. [Google Scholar] [CrossRef]
- Wu, X.W.; Wu, D.J.; Liu, X.J. Silver-doping induced lattice distortion in TiO2 nanoparticles. Chin. Phys. Lett. 2009, 7, 077809. [Google Scholar]
- Afifi, M.A.; Abdel-Aziz, M.M.; Yahia, I.S.; Fadel, M.; Wahab, L.A. Transport properties of polycrystalline TiO2 and Ti2O3 as semiconducting oxides. J. Alloy. Compd. 2008, 455, 92–97. [Google Scholar] [CrossRef]
- Ma, T.B.; Hu, Y.Z.; Wang, H. Role of atomic transverse migration in growth of diamond-like carbon films. Chin. Phys. 2007, 16, 2798–2802. [Google Scholar]
- Haase, F.; Kersten, H.; Lundin, D. Plasma characterization in reactive sputtering processes of Ti in Ar/O2 mixtures operated in metal, transition and poisoned modes: a comparison between direct current and high-power impulse magnetron discharges. Eur. Phys. J. D 2017, 71, 245. [Google Scholar] [CrossRef]
- Akazawa, H. Sputtering characteristics, crystal structures and transparent conductive properties of TiOxNy films deposited on α-Al2O3(0 0 0 1) and glass substrates. Appl. Surf. Sci. 2012, 263, 307–313. [Google Scholar] [CrossRef]
- Zhang, L.; Koka, R.V. A study on the oxidation and carbon diffusion of TiC in alumina–titanium carbide ceramics using XPS and Raman spectroscopy. Mater. Chem. Phys. 1998, 57, 23–32. [Google Scholar] [CrossRef]
- Lewin, E.; Persson, P.O.Å.; Lattemann, M.; Stüber, M.; Gorgoi, M.; Sandell, A.; Ziebert, C.; Schäfers, F.; Braun, W.; Halbritter, J.; et al. On the origin of a third spectral component of C1s XPS-spectra for nc-TiC/a-C nanocomposite thin films. Surf. Coat. Technol. 2008, 202, 3563–3570. [Google Scholar] [CrossRef]
- Kumar, P.M.; Badrinarayanan, S.; Sastry, M. Nanocrystalline TiO2 studied by optical, FTIR and X-ray photoelectron spectroscopy: correlation to presence of surface states. Thin Solid Films 2000, 358, 122–130. [Google Scholar] [CrossRef]
- Zalar, A.; Kovac, J.; Pracek, B.; Panjan, P.; Ceh, M. Ion sputtering rates of W-, Ti- and Cr-carbides studied at different Ar~+ ion incidence angles. Appl. Surf. Sci. 2008, 254, 6611–6618. [Google Scholar] [CrossRef]
- Aleksandrov, N.L.; Kindysheva, S.V.; Kirpichnikov, A.A.; Kosarev, I.N.; Starikovskii, A.Y. Plasma decay in N2, CO2 and H2O excited by high-voltage nanosecond discharge. J. Phys. D: Appl. Phys. 2007, 40, 4493. [Google Scholar] [CrossRef]
- Nikolett, O.; Zsolt, F.; Attila, S.; János, S.; Tamás, C.; Katalin, B. Ceramic TiC/a:C protective nanocomposite coatings: Structure and composition versus mechanical properties and tribology. Ceram. Int. 2016, 42, 12215–12220. [Google Scholar]
- Wu, H.; Jia, F.; Zhao, J.; Huang, S.; Wang, L.; Jiao, S.; Huang, H.; Jiang, Z. Effect of water-based nanolubricant containing nano-TiO2 on friction and wear behaviour of chrome steel at ambient and elevated temperatures. Wear 2019, 426–427, 792–804. [Google Scholar] [CrossRef]


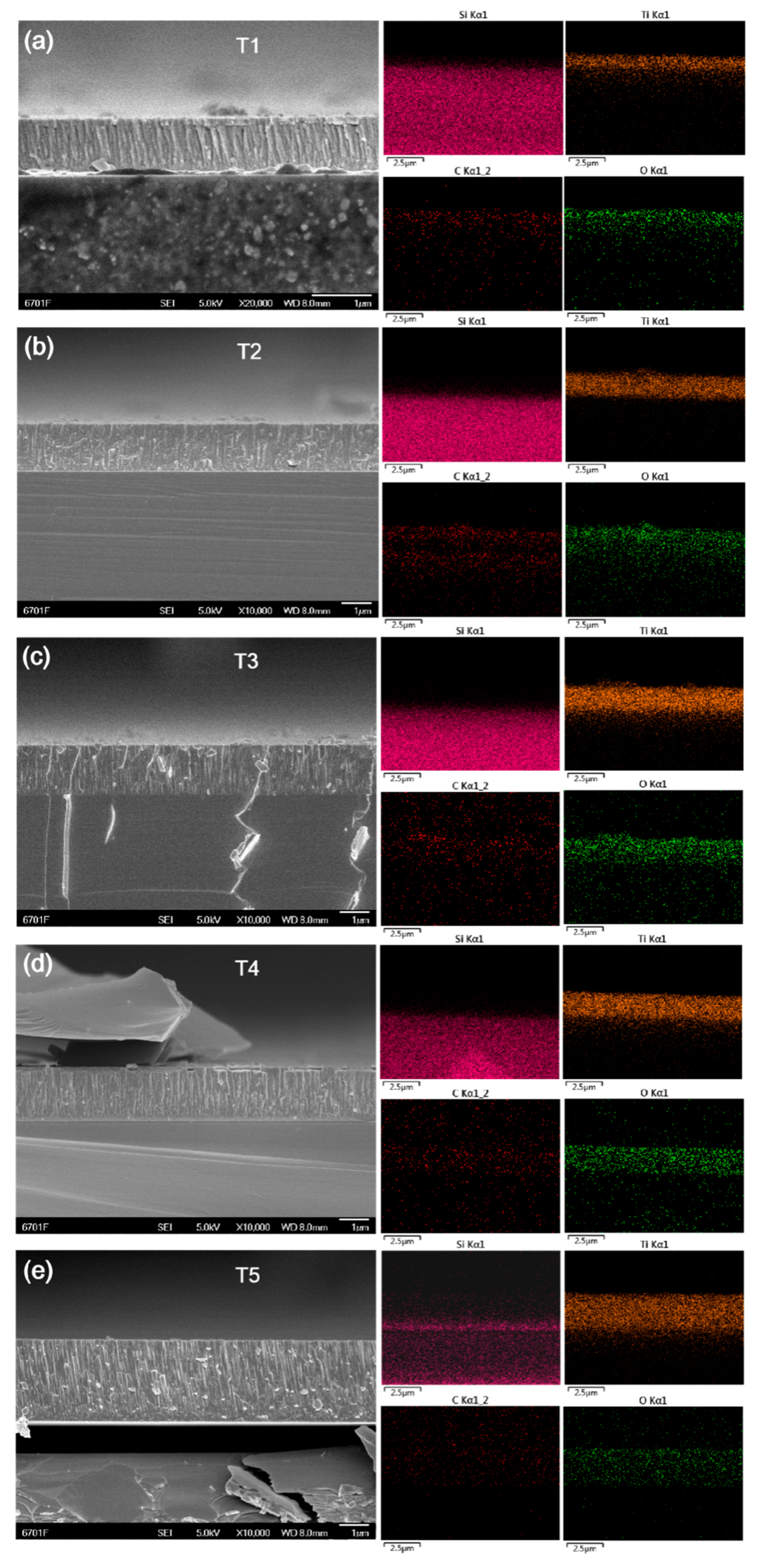
| Films | Ar (sccm) | CO2 (sccm) | Negative bias (V) | Target Current (A) | Deposited Times (h) |
|---|---|---|---|---|---|
| T1 | 50 | 10 | 150 | 0.65 | 1 |
| T2 | 50 | 10 | 150 | 0.80 | 1 |
| T3 | 50 | 10 | 150 | 0.95 | 1 |
| T4 | 50 | 10 | 150 | 1.1 | 1 |
| T5 | 50 | 10 | 150 | 1.25 | 1 |
| Films | Composition | Thickness (μm) | H3/E2 | Friction Coefficient | Current Density (μA/cm2) | Ti (at.%) |
|---|---|---|---|---|---|---|
| T1 | TiC0.41O1.76 | 0.95 | 0.08 | 0.091 | 1.830 | 32 |
| T2 | TiC0.27O1.69 | 1.58 | 0.14 | 0.085 | 0.033 | 34 |
| T3 | TiC0.19O1.87 | 1.66 | 0.28 | 0.065 | 0.024 | 33 |
| T4 | TiC0.29O1.23 | 1.97 | 0.25 | 0.071 | 0.340 | 40 |
| T5 | TiC0.25O1.40 | 2,75 | 0.16 | 0.080 | 257.4 | 38 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, K.; Wang, Z.; Jia, Q.; Zhang, B.; Mou, Z.; Zhang, J. The Utilization of Carbon Dioxide to Prepare TiCxOy Films with Low Friction and High Anti-Corrosion Properties. Coatings 2020, 10, 533. https://doi.org/10.3390/coatings10060533
Gao K, Wang Z, Jia Q, Zhang B, Mou Z, Zhang J. The Utilization of Carbon Dioxide to Prepare TiCxOy Films with Low Friction and High Anti-Corrosion Properties. Coatings. 2020; 10(6):533. https://doi.org/10.3390/coatings10060533
Chicago/Turabian StyleGao, Kaixiong, Zhaolong Wang, Qian Jia, Bin Zhang, Zhixing Mou, and Junyan Zhang. 2020. "The Utilization of Carbon Dioxide to Prepare TiCxOy Films with Low Friction and High Anti-Corrosion Properties" Coatings 10, no. 6: 533. https://doi.org/10.3390/coatings10060533





