Geomicrobial Investigations of Colored Outer Coatings from an Ethiopian Rock Art Gallery
Abstract
:1. Introduction
2. Materials and Methods
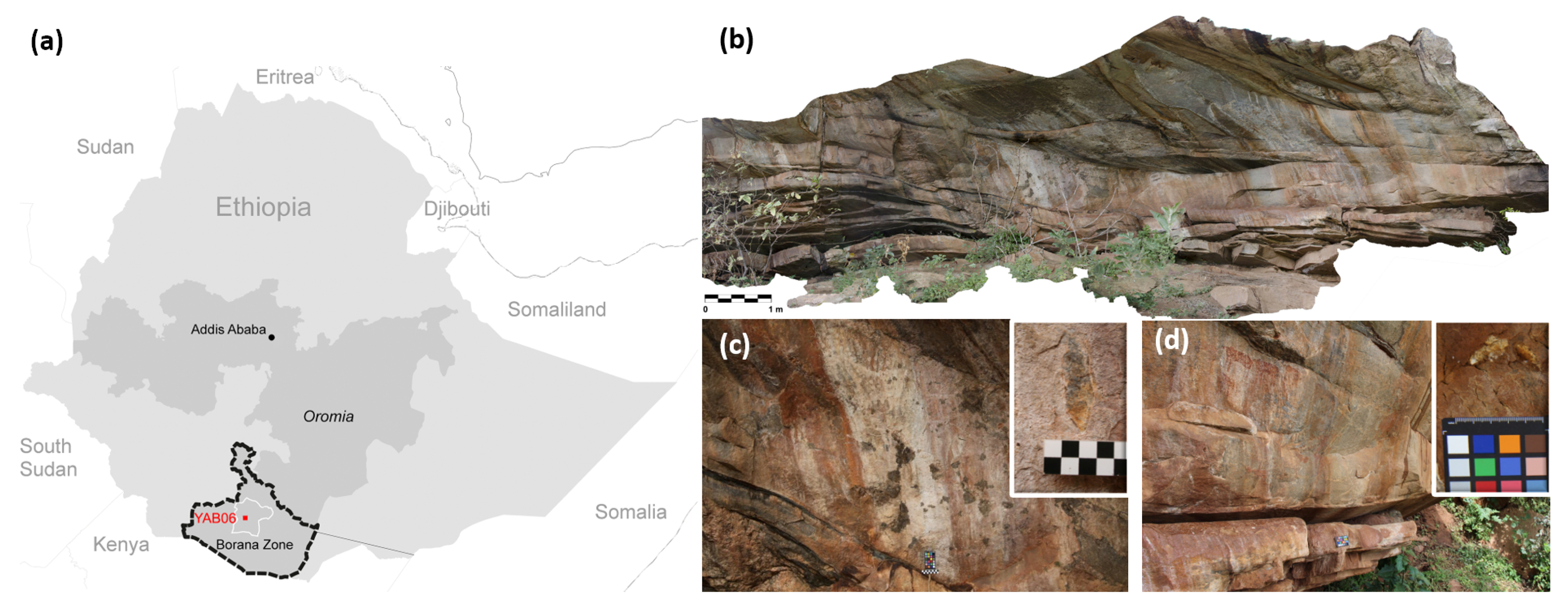
2.1. Site Description and Sampling
2.2. Microscopic Characterization of the Rock Substrate and Mineral Coating
2.3. Visualization of Biofilm Structure
2.4. DNA Extraction and Sequencing
3. Results
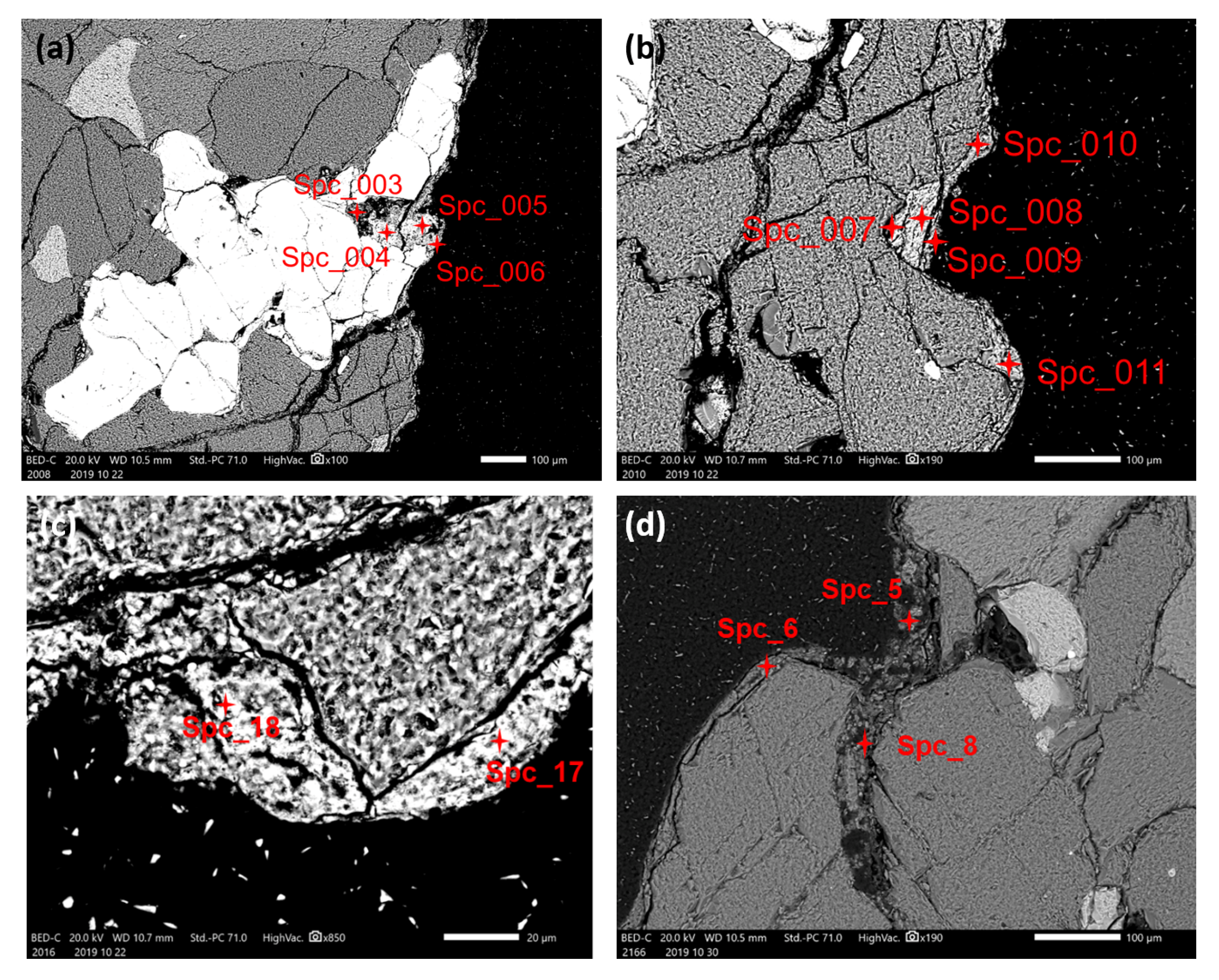
3.1. Optical Microscopy and SEM-EDS Analysis
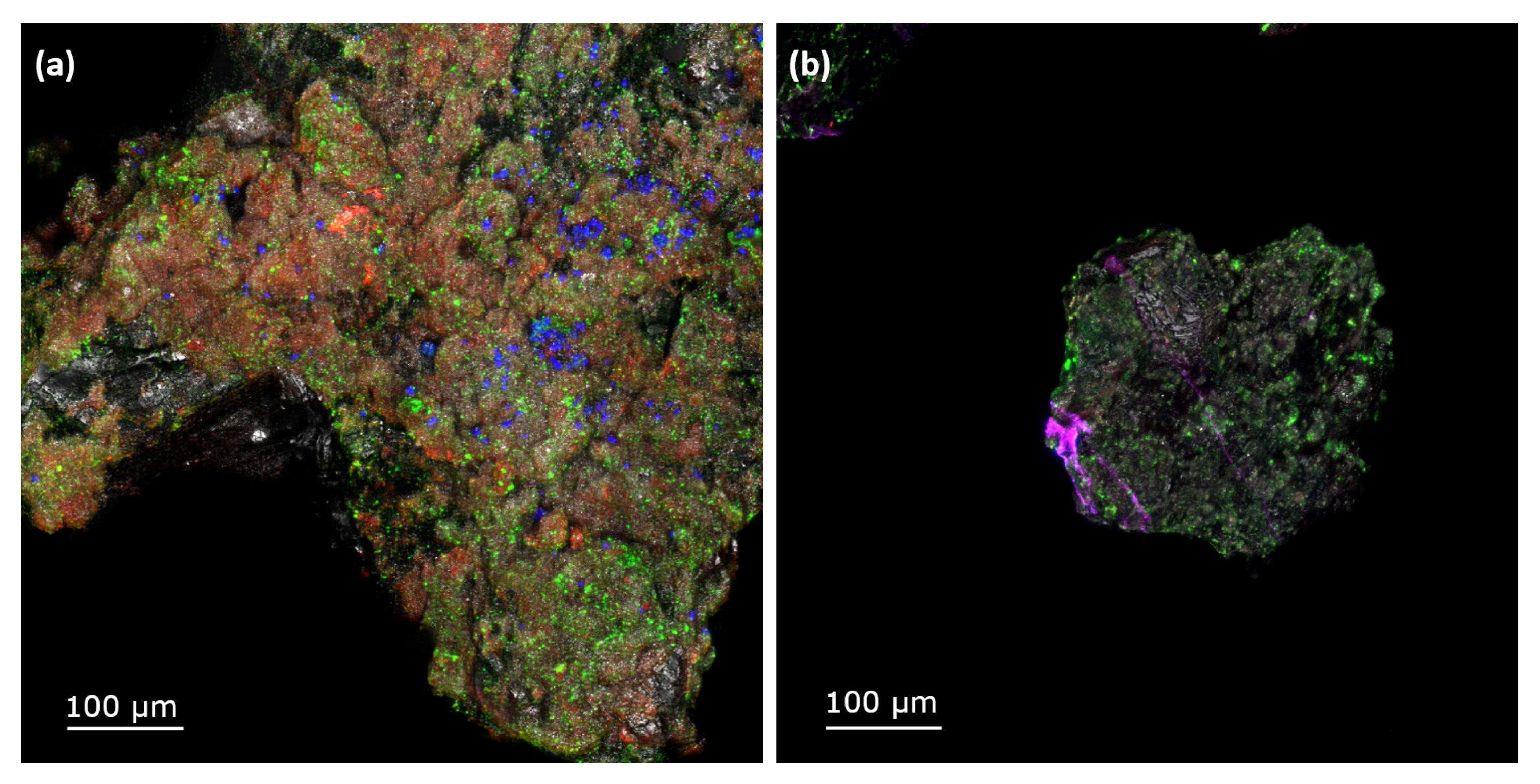
3.2. Biofilm Characterization
3.3. Bacterial Communities
4. Discussion
5. Conclusions
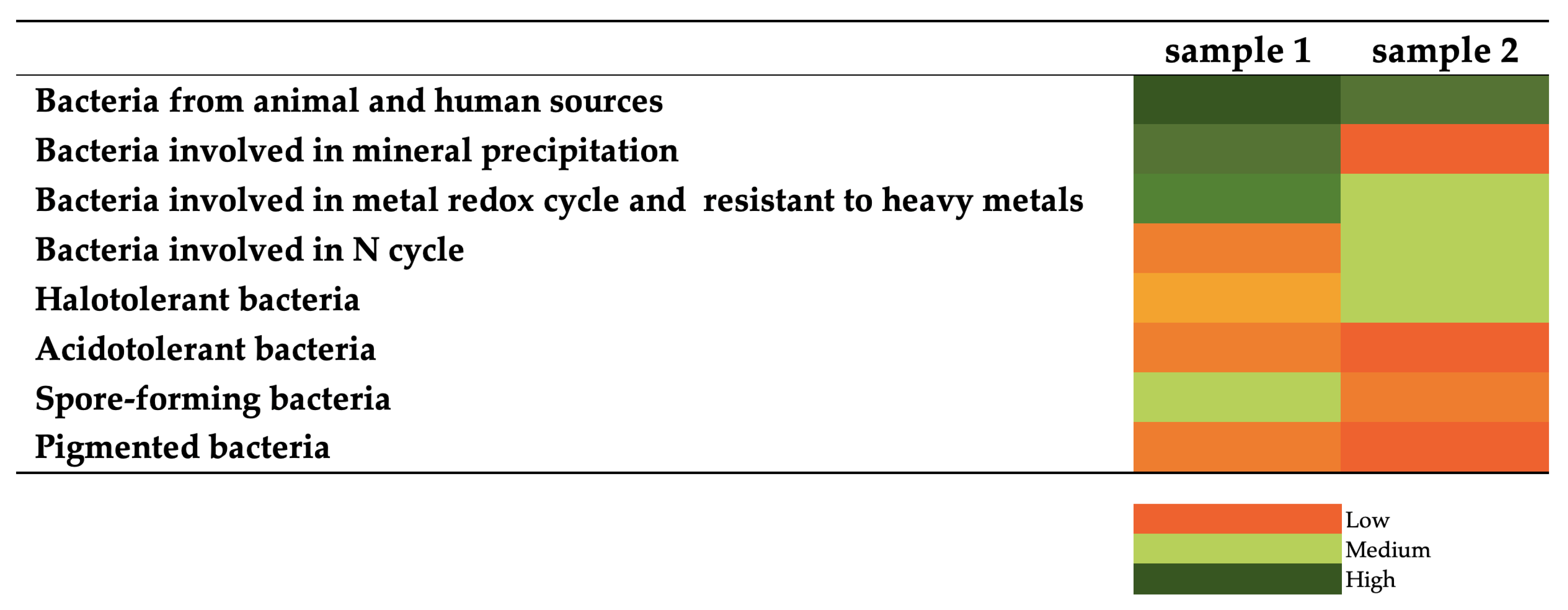
- The two coatings show distinct features, where the coating in sample 1 contains higher amounts of Ca and P than that of sample 2, which is likely related to the presence of organic matter.
- Heterotrophism is the most common energy-acquiring mechanism shared between the two coating communities. The widespread distribution of heterotrophic bacteria is likely to be derived from animal and human sources, which also provide important sources of organic materials generated by the herding activities around the rock art shelter.
- The core bacterial community of sample 1 is substantially different from sample 2, indicating that the microbiota is unique to the coating minerals. In fact, sample 1—the coating with the highest Fe, Ca and P content—hosts bacterial genera that are potentially involved in biomineralization processes, metal redox cycles and metal resistance. In contrast, sample 2 shows mainly pathogenic and commensal bacteria that are characteristic of the animal and human microbiota, and other microorganisms that are involved in nitrogen and metal biogeochemical cycles.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Whitley, D.S. Introduction to Rock Art Research, 2nd ed.; Informa UK Limited: Colchester, UK, 2011. [Google Scholar]
- Darvill, T. Open-Air Rock-Art Conservation and Management; Routledge: Abingdon-on-Thames, UK, 2014. [Google Scholar]
- Dorn, R. 4.5 Rock Coatings. In Treatise on Geomorphology; Elsevier BV: Amsterdam, The Netherlands, 2013; Volume 4, pp. 70–97. [Google Scholar]
- Hernanz, A.; Ruiz, J.F.; Madariaga, J.M.; Gavrilenko, E.; Maguregui, M.; De Vallejuelo, S.F.-O.; Martinez-Arkarazo, I.; Alloza-Izquierdo, R.; Baldellou-Martínez, V.; Viñas-Vallverdú, R.; et al. Spectroscopic characterisation of crusts interstratified with prehistoric paintings preserved in open-air rock art shelters. J. Raman Spectrosc. 2014, 45, 1236–1243. [Google Scholar] [CrossRef]
- Roberts, A.; Campbell, I.; Pring, A.; Bell, G.; Watchman, A.; Popelka-Filcoff, R.; Lenehan, C.; Gibson, C.; Franklin, N. A multidisciplinary investigation of a rock coating at Ngaut Ngaut (Devon Downs), South Australia. Aust. Archaeol. 2015, 80, 32–39. [Google Scholar] [CrossRef]
- Rousaki, A.; Vargas, E.; Vázquez, C.; Aldazábal, V.; Bellelli, C.; Calatayud, M.C.; Hajduk, A.; Palacios, Ò.; Moens, L.; Vandenabeele, P. On-field Raman spectroscopy of Patagonian prehistoric rock art: Pigments, alteration products and substrata. TrAC Trends Anal. Chem. 2018, 105, 338–351. [Google Scholar] [CrossRef] [Green Version]
- Mauran, G.; Lebon, M.; Détroit, F.; Caron, B.; Nankela, A.; Pleurdeau, D.; Bahain, J.-J. First in situ pXRF analyses of rock paintings in Erongo, Namibia: Results, current limits, and prospects. Archaeol. Anthr. Sci. 2019, 11, 4123–4145. [Google Scholar] [CrossRef]
- Šebela, S.; Miler, M.; Skobe, S.; Torkar, S.; Zupančič, N. Characterization of black deposits in karst caves, examples from Slovenia. Facies 2015, 61, 6. [Google Scholar] [CrossRef]
- Green, H.; Gleadow, A.; Finch, D.; Hergt, J.M.; Ouzman, S. Mineral deposition systems at rock art sites, Kimberley, Northern Australia—Field observations. J. Archaeol. Sci. Rep. 2017, 14, 340–352. [Google Scholar] [CrossRef]
- Gadd, G.M. Metals, minerals and microbes: Geomicrobiology and bioremediation. Microbiology 2009, 156, 609–643. [Google Scholar] [CrossRef]
- Villa, F.; Cappitelli, F. The Ecology of Subaerial Biofilms in Dry and Inhospitable Terrestrial Environments. Microorganisms 2019, 7, 380. [Google Scholar] [CrossRef] [Green Version]
- Kuhlman, K.R.; Fusco, W.G.; La Duc, M.T.; Allenbach, L.B.; Ball, C.L.; Kuhlman, G.M.; Anderson, R.C.; Erickson, I.K.; Stuecker, T.; Benardini, J.; et al. Diversity of Microorganisms within Rock Varnish in the Whipple Mountains, California. Appl. Environ. Microbiol. 2006, 72, 1708–1715. [Google Scholar] [CrossRef] [Green Version]
- Esposito, A.; Ahmed, E.; Ciccazzo, S.; Sikorski, J.; Overmann, J.; Holmström, S.J.M.; Brusetti, L. Comparison of Rock Varnish Bacterial Communities with Surrounding Non-Varnished Rock Surfaces: Taxon-Specific Analysis and Morphological Description. Microb. Ecol. 2015, 70, 741–750. [Google Scholar] [CrossRef]
- Mistri, A.; Mukherjee, A.; Watkin, E.L.J. Microbial Diversity and Mineralogical-Mechanical Properties of Calcitic Cave Speleothems in Natural and in Vitro Biomineralization Conditions. Front. Microbiol. 2018, 9, 40. [Google Scholar] [CrossRef]
- Bostick, B.C. Massive ore deposits from microscopic organisms. Geology 2019, 47, 191–192. [Google Scholar] [CrossRef] [Green Version]
- Meier, J.; Piva, A.; Fortin, D. Enrichment of sulfate-reducing bacteria and resulting mineral formation in media mimicking pore water metal ion concentrations and pH conditions of acidic pit lakes. FEMS Microbiol. Ecol. 2011, 79, 69–84. [Google Scholar] [CrossRef]
- Dorn, R.I. Desert Rock Coatings. In Geomorphology of Desert Environments; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2009; pp. 153–186. [Google Scholar]
- Dorn, R.I. Rock Coatings; Elsevier Science: Amsterdam, The Netherlands, 1998. [Google Scholar]
- Zerboni, A. Holocene rock varnish on the Messak plateau (Libyan Sahara): Chronology of weathering processes. Geomorphology 2008, 102, 640–651. [Google Scholar] [CrossRef]
- Gallinaro, M.; Zerboni, A.; Solomon, T.; Spinapolice, E.E. Rock Art between Preservation, Research and Sustainable Development—A Perspective from Southern Ethiopia. Afr. Archaeol. Rev. 2018, 35, 211–223. [Google Scholar] [CrossRef]
- Spinapolice, E.E.; Gallinaro, M.; Zerboni, A. New investigations in Southern Ethiopia (Yabelo and Gotera): Pleistocene and Holocene archaeological evidences. Sci. Dell’Antichità 2017, 23, 37–47. [Google Scholar]
- Hundie, G. The Emergence of Prehistoric Pastoralism in Southern ETHIOPIA. Ph.D. Thesis, University of Florida, Gainesville, FL, USA, 2001. [Google Scholar]
- Cremaschi, M.; Trombino, L.; Zerboni, A. Palaeosoils and Relict Soils; Elsevier BV: Amsterdam, The Netherlands, 2018; pp. 863–894. [Google Scholar]
- Rapin, A.; Pattaroni, C.; Marsland, B.J.; Harris, N.L. Microbiota analysis using an illumina MiSeq platform tosequence 16S rRNA genes. Curr. Protoc. Mouse Biol. 2017, 7, 100–129. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [Green Version]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013; Available online: http://www.r-project.org/ (accessed on 30 November 2019).
- Jacobs, J. Individual Based Rarefaction in R [online]. 2012. Available online: http://www.jennajacobs.org/R/rarefaction.html (accessed on 30 November 2019).
- Oksanen, J.; Kindt, R.; Legendre, P.; O’Hara, B.; Stevens, M.H.H.; Oksanen, M.J. The vegan package. Commun. Ecol. Package 2007, 10, 631–637. [Google Scholar]
- David, B.; McNiven, J. The Oxford Handbook of the Archaeology and Anthropology of Rock Art; Oxford University Press: Oxford, UK, 2018. [Google Scholar]
- Bonneau, A.; Staff, R.A.; Higham, T.; Brock, F.; Pearce, D.G.; Mitchell, P.J. Successfully Dating Rock Art in Southern Africa Using Improved Sampling Methods and New Characterization and Pretreatment Protocols. Radiocarbon 2016, 59, 659–677. [Google Scholar] [CrossRef] [Green Version]
- Zerboni, A.; Degli Esposti, M.; Wu, Y.-L.; Brandolini, F.; Mariani, G.S.; Villa, F.; Lotti, P.; Cappitelli, F.; Sasso, M.; Rizzi, A.; et al. Age, palaeoenvironment, and preservation of prehistoric petroglyphs on a boulder in the oasis of Salut (Northern Sultanate of Oman). Quat. Int. 2019. [Google Scholar] [CrossRef]
- Chalmin, E.; Castets, G.; Delannoy, J.-J.; David, B.; Barker, B.; Lamb, L.; Soufi, F.; Pairis, S.; Cersoy, S.; Martinetto, P.; et al. Geochemical analysis of the painted panels at the “Genyornis” rock art site, Arnhem Land, Australia. Quat. Int. 2017, 430, 60–80. [Google Scholar] [CrossRef]
- Krinsley, D.H.; Dorn, R.I.; Digregorio, B.E.; Langworthy, K.A.; Ditto, J. Rock varnish in New York: An accelerated snapshot of accretionary processes. Geomorphology 2012, 138, 339–351. [Google Scholar] [CrossRef]
- Krinsley, D.H.; Digregorio, B.; Dorn, R.I.; Razink, J.; Fisher, R. Mn-Fe-Enhancing Budding Bacteria in Century-Old Rock Varnish, Erie Barge Canal, New York. J. Geol. 2017, 125, 317–336. [Google Scholar] [CrossRef] [Green Version]
- Lang-Yona, N.; Maier, S.; Macholdt, D.S.; Müller-Germann, I.; Yordanova, P.; Rodríguez-Caballero, E.; Jochum, K.P.; Al-Amri, A.; Andreae, M.O.; Fröhlich-Nowoisky, J.; et al. Insights into microbial involvement in desert varnish formation retrieved from metagenomic analysis. Environ. Microbiol. Rep. 2018, 10, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Gorbushina, A. Live on the rocks. Environ. Microbiol. 2007, 9, 1613–1631. [Google Scholar] [CrossRef]
- Konhauser, K.; Fyfe, W.S.; Schultze-Lam, S.; Ferris, F.G.; Beveridge, T.J. Iron phosphate precipitation by epilithic microbial biofilms in Arctic Canada. Can. J. Earth Sci. 1994, 31, 1320–1324. [Google Scholar] [CrossRef]
- Larson, P.H.; Dorn, R.I. Painting Yosemite Valley: A Case Study of Rock Coatings Encountered at Half Dome. Phys. Geogr. 2012, 33, 165–182. [Google Scholar] [CrossRef] [Green Version]
- Marnocha, C.L.; Dixon, J.C. Endolithic bacterial communities in rock coatings from Kärkevagge, Swedish Lapland. FEMS Microbiol. Ecol. 2014, 90, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Schabereiter-Gurtner, C.; Saiz-Jimenez, C.; Piñar, G.; Lubitz, W.; Rã¶lleke, S.; Rölleke, S. Phylogenetic diversity of bacteria associated with Paleolithic paintings and surrounding rock walls in two Spanish caves (LlonÃn and La Garma). FEMS Microbiol. Ecol. 2004, 47, 235–247. [Google Scholar] [CrossRef]
- Irit, N.; Hana, B.; Yifat, B.; Esti, K.-W.; Ariel, K. Insights into bacterial communities associated with petroglyph sites from the Negev Desert, Israel. J. Arid. Environ. 2019, 166, 79–82. [Google Scholar] [CrossRef]
- Nir, I.; Barak, H.; Kramarsky-Winter, E.; Kushmaro, A. Seasonal diversity of the bacterial communities associated with petroglyphs sites from the Negev Desert, Israel. Ann. Microbiol. 2019, 69, 1079–1086. [Google Scholar] [CrossRef]
- Roldán, C.; Murcia-Mascaros, S.; López-Montalvo, E.; Vilanova, C.; Porcar, M. Proteomic and metagenomic insights into prehistoric Spanish Levantine Rock Art. Sci. Rep. 2018, 8, 10011. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, I.; Laiz, L.; Hermosin, B.; Caballero, B.; Incerti, C.; Saiz-Jimenez, C. Bacteria isolated from rock art paintings: The case of Atlanterra shelter (South Spain). J. Microbiol. Methods 1999, 36, 123–127. [Google Scholar] [CrossRef]
- Laiz, L.; Hermosin, B.; Caballero, B.; Saiz-Jimenez, C. Bacteria isolated from the rocks supporting prehistoricpaintings in two shelters from Sierra de Cazorla, Jaen, Spain. Aerobiologia 2000, 16, 119–124. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Li, F.; Lv, J. Morphology and formation mechanism in precipitation of calcite induced by Curvibacter lanceolatus strain HJ-1. J. Cryst. Growth 2017, 478, 96–101. [Google Scholar] [CrossRef]
- Kakoti, N.; Buragohain, M.; Sarmah, P.; Pegu, B.K. Arsenic Resistance Bacteria in Groundwater: A Review. Int. Res. J. Biological. Sci. 2020, 9, 47–49. [Google Scholar]
- Di Rienzi, S.; Sharon, I.; Wrighton, K.C.; Koren, O.; Hug, L.A.; Thomas, B.C.; Goodrich, J.K.; Bell, J.T.; Spector, T.D.; Banfield, J.F.; et al. The human gut and groundwater harbor non-photosynthetic bacteria belonging to a new candidate phylum sibling to Cyanobacteria. eLife 2013, 2, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.V.; Rentz, J.A.; Plaia, T.; Neubauer, S.C.; Merrill-Floyd, M.; Lilburn, T.; Bradburne, C.; Megonigal, J.P.; Emerson, D. Characterization of Neutrophilic Fe(II)-Oxidizing Bacteria Isolated from the Rhizosphere of Wetland Plants and Description of Ferritrophicum radicicola gen. nov. sp. nov., and Sideroxydans paludicola sp. nov. Geomicrobiol. J. 2007, 24, 559–570. [Google Scholar] [CrossRef]
- Mendoza, M.L.Z.; Lundberg, J.; Ivarsson, M.; Campos, P.; Nylander, J.A.A.; Sallstedt, T.; Dalen, L. Metagenomic Analysis from the Interior of a Speleothem in Tjuv-Ante’s Cave, Northern Sweden. PLoS ONE 2016, 11, e0151577. [Google Scholar] [CrossRef]
- Perry, T.D.; Klepac-Ceraj, V.; Zhang, X.V.; McNamara, C.J.; Polz, M.F.; Martin, S.T.; Berke, N.; Mitchell, R. Binding of Harvested Bacterial Exopolymers to the Surface of Calcite. Environ. Sci. Technol. 2005, 39, 8770–8775. [Google Scholar] [CrossRef] [PubMed]
- Konhauser, K.; Riding, R. Bacterial Biomineralization. In Fundamentals of Geobiology; Wiley: Hoboken, NJ, USA, 2012; pp. 105–130. [Google Scholar]
- Urzì, C. Biomineralization Processes on Rock and Monument Surfaces Observed in Field and in Laboratory Conditions. Geomicrobiol. J. 1999, 16, 39–54. [Google Scholar] [CrossRef]
- Jroundi, F.; Schiro, M.; Ruiz-Agudo, E.; Elert, K.; Martín-Sánchez, I.; Gonzalez-Muñoz, M.T.; Rodriguez-Navarro, C. Protection and consolidation of stone heritage by self-inoculation with indigenous carbonatogenic bacterial communities. Nat. Commun. 2017, 8, 279. [Google Scholar] [CrossRef] [Green Version]
- Perito, B.; Marvasi, M.; Barabesi, C.; Mastromei, G.; Bracci, S.; Vendrell, M.; Tiano, P. A Bacillus subtilis cell fraction (BCF) inducing calcium carbonate precipitation: Biotechnological perspectives for monumental stone reinforcement. J. Cult. Herit. 2014, 15, 345–351. [Google Scholar] [CrossRef]
- Soffritti, I.; Accolti, D.; Lanzoni, L.; Volta, A.; Mazzacane, S.; Caselli, E.; D’Accolti, M.; Bisi, M. he Potential Use of Microorganisms as Restorative Agents: An Update. Sustainability 2019, 11, 3853. [Google Scholar] [CrossRef] [Green Version]
- Fishman, M.R.; Giglio, K.; Fay, D.; Filiatrault, M.J. Physiological and genetic characterization of calcium phosphate precipitation by Pseudomonas species. Sci. Rep. 2018, 8, 10156. [Google Scholar] [CrossRef] [Green Version]
- Adetutu, E.; Thorpe, K.; Shahsavari, E.; Bourne, S.; Cao, X.; Fard, R.; Kirby, G.; Ball, A.S. Bacterial community survey of sediments at Naracoorte Caves, Australia. Int. J. Speleol. 2012, 41, 137–147. [Google Scholar] [CrossRef] [Green Version]
- Han, Z.; Yu, W.; Zhao, H.; Zhao, Y.; Tucker, M.E.; Yan, H. The Significant Roles of Mg/Ca Ratio, Cl− and SO42− in Carbonate Mineral Precipitation by the Halophile Staphylococcus epidermis Y2. Minerals 2018, 8, 594. [Google Scholar] [CrossRef] [Green Version]
- Marnocha, C.L.; Dixon, J.C. Bacterially facilitated rock-coating formation as a component of the geochemical budget in cold climates: An example from Kärkevagge, Swedish Lapland. Geomorphology 2014, 218, 45–51. [Google Scholar] [CrossRef]
- Rusznyák, A.; Akob, D.M.; Nietzsche, S.; Eusterhues, K.; Totsche, K.U.; Neu, T.R.; Frosch, T.; Popp, J.; Keiner, R.; Geletneky, J.; et al. Calcite Biomineralization by Bacterial Isolates from the Recently Discovered Pristine Karstic Herrenberg Cave. Appl. Environ. Microbiol. 2011, 78, 1157–1167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maciejewska, M.; Adam, D.; Naômé, A.; Martinet, L.; Tenconi, E.; Całusińska, M.; Delfosse, P.; Hanikenne, M.; Baurain, D.; Compère, P.; et al. Assessment of the Potential Role of Streptomyces in Cave Moonmilk Formation. Front. Microbiol. 2017, 8, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viti, C.; Pace, A.; Giovannetti, L. Characterization of Cr(VI)-Resistant Bacteria Isolated from Chromium-Contaminated Soil by Tannery Activity. Curr. Microbiol. 2003, 46, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Barns, S.M.; Cain, E.C.; Sommerville, L.; Kuske, C.R. Acidobacteria Phylum Sequences in Uranium-Contaminated Subsurface Sediments Greatly Expand the Known Diversity within the Phylum. Appl. Environ. Microbiol. 2007, 73, 3113–3116. [Google Scholar] [CrossRef] [Green Version]
- Marzan, L.W.; Hossain, M.; Mina, S.A.; Akter, Y.; Chowdhury, A.M.A. Isolation and biochemical characterization of heavy-metal resistant bacteria from tannery effluent in Chittagong city, Bangladesh: Bioremediation viewpoint. Egypt. J. Aquat. Res. 2017, 43, 65–74. [Google Scholar] [CrossRef]
- Crespo-Piazuelo, D.; Estellé, J.; Revilla, M.; Mesas, L.C.; Ramayo-Caldas, Y.; Óvilo, C.; Fernández, A.I.; Ballester, M.; Folch, J.M. Characterization of bacterial microbiota compositions along the intestinal tract in pigs and their interactions and functions. Sci. Rep. 2018, 8, 12727. [Google Scholar] [CrossRef] [PubMed]
- Takamatsu, D.; Ide, H.; Osaki, M.; Sekizaki, T. Identification of Facklamia sourekii from a Lactating Cow. J. Vet. Med. Sci. 2006, 68, 1225–1227. [Google Scholar] [CrossRef] [Green Version]
- Atienzar, A.I.C.; Gerber, P.F.; Opriessnig, T. Use of the rSpaA415 antigen indicates low rates of Erysipelothrix rhusiopathiae infection in farmed cattle from the United States of America and Great Britain. BMC Vet. Res. 2019, 15, 388. [Google Scholar] [CrossRef]
- Barton, H.; Taylor, N.; Kreate, M.; Springer, A.; Oehrle, S.; Bertog, J. The impact of host rock geochemistry on bacterial community structure in oligotrophic cave environments. Int. J. Speleol. 2007, 36, 93–104. [Google Scholar] [CrossRef] [Green Version]
- Cotta, M.; Whitehead, T.R.; Collins, M.D.; Lawson, P.A. Atopostipes suicloacale gen. nov., sp. nov., isolated from an underground swine manure storage pit. Anaerobe 2004, 10, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Wadud, S.; Michaelsen, A.; Gallagher, E.; Parcsi, G.; Zemb, O.; Stuetz, R.M.; Manefield, M. Bacterial and fungal community composition over time in chicken litter with high or low moisture content. Br. Poult. Sci. 2012, 53, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Hanchi, H.; Mottawea, W.; Sebei, K.; Hammami, R. The Genus Enterococcus: Between Probiotic Potential and Safety Concerns—An Update. Front. Microbiol. 2018, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Franz, C.M.A.P.; Van Belkum, M.J.; Holzapfel, W.H.; Abriouel, H.; Gálvez, A. Diversity of enterococcal bacteriocins and their grouping in a new classification scheme. FEMS Microbiol. Rev. 2007, 31, 293–310. [Google Scholar] [CrossRef] [PubMed]



| SiO2 | Al2O3 | FeO | CaO | MgO | P2O5 | SO3 | K2O | ||
|---|---|---|---|---|---|---|---|---|---|
| Sample 1 | Spc_003 | 3.45 | 1.01 | 1.21 | 33.93 | 5.03 | 25.47 | 1.53 | 0.84 |
| Sample 1 | Spc_004 | 5.22 | 0.77 | 6.60 | 36.99 | 2.72 | 29.57 | 0.76 | 0.69 |
| Sample 1 | Spc_005 | 3.44 | 0.55 | 1.97 | 41.13 | 2.00 | 33.62 | 0.75 | n.d. |
| Sample 1 | Spc_006 | 23.44 | 9.58 | 5.22 | 13.43 | 4.26 | 7.73 | 0.46 | 1.52 |
| Sample 1 | Spc_007 | 52.20 | 15.08 | n.d. | n.d. | n.d. | n.d. | n.d. | 11.45 |
| Sample 1 | Spc_008 | 52.64 | 15.28 | n.d. | n.d. | n.d. | n.d. | n.d. | 11.51 |
| Sample 1 | Spc_009 | 14.37 | 6.56 | 4.37 | 19.99 | 4.57 | 16.00 | 1.05 | 1.08 |
| Sample 1 | Spc_010 | 28.35 | 5.76 | 13.18 | 9.33 | 4.22 | 7.64 | 0.53 | 2.33 |
| Sample 1 | Spc_011 | 28.95 | 12.66 | 6.25 | 10.04 | 7.13 | 6.26 | 0.59 | 2.86 |
| Sample 1 | Spc_17 | 13.19 | 5.68 | 2.11 | 31.97 | 3.92 | 25.11 | 0.56 | 0.89 |
| Sample 1 | Spc_18 | 27.01 | 14.85 | 4.44 | 13.75 | n.d. | 14.64 | n.d. | 2.05 |
| Sample 2 | Spc_5 | 38.77 | 14.05 | 7.42 | 2.39 | 2.34 | 0.98 | 0.32 | 2.31 |
| Sample 2 | Spc_6 | 21.45 | 9.94 | 5.44 | 15.97 | 2.61 | 5.54 | 0.51 | 1.92 |
| Sample 2 | Spc_8 | 19.55 | 16.64 | 2.00 | 10.78 | n.d. | 2.29 | n.d. | 0.92 |
| Samples | Reads | Richness | Eveness | Shannon | Simpson |
|---|---|---|---|---|---|
| Sample 1 | 94137 | 141 | 0.60 | 4.26 | 0.02 |
| Sample 2 | 92380 | 34 | 0.33 | 1.70 | 0.27 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.-L.; Villa, F.; Mugnai, G.; Gallinaro, M.; Spinapolice, E.E.; Zerboni, A. Geomicrobial Investigations of Colored Outer Coatings from an Ethiopian Rock Art Gallery. Coatings 2020, 10, 536. https://doi.org/10.3390/coatings10060536
Wu Y-L, Villa F, Mugnai G, Gallinaro M, Spinapolice EE, Zerboni A. Geomicrobial Investigations of Colored Outer Coatings from an Ethiopian Rock Art Gallery. Coatings. 2020; 10(6):536. https://doi.org/10.3390/coatings10060536
Chicago/Turabian StyleWu, Ying-Li, Federica Villa, Gianmarco Mugnai, Marina Gallinaro, Enza Elena Spinapolice, and Andrea Zerboni. 2020. "Geomicrobial Investigations of Colored Outer Coatings from an Ethiopian Rock Art Gallery" Coatings 10, no. 6: 536. https://doi.org/10.3390/coatings10060536






